Abstract
Adult T-cell leukemia (ATL) occurs in a small population of human T-cell leukemia virus type 1 (HTLV-1)-infected individuals. Although the critical risk factor for ATL development is not clear, it has been noted that ATL is incidentally associated with mother-to-child infection, elevated proviral loads, and weakness in HTLV-1-specific T-cell immune responses. In the present study, using a rat system, we investigated the relationships among the following conditions: primary HTLV-1 infection, a persistent HTLV-1 load, and host HTLV-1-specific immunity. We found that the persistent HTLV-1 load in orally infected rats was significantly greater than that in intraperitoneally infected rats. Even after inoculation with only 50 infected cells, a persistent viral load built up to considerable levels in some orally infected rats but not in intraperitoneally infected rats. In contrast, HTLV-1-specific cellular immune responses were markedly impaired in orally infected rats. As a result, a persistent viral load was inversely correlated with levels of virus-specific T-cell responses in these rats. Otherwise very weak HTLV-1-specific cellular immune responses in orally infected rats were markedly augmented after subcutaneous reimmunization with infected syngeneic rat cells. These findings suggest that HTLV-1-specific immune unresponsiveness associated with oral HTLV-1 infection may be a potential risk factor for development of ATL, allowing expansion of the infected cell reservoir in vivo, but could be overcome with immunological strategies.
Human T-cell leukemia virus type 1 (HTLV-1) is causally associated with adult T-cell leukemia (ATL), an aggressive T-cell malignancy with a poor prognosis (9, 38, 41). Although the majority of HTLV-1-infected individuals remain asymptomatic during their lifetimes, a few percent of HTLV-1 carriers develop ATL after a long latency period (25, 45). HTLV-1-associated myelopathy or tropical spastic paraparesis (HAM/TSP), a chronic progressive neuromyelopathy, and other HTLV-1-related diseases are also associated with HTLV-1 infection (4, 19, 35). Genetic differences among HTLV-1 strains are not associated with the clinical outcomes of HTLV-1 infection (2, 23, 24).
In cohort studies of HTLV-1 carriers, it appeared that risk factors for ATL may include vertical HTLV-1 infection, gender (male > female), an increase in the number of abnormal lymphocytes that is associated with an increase in the HTLV-1 proviral load, and a low anti-Tax antibody level in serum (10-12). Genetic analysis indicated that ATL and HAM/TSP patients in an area of endemicity show significant segregation of HLA haplotypes (42). These observations indicate that the pathogenesis of HTLV-1 is more likely to be influenced by host factors.
Immunological studies have found a clear difference in HTLV-1-specific T-cell immune responses among HTLV-1-related diseases. HTLV-1-specific cytotoxic T lymphocytes (CTLs) are highly activated in HAM/TSP patients and can also be induced in asymptomatic carriers but only rarely in ATL patients (13, 15, 16, 18, 36). The HTLV-1 core, envelope, polymerase, Tax, Tof, and Rof proteins are known to be recognized by HTLV-1-specific CTLs (3, 14, 36, 37). Of these antigens, HTLV-1 Tax, a viral protein critical for T-cell immortalization, is a most popular target for HTLV-1-specific CTLs found in HTLV-1-infected individuals (13, 14). HTLV-1 Tax-specific CTLs are capable of lysing short-term-cultured ATL cells ex vivo (15, 16). In a recently established rat ATL model, HTLV-1-infected T-cell lymphomas expanded in vivo in the absence of T-cell immunity but regressed following administration of HTLV-1 Tax-specific CTLs (6, 7, 17, 32, 33). These findings strongly indicate that HTLV-1-specific CTLs contribute to anti-tumor surveillance in HTLV-1-infected individuals and suggest that insufficiency of T-cell immune responses to HTLV-1 may be a risk factor for development of ATL.
The exact reasons for the wide variety of levels of immune responses to HTLV-1 among HTLV-1-infected individuals are unclear. Segregation of HLA haplotypes in ATL patients suggests that weak immune responses may be associated with genetic factors (42). Another possibility is that weak immune responses in ATL patients are associated with vertical HTLV-1 infection. This possibility is suggested by the fact that ATL occurs mainly in vertically infected individuals but not in those who are infected later in life (39). Our previous finding that oral administration of HTLV-1 to rats induced much weaker HTLV-1-specific immune responses than intraperitoneal or intravenous infection (17, 20) also suggests that the conditions of primary infection may affect the host immune responses to HTLV-1.
In humans, the routes of HTLV-1 transmission are vertical transmission from mother to child, horizontal transmission from husband to wife, and parenteral transmission via blood transfusion or intravenous injection with contaminated needles (8, 21, 34, 40). Of these, mother-to-child transmission, especially through breast milk, is a major natural route in Japan (8). A number of infantile HTLV-1 carriers stay seronegative for HTLV-1 for a certain period of time (31), suggesting that immunological tolerance to HTLV-1 infection may be established in this period. Most vertically infected children seem to recover from their lack of humoral immunity to HTLV-1 by the age of 3 years (27), although the cellular immune responses of these children have not been carefully studied. It is not known to what extent immune unresponsiveness to HTLV-1 early in infection influences establishment of the HTLV-1-infected cell reservoir in vivo. Since the host immune response is a critical determinant of the control of persistently infecting viruses in vivo, a weaker immune response may result in a greater viral load. We suspect that vertical HTLV-1 infection, a weak T-cell immune response to HTLV-1, and a large HTLV-1 proviral load are closely related to each other.
In this study, using a rat model system, we investigated whether a persistent HTLV-1 proviral load is influenced by differences in host HTLV-1-specific immune responsiveness resulting from differences in conditions at primary HTLV-1 infection. We confirmed that HTLV-1-specific cellular and humoral immune responses of orally infected rats were markedly impaired compared to those of intraperitoneally infected rats. In contrast, we found that the HTLV-1 proviral load of orally infected rats was significantly greater than that of intraperitoneally infected rats. Moreover, subcutaneous reimmunization of orally infected rats with syngeneic HTLV-1-infected cells induced HTLV-1-specific immune responses. Our results strongly suggest that oral HTLV-1 infection induced unresponsiveness of HTLV-1-specific host immunity, which usually restricts the propagation of HTLV-1-infected cells, resulting in an increased proviral load, and that reimmunization with appropriate HTLV-1 antigens could restore HTLV-1-specific immune responses in infected individuals.
MATERIALS AND METHODS
Animals.
Three-week-old female F344/N Jcl-rnu/+ (F344 n/+) and WKAH/HKm Slc (WKA) rats were purchased from Clea Japan, Inc. (Tokyo, Japan), and the Shizuoka Animal Laboratory Center (Shizuoka, Japan), respectively. These rats were maintained at the experimental animal facilities of the Tokyo Medical and Dental University and treated in accordance with the regulations and guidelines of the Animal Care Committee of the university.
Cell lines.
An HTLV-1-producing human T-cell line, MT-2 (29), and an HTLV-1-infected rat T-cell line, FPM1 (26), derived from an F344 n/+ rat were cultured in RPMI 1640 medium (GIBCO Laboratories, Grand Island, N.Y.) with 10% heat-inactivated fetal calf serum (FCS; BioWhittaker, Walkersville, Md.), 100 IU of penicillin per ml, 100 μg of streptomycin per ml, and 2 mg of sodium bicarbonate per ml. G14 (33) is an interleukin-2-dependent, HTLV-1-negative CD8+ T-cell line established from an F344 n/+ rat in our laboratory. G14-Tax (33) is a stable transfectant of G14 with HTLV-1 Tax-expressing plasmids. G14 and G14-Tax were maintained in a medium containing 2-mercaptoethanol (10−5 M) and recombinant human interleukin-2 (Shionogi Pharmaceutical Co., Osaka, Japan) (10 U/ml).
Inoculation of rats with HTLV-1.
Various numbers of MT-2 cells were treated with 50 μg of mitomycin C (MMC) per ml at 37°C for 30 min, washed, and administered to 4-week-old female rats either orally or intraperitoneally. For oral infection, MMC-treated MT-2 cells in 0.5 ml of phosphate-buffered saline were directly administered into the esophagus through a feeder tube. For intraperitoneal infection, similarly treated MT-2 cells were percutaneously injected into the abdominal cavity. Peripheral blood samples were collected from all groups every 2 or 4 weeks after inoculation, and the presence of HTLV-1 provirus in peripheral blood cells and levels of antibodies to HTLV-1 in serum were determined. HTLV-1 provirus in the spleen was quantified at necropsy. We confirmed that all MMC-treated MT-2 cells died in 5 days after MMC treatment in vitro.
Detection of HTLV-1 provirus in infected rats.
To detect HTLV-1 provirus in peripheral blood cells and spleen, genomic DNA samples prepared from these tissues by sodium dodecyl sulfate-proteinase K digestion, followed by phenol-chloroform extraction, were subjected to nested PCR as described previously (1). The outer set of primers for detection of HTLV-1 provirus was pX1 (5′-CCC ACT TCC CAG GGT TTG GAC AGA GTC TTC-3′) and pX4 (5′-GGG GAA GGA GGG GAG TCG AGG GAT AAG GAA-3′), and the inner set of primers was pX2 (5′-CGG ATA CCC AGT CTA CGT GTT TGG AGA CTG T-3′) and pX3 (5′-GAG CCG ATA ACG CGT CCA TCG ATG GGG TCC-3′). In the first PCR, a 0.4 μM concentration of a primer set for rat glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (5′-ACC ACA GTC CAT GCC ATC AC-3′ and 5′-TCC ACC ACC CTG TTG CTG TA-3′) was included in the same reaction mixture as an internal control. The PCR was started from the activation step of Taq DNA polymerase (TOYOBO Inc., Nagoya, Japan) (95°C, 2 min); 35 cycles of denaturation (95°C, 1 min), annealing (60°C, 1 min), and extension (72°C, 1 min) were performed; and the PCR was finalized by elongation of the product (72°C, 5 min). For a nested PCR, an aliquot of the first PCR product was subjected to another 35 PCR cycles with the inner set of primers. The final PCR products were visualized by ethidium bromide staining following 2% agarose gel electrophoresis.
Quantification of HTLV-1 proviral load by LightCycler-based real-time PCR.
The HTLV-1 proviral loads in the spleen cells of HTLV-1-infected rats were quantified by real-time PCR on a LightCycler PCR Instrument (Roche Diagnostics, Mannheim, Germany). For each test sample, 500 ng of genomic DNA extracted from the spleen was used as the template. The PCR was performed with the QuantiTect SYBR Green PCR kit (QIAGEN K.K, Tokyo, Japan) in accordance with the manufacturer's instructions. Briefly, 20 μl of a PCR mixture in a capillary tube containing each HTLV-1 pX-specific inner primer pair at 0.5 μM, 1× QuantiTect SYBR Green PCR Master Mix, and 500 ng of genomic DNA was subjected to 50 cycles of denaturation (95°C, 15 s), annealing (55°C, 10 s), extension (72°C, 10 s), and denaturation of primer-dimers (82°C, 10 s) following an initial Taq polymerase activation step (95°C, 15 min). The copy numbers of HTLV-1 provirus in the samples were estimated from the standard regression curve with the LightCycler Software version 3 (Roche Diagnostics). The standard curve for HTLV-1 provirus was obtained by PCR data for 5 × 100 to 5 × 103 copies of pCR-pX1-4 plasmids serially diluted with genomic DNA from the spleen of a naive rat. The pCR-pX1-4 plasmids were constructed by inserting a PCR fragment amplified with pX1 and pX4 from the genomic DNA of MT-2 cells into a pCR2.1 vector (Invitrogen, Groningen, The Netherlands) by TA cloning. To correct for differences among the samples, relative HTLV-1 provirus copy numbers were calculated as raw values divided by the amount of GAPDH in the same sample. The number of HTLV-1 provirus DNA copies in MT-2 cells measured by this method was 12.6 ± 3.0/cell.
Detection of antibodies to HTLV-1 antigens.
The titers of antibodies against HTLV-1 antigens in the sera of infected rats were determined by the particle agglutination method using Serodia HTLV-1 (Fuji Rebio Inc., Tokyo, Japan).
Secondary transmission of HTLV-1 in rats.
At 20 weeks after oral or intraperitoneal inoculation of MMC-treated MT-2 cells into F344 n/+ rats, splenic T cells isolated from the infected rats were treated with or without MMC and intraperitoneally injected into 4-week-old syngeneic F344 n/+ rats or allogeneic WKA rats, respectively (2 × 107 cells/rat). Spleen cells were collected from these rats at 8 to 10 weeks after inoculation, and genomic DNA samples were extracted from the spleen cells and used as the template for a nested PCR to detect HTLV-1 provirus. The PCR was carried out as described above.
T-cell proliferation assay.
Splenic T cells from naive and HTLV-1-infected rats were enriched with a nylon-wool column and used as responder cells. Syngeneic G14 and G14-Tax cells were treated with 1% formalin in phosphate-buffered saline for 30 min, washed, and used as stimulator cells. Responder cells (105/well) and stimulator cells (5 × 104/well) were cultured in medium containing 10% FCS in a 96 well round-bottom culture plate at 37°C for 96 h. These cultures were pulsed with [3H]thymidine at a concentration of 37 kBq/well for the last 12 h to examine T-cell proliferation. Cells were harvested with a Micro 96 Harvester (Skatron, Lier, Norway), and [3H]thymidine incorporation was measured in a microplate beta counter (Micro Beta Plus; Wallac, Turku, Finland). To normalize for differences among experiments, a proliferation index was calculated as counts per minute of the sample well divided by the counts per minute of the control well of naive splenic T cells without stimulator cells in the same experiments. An HTLV-1-specific proliferation index was calculated as (counts per minute of the sample with G14-Tax − counts per minute of the sample with G14)/counts per minute of the control well.
IFN-γ production assay.
Splenic T cells (105/well) from naive and HTLV-infected rats were cultured without or with formalin-fixed G14, G14-Tax, or FPM1 cells (5 × 104/well) in a microtiter plate with medium containing 10% FCS for 6 days. The concentrations of gamma interferon (IFN-γ) in the supernatants were measured by enzyme-linked immunosorbent assay (ELISA) using Cytoscreen Rat IFN-γ ELISA kits (BioSource International, Inc.) in accordance with the manufacturer's instructions.
Statistics analysis.
The Mann-Whitney test was used to determine differences between the HTLV-1 proviral loads of orally and intraperitoneally infected rats. The Spearman correlation test was used to examine the relationship between HTLV-1 proviral loads and virus-specific T-cell proliferative responses.
RESULTS
Establishment of a persistent infection in rats with various numbers of infected cells.
We initially tried to determine the minimal number of MT-2 cells needed to establish an HTLV-1 infection in rats. Various numbers, ranging from 1 × 101 to 5 × 107, of MMC-treated MT-2 cells were inoculated intraperitoneally or orally into 4-week-old immunocompetent F344 n/+ or WKA rats. The results are summarized in Table 1. All F344 n/+ rats inoculated with 5 × 101 to 5 × 107 MMC-treated MT-2 cells were persistently infected with HTLV-1 regardless of the route of inoculation (experiments 1 to 4). Establishment of HTLV-1 infection by a small number of MT-2 cells was a phenomenon not restricted to strain F344 n/+ but also observed in WKA rats (experiments 5 and 6). Although some WKA rats inoculated with 1 × 102 to 1 × 104 MMC-treated MT-2 cells (4 of 24 rats) failed to carry detectable levels of HTLV-1 proviruses, most inoculated with 1 × 101 to 1 × 106 MMC-treated MT-2 cells (20 of 24 rats) were persistently infected. HTLV-1-specific antibody responses were detectable only in rats intraperitoneally inoculated with relatively large numbers of MT-2 cells. These results indicate that an HTLV-1 infection can be established in rats with a very small number of HTLV-1-infected cells and that the number of MT-2 cells required for establishment of a persistent HTLV-1 infection is much lower than that required for systemic HTLV-1-specific antibody responses.
TABLE 1.
Establishment of persistent HTLV-1 infection in rats inoculated with HTLV-1-infected cells
| Expt no. | Rat straina | No. of MT-2 cells inoculated | Type of MT-2 cells | Route of inoculationb | Detection of HTLV-I provirusc | HTLV-1-specific antibody responsed | Time (wk)e after inoculation |
|---|---|---|---|---|---|---|---|
| 1 | F344 n/+ | 5 × 107 | MMC treated | i.p. | 2/2 | 2/2 | 9, 26 |
| 5 × 101 | 2/2 | 0/2 | 9, 26 | ||||
| 0 | 0/2 | 0/2 | 9, 26 | ||||
| 2 | F344 n/+ | 2 × 107 | Live | i.p. | 6/6 | 6/6 | 12, 29, 31 |
| 5 × 107 | Live | p.o. | 6/6 | 0/6 | 10, 12, 15, 31 | ||
| 3 | F344 n/+ | 5 × 107 | Live | p.o. | 3/3 | 1/3 | 20, 21, 52 |
| 4 | F344 n/+ | 5 × 107 | MMC treated | i.p. | 3/3f | 2/3f | 14, 20 |
| 5 × 101 | 4/4 | 0/4 | 14, 18, 20 | ||||
| 5 × 107 | MMC treated | p.o. | 4/4 | 0/4 | 14, 18, 20 | ||
| 5 × 101 | 4/4 | 0/4 | 14, 18, 20 | ||||
| 0 | 0/4 | 0/4 | 14, 18, 20 | ||||
| 5 | WKA | 5 × 107 | MMC treated | i.p. | 2/2 | 1/2 | 17, 30 |
| 5 × 101 | 2/2 | 0/2 | 17, 30 | ||||
| 0 | 0/2 | 0/2 | 17, 30 | ||||
| 6 | WKA | 1 × 106 | MMC treated | i.p. | 4/4 | 1/4 | 4, 8, 18, 48 |
| 1 × 105 | 4/4 | 4/4 | 4, 8, 18, 48 | ||||
| 1 × 104 | 3/4 | 1/4 | 4, 8, 18, 48 | ||||
| 1 × 103 | 3/4 | 0/4 | 4, 8, 18, 48 | ||||
| 1 × 102 | 2/4 | 0/4 | 4, 8, 18, 48 | ||||
| 1 × 101 | 0/4 | 0/4 | 4, 8, 18, 48 | ||||
| 0 | 0/4 | 0/4 | 4, 8, 18, 48 |
Four-week-old female rats of the F344/N Jcl-rnu/+ (F344 n/+) and WKAH/HKm Slc (WKA) strains were used.
i.p., intraperitoneal inoculation; p.o., oral inoculation.
Number of rats positive for HTLV-1 provirus by PCR at the time of necropsy/number of rats tested.
Number of rats with a positive anti-HTLV-1 antibody response by the particle agglatination method at the time of necropsy/number of rats tested.
Number of weeks between HTLV-1 infection and necropsy.
One of the four rats initially set up was accidentally lost during the experiments.
To confirm the presence of transmissible virus in rats inoculated with 5 × 101 MMC-treated MT-2 cells, we further transferred splenic T cells from these rats to other rats 20 weeks after the initial MT-2 inoculation. As shown in Table 2, HTLV-1 provirus became constantly detectable in the secondary syngeneic rats to which MMC-treated splenic T cells of the initial rats had been transferred within 8 to 10 weeks after the transfer. Even when the splenic T cells were given to allogeneic strain WKA rats, HTLV-1 provirus become detectable, indicating that the presence of HTLV-1 provirus in these rats was due to virus transmission and not to survival of the transferred cells.
TABLE 2.
Presence of transmissible HTLV-1 in rats infected with MMC-treated MT-2 cellsa
| Expt. no. | Initially infected rat
|
Splenic T cells used for HTLV-1 transmission
|
Route of inoculation | Rat with secondary transmission
|
||||
|---|---|---|---|---|---|---|---|---|
| Strain | No. of MMC-treated MT-2 cells | Route of inoculationb | Type of cells | No. of cells | Strain | Establishment of HTLV-1 infectionc | ||
| 1 | F344 n/+ | 5 × 107 | i.p. | Live | 2 × 107 | i.p. | WKA | Yes |
| 5 × 101 | i.p. | Live | 2 × 107 | i.p. | WKA | Yes | ||
| 5 × 107 | p.o. | Live | 2 × 107 | i.p. | WKA | Yes | ||
| 5 × 101 | p.o. | Live | 2 × 107 | i.p. | WKA | Yes | ||
| 2 | F344 n/+ | 5 × 107 | i.p. | MMC treated | 2 × 107 | i.p. | F344 n/+ | Yes |
| 5 × 101 | i.p. | MMC treated | 2 × 107 | i.p. | F344 n/+ | Yes | ||
| 5 × 107 | p.o. | MMC treated | 2 × 107 | i.p. | F344 n/+ | Yes | ||
| 5 × 101 | p.o. | MMC treated | 2 × 107 | i.p. | F344 n/+ | Yes | ||
Splenic T cells of F344 n/+ rats initially inoculated with various numbers of MMC-treated MT-2 cells were isolated at 20 weeks after inoculation and transferred into the second rats indicated.
i.p., intraperitoneal inoculation; p.o., oral inoculation.
Establishment of HTLV-1 infection was evaluated by a nested PCR specific for the HTLV-1 pX region at 8 to 10 weeks after transfer of splenic T cells of the initially infected rats.
Proviral load in spleen cells of rats persistently infected with HTLV-1.
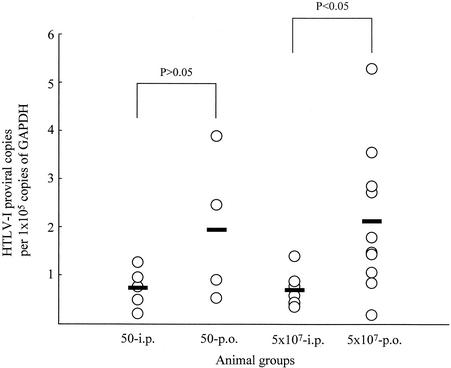
Next, we assessed effects of the initial viral amount and the route of HTLV-1 infection on the proviral load during the persistent phase of infection. Quantification of the proviral load in rats orally or intraperitoneally inoculated with 5 × 101 or 5 × 107 MMC-treated MT-2 cells was carried out by LightCycler-based real-time PCR using HTLV-1 pX-specific primers. Since HTLV-1 proviruses were more frequently detectable in the spleen than in the other organs tested (data not shown), we used spleen cells for quantification of HTLV-1 provirus. The results are shown in Fig. 1. Among rats inoculated with 5 × 107 MMC-treated MT-2 cells, although individual differences existed, the average HTLV-1 proviral load in orally infected rats was greater than that in intraperitoneally infected rats (P < 0.05). The number of MT-2 cells given at primary infection did not markedly affect the proviral load within either the orally or the intraperitoneally infected group. This observation suggests that the route of infection more strongly influences the persistent HTLV-1 load in the host than does the amount of virus delivered.
FIG. 1.
HTLV-1 proviral loads of rats intraperitoneally or orally inoculated with HTLV-1. HTLV-1 proviral loads in spleen cells of rats intraperitoneally (i.p.) or orally (p.o.) inoculated with 5 × 101 or 5 × 107 MMC-treated MT-2 cells were quantified by LightCycler-based real-time PCR. The animals analyzed here were the same as those used in experiments 1, 2, and 4 of Table 1. The relative copy number of HTLV-1 proviruses per 105 copies of GAPDH in each rat is indicated. The thick bars show the average proviral loads. The statistical significance of differences was determined with the Mann-Whitney test.
HTLV-1-specific antibody responses in HTLV-1-infected rats.
We then examined the effects of the amount of HTLV-1 at primary infection and the route of infection on immune responses to HTLV-1. Figure 2 shows antibody responses to HTLV-1 in the sera of rats (included in experiments 1 and 4 of Table 1) orally or intraperitoneally inoculated with 5 × 101 or 5 × 107 MMC-treated MT-2 cells. In four of the five rats given 5 × 107 MMC-treated MT-2 cells intraperitoneally, anti-HTLV-1 antibodies became detectable at 2 weeks after inoculation, gradually increased in number until 12 weeks after inoculation, and then maintained steady levels. However, the remaining rat in that group exhibited no detectable antibody response during the observation period. None of the rats orally given 5 × 107 MMC-treated MT-2 cells exhibited an HTLV-1-specific antibody response. The rats given 5 × 101 MMC-treated MT-2 cells exhibited no antibody responses, regardless of the route of infection. These results indicate that a small amount of HTLV-1 infection, as well as oral infection, induced persistent HTLV-1 infection with no HTLV-1-specific antibody responses.
FIG. 2.
HTLV-1-specific antibody responses of rats inoculated with MMC-treated MT-2 cells. F344 n/+ rats at 4 weeks of age were intraperitoneally (i.p.) (a, c) or orally (p.o.) (b, d) inoculated with 5 × 101 (c, d) or 5 × 107 (a, b) MMC-treated MT-2 cells. Anti-HTLV-1 antibody titers in sera were monitored by the particle agglutination method. Each symbol represents an individual rat. An antibody titer of <16 indicates an undetectable response.
HTLV-1-specific T-cell responses in HTLV-1-infected rats.
Next, we assessed the effects of the initial dose and the route of HTLV-1 infection on cellular immune responses to HTLV-1. To detect HTLV-1-specific T-cell proliferative responses, splenic T cells from HTLV-1-infected rats included in experiment 4 of Table 1 were incubated with formalin-treated G14-Tax, a syngeneic rat T-cell line expressing HTLV-1 Tax, and [3H]thymidine incorporation was measured. As shown in Fig. 3, T cells from all three rats intraperitoneally inoculated with 5 × 107 MMC-treated MT-2 cells exhibited generally high levels of proliferation and the proliferative response against G14-Tax cells was significantly stronger than that against the parent G14 cells, which were negative for HTLV-1 antigens. In contrast, rats orally infected with 5 × 107 MMC-treated MT-2 cells exhibited no detectable HTLV-1 Tax-specific proliferative response, similar to uninfected rats. These observations are consistent with those noted in our previous report (20). In rats inoculated with 5 × 101 MMC-treated MT-2 cells, HTLV-1 Tax-specific proliferative responses varied among individuals regardless of the route of infection. Some exhibited almost no response, while others exhibited moderate HTLV-1 Tax-specific responses (Fig. 3).
FIG. 3.
HTLV-1 Tax-specific T-cell proliferative responses of MMC-treated MT-2-inoculated rats. T-cell-enriched spleen cells from rats intraperitoneally (i.p.) (a, c) or orally (p.o.) (b, d) inoculated with 5 × 107 (a, b) or 5 × 101 (c, d) MMC-treated MT-2 cells were incubated without (open bars) or with formalin-treated G14 (hatched bars) or G14-Tax (closed bars) cells, and [3H]thymidine incorporation was measured. To normalize for differences among four experiments, values are given as proliferation indexes (determined as described in Materials and Methods). The means of triplicate cultures ± the standard deviations are indicated. *, not determined because of accidental death.
We also examined the IFN-γ-producing ability of splenic T cells from HTLV-1-infected rats in response to formalin-treated G14, G14-Tax, and FPM1, a syngeneic rat HTLV-1-infected cell line. As shown in Fig. 4, the IFN-γ-producing ability of T cells from rats intraperitoneally inoculated with 5 × 107 MMC-treated MT-2 cells was generally high, but they produced marked levels of IFN-γ against G14-Tax and FPM1 cells. T cells of rats inoculated with 50 MMC-treated MT-2 cells also produced a low level of IFN-γ upon FPM1 stimulation. However, the responses of T cells from rats orally inoculated with 5 × 107 or 50 MMC-treated MT-2 cells were indistinguishable from those in naive rats. Thus, the HTLV-1-specific cellular immune response in orally infected rats resulted in insufficient IFN-γ production, as well as in the T-cell proliferative response.
FIG. 4.
HTLV-1-specific IFN-γ production by T cells in MMC-treated MT-2-inoculated rats. T-cell-enriched spleen cells isolated from rats intraperitoneally (i.p.) or orally (p.o.) inoculated with 5 × 107 or 5 × 101 MMC-treated MT-2 cells were incubated without (open bars) or with formalin-treated G14 (hatched bars), G14-Tax (closed bars), or FPM1 (dotted bars) cells for 6 days, and IFN-γ produced in the supernatants was measured by ELISA. The means of duplicate cultures ± the standard deviations are indicated. The results shown are representative of two independent experiments.
Relationship of HTLV-1 proviral load with HTLV-1-specific T-cell proliferative responses.
We then examined the relationship between the HTLV-1 proviral load and T-cell proliferative responses to HTLV-1 in persistent infection. HTLV-1 proviral loads normalized to the GAPDH and HTLV-1-specific T-cell proliferation index of each rat orally or intraperitoneally inoculated with 5 × 107 or 5 × 101 MMC-treated MT-2 cells are plotted in Fig. 5. The rat carrying the lightest viral load exhibited the strongest T-cell response and had been intraperitoneally inoculated with 5 × 107 cells. Surprisingly, the rat with the greatest viral load had been orally inoculated with 5 × 101 cells and exhibited a very weak T-cell response. There was a negative correlation between the HTLV-1 proviral load and HTLV-1-specific T-cell proliferation, especially in rats inoculated with 5 × 107 MMC-treated MT-2 cells (r = −0.893; P < 0.05), but also for all of the groups of rats tested (r = −0.575; P < 0.05). These results indicate that the HTLV-1-specific T-cell immune response plays an important role in limiting the expansion of HTLV-1 or HTLV-1-infected cells in vivo.
FIG. 5.
Inverse correlation between HTLV-1 proviral loads and HTLV-1 Tax-specific T-cell proliferative responses. The relationship between the HTLV-1 proviral load and HTLV-1 Tax-specific T-cell proliferative responses of rats inoculated intraperitoneally (circles) or orally (triangles) with 5 × 101 (open symbols) or 5 × 107 (closed symbols) MMC-treated MT-2 cells was examined. The relative copy number of HTLV-1 provirus in spleen cells and the HTLV-1 Tax-specific T-cell proliferation index of each rat are plotted on the vertical and horizontal axes, respectively. The HTLV-1 Tax-specific proliferation index was calculated as described in Materials and Methods. Statistical significance was determined with the Spearman correlation test.
Recovery of HTLV-1-specific T-cell proliferative responses in orally HTLV-1-infected rats by reimmunization.
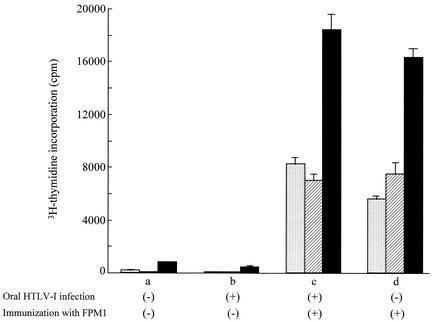
Rats orally infected with 5 × 107 MMC-treated MT-2 cells exhibited immune unresponsiveness to HTLV-1. It is well established that immune tolerance to orally administered protein antigen cannot be abrogated by a challenge with the same antigen (28, 43). Accordingly, we examined whether HTLV-1-specific T-cell responses could be induced in orally HTLV-1-infected rats by reimmunization. A syngeneic rat HTLV-1-infected cell line, FPM1, was used as the immunogen. MMC-treated FPM1 cells were subcutaneously inoculated into F344 n/+ rats that had been orally infected with 5 × 107 MMC-treated MT-2 cells 8 weeks before. Four to 5 weeks after reimmunization, HTLV-1-specific T-cell proliferation was evaluated in these rats. The results are shown in Fig. 6. T cells enriched from spleen cells of the orally HTLV-1-infected rats without reimmunization exhibited no significant HTLV-1-specific proliferation, nor did naive control rats. In contrast, following reimmunization, all of the rats exhibited strong HTLV-1-specific T-cell proliferative responses that were comparable to those of control rats that had been simply immunized with MMC-treated FPM1 cells. Thus, the immune unresponsiveness of orally HTLV-1-infected rats could be abrogated by reimmunization with syngeneic HTLV-1-infected cells.
FIG. 6.
Recovery of HTLV-1 Tax-specific T-cell proliferative responses in orally infected rats by reimmunization. Naive control rats (a, d) and rats orally infected with 5 × 107 MMC-treated MT-2 cells 8 weeks previously (b, c) were subcutaneously reimmunized without (a, b) or with (c, d) 2 × 107 MMC-treated FPM1 cells. T-cell-enriched spleen cells from these rats were isolated at 4 weeks after reimmunization, and [3H]thymidine incorporation was measured following inoculation without (dotted bar) or with formalin-treated G14 (hatched bar) or G14-Tax (closed bar) cells. The values shown are mean counts per minute of triplicate cultures ± the standard deviations. Similar results were obtained with three other pairs of orally infected rats with or without reimmunization with FPM1 cells.
DISCUSSION
In the present study, we demonstrated that orally HTLV-1-infected rats exhibited weaker HTLV-1-specific cellular immune responses with a greater persistent HTLV-1 load than did intraperitoneally infected rats. The finding of an inverse correlation between the cellular immune response and the persistent viral load suggests that the magnitude of the host immune response at primary infection may be a critical determinant of the persistent HTLV-1 level in vivo thereafter. The incidental association of the human ATL population with vertical HTLV-1 infection, a large viral load, and a weak T-cell response to HTLV-1 exhibits a striking similarity to the profile of oral HTLV-1 infection demonstrated in the present study. Since oral infection is a major route of vertical HTLV-1 infection, similar immune unresponsiveness to HTLV-1 and subsequent enlargement of the HTLV-1-infected cell population might also occur in humans, providing conditions favoring the evolution of infected cells toward more malignant phenotypes.
Cellular immunity is a critical mechanism of host defense against viruses and tumors. In our previous study, syngeneic HTLV-1-transformed tumor cells expanded in immunocompetent rats following administration of neutralizing antibodies to CD80 and CD86, which were able to block costimulatory signals necessary for antigen-specific T-cell immune responses (7). Withdrawal of these antibodies induced tumor regression in this model, which was associated with the appearance of active T-cell immunity against HTLV-1. These findings, together with the inverse correlation between HTLV-1-specific cellular immunity and the HTLV-1 proviral load demonstrated in the present study, indicate that HTLV-1-specific T-cell immunity actively controls the expansion of HTLV-1-infected cells in vivo.
Humoral immune responses to HTLV-1 were observed only in rats intraperitoneally inoculated with relatively large amounts of HTLV-1. This is due in part to insufficient expression of HTLV-1 structural proteins in rat HTLV-1-infected cells because of Rex dysfunction (5, 26). However, Tax can be expressed in rat, as well as human, cells. Since Tax is a major target antigen recognized by both human and rat cellular immune systems (6), cellular immune responses should more directly reflect the host immune status against HTLV-1 in rats.
Among intraperitoneally HTLV-1-infected rats, those inoculated with 5 × 101 MT-2 cells exhibited weaker cellular immune responses to HTLV-1 than those inoculated with 5 × 107 MT-2 cells. The viral dose at the primary HTLV-1 infection may be another determinant of host immunity. This raised the possibility that the immune unresponsiveness of rats orally inoculated with 5 × 107 cells might have been due to the small initial amount of HTLV-1 actually absorbed through the intestinal wall. However, the persistent viral loads in the orally infected rats were much greater than those in the rats intraperitoneally inoculated with 5 × 101 cells. Therefore, scarcity of viral antigens in vivo may explain the poor immune responses of intraperitoneally infected rats but not those of orally infected rats. In some of the rats orally inoculated with 5 × 101 cells, high levels of viral persistence were observed. This suggests that even when the initial number of HTLV-1-infected cells was extremely small, once such cells began to grow in vivo, the orally infected hosts might not have been able to control them.
It is known that oral administration of protein antigens induces peripheral tolerance in which host immunity does not respond to challenge administration of the fed antigen (28, 43). In this study, we found that HTLV-1-specific T-cell responses in orally HTLV-1-infected rats were restored by reimmunization with rat HTLV-1-infected syngeneic cells. Therefore, the immune unresponsiveness of orally HTLV-1-infected rats may differ from the typical observed oral tolerance of noninfectious protein antigens. Alternatively, the syngeneic HTLV-1-infected cells used as a challenge immunogen could be antigen-presenting cells strong enough to break T-cell anergy. The precise mechanisms of immune unresponsiveness in oral HTLV-1 infection remain to be clarified.
In humans, infants born to HTLV-1-carrying mothers are fed about 108 HTLV-1-infected cells before weaning (22) and a number of infantile carriers stay seronegative for HTLV-1 for a certain period of time (31). Since most of these HTLV-1-infected individuals exhibit seroconversion in a few years, T-cell immune unresponsiveness to HTLV-1 might also recover spontaneously later in life. In fact, many HTLV-1 carriers exhibit HTLV-1-specific T-cell responses. Once T-cell immunity to HTLV-1 is established, the magnitude of the immune response would positively correlate with the pre-existing viral load in vivo (30, 44). The presence of a large viral load and elevated levels of HTLV-1-specific immunity in HAM/TSP patients might be explained by T-cell immune conversion long after the establishment of a persistent viral load following vertical infection. Nevertheless, a small population of adult HTLV-1-infected individuals exhibits low T-cell responses to HTLV-1 despite an abundant viral load, as seen in ATL patients. Although the exact mechanism of development of ATL is still unclear, our results indicate that immune unresponsiveness to HTLV-1 is one of the risk factors for it and allows expansion of HTLV-1-infected cells. In this regard, reactivation of HTLV-1-specific T-cell immunity by vaccines might be of benefit. In the present study, we showed that HTLV-1-specific cellular immune unresponsiveness associated with oral infection was reversible and could be broken by reimmunization with syngeneic rat HTLV-1-expressing cells, a finding that may encourage the development of prophylactic approaches against ATL development.
In conclusion, oral HTLV-1 infection induced unresponsiveness of HTLV-1-specific host immunity associated with an increased persistent HTLV-1 viral load, a potential risk factor for ATL development. Prophylactic immunization may be one method of evading this risk.
Acknowledgments
We thank Mitsuhiko Yanagisawa and Shu Endo for structural assistance with the maintenance of animals at the P3 level facilities.
This work was supported in part by grants from the Ministry of Education, Science, Culture and Sports of Japan and the Japan Science and Technology Corporation.
REFERENCES
- 1.Aono, Y., J. Imai, K. Tominaga, S. Orita, A. Sato, and H. Igarashi. 1992. Rapid, sensitive, specific, and quantitative detection of human T-cell leukemia virus type 1 sequence in peripheral blood mononuclear cells by an improved polymerase chain reaction method with nested primers. Virus Genes 6:159-171. [DOI] [PubMed] [Google Scholar]
- 2.Daenke, S., S. Nightingale, J. K. Cruickshank, and C. R. Bangham. 1990. Sequence variants of human T-cell lymphotropic virus type I from patients with tropical spastic paraparesis and adult T-cell leukemia do not distinguish neurological from leukemic isolates. J. Virol. 64:1278-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elovaara, I., S. Koenig, A. Y. Brewah, R. M. Woods, T. Lehky, and S. Jacobson. 1993. High human T cell lymphotropic virus type 1 (HTLV-1)-specific precursor cytotoxic T lymphocyte frequencies in patients with HTLV-1-associated neurological disease. J. Exp. Med. 177:1567-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gessain, A., F. Barin, J. C. Vernant, O. Gout, L. Maurs, A. Calender, and G. de The. 1985. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet ii:407-410. [DOI] [PubMed]
- 5.Hakata, Y., M. Yamada, and H. Shida. 2001. Rat CRM1 is responsible for the poor activity of human T-cell leukemia virus type 1 Rex protein in rat cells. J. Virol. 75:11515-11525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanabuchi, S., T. Ohashi, Y. Koya, H. Kato, A. Hasegawa, F. Takemura, T. Masuda, and M. Kannagi. 2001. Regression of human T-cell leukemia virus type I (HTLV-I)-associated lymphomas in a rat model: peptide-induced T-cell immunity. J. Natl. Cancer Inst. 93:1775-1783. [DOI] [PubMed] [Google Scholar]
- 7.Hanabuchi, S., T. Ohashi, Y. Koya, H. Kato, F. Takemura, K. Hirokawa, T. Yoshiki, H. Yagita, K. Okumura, and M. Kannagi. 2000. Development of human T-cell leukemia virus type 1-transformed tumors in rats following suppression of T-cell immunity by CD80 and CD86 blockade. J. Virol. 74:428-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hino, S., K. Yamaguchi, S. Katamine, H. Sugiyama, T. Amagasaki, K. Kinoshita, Y. Yoshida, H. Doi, Y. Tsuji, and T. Miyamoto. 1985. Mother-to-child transmission of human T-cell leukemia virus type-I. Jpn J. Cancer Res. 76:474-480. [PubMed] [Google Scholar]
- 9.Hinuma, Y., K. Nagata, M. Hanaoka, M. Nakai, T. Matsumoto, K. I. Kinoshita, S. Shirakawa, and I. Miyoshi. 1981. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc. Natl. Acad. Sci. USA 78:6476-6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hisada, M., A. Okayama, S. Shioiri, D. L. Spiegelman, S. O. Stuver, and N. E. Mueller. 1998. Risk factors for adult T-cell leukemia among carriers of human T-lymphotropic virus type I. Blood 92:3557-3561. [PubMed] [Google Scholar]
- 11.Hisada, M., A. Okayama, D. Spiegelman, N. E. Mueller, and S. O. Stuver. 2001. Sex-specific mortality from adult T-cell leukemia among carriers of human T-lymphotropic virus type I. Int. J. Cancer 91:497-499. [DOI] [PubMed] [Google Scholar]
- 12.Hisada, M., A. Okayama, N. Tachibana, S. O. Stuver, D. L. Spiegelman, H. Tsubouchi, and N. E. Mueller. 1998. Predictors of level of circulating abnormal lymphocytes among human T-lymphotropic virus type I carriers in Japan. Int. J. Cancer 77:188-192. [DOI] [PubMed] [Google Scholar]
- 13.Jacobson, S., H. Shida, D. E. McFarlin, A. S. Fauci, and S. Koenig. 1990. Circulating CD8+ cytotoxic T lymphocytes specific for HTLV-I pX in patients with HTLV-I associated neurological disease. Nature 348:245-248. [DOI] [PubMed] [Google Scholar]
- 14.Kannagi, M., S. Harada, I. Maruyama, H. Inoko, H. Igarashi, G. Kuwashima, S. Sato, M. Morita, M. Kidokoro, M. Sugimoto, S. Funahashi, M. Osame, and H. Shida. 1991. Predominant recognition of human T cell leukemia virus type I (HTLV-I) pX gene products by human CD8+ cytotoxic T cells directed against HTLV-I-infected cells. Int. Immunol. 3:761-767. [DOI] [PubMed] [Google Scholar]
- 15.Kannagi, M., S. Matsushita, and S. Harada. 1993. Expression of the target antigen for cytotoxic T lymphocytes on adult T-cell-leukemia cells. Int. J. Cancer 54:582-588. [DOI] [PubMed] [Google Scholar]
- 16.Kannagi, M., S. Matsushita, H. Shida, and S. Harada. 1994. Cytotoxic T cell response and expression of the target antigen in HTLV-I infection. Leukemia 8(Suppl. 1):S54-S59. [PubMed] [Google Scholar]
- 17.Kannagi, M., T. Ohashi, S. Hanabuchi, H. Kato, Y. Koya, A. Hasegawa, T. Masuda, and T. Yoshiki. 2000. Immunological aspects of rat models of HTLV type 1-infected T lymphoproliferative disease. AIDS Res. Hum. Retrovir. 16:1737-1740. [DOI] [PubMed] [Google Scholar]
- 18.Kannagi, M., K. Sugamura, K. Kinoshita, H. Uchino, and Y. Hinuma. 1984. Specific cytolysis of fresh tumor cells by an autologous killer T cell line derived from an adult T cell leukemia/lymphoma patient. J. Immunol. 133:1037-1041. [PubMed] [Google Scholar]
- 19.Kaplan, J. E., M. Osame, H. Kubota, A. Igata, H. Nishitani, Y. Maeda, R. F. Khabbaz, and R. S. Janssen. 1990. The risk of development of HTLV-I-associated myelopathy/tropical spastic paraparesis among persons infected with HTLV-I. J. Acquir. Immune Defic. Syndr. 3:1096-1101. [PubMed] [Google Scholar]
- 20.Kato, H., Y. Koya, T. Ohashi, S. Hanabuchi, F. Takemura, M. Fujii, H. Tsujimoto, A. Hasegawa, and M. Kannagi. 1998. Oral administration of human T-cell leukemia virus type 1 induces immune unresponsiveness with persistent infection in adult rats. J. Virol. 72:7289-7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinoshita, K., T. Amagasaki, S. Hino, H. Doi, K. Yamanouchi, N. Ban, S. Momita, S. Ikeda, S. Kamihira, M. Ichimaru, S. Katamine, T. Miyamoto, Y. Tsuji, T. Ishimaru, T. Yamabe, M. Ito, S. Kamura, and T. Tsuda. 1987. Milk-borne transmission of HTLV-I from carrier mothers to their children. Jpn. J. Cancer Res. 78:674-680. [PubMed] [Google Scholar]
- 22.Kinoshita, K., K. Yamanouchi, S. Ikeda, S. Momita, T. Amagasaki, H. Soda, M. Ichimaru, R. Moriuchi, S. Katamine, T. Miyamoto, and S. Hino. 1985. Oral infection of a common marmoset with human T-cell leukemia virus type-I (HTLV-I) by inoculating fresh human milk of HTLV-I carrier mothers. Jpn. J. Cancer Res. 76:1147-1153. [PubMed] [Google Scholar]
- 23.Kinoshita, T., A. Tsujimoto, and K. Shimotohno. 1991. Sequence variations in LTR and env regions of HTLV-I do not discriminate between the virus from patients with HTLV-I-associated myelopathy and adult T-cell leukemia. Int. J. Cancer 47:491-495. [DOI] [PubMed] [Google Scholar]
- 24.Komurian, F., F. Pelloquin, and G. de The. 1991. In vivo genomic variability of human T-cell leukemia virus type I depends more upon geography than upon pathologies. J. Virol. 65:3770-3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kondo, T., H. Kono, N. Miyamoto, R. Yoshida, H. Toki, I. Matsumoto, M. Hara, H. Inoue, A. Inatsuki, T. Funatsu, N. Yamano, F. Bando, E. Iwao, I. Miyoshi, Y. Hinuma, and M. Hanaoka. 1989. Age- and sex-specific cumulative rate and risk of ATLL for HTLV-I carriers. Int. J. Cancer 43:1061-1064. [DOI] [PubMed] [Google Scholar]
- 26.Koya, Y., T. Ohashi, H. Kato, S. Hanabuchi, T. Tsukahara, F. Takemura, K. Etoh, M. Matsuoka, M. Fujii, and M. Kannagi. 1999. Establishment of a seronegative human T-cell leukemia virus type 1 (HTLV-1) carrier state in rats inoculated with a syngeneic HTLV-1-immortalized T-cell line preferentially expressing Tax. J. Virol. 73:6436-6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kusuhara, K., S. Sonoda, K. Takahashi, K. Tokugawa, J. Fukushige, and K. Ueda. 1987. Mother-to-child transmission of human T-cell leukemia virus type I (HTLV-I): a fifteen-year follow-up study in Okinawa, Japan. Int. J. Cancer 40:755-757. [DOI] [PubMed] [Google Scholar]
- 28.MacDonald, T. T. 1998. T cell immunity to oral allergens. Curr. Opin. Immunol. 10:620-627. [DOI] [PubMed] [Google Scholar]
- 29.Miyoshi, I., I. Kubonishi, S. Yoshimoto, T. Akagi, Y. Ohtsuki, Y. Shiraishi, K. Nagata, and Y. Hinuma. 1981. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature 294:770-771. [DOI] [PubMed] [Google Scholar]
- 30.Nagai, M., R. Kubota, T. F. Greten, J. P. Schneck, T. P. Leist, and S. Jacobson. 2001. Increased activated human T cell lymphotropic virus type I (HTLV-I) Tax11-19-specific memory and effector CD8+ cells in patients with HTLV-I-associated myelopathy/tropical spastic paraparesis: correlation with HTLV-I proviral load. J. Infect. Dis. 183:197-205. [DOI] [PubMed] [Google Scholar]
- 31.Nakano, S., Y. Ando, K. Saito, I. Moriyama, M. Ichijo, T. Toyama, K. Sugamura, J. Imai, and Y. Hinuma. 1986. Primary infection of Japanese infants with adult T-cell leukaemia-associated retrovirus (ATLV): evidence for viral transmission from mothers to children. J. Infect. 12:205-212. [DOI] [PubMed] [Google Scholar]
- 32.Ohashi, T., S. Hanabuchi, H. Kato, Y. Koya, F. Takemura, K. Hirokawa, T. Yoshiki, Y. Tanaka, M. Fujii, and M. Kannagi. 1999. Induction of adult T-cell leukemia-like lymphoproliferative disease and its inhibition by adoptive immunotherapy in T-cell-deficient nude rats inoculated with syngeneic human T-cell leukemia virus type 1-immortalized cells. J. Virol. 73:6031-6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohashi, T., S. Hanabuchi, H. Kato, H. Tateno, F. Takemura, T. Tsukahara, Y. Koya, A. Hasegawa, T. Masuda, and M. Kannagi. 2000. Prevention of adult T-cell leukemia-like lymphoproliferative disease in rats by adoptively transferred T cells from a donor immunized with human T-cell leukemia virus type 1 Tax-coding DNA vaccine. J. Virol. 74:9610-9616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okochi, K., H. Sato, and Y. Hinuma. 1984. A retrospective study on transmission of adult T cell leukemia virus by blood transfusion: seroconversion in recipients. Vox Sang. 46:245-253. [DOI] [PubMed] [Google Scholar]
- 35.Osame, M., K. Usuku, S. Izumo, N. Ijichi, H. Amitani, A. Igata, M. Matsumoto, and M. Tara. 1986. HTLV-I associated myelopathy, a new clinical entity. Lancet i:1031-1032. [DOI] [PubMed]
- 36.Parker, C. E., S. Daenke, S. Nightingale, and C. R. Bangham. 1992. Activated, HTLV-1-specific cytotoxic T-lymphocytes are found in healthy seropositives as well as in patients with tropical spastic paraparesis. Virology 188:628-636. [DOI] [PubMed] [Google Scholar]
- 37.Pique, C., A. Ureta-Vidal, A. Gessain, B. Chancerel, O. Gout, R. Tamouza, F. Agis, and M. C. Dokhelar. 2000. Evidence for the chronic in vivo production of human T cell leukemia virus type I Rof and Tof proteins from cytotoxic T lymphocytes directed against viral peptides. J. Exp. Med. 191:567-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poiesz, B. J., F. W. Ruscetti, A. F. Gazdar, P. A. Bunn, J. D. Minna, and R. C. Gallo. 1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 77:7415-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tajima, K., and Y. Hinuma. 1992. Epidemiology of HTLV-I/II in Japan and the world. Gann Monogr. Cancer Res. 39:129. [Google Scholar]
- 40.Tajima, K., S. Tominaga, T. Suchi, T. Kawagoe, H. Komoda, Y. Hinuma, T. Oda, and K. Fujita. 1982. Epidemiological analysis of the distribution of antibody to adult T-cell leukemia-virus-associated antigen: possible horizontal transmission of adult T-cell leukemia virus. Gann 73:893-901. [PubMed] [Google Scholar]
- 41.Uchiyama, T., J. Yodoi, K. Sagawa, K. Takatsuki, and H. Uchino. 1977. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood 50:481-492. [PubMed] [Google Scholar]
- 42.Usuku, K., S. Sonoda, M. Osame, S. Yashiki, K. Takahashi, M. Matsumoto, T. Sawada, K. Tsuji, M. Tara, and A. Igata. 1988. HLA haplotype-linked high immune responsiveness against HTLV-I in HTLV-I-associated myelopathy: comparison with adult T-cell leukemia/lymphoma. Ann. Neurol. 23(Suppl.):S143-S150. [DOI] [PubMed] [Google Scholar]
- 43.Weiner, H. L. 2001. Oral tolerance: immune mechanisms and the generation of Th3-type TGF-beta-secreting regulatory cells. Microbes Infect. 3:947-954. [DOI] [PubMed] [Google Scholar]
- 44.Wodarz, D., S. E. Hall, K. Usuku, M. Osame, G. S. Ogg, A. J. McMichael, M. A. Nowak, and C. R. Bangham. 2001. Cytotoxic T-cell abundance and virus load in human immunodeficiency virus type 1 and human T-cell leukaemia virus type 1. Proc. R. Soc. Lond. B Biol. Sci. 268:1215-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong-Staal, F., and R. C. Gallo. 1985. Human T-lymphotropic retroviruses. Nature 317:395-403. [DOI] [PubMed] [Google Scholar]