Abstract
OBJECTIVE
California law (Grant H. Kenyon Prostate Cancer Detection Act) requires physicians to inform all patients older than aged 50 years who receive a prostate examination about the availability of the prostate-specific antigen (PSA) test. Physicians are not given guidance on how this information should be presented. We sought to evaluate the effects upon PSA screening rates of informing patients about PSA testing by 2 different techniques.
DESIGN
Factorial comparison of discussion versus video formats for presenting information about the PSA test.
SETTING
Patients were recruited through the Health Appraisal screening program in the Department for Preventive Medicine, Kaiser Permanente, San Diego, Calif.
PARTICIPANTS
Male patients undergoing health appraisal screening participated in 1 of 4 groups providing information about PSA screening: usual care ( n =43), discussion about risks and benefits of PSA ( n =45), shared decision-making video ( n =46), or video plus discussion ( n =42). Participants were sequentially assigned to 1 of the 4 groups.
RESULTS
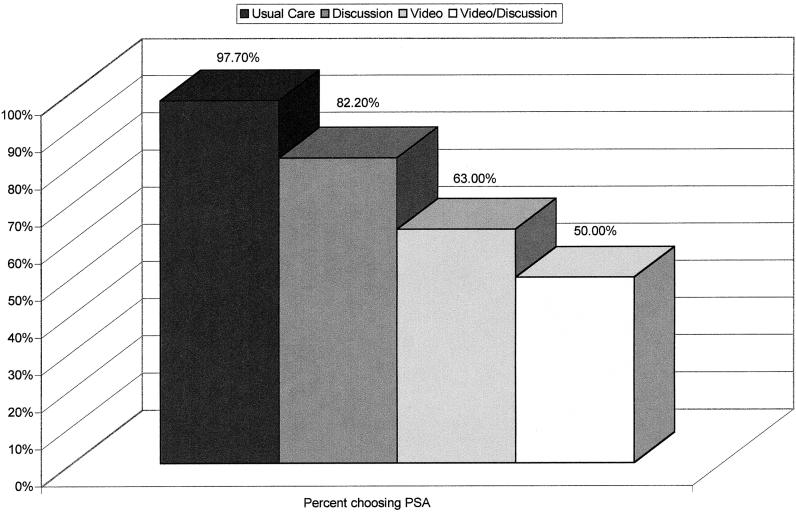
No significant differences in demographics or family history was demonstrated between the groups at the time of group assignment. Participants in the intervention groups rated the information as clear, balanced, and fair. There were significant differences in the number of men requesting a PSA test, with the highest rate in the usual care group (97.7%), followed by discussion (82.2%), video (60.0%), and video plus discussion (50.0%).
CONCLUSION
Providing information about PSA screening in the form of video or discussion is feasible and significantly alters PSA screening rates.
Keywords: PSA, shared decision making, doctor-patient relationship
On May 20, 1997 the California Legislature enacted the Grant H. Kenyon Prostate Cancer Detection Act. This law requires any physician in the state California examining a patient's prostate to offer information about the availability of diagnostic procedures, including but not limited to the prostate-specific antigen (PSA) test. The physician is required to provide such information if the patient is aged more than 50 years, manifests clinical symptomatology, or is at an increased risk of prostate cancer. The physician is also required to provide information if it is “medically necessary” in the opinion of the physician and surgeon. Under this law, failure to provide information about the availability of the PSA test is considered unprofessional conduct.
Although well intended, the California statute does not recognize the substantial controversy concerning the value of the PSA screening.1 While early detection of cancer is generally prudent, there is no conclusive evidence to show that using a PSA test in addition to a digital rectal examination leads to a reduction in prostate cancer mortality.2 Many older men with prostate cancer die of other causes; moreover, treatment for prostate cancer can significantly reduce quality of life by causing impotence, incontinence, rectal injury, and, in rare cases, death.3,4
Recognizing these uncertainties, the American Cancer Society and the American Urological Association recently revised their clinical guidelines concerning PSA testing.5,6 Both organizations abandoned the recommendation that all men aged more than 50 years be routinely screened, and now recommend that physicians attempt to give patients balanced information about the possible risks and benefits of PSA testing.
How should information about PSA testing be presented to patients? Shared decision making can be used to engage patients in decisions involving considerable uncertainty.7 In shared decision making, the patient and physician discuss available treatment options and make a mutually agreeable choice that reflects the health preferences of the patient.8 Shared decision-making videotapes have become available as mechanisms for informing patients about difficult choices. Because personal counseling is difficult to complete within the confines of an average office visit, shared decision-making videos offer physicians a useful alternative. Moreover, in addition to exposure to information, discussion may also help clarify personal decisions. The present study evaluates the impact of shared decision-making videos and discussion upon the use of PSA screening.
We postulated the following a priori hypotheses:
Individuals participating in any shared decision-making intervention would show lower rates of PSA screening than participants who did not.
Discussion will enhance the effect of the video, resulting in lower PSA screening rates for those in the combined condition.
Individuals participating in any shared decision-making intervention would show increased knowledge about prostate cancer and PSA screening.
Individuals participating in any shared decision-making intervention would show lower confidence in their screening decisions.
Individuals participating in any shared decision-making intervention would be less likely to endorse physician-based medical decision making.
METHODS
Design and Procedure
Patients were recruited through the Health Appraisal screening program in the Department of Preventive Medicine, Kaiser Permanente, San Diego, Calif. The Health Appraisal screening program offers annual physical evaluations for preventive care purposes to Kaiser Permanente members. In addition, individuals who are not members of Kaiser Permanente can receive a physical evaluation on a fee-for-service basis. The Health Appraisal Clinic is unique in the way that it structures the comprehensive biopsychosocial examination patients receive. Patients complete the examination in 2 visits; the second visit occurs 2 weeks after the first. During the first visit, patients complete numerous preliminary examinations including a hearing and vision test, vital signs, necessary x-rays, and a blood draw. During the second visit, patients receive an in-depth medical history and physical examination that integrates the laboratory and other findings from the first visit. Patients do not receive primary care in the Health Appraisal Clinic.
The study used a 2×2 factorial comparison of discussion and video formats for presenting men information about PSA testing. Therefore, each level of either intervention condition is represented at each level of the other intervention condition, resulting in 4 groups.
Usual care ( n =43): Consistent with the usual procedure at the Health Appraisal screening program, participants were told there was a blood test which could be used to detect prostate cancer and were asked whether or not they would like a PSA test as part of their comprehensive physical evaluation. They were told that the PSA test is a screening test for prostate cancer but were not given any other specific information.
Discussion ( n =45): Participants listened to a lecture about possible risks and benefits of PSA testing, as well as information about the nature of prostate cancer, and risks and benefits of treatment options for prostate cancer. The lecture closely followed the content of the videotape The PSA Decision: What You Need to Know(PSA video), developed by the Foundation for Informed Medical Decision Making.9 The lecture took between 25 and 30 minutes. Following the lecture, participants were invited to ask questions and discuss the lecture content.
Video ( n =46): Participants viewed the 25-minute PSA video. The videotape was previously evaluated and described by Flood et al.9
Video and Discussion ( n =42): Participants viewed the 25-minute PSA video. Following the videotape, participants were given an opportunity to ask questions and discuss the content of the videotape with a moderator. Group discussions following the video averaged 7 minutes in length.
Participants in the usual care group were approached by the first author. Similarly, the lectures and discussions in the discussion group and the discussions in the video and discussion group were led by the first author, following an extensive review of the literature and the PSA video.
Participants were sequentially accrued into the 4 groups over a period of 19 consecutive weeks. Recruitment began with the usual care group, followed by the discussion group, the video group, and the video and discussion group. Accrual into each group began once the previous group had completed recruitment. Recruitment lasted 2.5, 5.5, 6, and 5 weeks for groups 1 to 4, respectively. Target sample size for each study group was 40 to 45 participants. Targets were based on statistical power calculations assuming a medium to large effect size.10 Participation in the intervention groups took place in small groups. Comparing the 3 intervention groups, there were no differences in size of the groups. Groups averaged 2.11 participants (SD, 1.10; range, 1 to 5 participants).
For the usual care group, participants were recruited during the first part of their 2-part physical evaluation at the Health Appraisal Clinic. All men aged more than 50 years were asked to participate. One individual refused participation. For the remaining 3 groups, recruitment began 2 days prior to the actual appointments for the first part of the evaluation at the Health Appraisal Clinic. All men aged more than 50 years were telephoned and told briefly about the PSA test, and that, should they be interested, there was an opportunity to learn more about the PSA test prior to deciding whether or not to have the test. This required them to report to the clinic 1 hour prior to their actual appointment to participate in the discussion and/or view the video.
In the discussion group, 111 men were contacted; 40.5% refused participation, and 13.5% could not be reached. In the video group, 117 men were contacted; 40.2% refused participation, and 12.8% could not be reached. In the video and discussion group, 129 men were contacted; 42.6% refused participation, and 17.4% could not be reached. Of those men who indicated an interest, 86.3%, 83.6%, and 82.4%, respectively, came to participate in the discussion, video, and video and discussion groups. None of the differences in recruitment rates were statistically significant. All men were told that receiving a PSA test as part of their physical evaluation was not contingent upon participating in the intervention, and that they would receive a PSA test if they requested it.
Measures
All participants completed a brief questionnaire. Participants in the intervention groups completed the questionnaire following the intervention. The questionnaire asked participants to indicate: (1) demographic information, including age, marital status, education, and ethnicity; (2) whether or not they would like a PSA test; (3) confidence in the personal PSA decision; (4) number of previous PSA tests; (5) concern about prostate cancer; (6) personal, family, and friends' history of cancer; and (7) who should choose medical treatments for a patient. A questionnaire developed by Flood and colleagues that tests knowledge about prostate cancer and PSA screening9 was also completed by the participants.
Individuals requesting a PSA test were given a lab slip that was attached to their blood samples prior to shipping for analysis, indicating a need for analysis of PSA levels in the specimen. Confidence in the personal PSA decision was rated on a scale of 0 (not at all confident) to 10 (completely confident). There is considerable medical uncertainty about the value of PSA screening.1–4 This item was included to evaluate the hypothesis that providing information increases patient uncertainty. Concern about prostate cancer was rated on a scale of 0 (not at all concerned) to 4 (extremely concerned). Items regarding personal, family, and friends' history of cancer were added in order to rule out any potential influence of these variables upon PSA screening choices. Who should choose medical treatments for a patient was rated on a scale of 1 (doctor only) to 5 (you only), with 3 indicating “both.” This item was added in order to evaluate the hypothesis that individuals who are informed about the importance of personal preferences in medical decision making would be less likely to endorse physician-based medical decision making.
Participants in the intervention groups were also asked to rate the amount of information provided, the clarity of information, the length of the respective presentations, how balanced and fair the presentations were, how they generally felt about receiving information in this format, and whether or not their attitudes had changed as a result of participating in the respective intervention.
Participants
One hundred seventy-six men participated in the study. There were no differences in participant's age; education; marital status; ethnicity; personal, family, or friends' history of cancer; or the number of previous PSA tests. Descriptive data are shown in Table 1.
Table 1.
Participant Demographics
| Usual Care ( n = 43) | Discussion ( n = 45) | Video ( n = 46) | Video and Discussion ( n = 42) | |
|---|---|---|---|---|
| Age, Mean, y (SD) | 64.09 (10.39) | 63.11 (8.04) | 64.26 (8.99) | 63.62 (9.57) |
| Marital Status (% married) | 76.3 | 76.7 | 77.8 | 87.8 |
| Education, % | ||||
| H.S. or less | 14.0 | 28.8 | 19.5 | 23.8 |
| College | 67.4 | 46.7 | 63.1 | 50.0 |
| Graduate degree | 17.6 | 24.5 | 17.4 | 26.2 |
| Ethnicity, % | ||||
| African American | 9.3 | 11.1 | 2.2 | 0.0 |
| Hispanic | 2.3 | 13.3 | 8.7 | 4.8 |
| Asian | 7.0 | 4.4 | 4.3 | 9.5 |
| Native American | 4.7 | 2.2 | 2.2 | 0.0 |
| Caucasian | 76.7 | 68.9 | 82.6 | 85.7 |
| Number of previous PSA tests, Mean (SD) | ||||
| Overall: Mode = 0; 73.7% never had PSA test | 1.16 (2.66) | 1.11 (2.22) | 1.02 (2.35) | .69 (1.92) |
| Number of individuals with personal history of cancer, % | 9.3 | 9.1 | 15.6 | 9.5 |
| Number of individuals with family history of cancer, % | 44.2 | 68.2 | 55.6 | 47.5 |
| Number of individuals with friends with history of cancer, % | 66.7 | 69.8 | 75.0 | 59.5 |
Statistical Analysis
The effect of the interventions on the rate of PSA testing was examined using logistic regression. We report changes in χ2as well as odds ratios with 95% confidence intervals. Odds ratios below 1.0 indicate an increased likelihood of requesting a PSA test. Odds ratios above 1.0 indicate a decreased likelihood of requesting a PSA test. Prior to running logistic regression models, we compared patients choosing a PSA test with those not choosing a PSA test on a number of demographic variables using t tests and χ2. Any confounding variables were included as covariates in the logistic regression models. Categorical variables with more than 2 levels were analyzed with Pearson χ2tests. Continuous dependent variables were analyzed with univariate analysis of variance. Type I error was controlled in post hoc tests using Tukey's method for all possible pairwise comparisons.11 The presence of a significant interaction effect in logistic regression or analysis of variance analyses indicates that an independent variable has differential effects at different levels of another independent variable. In this case, an interaction might indicate that the effect of discussion depends on exposure to the video.
RESULTS
Participant Ratings of Interventions
There were no differences in how participants rated the amount of information provided in discussion, video, or video and discussion. Overall, 88.5% rated the amount of information “about right.” There was a difference in the clarity rating of the presentation. In the video group, 6.8% of participants rated the presentation as “poor” or “fair,” compared with 0% in the discussion and video and discussion groups. In the discussion and video and discussion groups, 75.0% and 66.6%, respectively, rated the clarity as “very good” or “excellent,” compared with 43.2% in the video group (χ28=16.32, P <.05). There were no differences in rating the length of the presentation. Overall, 95.4% of participants rated the length about right. Similarly, there were no differences in rating the presentation as balanced and fair. Overall, 81.5% of participants considered the presentation “completely balanced,” 7.7% felt it was slanted in favor of having of having a PSA test, and 10.8% felt it was slanted in favor of not having a PSA test. Differences in how participants felt in general about men participating in these types of interventions were nonsignificant. Overall, 70.2% felt “somewhat positive” or “very positive,” 29.0% felt neutral, and only 0.8% felt negative about participating in these types of interventions. There were no associations between how the presentations were rated and whether or not participants chose to request a PSA test.
Prostate-specific Antigen Decisions, Confidence, and Concern
Participants' willingness to have a PSA test is illustrated in Figure 1. Prior to running logistic regression analyses, we examined the degree to which the choice to have a PSA test was associated with age, ethnicity, education, marital status, personal, family or friends' history of cancer, and the number of previous PSA tests. There were no differences among any of these variables depending on the PSA choice, except for the number of previous PSA tests. Specifically, men who chose to have a PSA test reported an average of 1.27 (SD, 2.60) previous PSA tests, compared with 0.26 (SD, 0.68) previous PSA tests among those who chose not to have a PSA test ( t173=2.65, P <.01). Hence, the number of previous PSA tests was included in the logistic regression model as a covariate (Δχ12=10.12, P <.01; Exp(B)=.65; 95% CI, 0.45 to 0.96). There were main effects of discussion (Δχ12=3.97, P <.05; Exp(B)=2.35; 95% CI, 1.10 to 5.06) and video (Δχ12=25.87, P <.001; Exp(B)=7.24; 95% CI, 3.12 to 16.82) on the choice of whether or not to have a PSA test. There was no interaction effect. The final model was significant (χ32=39.97, P <.0001) and classified 75.4% of participants correctly. Individual group-by-group comparisons yielded a significant difference in the rate of PSA tests comparing the usual care with the discussion group (χ12=5.72, P <.05), and comparing the discussion with the video group (χ12=4.20, P <.05). The difference between the video group and the video and discussion group was not significant.
FIGURE 1.
Number of men choosing the prostate-specific antigen (PSA) test.
We found a significant interaction effect (F 1,170=9.30, P <.01) of intervention group on confidence about the PSA decision, indicating that any intervention significantly reduced the confidence men had in their decision. Men in the usual care group showed the highest confidence in their decision (mean, 9.41±1.70), compared with men in the intervention groups whom all showed similarly lower confidence in their decision (discussion group mean, 7.89±1.88; video group mean, 7.25±2.42; video and discussion group mean, 7.67±2.26). The differences in confidence between the intervention groups were non-significant. In contrast, using Tukey post hoc tests to control type I error, pairwise comparisons of the control group with each intervention group were all statistically significant ( P <.01). There was a weak but significant correlation between men's confidence in their decision and the number of previous PSA tests they reported ( R =.18, P <.05).
Participants in the usual care group expressed more concern about prostate cancer than men in the intervention groups. In the usual care group, 34.9% of men indicated that they were “considerably” or “extremely” concerned about prostate cancer. In the discussion, video, and video/discussion groups, the proportion of men expressing this degree of concern was 13.4%, 13.3%, and 16.7%, respectively (χ122=21.25, P <.05).
Knowledge About Prostate Cancer and Prostate-specific Antigen Testing
We found a significant interaction effect of the interventions on men's knowledge about prostate cancer and PSA testing (F 1,146=20.57, P <.0001). Specifically, participating in any intervention significantly increased knowledge compared to the usual care group (number of correct responses to 5 questions: usual care group mean, 1.62±1.10; discussion group mean, 3.90±1.24; video group mean, 3.38±1.36; video and discussion group mean, 3.82±1.25). Controlling for type I error with Tukey's method for all possible pairwise comparisons, we found significant differences when comparing the usual care group with each intervention (all significant at P <.001), but no differences comparing any of the intervention groups with each other. Responses to the individual questions by intervention group are shown in Table 2.
Table 2.
Responses to Questions about Prostate Cancer and PSA Testing by Intervention Group.
| Percent Choosing Most Accurate Response | ||||||
|---|---|---|---|---|---|---|
| Question | Usual Care | Discussion | Video | Video and Discussion | Statistic | |
| How many men with prostate cancer experience symptoms? | ||||||
| Choices: | Most or all do | |||||
| About half do | ||||||
| Most do not* | 54.1 | 79.5 | 68.4 | 84.6 | χ2(3) = 10.51, P < .05 | |
| At this time, how sure are you about whether treatment of prostate cancer can extend a man's life? | ||||||
| Choices: | Very sure it can | |||||
| Fairly it can | ||||||
| Not sure either way* | ||||||
| Fairly sure it cannot | ||||||
| Very sure it cannot | 18.6 | 84.1 | 60.9 | 73.8 | χ2(3) = 44.46, P < .001 | |
| What is the chance that treatment for prostate cancer will result in side effects? | ||||||
| Choices: | There is a big chance* | |||||
| There is a small chance | ||||||
| There are usually no problems | 42.1 | 53.3 | 55.6 | 61.9 | Not significant | |
| When men have elevated PSA test results, would you say…. | ||||||
| Choices: | All have prostate cancer | |||||
| Most have prostate Cancer | ||||||
| About half have prostate Cancer | ||||||
| Most do not have prostate Cancer* | ||||||
| No one has prostate Cancer | 10.8 | 72.7 | 72.7 | 80.5 | χ2(3) = 50.73, P <.001 | |
| Of all men who are diagnosed as having prostate cancer, would you say… | ||||||
| Choices: | Most die of prostate Cancer | |||||
| About half die of prostate Cancer | ||||||
| Most die of something else* | 42.5 | 88.6 | 81.8 | 82.5 | χ2(3)=28.71, p<.001 | |
Indicates most accurate response.
Attitudes About Who Should Make Medical Decisions
There was a significant association between intervention group and preferences for patient involvement in medical decision making ((χ122=58.67, P <.0001). In all 4 groups, the majority of patients wanted to share decisions with their physicians. In the usual care group, 46.5% wanted to share decisions, 54.5% wanted to share decisions in the discussion only group, 66.7% wanted to share decisions in the video only group, and 70.0% wanted to share decisions in the video and discussion group. However, while 48.8% of patients in the usual care group wanted physicians to be the primary or only decision maker, only 2.3%, 4.4%, and 7.5%, respectively, in the discussion, video, and video and discussion groups wanted physicians to be the primary or only decision maker.
Choice of Treatment
Participants were asked which type of treatment they would choose if they were diagnosed with prostate cancer. Participants in the informational intervention groups were more likely than participants in the usual care group to choose watchful waiting over surgery or radiation treatment (χ92=30.53, P <.001). Only 35.7% of participants in the usual care group chose watchful waiting compared with 81.8%, 72.7%, and 67.5% of participants in the discussion, video, and video and discussion groups, respectively.
DISCUSSION
Three previous studies have examined methods for providing patients information about PSA testing. Wolf et al. used a script read aloud to patients, which detailed the potential risks and benefits of PSA testing. Compared with a control group, men who heard this script requested significantly fewer PSA tests.12 Flood et al. provided patients with a 25-minute videotape about the risks and benefits of PSA testing, and found that men viewing the tape were less likely to request a PSA test and showed increased knowledge about prostate cancer and screening.9 Volk et al. evaluated a videotape to facilitate shared decision making in a randomized, controlled trial, and also found that participants viewing the tape were less likely to request a PSA test, and showed increased knowledge about relevant issues.13 These studies suggest that patients are less enthusiastic about the PSA test when given detailed information and that these methods increase patients' knowledge about relevant issues.
The present study directly compares 2 different methods for presenting patients information about a clinical decision. Several findings are noteworthy. First, similar to previous studies, men who were given detailed information about the PSA test were more likely to refuse the test, compared with men who were not provided such information. Men viewing the video requested fewer PSA tests than men who were given the same information in discussion format, even though knowledge about prostate cancer was similar in all intervention groups. More importantly, men who participated in an intervention were more knowledgeable about prostate cancer than men in usual care.
Despite lower clarity ratings in the video group, participants demonstrated similar knowledge to the other intervention groups. Hence, the interventions succeeded in educating men about the nuances and complexities of PSA testing. The interventions also reduced overall concern about prostate cancer, reflecting the fact that many men with prostate cancer do not experience morbidity or mortality as a result of the disease. Nonetheless, the confidence that men expressed in their decisions was lowered for all 3 intervention groups, when compared with the usual care group. Given the current uncertainty about the benefits of PSA testing, this lowered confidence may be more consistent with current literature than the confidence in the PSA test expressed by the usual care group.1–4 Many men who participated in this study shared the common belief that the PSA test was an easy way to address a potentially life-threatening problem, and most were attracted to the PSA test since they believed it to be a simple blood test. The interventions clearly resulted in changes for these perceptions. Men learned that while the initial test is simple, the implications of the results can be very complex, underscoring the importance of personal preferences when choosing between uncertain outcomes. We also found a significant association between participating in an intervention and judgments about who should make medical decisions. Although the majority of patients in all 4 groups expressed the belief that medical decisions should be shared between physician and patient, there was a clear shift away from leaving the physician with the authority to make clinical decisions to giving that authority to the patient. Barry et al. argue that many patients need to be educated about the uncertainty involved in the medical decision-making process.14 Because of these uncertainties, patient input in the decision-making process is important in order to reach individually desired health states. When patients are given information about a medical problem in a manner that invites their participation, most welcome the opportunity. This contradicts the findings in the shared decision-making literature that older patients are less interested in decision making.8 The mean age of men in this study was 63.8 years.
Wennberg et al. have observed large nationwide variations in the use of different surgical procedures.15 These differences are attributed to hospital capacities, as well as uncertainty about the value of some surgical procedures. Wennberg's group argues that shared decision making would reduce the number of surgical procedures. A recent study examining the use of a shared decision-making program for patients with ischemic heart disease found that patients who received detailed tailored information requested fewer revascularizations than patients assigned to the control group.16 Findings from the present study suggest that asymptomatic men faced with hypothetical prostate cancer are more likely to choose watchful waiting, when given balanced information. Thus, these results lend indirect support to Wennberg's hypothesis.
There are several important limitations to this study. First, it involved a single clinic. It is not clear to what extent these findings would replicate in other clinics. Additionally, individuals attending the Health Appraisal screening program may not be representative of the general population. Completing a physical evaluation for preventive screening purposes may indicate a greater general concern about maintaining good health.
An important weakness of the study is that it was not randomized. In order to prevent potential contamination of conditions, participants were sequentially accrued into the 4 study groups. From a logistical point of view, a randomized study would have presented substantial challenges within a busy practice setting. While recruitment of the usual care group differed substantially from the other 3 groups, we believe that this group provides an approximation of what can be expected from individuals who present for a preventive screening physical evaluation and are not given detailed information about potential risks and benefits of PSA screening. Although this differential recruitment procedure may have introduced bias, we nonetheless feel that the comparison between the usual care group and the intervention groups is a meaningful one. Accrual of participants was similar in all 3 intervention conditions. All consecutive patients were approached, and participation rates were approximately equal. Analysis of pretreatment information (see Table 1) demonstrated that the 4 groups appear equivalent. We view the sequential accrual of participants into conditions as a reasonable compromise in a busy practice setting where true randomization is difficult. However, replication of the study using true randomization may be warranted. Moreover, further research is needed to evaluate other potential methods of providing patients information that present fewer logistical difficulties. For example, patients may be able to access information on an Internet site prior to coming to a clinic appointment.
The ethnic composition of the sample was fairly homogenous; the majority of participants were white. Since African-American men are at higher risk of prostate cancer, it is important that further research examine the use of these types of interventions with more ethnically varied samples.5 Some readers may also question how willing patients are to spend an additional 25 minutes during a visit to their physician to view a video about PSA testing or listen to a lecture of similar length. Given our findings, we would argue that most men, although perhaps initially reluctant, ultimately would welcome the information provided. In the present study, fewer than 1% of participants felt negative about having participated in the intervention.
One concern about the presentations is that they may have been biased against PSA testing. However, the majority of participants felt that the presentations were balanced and fair. Similar numbers of participants felt that the presentations were as balanced in favor of having a PSA test or as in not having a PSA test. These findings are very similar to the ratings provided by participants in the studies by Flood et al., and Volk et al., who viewed the same video.9,13 Although eliminating bias may never be fully accomplished, the Foundation for Informed Medical Medical Decision Making engaged in extensive exercises to reduce bias. The general procedure in developing shared decision-making videos begins with a team developing a specification of the targeted medical problem and the available treatment options. Program content is determined by individuals from academic medicine through literature review and meta-analysis. Using a series of focus groups, the team defines the structure of the decision-making problem, develops a program content summary, conducts an external content review, analyzes and resolves issues raised, designs the program, and develops supporting documentation. Programs are updated as needed and a team is appointed to monitor developments that would necessitate revisions.17 The PSA shared decision-making program was developed in collaboration with Massachusetts General Hospital-Dartmouth Patient Outcomes Research Team. The research team included both internists and representatives from the American Urological Association. Leaders in both urology and internal medicine reviewed the video in attempts to address balance and fairness.9,17 Similar methods for developing decision aids for clinical medicine have been described by other researchers.18
There have been few systematic studies of shared decision making in clinical medicine.8 Future research is needed to compare alternative approaches for facilitating shared decision making for health care decisions where the value of the alternatives is uncertain. The present study addressed an issue that only pertains to men. It is unknown whether men and women respond differently to these types of interventions for health conditions affecting both sexes. Thirty-seven percent of men contacted for participation in the intervention groups ultimately participated. New innovative methods are needed to increase patient participation in decisions that involve uncertainty.
In summary, the present study provides compelling evidence that men, when given detailed and balanced information, are far less enthusiastic about the PSA test than some policy makers advocating its use. The study also demonstrates the feasibility of providing men information in different formats within a busy practice setting. Patients welcome the opportunity to participate in their own medical decision making, and feel positive about these types of interventions. Shared decision-making tools are an accepted and effective way of reducing the physician's burden to provide and explain large amounts of complex information. New applications of these types of tools are sorely needed.
Acknowledgments
Supported in part by grant TPRH-98-119-01 from the American Cancer Society. Gratitude is expressed to Kathy Peterman, Donna Lupinacci, Kathy Jakstis, and the staff at Health Appraisal Clinic, Kaiser Permanente, San Diego, without whom this study would not have been possible.
REFERENCES
- 1.Boyle P. Prostate-specific antigen (PSA) testing as screening for prostate cancer: the current controversy. Ann Oncol. 1998;9:1263–4. doi: 10.1023/a:1008435911379. [DOI] [PubMed] [Google Scholar]
- 2.Barry MJ. PSA screening for prostate cancer: the current controversy — a viewpoint. Ann Oncol. 1998;9:1279–82. doi: 10.1093/oxfordjournals.annonc.a010952. [DOI] [PubMed] [Google Scholar]
- 3.Johansson JE, Holmberg L, Johansson S, Bergström R, Adami HO. Fifteen-year survival in prostate cancer. A prospective, population-based study in Sweden. JAMA. 1997;277:467–71. [PubMed] [Google Scholar]
- 4.The American Urological Association Prostate Cancer Clinical Guidelines Panel. Report on the Management of Clinically Localized Prostate Cancer. Baltimore, Md: American Urological Association Inc; 1995. [PubMed] [Google Scholar]
- 5.American Cancer Society Prostate Cancer Screening Guidelines. Cancer Facts and Figures 1997. Atlanta, Ga: American Cancer Society; 1997. [Google Scholar]
- 6.Executive Committee Report. Early Detection of Prostate Cancer. Baltimore, Md: American Urologic Association; 1997. [Google Scholar]
- 7.Kaplan RM. Shared medical decision-making: a new paradigm for behavioral medicine. Ann Behav Med. 1999;21:3–11. doi: 10.1007/BF02895027. [DOI] [PubMed] [Google Scholar]
- 8.Frosch DL, Kaplan RM. Shared decision-making in clinical medicine: past research and future directions. Am J Prev Med. 1999;17:285–94. doi: 10.1016/s0749-3797(99)00097-5. [DOI] [PubMed] [Google Scholar]
- 9.Flood AB, Wennberg JE, Nease RF, Fowler FJ, Ding J, Hynes LM. The importance of patient preference in the decision to screen for prostate cancer. Prostate Patient Outcomes Research Team. J Gen Intern Med. 1996;11:342–9. doi: 10.1007/BF02600045. [DOI] [PubMed] [Google Scholar]
- 10.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 11.Maxwell SE. Designing Experiments and Analyzing Data: A Model Comparison Perspective. Belmont, Calif: Wadsworth Publishers; 1990. [Google Scholar]
- 12.Wolf AM, Nasser JF, Wolf AM, Schorling JB. The impact of informed consent on patient interest in prostate-specific antigen screening. Arch Intern Med. 1996;156:1333–6. [PubMed] [Google Scholar]
- 13.Volk RJ, Cass AR, Spann SJ. A randomized controlled trial of shared decision making for prostate cancer screening. Arch Fam Med. 1999;8:333–40. doi: 10.1001/archfami.8.4.333. [DOI] [PubMed] [Google Scholar]
- 14.Barry MJ, Fowler FJ, Mulley AG, Henderson JV, Wennberg JE. Patient reactions to a program designed to facilitate patient participation in treatment decisions for benign prostatic hyperplasia. Med Care. 1995;33:771–82. doi: 10.1097/00005650-199508000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Dartmouth Medical School, Center for the Evaluative Clinical Sciences. The Dartmouth Atlas of Health Care 1998. Dartmouth: American Hospital Association; 1998. [Google Scholar]
- 16.Morgan MW, Deber RB, Llewellyn-Thomas HA, et al. Randomized, controlled trial of an interactive videodisc decision aid for patients with ischemic heart disease. J Gen Intern Med. 2000;15:685–93. doi: 10.1046/j.1525-1497.2000.91139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kasper JF, Mulley AG, Wennberg JE. Developing shared decision-making programs to improve the quality of health care. QRB Qual Rev Bull. 1992;18:183–90. doi: 10.1016/s0097-5990(16)30531-0. [DOI] [PubMed] [Google Scholar]
- 18.Man-Son-Hing M, Laupacis A, O'Connor AM, Hart RG, Feldman G, Blackshear JL, Anderson DC. Development of a decision aid for patients with atrial fibrillation who are considering antithrombotic therapy. J Gen Intern Med. 2000;15:723–30. doi: 10.1046/j.1525-1497.2000.90909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]