Abstract
Evidence suggests the estrogens may play a role in various mental and neurodegenerative diseases. We review the evidence implicating estradiol in schizophrenia and Parkinson's disease. Epidemiologic and clinical studies on the effects of estrogens in schizophrenia are surveyed, and animal studies and in vitro models of the modulatory effects of estrogens on neurotransmitters associated with schizophrenia (i.e., dopamine, serotonin, glutamate) are reviewed. Epidemiologic and clinical data suggesting a role for estrogens in Parkinson's disease and in vivo and in vitro models demonstrating neuroprotective effects of estrogens are then examined. Despite the numerous animal studies on the effects of estrogens in the brain, clinical data are sparse and often contradictory. Compounds with more specific and potent estrogenic activity in the brain are required to further research efforts in this area. Possible candidates are the selective estrogen receptor modulators (SERMs), whose agonist or antagonist properties depend on the target tissue. The effects of various SERMs in the brain are reviewed, and our novel findings on the effects of SERMs on 5-HT2A receptors in the rat cortex and nucleus accumbens are presented. We suggest that drugs with estrogenic activity in the brain may have therapeutic potential, either by modulating brain neurotransmission or through neuroprotective activity.
Medical subject headings: dopamine; estradiol; estrogens; glutaminc acid; models, animal; neuroprotective agents; Parkinson disease; raloxifene; schizophrenia; selective estrogen receptor modulators; serotonin; tamoxifen
Abstract
Les estrogènes semblent intervenir dans les maladies mentales et neurodégénératives. Cet article passe en revue les preuves de l'intervention de l'estradiol dans la schizophrénie et dans la maladie de Parkinson. On examine les données épidémiologiques et cliniques sur les effets des estrogènes dans la schizophrénie, ainsi que des études animales et des modèles in vitro des effets neuromodulateurs des estrogènes sur les neurotransmetteurs associés à la schizophrénie (par ex., dopamine, sérotonine et glutamate). On examine ensuite les données épidémiologiques et cliniques suggérant une médiation des estrogènes dans la maladie de Parkinson et des modèles in vivo et in vitro démontrant les effets neuroprotecteurs des estrogènes. En dépit de nombreuses études animales rapportant les effets des estrogènes sur le cerveau, les résultats cliniques sont peu nombreux et souvent contradictoires. Il faudra trouver des composés ayant une activité estrogénique plus puissante et plus spécifique sur le cerveau pour faire progresser les recherches dans ce domaine. Les composés possibles sont les modulateurs spécifiques des récepteurs des estrogènes (SERM), qui ont une activité agoniste ou antagoniste selon les tissus visés. Les auteurs passent en revue les effets des SERM sur le cerveau et présentent leurs découvertes récentes des effets des SERM sur les récepteurs 5-HT2A du cortex et du noyau accumbens chez le rat. Nous suggérons que les médicaments ayant une activité estrogénique dans le cerveau pourraient avoir un potentiel thérapeutique soit en modulant la neurotransmission au cerveau, soit par une activité neuroprotectrice.
Introduction
The sex steroid hormones (estrogens and androgens) have been shown to exert profound effects on brain differentiation, neural plasticity and central neurotransmission during development.1,2 Accumulating evidence supports a modulatory role of these steroids in the brain3 and, more recently, their prime importance in the normal maintenance of brain function during aging.4 Beneficial effects of sex steroids in several mental diseases, such as schizophrenia, depression, premenstrual syndrome, postnatal depression and Gilles de la Tourette's syndrome, have also been reported.5,6 Estradiol may also be beneficial in neurodegenerative diseases such as Alzheimer's (AD).7,8,9,10 With the increased life expectancy and the average age of menopause remaining constant, women can now expect to live up to one half of their adult lives after menopause.11 Women represent more than half the population, and more than 60 million women in the world now use oral contraceptives.12 The recent determination that long-term use of oral contraceptives is safe, the lower steroid concentrations now in use and their reported safety for women over the age of 35 years of age12 support the possible use of sex steroids to improve drug treatments and the search for new applications as drugs for central nervous system (CNS) disorders.
The importance of estrogen in the development and maintenance of the female reproductive system has led to the pharmaceutical development of a variety of steroidal and nonsteroidal compounds that interact with the estrogen receptor as contraceptives and are used to treat breast cancer, uterine dysfunction and other reproductive disorders. A role of these estrogen receptor-directed drugs in nontarget tissues such as the skeleton, the cardiovascular system and the CNS has also been recognized.13,14 Hormone replacement therapy, when given after menopause, is reported to be beneficial for osteoporosis, coronary artery disease, depression15 and AD.7,9 However, hormone replacement therapy also has less desirable effects, such as increasing risk of breast and endometrial cancer.16
Given that the number of women at midlife and beyond who may seek professional advice regarding hormone replacement therapy is expected to double over the next 2 decades,17,18 finding new drugs that effectively reduce the symptoms associated with menopause and do not increase the cancer risk, is of great interest. Tamoxifen, a mixed estrogen agonist-antagonist, currently used in the prevention and treatment of breast cancer, is a first-generation selective estrogen receptor modulator (SERM); it acts as an estrogen antagonist in mammary tissue but mimics the effects of estrogen in other tissues.19 Raloxifene, a second-generation SERM, acts as an estrogen antagonist in mammary and uterine tissues and an agonist in bone and cholesterol metabolism.19 The activities of tamoxifen and raloxifene have been reviewed with respect to their effects in nontraditional target tissues (i.e., skeletal and cardiovascular systems),20 but there is currently little relevant biological data available to support the use of these compounds for CNS diseases.
We review the effects of estrogens on mental and neurodegenerative diseases, with a focus on schizophrenia and PD because of their prevalence and the documented effects of steroids in these diseases. We review data from clinical studies and molecular investigations on the effects of estrogens in the brain and present novel findings with SERMs. We suggest drugs with estrogenic activity in the brain may have therapeutic potential, either by modulating brain neurotransmission or through neuroprotective activity.
Estrogens and schizophrenia
Schizophrenia is a mental illness characterized by episodic positive symptoms such as delusions, hallucinations, paranoia and psychosis and may include persistent negative symptoms such as flattened affect, impaired attention, social withdrawal and cognitive impairment.21,22 Epidemiologic and clinical evidence suggests an influence of estrogens on the vulnerability threshold for schizophrenia.23,24 Although early studies suggested the incidence of schizophrenia in men and women was about equal,25,26 more recent studies indicate incidence rates are higher in men.27,28
It has been suggested that estrogen may act as a protective factor in women; the age of onset of schizophrenia is significantly later in women than in men, with a second peak of onset larger and later in women after 40–45 years of age.22,29 As well, levels of psychopathology have been observed to fluctuate with phases of the menstrual cycle.30,31 In women with schizophrenia, relapse rates are higher when estrogen levels are low during the menstrual cycle, whereas relapse is low when estrogen levels are high.32,33 Higher rates of relapse in women with schizophrenia are also observed during the postpartum period (low estrogens),34 whereas relapse is low during pregnancy (high estrogens).33
Sex differences in the clinical expression of schizophrenia have also been observed. For example, women with schizophrenia have a higher prevalence of auditory hallucinations than men.35,36,37 Women with schizophrenia have been described as “hallucinatory, illusionary,” with symptoms mimicking affective disorders, whereas men tend to become “dull, autistic” and have an amotivational syndrome with loss of organization and regulation.38,39,40 Women appear to require lower doses of neuroleptics than men in the acute phase of illness and in the maintenance stage41 and respond better to both psychosocial and pharmacological treatments.42 In a long-term follow-up study, women tended, more than men, to deteriorate in the perimenopausal period when there is a marked fall in estrogen levels.43 Overall, men with schizophrenia have an earlier age of onset, are admitted to hospital earlier and demonstrate a more typical picture and poorer prognosis than women.44
There have been few treatment studies investigating the effects of estrogen in patients with schizophrenia. One study assessed the response of women with acute psychotic symptoms to either an 8-week therapy of estradiol and a neuroleptic or the neuroleptic treatment alone.45 The results suggested that estradiol may have antipsychotic properties or act as a catalyst for neuroleptic responsiveness in women with schizophrenia. A case study also reported a beneficial effect of estradiol on psychotic symptoms in a woman with recurrent psychotic and affective symptoms.46 It has been suggested that the beneficial effects of estrogen in schizophrenia may be associated with a modulation of dopaminergic activity.24 After menopause, women require higher doses of neuroleptics, the risk for dyskinesias increases and more severe forms of dyskinesias are more common.47 Some clinical studies report beneficial effects of estradiol48,49,50,51,52 in women with tardive dyskinesia, whereas others do not.53,54,55
Despite abundant clinical and epidemiologic literature on the beneficial effect of endogenous estrogens, there are few studies of estrogen therapy in schizophrenia and contradictory findings for tardive dyskinesia. Although the effects of estrogens on cognitive impairment in schizophrenia have not been systematically reported, improvement has been reported with hormone replacement therapy in normal women.56 This warrants further studies with drugs with estrogen-like potency that are more selective for the brain.
Estrogenic modulation of neurotransmitters implicated in schizophrenia
Schizophrenia has long been attributed to abnormal hyperactivity of central dopaminergic mechanisms on the basis of the observation that drugs effective in treating this illness share the common feature of blocking dopamine (DA) receptors.57,58 With the advance of brain imaging techniques, direct evidence suggestive of dysfunctional dopaminergic transmission in schizophrenia has emerged. This includes measures of striatal [18F]fluorodopa or [11C]dopa accumulation in patients with schizophrenia59,60,61,62 and synaptic dopamine concentrations measured indirectly by a decrease in binding potential of [11C]raclopride or [123I]IBZM after amphetamine challenge.63,64,65 A recent single-photon emission computed tomography study demonstrated increased baseline occupancy of D2 receptors by dopamine in schizophrenia.66 This finding provides direct evidence of increased stimulation of D2 receptors by dopamine in schizophrenia, consistent with phasic activity of dopaminergic neurons.
In experimental animals, sex differences in brain dopaminergic activity, as well as estrous cycle variations, acute effects of a physiological dose of estrogens and chronic effects of high doses of this hormone have been reported.6,67 Estradiol may modulate dopaminergic activity at various steps of DA transmission (i.e., DA release and metabolism, pre- and postsynaptic DA receptors and transporter).6 Both pro- and antidopaminergic effects of estrogens have been documented in animals. These opposing activities may be related to factors such as sex-specific responses, dose of estrogen, short- versus long-term administration and time after administration.6,67 A single low dose of estradiol and estrous cycle variations support a stimulatory role of estrogen on dopaminergic activity. This stimulatory effect on DA release, metabolism, receptor, transporter and behaviour is rapid and well documented in the striatum and nucleus accumbens of adult rats. It is stereospecific and, in vitro, shown to be antagonized by the pure estrogen receptor antagonist ICI 182,780 but not by tamoxifen, which has mixed agonist and antagonist activity at this receptor.67 Changes in DA receptors and transporter after estradiol are not paralleled by changes of their expression.6,68 The effects are rapid, as fast as 2 minutes for the stereospecific effect of estradiol on DA release in the nucleus accumbens.69 Furthermore, estrogen was shown within seconds to decrease L-type Ca2+ currents in striatal neurons, and this was also observed with estrogen conjugated with bovine serum albumin to prevent entry in the cells.70 Morphology of rat striatal membranes, as measured by freeze fracture, varies during the estrous cycle, and this is correlated with striatal estradiol and progesterone concentrations.71 The collective evidence suggests that it is doubtful that these rapid effects of estrogen in the striatum are mediated by genome-activating estrogen receptors, but likely occur at the membrane surface.6,72,73 Additional support for nongenomic mechanisms comes from a study demonstrating that long-term administration of high doses of estradiol increases the density of DA receptors and transporters.6 It has been suggested that the hormonal modulation of the striatum may have evolved to facilitate reproductive success in female rats by enhancing pacing behaviour.67 In humans, the hormonal modulation of the striatum may have relevance in addiction and mental disorders associated with abnormalities in this brain region.
The efficacy of atypical antipsychotics such as clozapine and risperidone, which are mainly serotonergic (5-HT) 2A receptor antagonists,74 has led to theories of serotonergic involvement in schizophrenia. This system, in addition to the DA system, may be important for an optimal antipsychotic response.75 Several studies have reported a decrease in 5-HT2A receptor density in the frontal cortex of patients with schizophrenia,76,77,78 although others report no change.79,80 In a study of postmenopausal women using positron emission tomography, estradiol and progesterone administration increased 5-HT2A receptor binding potential in widespread areas of the cerebral cortex.81 Studies in rats have also shown that estradiol modulates brain serotonergic activity, increasing 5-HT2A receptors and the serotonin transporter.5 Short- and long-term estradiol treatments increase the density of 5-HT2A receptors, mainly in the anterior cingulate and anterior frontal cortices.82,83
An alternative explanation for the pathogenesis of schizophrenia, the glutamate dysfunction hypothesis, originated from the observation that phencyclidine, a noncompetitive antagonist of the N-methyl-D-aspartate (NMDA) glutamate receptor, produces a psychotomimetic state in normal humans that is similar to schizophrenic psychosis.84 Ketamine and MK-801, 2 other noncompetitive antagonists of NMDA receptors, also produce schizophrenic symptoms85,86,87 and exacerbate psychosis in patients with schizophrenia.88 Several glutamate-related neurochemical abnormalities are reported in postmortem tissue of patients with schizophrenia89 that provide evidence that schizophrenia is associated with reduced glutamate release in the hippocampus and frontal cortex.
Recently, we demonstrated that estradiol has regionally specific effects on glutamate receptors in the rat brain. Estradiol treatment increases NMDA receptors in the CA1 region and dentate gyrus of the hippocampus and decreases these receptors in the frontal cortex and dorsal part of the caudate putamen.90 In addition, estradiol has no effect on AMPA receptor binding density in the hippocampus, but it decreases receptor density in the frontal cortex, caudate putamen and nucleus accumbens.90 These results confirm and extend those previously published on the effect of short-term treatment with estradiol on rat hippocampal NMDA and AMPA receptors.91,92,93 In schizophrenia, disruption of glutamate-mediated transmission involving the NMDA receptor is suspected to occur in the intrahippocampal circuitry, interrupting information flow within hippocampus and diminishing excitatory neuronal activity in its glutamatergic efferent pathway.89 Recently, this concept has been supported by the development of mice with reduced NMDA expression that display behaviours related to schizophrenia.94 These behavioural alterations are similar to those observed in pharmacologically induced animal models of schizophrenia and can be improved with haloperidol or clozapine treatment.94
Estrogens and Parkinson's disease
Parkinson's disease (PD), the second most common neurodegenerative disorder, is mainly characterized by the progressive and selective depletion of DA synthesizing neurons in the substantia nigra pars compacta.95 The regulation of dopaminergic neurotransmission in the basal ganglia by estrogens is now well established.6,67,96 DA synthesis and release, DA receptor, DA uptake site, as well as catechol-o-methyl transferase (COMT) expression have been shown to be regulated by ovarian steroids.6,67,97
The influence of sex steroids on movement disorders, either during the menstrual cycle or after hormone therapy in postmenopausal women, has been investigated.6,96,98,99 Estrogens, in particular, have been shown to modulate symptoms of PD and levodopa-induced dyskinesias.6,100,101,102 Many of these studies are based on the effect estrogen replacement therapy has on the response of parkinsonian patients to standard levodopa therapy and generally support an antidopaminergic effect of estrogens on parkinsonian symptoms.101 However, a recent study suggests that estrogen replacement therapy might be beneficial to women with early PD before the initiation of levodopa.103 Estrogen was reported to improve motor disability in postmenopausal women with PD with motor fluctuations.104 However, a slight antiparkinsonian effect105 and no effect106 of 17β-estradiol treatment has been reported in clinical trials. Hence, in humans, the issue of whether estrogens stimulate or inhibit the dopaminergic system is still open. Animal studies also support both pro- and antidopaminergic activities of estrogens; it is possible that these effects might be dissociated with SERMs. The effect of newer SERMs such as raloxifene on parkinsonism clearly deserves investigation.
In addition to the modulatory effect of estrogens on dopaminergic activity, evidence also suggests estrogens may possess neuroprotective activity in PD. Although numerous studies report a greater prevalence and incidence of PD in men than in women,107,108,109,110,111,112,113,114,115 no such sex difference has also been reported.116 As well, sex differences have been reported for the evolution of symptoms and response to levodopa treatment.117 Moreover, estrogen replacement therapy may decrease the risk of developing dementia.113
Current concepts of the cause of PD suggest a role for both genetic and environmental influences.118,119,120,121 Common to a variety of potential causes of nigral cell degeneration in PD is the involvement of oxidative stress, exitotoxicity and metabolic compromise.118,122 Potential neuroprotective agents such as ovarian steroids may act at multiple levels to exert their effects, both by genomic action on transcription of genes related to cell survival and antiapoptotic proteins and nongenomic actions such as a NMDA receptor antagonists, antioxidants, interaction with the MAPK signalling pathway and the phosphatidylinositol 3-kinase cascade.123,124
Estrogens may be neurotoxic during development and neuroprotective in aging.125 In primates, estrogen was shown to be essential to maintain nigrostriatal neurons,126 intact females having a higher density of dopamine cells than males and ovariectomized females. Estradiol replacement early after ovariectomy prevented the neuronal loss.126
The potential of 17β-estradiol to alter neuronal survival may depend on the estrogen receptor subtype present, with the α subtype having a neuroprotective effect and the β subtype mediating the induction of apoptosis in neuronal cells.127 Nielsen and colleagues127 also observed that in cells expressing both estrogen receptor subtypes, estradiol was neuroprotective, and that estrogen-induced apoptosis (through β subtype) required the expression of Fas- and Fas ligand (FasL) proteins, since the absence of FasL in neurons prevented this effect. These authors note that the microenvironments in the brain play an important role in the response to estradiol and may partly explain the different responses of developing and aging brains.
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is a neurotoxin that selectively destroys dopaminergic neurons of the substantia nigra pars compacta in humans and experimental animals.128,129,130 MPTP is therefore a useful drug to investigate the neurodegeneration process associated with idiopathic PD.130 Sex differences are observed in mice bearing MPTP-induced nigrostriatal lesions; the neurotoxic effect of MPTP and methamphetamine is greater in male than in female mice.131,132 Studies show that estrogen pretreatment prevents the dramatic depletion of striatal DA induced by MPTP132,133,134,135,136 and methamphetamine.137
Neuroprotection of dopaminergic neurons against degeneration is a possible explanation of the effect of estrogens on striatal DA.138 Changes in the metabolism of residual neurons to increase available DA in the striatum may alternatively explain these data. For example, 17β-estradiol, but not 17α-estradiol, rapidly induces a dose-dependent increase in striatal tyrosine hydroxylase activity in ovariectomized rats.139 Reduction of COMT activity may also be a mechanism by which estrogen increases DA striatal content in MPTP-treated mice.97
Other data also suggest a neuroprotective action of estrogens. The glial fibrillary acidic protein (GFAP) is localized in the astrocyte and used as a marker of neuronal damage.132 Miller et al132 observed that estrogen replacement reduces the GFAP elevation induced by MPTP, and this may imply actual neuroprotection.132 Low endogenous levels of estrogens in intact female mice appear to be sufficient to provide the neuroprotection observed.132
The effect of estradiol may be indirect and involve other substances, such as the neurotrophins brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF), whose secretion is amplified by estrogens.140,141 For instance, the proto-oncogene Bcl-2 protects neurons from MPTP neurotoxicity, perhaps by mechanisms which involve inhibition of apoptosis and antioxidant activity.142,143 Estrogens increase the number of Bcl-2 immunoreactive cells in rat brain, and this may be linked to its possible neuroprotective activity.144
The beneficial effects of estrogens that were observed with 17β-estradiol and not with 17α-estradiol suggest a stereospecific effect, possibly via the classical intracellular estrogen receptor.135 Expression of mRNA transcripts for both α and β estrogen receptors was detected in the substantia nigra,145 whereas immunohistochemical and binding studies have not confirmed the presence of any estrogen receptor protein in this structure.146 Classical estrogen receptors are thought to be located within the cells, but recent evidence suggests that the existence of membrane-bound estrogen receptors should not be ruled out.70,147,148 A stereospecific effect of 17β-estradiol (and a membrane impermeable estrogen – bovine serum albumin construct) to stimulate neurite growth of midbrain dopaminergic neurons, which is inhibited by antagonists of cAMP/protein kinase A and calcium signalling pathways but not by the estrogen receptor antagonist ICI 182,780, was recently reported.149 These results support nongenomic membrane effects of estradiol.
Cell culture studies reveal neuroprotective action of 17β-estradiol and 17α-estradiol against various insults.150,151,152,153 Tamoxifen, used for its estrogen receptor antagonist activity, did not block estradiol's protection from oxidative stress in mesencephalic dopaminergic neurons.152 By contrast, the same authors noted that the estrogen antagonist ICI 182,780 did oppose the antiapoptotic effect of estradiol caused by bleomycin sulfate or buthionine sulfoximine.153
Estrogen receptor-independent mechanisms, such as antioxidant properties, have been proposed for the possible action of ovarian steroids in these in vitro models. Oxidative stress may contribute to dopaminergic cell death in PD,118 but clinical studies based on antioxidative strategies have generally been inconclusive.154 The concentration of estrogens used in these in vitro studies are in the micromolar range and are higher than those generally found in the brain.71 At these higher concentrations, the stereospecificity of the mechanism of action of estrogens may be lost.
It has been suggested that estrogen may act on dopaminergic neurons by hampering DA uptake and release.155,156,157,158 More specifically, studies show that estradiol inhibits striatal DA uptake by decreasing the affinity of the transporter for DA.155 This has also been proposed as a mechanism whereby MPTP-induced dopaminergic cell destruction is prevented because MPP+, the active form of MPTP, enters dopaminergic cells via the DA uptake site before initiating the cell death process.130,155,156 This is not supported by our recent findings where DA concentrations are spared, whereas DA transporter-specific binding and expression remains unchanged after estrogen treatment in MPTP mice versus saline-treated MPTP animals.135
Well-designed large clinical trials might help to determine the neuroprotective activity of estrogens and other estrogen receptor-directed drugs such as SERMs. However, neuroprotection is difficult to assess in clinical studies in which a symptomatic effect is hard to distinguish from a real neuroprotective effect.154 The need for reliable biological markers for nigrostriatal degeneration is evident, and brain imaging tools, such as single photon emission computed tomography and PET, may provide a satisfactory solution.159
Selective estrogen receptor modulators
Clinical studies on the effect of tamoxifen in patients with schizophrenia are lacking, and animal studies on the effects of tamoxifen in brain areas associated with mental and neurodegenerative diseases are much less abundant than they are for estradiol. Tamoxifen, but not estradiol, is a weak competitive inhibitor of the DA antagonist site of D2 receptors in striatal membranes.160,161 Ferretti et al162 found, in animals treated with estradiol, tamoxifen antagonizes both increases in [3H]spiperone binding to D2 receptors and stereotyped behaviour induced by apomorphine. Tamoxifen was also reported to mimic some of estradiol's activities on dopaminergic systems. In the pituitary, tamoxifen increases adenohypophysal DA receptors labelled with [3H]spiperone.162 McDermott et al163,164,165,166 investigated the interactive effects of tamoxifen and estrogen upon the nigrostriatal dopaminergic system. Their findings suggest that tamoxifen, like estradiol, can alter DA output through direct, nongenomic effects on striatal neurons. In addition, an indirect effect of tamoxifen on dopaminergic activity was demonstrated when tamoxifen reduced nigral glutamic acid decarboxylase activity.167 Tamoxifen also increases striatal extracellular levels of DA and 3,4-dihydroxyphenylacetic acid (DOPAC) in freely moving male rats,168 and was found to increase synaptic density in the hippocampus of ovariectomized rats.169 It was also shown to block the effect of estradiol on dendritic spines of hippocampal neurons.170
An early safety assessment of raloxifene's effects on cognition and mood in postmenopausal women participating in a randomized, double-blind osteoporosis treatment trial was reported.171 After 12 months of treatment, no clinically significant effect of raloxifene on cognitive performance was observed.171 The penetration of raloxifene in blood–brain barrier protected regions is low and may render the detection of any brain effect difficult at the dosage currently used in practice.172 In rats, Wu et al172 report that systemic administration of raloxifene reverses the ovariectomy-induced reduction in choline acetyltransferase activity in the hippocampus. CI-628, a nonsteroidal estrogen antagonist which readily crosses the blood–brain barrier, prevented estrogen induction of spines on CA1 pyramidal neurons, without having any agonist effects of its own.173 In contrast, CI-628 has an estrogen-like agonist effect on brain monoamine oxidase (MAO) (reduction of activity) and choline acetyltransferase (increase of activity).174 The raloxifene analog LY117018 has an estrogen-like action on neuroendocrine opiatergic pathways and on allopregnanolone concentrations when administered alone in ovariectomized rats, whereas it exerts an antiestrogen effect in fertile or ovariectomized rats treated with 17β-estradiol.175,176
We previously reported that estradiol increases 5-HT2A receptor density and expression in rat brain83 and then sought the specificity of this hormonal modulation. Raloxifene mimics the effect of estradiol to increase 5-HT2A receptor binding and its mRNA levels. (Fig. 1). Of the 18 brain regions investigated, this was specific to the anterior cingulate (Fig. 2) and anterior frontal cortices (Fig. 3), the cortical nucleus of the amygdala, the nucleus accumbens (Fig. 4) and the striatum.177 More specifically, in the cortical areas, the hormonal effect was observed in the anterior, but not the posterior part, as shown for lamina IV of the cingulate cortex for 5-HT2A mRNA levels in rats after ovariectomy and chronic estradiol or raloxifene treatment (Fig. 2). In the anterior frontal cortex, the effect of ovariectomy and estradiol or raloxifene treatment in rats on 5-HT2A mRNA levels was observed in lamina II and IV (Fig. 3). The effect of ovariectomy and hormone treatment in rats on [3H]ketanserine-specific binding to 5-HT2A receptors was more pronounced in the shell than the core of the nucleus accumbens (Fig. 4), the shell being more related to the mesolimbic system.178
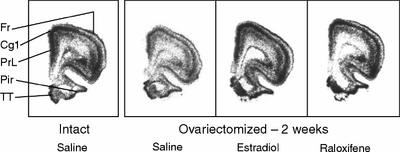
Fig. 1: Examples of in situ hybridization of 5-HT2A receptors mRNA in the rat brain showing the effect in frontal cortex of ovariectomy and a 2-week treatment of 17β-estradiol (1 silastic implant) or raloxifene (250 μg). Coronal brain sections (30 μm thick) of frontal cortex (4.7–3.7 mm from bregma211) are shown. Fr = frontal cortex, Cg1= cingulate cortex, PrL = prelimbic cortex, Pir = piriform cortex, TT = Taenia tecta.
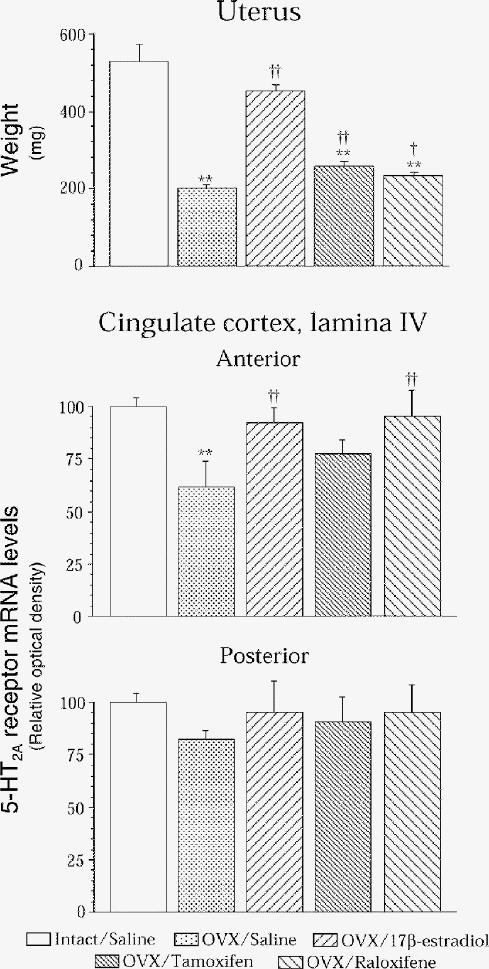
Fig. 2: Uterine weight and in situ hybridization of brain 5-HT2A receptors in the anterior and posterior cingulate cortex of intact rats that received vehicle (once daily, subcutaneously) for 2 weeks, as well as ovariectomized (OVX) rats treated for 2 weeks with vehicle (once daily, subcutaneously) or with 17β-estradiol (1 silastic implant), tamoxifen (250 μg) or raloxifene (250 μg). Mean in situ hybridization results are expressed as percentage of relative optical density control values (and standard errors of the means [SEM]) of 4–6 rats per group. Control values in relative optical density were 0.42 (SEM 0.02) (upper panel) and 0.33 (SEM 0.02) (lower panel). **p < 0.005 v. intact/saline; †p < 0.05 and ††p < 0.01 v. OVX/saline.
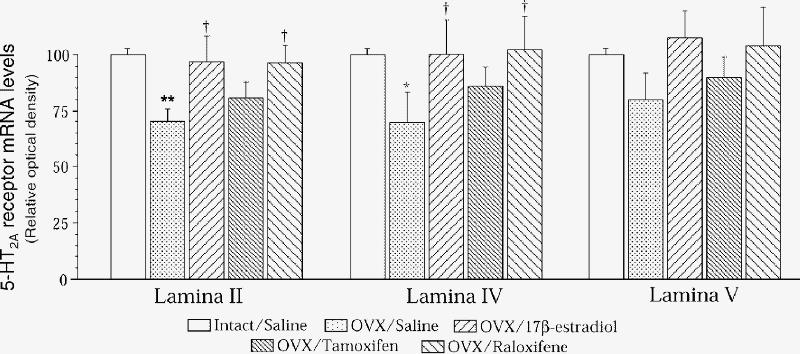
Fig. 3: In situ hybridization of 5-HT2A receptors in lamina II, IV and V of the anterior frontal cortex of intact rats that received vehicle (once daily, subcutaneously) for 2 weeks, as well as ovariectomized (OVX) rats treated for 2 weeks with vehicle (once daily, subcutaneously) or with 17β-estradiol (1 silastic implant), tamoxifen (250 μg) or raloxifene (250 μg). Results are expressed as percentage of control values and SEMs of 4 rats per group. Control values in relative optical density were 0.27 (SEM 0.02) for lamina II, 0.33 (SEM 0.01) for lamina IV and 0.23 (SEM 0.01) for lamina V. *p < 0.05 and **p < 0.005 v. intact/saline; †p < 0.05 v. OVX/saline.
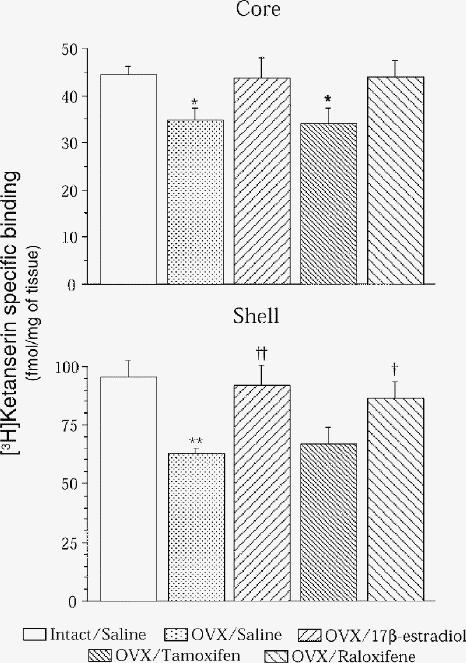
Fig. 4: [3H]Ketanserin-specific binding to 5-HT2A receptors in the core and shell of the nucleus accumbens of intact rats that received vehicle (once daily, subcutaneously) for 2 weeks, as well as ovariectomized (OVX) rats treated for 2 weeks with vehicle (once daily, subcutaneously) or with 17β-estradiol (1 silastic implant), tamoxifen (250 μg) or raloxifene (250 μg). Results are means and SEMs of 6 rats per group. *p < 0.05 and **p < 0.005 v. intact/saline; †p < 0.05 and ††p < 0.01 v. OVX/saline.
In general, tamoxifen did not significantly increase 5-HT2A receptor expression, except in one part of the anterior cingulate cortex and binding in the striatum.177 Considering the short- versus the long-term treatment and the dose used, this is in overall agreement with the study of Sumner et al.179 This suggests that raloxifene, more than tamoxifen, acts as an estrogen agonist at brain 5-HT2A receptors and that it is possible to have brain estrogenic agonist activity dissociated from agonist activity in the periphery. Indeed, whereas raloxifene displays as estradiol activity to increase 5-HT2A receptor density and expression, this effect can be dissociated from the activity to stimulate the uterus.
Tamoxifen and raloxifene also modulate brain NMDA and AMPA receptors. These SERMs increase NMDA-specific binding in the hippocampus but decrease binding in the frontal cortex, striatum and nucleus accumbens.180 As with estradiol treatment, AMPA receptor-specific binding is decreased by tamoxifen and raloxifene.181 Using specific ligands for binding autoradiography of subunits of NMDA receptors and specific probes for subunits measured by in situ hybridization, it was shown that estradiol and SERMs modulate NR1 and NR2B subunits, whereas the NR1/2A subunit remains unchanged.180
Clinical data are not yet available for a possible neuroprotective activity of SERMs. At this point, preclinical as well as clinical experiments are needed to assess the potential neuroprotective effects of the available SERMs and make comparisons with what is known with estradiol. Tamoxifen may stimulate DA liberation in the striatum of rats through direct, nongenomic action.163,164,165,182 An in vivo microdialysis study revealed that tamoxifen administration increased DA and DOPAC in the striatum of freely moving rats.168 The effect of a 40-day treatment of ovariectomized rats with tamoxifen, either alone or in combination with estradiol, showed that estradiol amplified amphetamine-stimulated DA release, and this effect was blocked by tamoxifen.166 This suggests that, in the dopaminergic nigrostriatal system, tamoxifen may act as an estradiol agonist for short-term nongenomic effects, but may block estradiol in regards to long-term activity. In addition, the neuroprotective effect of estradiol from glutamate neurotoxicity was blocked by tamoxifen in primary cortical neurons,183 but it was not affected by tamoxifen in mesencephalic dopaminergic neurons in culture.152 Studies in mice show that tamoxifen does not protect from MPTP- or methamphetamine-induced striatal DA decrease and it may block the protective effect of estrogens.184,185 On the other hand, we have shown that raloxifene prevents the MPTP-induced striatal depletion of DA and its metabolites DOPAC and homovanillic acid.136 In NGF-primed PC12 cells expressing both estrogen α and β receptors, raloxifene-induced neurite outgrowth, and the combined treatment of estradiol and raloxifene produced a statistically greater effect than either agent alone.186 These findings on dopaminergic toxicity and those reported above for 5-HT2A receptors suggest that raloxifene has more brain estrogenic agonist activity than tamoxifen, the latter compound displaying both agonist and antagonist activities in the CNS. For brain NMDA and AMPA receptors, both tamoxifen and raloxifene have similar estrogenic activity.
Discussion
Effects of estrogens on the brain are reported in numerous epidemiological, clinical as well as animal and in vitro cell culture studies. Some possible explanations for the contradictory or discordant findings of these studies are discussed below.
In studies on sex differences in mental and neurodegenerative diseases, estrogens and androgens are often the focus and the other major ovarian steroid progesterone receives less attention. Progesterone may also play a role in the sex differences observed, however. Progestatives alone are in use as contraceptives,187,188 although it is less common than contraceptives combining estrogens and progestatives.187 Animal studies with progesterone often use an estrogen priming,73,189,190,191 although clear evidence of an effect of progesterone alone on neurotransmitter activity is observed in several animal models.192,193,194 In hormone replacement therapies in humans, estrogens are often associated with progestatives to avoid sustained uterine stimulation. The brain effects observed are likely due to both estrogens and progesterone. In animal studies, progesterone can potentiate,73,195 leave unchanged196 or antagonize197,198,199 the effect of estrogens on various neurotransmitter activities. In human studies, an adverse effect of progestins on mood, including increasing depression scores, has been reported.200,201,202,203 With the availability of SERMs that do not stimulate the uterus, it will be possible to probe estrogenic activity in the brain without the need of a progestative in the treatment. Nevertheless, the effect of progestatives on the brain is beyond the scope of this paper.
A review of the literature shows multiple activities of estrogens on brain neurotransmitters involving various genomic and nongenomic mechanisms. For example, genomic mechanisms involving an estrogen receptor either of the α or β subtype likely involve a stereospecific activity, 17α-estradiol being much less active than the active natural 17β-estradiol; this is noted in the neuroprotection of DA neurons in mice from MPTP toxicity.135,136 In contrast, nongenomic mechanisms involving the antioxidant activity of estradiol in protection against various sources of neurotoxicity in cell cultures150,204,205 are associated with the presence of a hydroxyl group in the C3 position. In assessing estrogenic activity through an estrogen receptor, a standard pharmacological approach is to probe if an estrogen receptor antagonist opposes the estrogenic effect. This is somewhat difficult for in vivo experiments at present because of the lack of pure antiestrogen compounds devoid of estrogenic activity for the brain. Reasons for this include the fact that agonist or antagonist effects in the brain are not yet possible to predict, several existing antagonists do not cross the blood–brain barrier (some of them were designed not to do so) and the design of such compounds may be of less therapeutic interest than would be the case for SERMs.13
Dose-related biphasic effects of estradiol are observed in animal studies.70,206,207 Some effects are lost at higher concentrations,206,207 whereas others require high doses.151,152,204 This could reflect an adaptation of the brain to fluctuating levels of estradiol during the menstrual cycle and the very high levels of the steroid during pregnancy.208 These dose-related effects could be taken as an advantage to possibly dissociate some activities in the brain. It is not known how SERMs will behave in this respect or if biphasic dose-related effects will be found, even for those compounds devoid of antagonistic activity in the target tissue.
For the CNS, the ideal estrogen-like compound would have activity in the brain and none in the periphery. Is it possible to find such a compound? SERMs have gone some way in that direction, and 17α-estradiol has shown activity similar to 17β-estradiol in models in vitro,151,209 although it is inactive in others in vivo.135,136 Estrogen receptor β-selective ligands are also likely to be fairly specific to brain since this receptor is localized in brain regions associated with learning and memory and is much less abundant in the uterus and pituitary than the α subtype.210 Although a variety of synthetic and naturally occurring estrogenic compounds (including phytoestrogens and environmental estrogenic compounds) were found to have similar affinity for the α and β subtypes, some phytoestrogens, such as genistein and coumestrol, have higher binding affinity for estrogen receptor β.210 At this point in human studies, it is important to learn as much as possible about the brain effect of SERMs and other estrogenic compounds (e.g., phytoestrogens, xenoestrogen, DHEA) that are given for traditional indications and to pursue animal studies with such compounds as well.
Clinical data should be gathered in patients with schizophrenia and PD who are taking raloxifene for osteoporosis or tamoxifen for breast cancer. Research on brain hormonal effects is not simple but is quite relevant. Indeed, schizophrenia generates considerable health costs and the incidence of neurodegenerative diseases such as Alzheimer's disease and PD is increasing with the aging population. Hence, research on the effects of estrogenic-like compounds in the brain has important social and financial implications.
Acknowledgments
We thank Dr. F. Labrie for the gift of raloxifene. This research was supported by a grant from the Canadian Institutes of Health Research to T.D.P.
Footnotes
Competing interests: None declared.
Correspondence to: Dr. Thérèse Di Paolo, Oncology and Molecular Endocrinology Research Centre, Laval University Medical Centre (CHUL), 2705 Laurier Blvd., Sainte-Foy QC G1V 4G2; fax 418 654-2761; therese.dipaolo@crchul.ulaval.ca
Submitted Aug. 29, 2000 Revised Mar. 27, 2001 Accepted Apr. 9, 2001
References
- 1.Kawata M. Roles of steroid hormones and their receptors in structural organization in the nervous system. Neurosci Res 1995; 24:1-46. [DOI] [PubMed]
- 2.McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev 1999;20:279-307. [DOI] [PubMed]
- 3.Sherwin BB. Estrogen effects on cognition in menopausal women. Neurology 1997;48:S21-6. [DOI] [PubMed]
- 4.Simpkins JW, Singh M, Bishop J. The potential role for estrogen replacement therapy in the treatment of the cognitive decline and neurodegeneration associated with Alzheimer's disease. Neurobiol Aging 1994;15:S195-7. [DOI] [PubMed]
- 5.Fink G, Sumner BE, McQueen JK, Wilson H, Rosie R. Sex steroid control of mood, mental state and memory. Clin Exp Pharmacol Physiol 1998;25:764-75. [DOI] [PubMed]
- 6.Di Paolo T. Modulation of brain dopamine transmission by sex steroids. Rev Neurosci 1994;5:27-41. [DOI] [PubMed]
- 7.Henderson VW. The epidemiology of estrogen replacement therapy and Alzheimer's disease. Neurology 1997;48:S27-35. [DOI] [PubMed]
- 8.Inestrosa NC, Marzolo MP, Bonnefont AB. Cellular and molecular basis of estrogen's neuroprotection. Potential relevance for Alzheimer's disease. Mol Neurobiol 1998;17:73-86. [DOI] [PubMed]
- 9.Tang MX, Jacobs D, Stern Y, Marder K, Schofield P, Gurland B, et al. Effect of oestrogen during menopause on risk and age at onset of Alzheimer's disease. Lancet 1996;348:429-32. [DOI] [PubMed]
- 10.Yaffe K, Ettinger B, Pressman A, Seeley D, Whooley M, Schaefer C, et al. Neuropsychiatric function and dehydroepiandrosterone sulfate in elderly women: a prospective study. Biol Psychiatry 1998;43:694-700. [DOI] [PubMed]
- 11.Vliet EL, Davis VL. New perspectives on the relationship of hormone changes to affective disorders in the perimenopause. NAACOGGS Clin Issu Perinat Womens Health Nurs 1991;2:453-71. [PubMed]
- 12.Tierney MC, Luine VN. Effects of estrogens and SERMs on the central nervous system. J Soc Obstet Gynaecol Can 1997;19:46-56.
- 13.Grese TA, Pennington LD, Sluka JP, Adrian MD, Cole HW, Fuson TR, et al. Synthesis and pharmacology of conformationally restricted raloxifene analogues: highly potent selective estrogen receptor modulators. J Med Chem 1998;41:1272-83. [DOI] [PubMed]
- 14.Ciocca DR, Roig LM. Estrogen receptors in human nontarget tissues: biological and clinical implications. Endocr Rev 1995; 16: 35-62. [DOI] [PubMed]
- 15.Epperson CN, Wisner KL, Yamamoto B. Gonadal steroids in the treatment of mood disorders. Psychosom Med 1999;61:676-97. [DOI] [PubMed]
- 16.Sismondi P, Biglia N, Giai M, Ponzone R, Roagna R, Sgro L, et al. HRT, breast and endometrial cancers: strategies and intervention options. Maturitas 1999;32:131-9. [DOI] [PubMed]
- 17.Derzko C. Hormone replacement therapy: helping women make an informed choice (Part I). J Soc Obstet Gynaecol Can 1997; 19(Suppl):1-10.
- 18.Derzko C. Hormone replacement therapy: helping women make an informed choice (Part II). J Soc Obstet Gynaecol Can 1997; 19(Suppl):11-20.
- 19.Sato M, Rippy MK, Bryant HU. Raloxifene, tamoxifen, nafoxidine, or estrogen effects on reproductive and nonreproductive tissues in ovariectomized rats. FASEB J 1996;10:905-12. [DOI] [PubMed]
- 20.Grese TA, Dodge JA. Selective estrogen receptor modulators (SERMs). Curr Pharm Des 1998;4:71-92. [PubMed]
- 21.Ban TA, Guy W, Wilson WH. Description and distribution of the subtypes of chronic schizophrenia based on Leonhard's classification. Psychiatr Dev 1984;2:179-99. [PubMed]
- 22.Lindamer LA, Lohr JB, Harris MJ, Jeste DV. Gender, estrogen, and schizophrenia. Psychopharmacol Bull 1997;33:221-8. [PubMed]
- 23.Hafner H, Riecher A, Maurer K, Loffler W, Munk-Jorgensen P, Stromgren E. How does gender influence age at first hospitalization for schizophrenia? A transnational case register study. Psychol Med 1989;19:903-18. [DOI] [PubMed]
- 24.Hafner H, Behrens S, De Vry J, Gattaz WF. Oestradiol enhances the vulnerability threshold for schizophrenia in women by an early effect on dopaminergic neurotransmission. Evidence from an epidemiological study and from animal experiments. Eur Arch Psychiatry Clin Neurosci 1991;241:65-8. [DOI] [PubMed]
- 25.Dohrenwend BP, Dohrenwend BS. Social and cultural influences on psychopathology. Annu Rev Psychol 1974;25:417-52. [DOI] [PubMed]
- 26.Lewine R, Burbach D, Meltzer HY. Effect of diagnostic criteria on the ratio of male to female schizophrenic patients. Am J Psychiatry 1984;141:84-7. [DOI] [PubMed]
- 27.Iacono WG, Beiser M. Where are the women in first-episode studies of schizophrenia? Schizophr Bull 1992;18:471-80. [DOI] [PubMed]
- 28.Salem JE, Kring AM. The role of gender differences in the reduction of etiologic heterogeneity in schizophrenia. Clin Psychol Rev 1998;18:795-819. [DOI] [PubMed]
- 29.Hambrecht M, Maurer K, Häfner H. Evidence for a gender bias in epidemiological studies of schizophrenia. Schizophr Res 1992; 8:223-31. [DOI] [PubMed]
- 30.Seeman MV. The role of estrogen in schizophrenia. J Psychiatry Neurosci 1996;21:123-7. [PMC free article] [PubMed]
- 31.Hendrick V, Altshuler LL, Burt VK. Course of psychiatric disorders across the menstrual cycle. Harv Rev Psychiatry 1996;4:200-7. [DOI] [PubMed]
- 32.Seeman MV, Lang M. The role of estrogens in schizophrenia gender differences. Schizophr Bull 1990;16:185-94. [DOI] [PubMed]
- 33.Chang SS, Renshaw DC. Psychosis and pregnancy. Compr Ther 1986; 12:36-41. [PubMed]
- 34.Kendell RE, Chalmers JC, Platz C. Epidemiology of puerperal psychoses [published erratum appears in Br J Psychiatry 1987; 151: 135] Br J Psychiatry 1987;150:662-73. [DOI] [PubMed]
- 35.Cetingok M, Chu CC, Park DB. The effect of culture on the sex differences in schizophrenia. Int J Soc Psychiatry 1990;36:272-9. [DOI] [PubMed]
- 36.Marneros A. Frequency of occurrence of Schneider's first rank symptoms in schizophrenia. Eur Arch Psychiatry Neurol Sci 1984; 234:78-82. [DOI] [PubMed]
- 37.Rector NA, Seeman MV. Auditory hallucinations in women and men. Schizophr Res 1992;7:233-6. [DOI] [PubMed]
- 38.Flor-Henry P. Influence of gender in schizophrenia as related to other psychopathological syndromes. Schizophr Bull 1990; 16: 211-27. [DOI] [PubMed]
- 39.Goldstein JM, Link BG. Gender and the expression of schizophrenia. J Psychiatr Res 1988;22:141-55. [DOI] [PubMed]
- 40.Lewine R. Schizophrenia: an amotivational syndrome in men. Can J Psychiatry 1985;30:316-8. [DOI] [PubMed]
- 41.Salokangas RK. Gender and the use of neuroleptics in schizophrenia. Further testing of the oestrogen hypothesis. Schizophr Res 1995;16:7-16. [DOI] [PubMed]
- 42.Tamminga CA. Gender and schizophrenia. J Clin Psychiatry 1997;58:33-7. [PubMed]
- 43.Opjordsmoen S. Long-term clinical outcome of schizophrenia with special reference to gender differences. Acta Psychiatr Scand 1991;83:307-13. [DOI] [PubMed]
- 44.Feighner JP, Robins E, Guze SB, Woodruff RA, Jr, Winokur G, Munoz R. Diagnostic criteria for use in psychiatric research. Arch Gen Psychiatry 1972;26:57-63. [DOI] [PubMed]
- 45.Kulkarni J, de Castella A, Smith D, Taffe J, Keks N, Copolov D. A clinical trial of the effects of estrogen in acutely psychotic women. Schizophr Res 1996;20:247-52. [DOI] [PubMed]
- 46.Korhonen S, Saarijarvi S, Aito M. Successful estradiol treatment of psychotic symptoms in the premenstrual phase: a case report. Acta Psychiatr Scand 1995;92:237-8. [DOI] [PubMed]
- 47.Yassa R, Jeste DV. Gender differences in tardive dyskinesia: a critical review of the literature. Schizophr Bull 1992;18:701-15. [DOI] [PubMed]
- 48.Bédard PJ, Langelier P, Dankova J, Villeneuve A, Di Paolo T, Barden N, et al. Estrogens, progesterone and the extrapyramidal system. In: Poirier LJ, Sourkes TL, Bédard PJ, editors. Advances in Neurology. New York: Raven Press; 1979. p. 411-22.
- 49.Glazer WM, Naftolin F, Moore DC, Bowers MB, MacLusky NJ. The relationship of circulating estradiol to tardive dyskinesia in men and postmenopausal women. Psychoneuroendocrinology 1983; 8:429-34. [DOI] [PubMed]
- 50.Villeneuve A, Langelier P, Bédard P. Estrogens, dopamine and dyskinesias. Can Psychiatr Assoc J 1978;23:68-70. [DOI] [PubMed]
- 51.Villeneuve A, Cazejust T, Cote M. Estrogens in tardive dyskinesia in male psychiatric patients. Neuropsychobiology 1980;6:145-51. [DOI] [PubMed]
- 52.Turrone P, Seeman MV, Silvestri S. Estrogen receptor activation and tardive dyskinesia. Can J Psychiatry 2000;45:288-90. [DOI] [PubMed]
- 53.Glazer WM, Naftolin F, Morgenstern H, Barnea ER, MacLusky NJ, Brenner LM. Estrogen replacement and tardive dyskinesia. Psychoneuroendocrinology 1985;10:345-50. [DOI] [PubMed]
- 54.Gordon JH, Borison RL, Diamond BI. Modulation of dopamine receptor sensitivity by estrogen. Biol Psychiatry 1980;15:389-96. [PubMed]
- 55.Koller WC, Barr A, Biary N. Estrogen treatment of dyskinetic disorders. Neurology 1982;32:547-9. [DOI] [PubMed]
- 56.Hogervorst E, Williams J, Budge M, Riedel W, Jolles J. The nature of the effect of female gonadal hormone replacement therapy on cognitive function in post-menopausal women: a meta-analysis. Neuroscience 2000;101:485-512. [DOI] [PubMed]
- 57.Carlsson A, Hansson LO, Waters N, Carlsson ML. Neurotransmitter aberrations in schizophrenia: new perspectives and therapeutic implications. Life Sci 1997;61:75-94. [DOI] [PubMed]
- 58.Seeman P, Lee T, Chau-Wong M, Wong K. Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature 1976; 261: 717-9. [DOI] [PubMed]
- 59.Reith J, Benkelfat C, Sherwin A, Yasuhara Y, Kuwabara H, Andermann F, et al. Elevated dopa decarboxylase activity in living brain of patients with psychosis. Proc Natl Acad Sci U S A 1994; 91:11651-4. [DOI] [PMC free article] [PubMed]
- 60.Hietala J, Syvalahti E, Vuorio K, Rakkolainen V, Bergman J, Haaparanta M, et al. Presynaptic dopamine function in striatum of neuroleptic-naive schizophrenic patients. Lancet 1995; 346: 1130-1. [DOI] [PubMed]
- 61.Hietala J, Syvalahti E, Vilkman H, Vuorio K, Rakkolainen V, Bergman J, et al. Depressive symptoms and presynaptic dopamine function in neuroleptic-naive schizophrenia. Schizophr Res 1999;35:41-50. [DOI] [PubMed]
- 62.Lindstrom LH, Gefvert O, Hagberg G, Lundberg T, Bergstrom M, Hartvig P, et al. Increased dopamine synthesis rate in medial prefrontal cortex and striatum in schizophrenia indicated by L-(beta-11C) DOPA and PET. Biol Psychiatry 1999;46:681-8. [DOI] [PubMed]
- 63.Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D'Souza CD, Erdos J, et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci U S A 1996; 93: 9235-40. [DOI] [PMC free article] [PubMed]
- 64.Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A, et al. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci U S A 1997;94:2569-74. [DOI] [PMC free article] [PubMed]
- 65.Abi-Dargham A, Gil R, Krystal J, Baldwin RM, Seibyl JP, Bowers M, et al. Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort. Am J Psychiatry 1998; 155:761-7. [DOI] [PubMed]
- 66.Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, et al. From the cover: increased baseline occupancy of D2 receptors by dopamine in schizophrenia, Proc Natl Acad Sci U S A 2000;97:8104-9. [DOI] [PMC free article] [PubMed]
- 67.Becker JB. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol Biochem Behav 1999; 64:803-12. [DOI] [PubMed]
- 68.Rivest R, Falardeau P, Di Paolo T. Brain dopamine transporter: gender differences and effect of chronic haloperidol. Brain Res 1995; 692:269-72. [DOI] [PubMed]
- 69.Thompson TL, Moss RL. Estrogen regulation of dopamine release in the nucleus accumbens: genomic-and nongenomic-mediated effects. J Neurochem 1994;62:1750-6. [DOI] [PubMed]
- 70.Mermelstein PG, Becker JB, Surmeier DJ. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J Neurosci 1996;16:595-604. [DOI] [PMC free article] [PubMed]
- 71.Morissette M, Garcia-Segura LM, Belanger A, Di Paolo T. Changes of rat striatal neuronal membrane morphology and steroid content during the estrous cycle. Neuroscience 1992; 49: 893-902. [DOI] [PubMed]
- 72.Toran-Allerand CD, Singh M, Setalo G Jr. Novel mechanisms of estrogen action in the brain: new players in an old story. Front Neuroendocrinol 1999;20:97-121. [DOI] [PubMed]
- 73.Becker JB, Rudick CN. Rapid effects of estrogen or progesterone on the amphetamine-induced increase in striatal dopamine are enhanced by estrogen priming: a microdialysis study. Pharmacol Biochem Behav 1999;64:53-7. [DOI] [PubMed]
- 74.Schotte A, Janssen PF, Megens AA, Leysen JE. Occupancy of central neurotransmitter receptors by risperidone, clozapine and haloperidol, measured ex vivo by quantitative autoradiography. Brain Res 1993;631:191-202. [DOI] [PubMed]
- 75.Kapur S, Remington G. Serotonin-dopamine interaction and its relevance to schizophrenia. Am J Psychiatry 1996;153:466-76. [DOI] [PubMed]
- 76.Dean B, Hayes W. Decreased frontal cortical serotonin-2A receptors in schizophrenia. Schizophr Res 1996;21:133-9. [DOI] [PubMed]
- 77.Dean B, Hayes W, Hill C, Copolov D. Decreased serotonin2A receptors in Brodmann's area 9 from schizophrenic subjects. A pathological or pharmacological phenomenon? Mol Chem Neuropathol 1998;34:133-45. [DOI] [PubMed]
- 78.Dean B, Hussain T, Hayes W, Scarr E, Kitsoulis S, Hill C, et al. Changes in serotonin2A and GABA(A) receptors in schizophrenia: studies on the human dorsolateral prefrontal cortex. J Neurochem 1999;72:1593-9. [DOI] [PubMed]
- 79.Dean B, Hayes W, Opeskin K, Naylor L, Pavey G, Hill C, et al. Serotonin2 receptors and the serotonin transporter in the schizophrenic brain. Behav Brain Res 1996;73:169-75. [DOI] [PubMed]
- 80.Burnet PW, Chen CP, McGowan S, Franklin M, Harrison PJ. The effects of clozapine and haloperidol on serotonin-1A, -2A and -2C receptor gene expression and serotonin metabolism in the rat forebrain. Neuroscience 1996;73:531-40. [DOI] [PubMed]
- 81.Moses EL, Drevets WC, Smith G, Mathis A, Kalro BN, Butters MA, et al. Effects of estradiol and progesterone administration on human serotonin 2A receptor binding: a PET study. Biol Psychiatry 2000;48:854-60. [DOI] [PubMed]
- 82.Fink G, Sumner BE. Oestrogen and mental state. Nature 1996; 383:306. [DOI] [PubMed]
- 83.Cyr M, Bosse R, Di Paolo T. Gonadal hormones modulate 5-hydroxytryptamine2A receptors: emphasis on the rat frontal cortex. Neuroscience 1998;83:829-36. [DOI] [PubMed]
- 84.Luby ED, Cohen BD, Rosenbaum F, Gottlieb J, Kelley R. Study of a new schizophrenomimetic drug. Sernyl Arch Neurol Psychiatry 1959;81:363-9. [DOI] [PubMed]
- 85.Ellison G. The N-methyl-D-aspartate antagonists phencyclidine, ketamine and dizocilpine as both behavioral and anatomical models of the dementias. Brain ResBrain Res Rev 1995; 20:250-67. [DOI] [PubMed]
- 86.Malhotra AK, Pinals DA, Adler CM, Elman I, Clifton A, Pickar D, et al. Ketamine-induced exacerbation of psychotic symptoms and cognitive impairment in neuroleptic-free schizophrenics. Neuropsychopharmacology 1997;17:141-50. [DOI] [PubMed]
- 87.Abi-Saab WM, D'Souza DC, Moghaddam B, Krystal JH. The NMDA antagonist model for schizophrenia: promise and pitfalls. Pharmacopsychiatry 1998;31(Suppl 2):104-9. [DOI] [PubMed]
- 88.Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia [see comments]. Am J Psychiatry 1991; 148: 1301-8. [DOI] [PubMed]
- 89.Tamminga CA. Schizophrenia and glutamatergic transmission. Crit Rev Neurobiol 1998;12:21-36. [DOI] [PubMed]
- 90.Cyr M, Ghribi O, Di Paolo T. Regional and selective effects of oestradiol and progesterone on NMDA and AMPA receptors in the rat brain. J Neuroendocrinol 2000;12:445-52. [DOI] [PubMed]
- 91.Weiland NG. Estradiol selectively regulates agonist binding sites on the N-methyl-D-aspartate receptor complex in the CA1 region of the hippocampus. Endocrinology 1992;131:662-8. [DOI] [PubMed]
- 92.Gazzaley AH, Weiland NG, McEwen BS, Morrison JH. Differential regulation of NMDAR1 mRNA and protein by estradiol in the rat hippocampus. J Neurosci 1996;16:6830-8. [DOI] [PMC free article] [PubMed]
- 93.Woolley CS, Weiland NG, McEwen BS, Schwartzkroin PA. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: correlation with dendritic spine density. J Neurosci 1997;17:1848-59. [DOI] [PMC free article] [PubMed]
- 94.Mohn AR, Gainetdinov RR, Caron MG, Koller BH. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell 1999;98:427-36. [DOI] [PubMed]
- 95.Hornykiewicz O. The neurochemical basis of the pharmacology of Parkinson's disease. In: Calne DB, editor. Handbook of experimental pharmacology. New York: Springer Verlag; 1989. p. 185-204.
- 96.Van Hartesveldt C, Joyce JN. Effects of estrogen on the basal ganglia. Neurosci Biobehav Rev 1986;10:1-14. [DOI] [PubMed]
- 97.Xie T, Ho SL, Ramsden D. Characterization and implications of estrogenic down-regulation of human catechol-O-methyltransferase gene transcription. Mol Pharmacol 1999;56:31-8. [DOI] [PubMed]
- 98.Quinn NP, Marsden CD. Menstrual-related fluctuations in Parkinson's disease. Mov Disord 1986;1:85-7. [DOI] [PubMed]
- 99.Mizuta E, Yamasaki S, Nakatake M, Kuno S. Neuroleptic malignant syndrome in a parkinsonian woman during the premenstrual period. Neurology 1993;43:1048-9. [DOI] [PubMed]
- 100.Bedard P, Langelier P, Villeneuve A. Oestrogens and extrapyramidal system. Lancet 1977;2:1367-8. [DOI] [PubMed]
- 101.Session DR, Pearlstone MM, Jewelewicz R, Kelly AC. Estrogens and Parkinson's disease. Med Hypotheses 1994;42:280-2. [DOI] [PubMed]
- 102.Giladi N, Honigman S. Hormones and Parkinson's disease. Neurology 1995;45:1028-9. [DOI] [PubMed]
- 103.Saunders-Pullman R, Gordon-Elliott J, Parides M, Fahn S, Saunders HR, Bressman S. The effect of estrogen replacement on early Parkinson's disease. Neurology 1999;52:1417-21. [DOI] [PubMed]
- 104.Tsang KL, Ho SL, Lo SK. Estrogen improves motor disability in parkinsonian postmenopausal women with motor fluctuations. Neurology 2000;54:2292-8. [DOI] [PubMed]
- 105.Blanchet PJ, Fang J, Hyland K, Arnold LA, Mouradian MM, Chase TN. Short-term effects of high-dose 17beta-estradiol in postmenopausal PD patients: a crossover study. Neurology 1999; 53:91-5. [DOI] [PubMed]
- 106.Strijks E, Kremer JA, Horstink MW. Effects of female sex steroids on Parkinson's disease in postmenopausal women. Clin Neuropharmacol 1999;22:93-7. [DOI] [PubMed]
- 107.Diamond SG, Markham CH, Hoehn MM, McDowell FH, Muenter MD. An examination of male-female differences in progression and mortality of Parkinson's disease. Neurology 1990; 40:763-6. [DOI] [PubMed]
- 108.Kurtzke JF,Goldberg ID. Parkinsonism death rates by race, sex, and geography. Neurology 1988;38:1558-61. [DOI] [PubMed]
- 109.Mutch WJ, Dingwall-Fordyce I, Downie AW, Paterson JG, Roy SK. Parkinson's disease in a Scottish city. Br Med J (Clin Res Ed) 1986;292:534-6. [DOI] [PMC free article] [PubMed]
- 110.Mayeux R, Denaro J, Hemenegildo N, Marder K, Tang MX, Cote LJ, et al. A population-based investigation of Parkinson's disease with and without dementia. Relationship to age and gender. Arch Neurol 1992;49:492-7. [DOI] [PubMed]
- 111.Bower JH, Maraganore DM, McDonnell SK, Rocca WA. Incidence and distribution of parkinsonism in Olmsted County, Minnesota, 1976-1990. Neurology 1999;52:1214-20. [DOI] [PubMed]
- 112.Li SC, Schoenberg BS, Wang CC, Cheng XM, Rui DY, Bolis CL, et al. A prevalence survey of Parkinson's disease and other movement disorders in the People's Republic of China. Arch Neurol 1985;42:655-7. [DOI] [PubMed]
- 113.Marder K, Tang MX, Mejia H, Alfaro B, Cote L, Louis E, et al. Risk of Parkinson's disease among first-degree relatives: A community-based study. Neurology 1996;47:155-60. [DOI] [PubMed]
- 114.Baldereschi M, Di Carlo A, Rocca WA, Vanni P, Maggi S, Perissinotto E, et al. Parkinson's disease and parkinsonism in a longitudinal study: two-fold higher incidence in men. ILSA Working Group. Italian Longitudinal Study on Aging. Neurology 2000;55:1358-63. [DOI] [PubMed]
- 115.Bower JH, Maraganore DM, McDonnell SK, Rocca WA. Influence of strict, intermediate, and broad diagnostic criteria on the age- and sex-specific incidence of Parkinson's disease. Mov Disord 2000;15:819-25. [DOI] [PubMed]
- 116.de Rijk MC, Breteler MM, Graveland GA, Ott A, Grobbee DE, van der Meche FG, et al. Prevalence of Parkinson's disease in the elderly: the Rotterdam Study. Neurology 1995;45:2143-6. [DOI] [PubMed]
- 117.Lyons KE, Hubble JP, Troster AI, Pahwa R, Koller WC. Gender differences in Parkinson's disease. Clin Neuropharmacol 1998;21:118-21. [PubMed]
- 118.Jenner P, Olanow CW. Understanding cell death in Parkinson's disease. Ann Neurol 1998;44:S72-84. [DOI] [PubMed]
- 119.Polymeropoulos MH. Genetics of Parkinson's disease. Ann N Y Acad Sci 2000;920:28-32. [DOI] [PubMed]
- 120.Priyadarshi A, Khuder SA, Schaub EA, Shrivastava S. A meta-analysis of Parkinson's disease and exposure to pesticides. Neurotoxicology 2000;21:435-40. [PubMed]
- 121.Riess O, Kuhn W, Kruger R. Genetic influence on the development of Parkinson's disease. J Neurol 2000;247(Suppl 2):69-74. [DOI] [PubMed]
- 122.Alexi T, Borlongan CV, Faull RL, Williams CE, Clark RG, Gluckman PD, et al. Neuroprotective strategies for basal ganglia degeneration: Parkinson's and Huntington's diseases. Prog Neurobiol 2000;60:409-70. [DOI] [PubMed]
- 123.Marsden CD, Olanow CW. The causes of Parkinson's disease are being unraveled and rational neuroprotective therapy is close to reality. Ann Neurol 1998;44(3 Suppl 1):S189-96. [DOI] [PubMed]
- 124.Honda K, Sawada H, Kihara T, Urushitani M, Nakamizo T, Akaike A, et al. Phosphatidylinositol 3-kinase mediates neuroprotection by estrogen in cultured cortical neurons. J Neurosci Res 2000;60:321-7. [DOI] [PubMed]
- 125.Scallet AC. Estrogens: neuroprotective or neurotoxic? Ann N Y Acad Sci 1999;890:121-32. [DOI] [PubMed]
- 126.Leranth C, Roth RH, Elswoth JD, Naftolin F, Horvath TL, Redmond DE. Estrogen is essential for maintaining nigrostriatal dopamine neurons in primates: implications for Parkinson's disease and memory. J Neurosci 2000;20:8604-9. [DOI] [PMC free article] [PubMed]
- 127.Nilsen J, Mor G, Naftolin F. Estrogen-regulated developmental neuronal apoptosis is determined by estrogen receptor subtype and the Fas/Fas ligand system. J Neurobiol 2000;43:64-78. [DOI] [PubMed]
- 128.Langston JW, Forno LS, Rebert CS, Irwin I. Selective nigral toxicity after systemic administration of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyrine (MPTP) in the squirrel monkey. Brain Res 1984;292:390-4. [DOI] [PubMed]
- 129.Heikkila RE, Hess A, Duvoisin RC. Dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine in mice. Science 1984;224:1451-3. [DOI] [PubMed]
- 130.Przedborski S, Jackson-Lewis V. Mechanisms of MPTP toxicity. Mov Disord 1998;13:35-8. [PubMed]
- 131.Wagner GC, Tekirian TL, Cheo CT. Sexual differences in sensitivity to methamphetamine toxicity. J Neural Transm Gen Sect 1993; 93:67-70. [DOI] [PubMed]
- 132.Miller DB, Ali SF, O'Callaghan JP, Laws SC. The impact of gender and estrogen on striatal dopaminergic neurotoxicity. Ann N Y Acad Sci 1998;844:153-65. [PubMed]
- 133.Dluzen DE, McDermott JL, Liu B. Estrogen alters MPTP-induced neurotoxicity in female mice: effects on striatal dopamine concentrations and release. J Neurochem 1996;66:658-66. [DOI] [PubMed]
- 134.Dluzen DE, McDermott JL, Liu B. Estrogen as a neuroprotectant against MPTP-induced neurotoxicity in C57/B1 mice. Neurotoxicol Teratol 1996;18:603-6. [DOI] [PubMed]
- 135.Callier S, Morissette M, Grandbois M, Di Paolo T. Stereospecific prevention by 17beta-estradiol of MPTP-induced dopamine depletion in mice. Synapse 2000;37:245-51. [DOI] [PubMed]
- 136.Grandbois M, Morissette M, Callier S, Di Paolo T. Ovarian steroids and raloxifene prevent MPTP-induced dopamine depletion in mice. Neuroreport 2000;11:343-6. [DOI] [PubMed]
- 137.Yu L, Liao PC. Estrogen and progesterone distinctively modulate methamphetamine-induced dopamine and serotonin depletions in C57BL/6J mice. J Neural Transm 2000;107:1139-47. [DOI] [PubMed]
- 138.Sawada H, Shimohama S. Neuroprotective effects of estradiol in mesencephalic dopaminergic neurons. Neurosci Biobehav Rev 2000; 24:143-7. [DOI] [PubMed]
- 139.Pasqualini C, Olivier V, Guibert B, Frain O, Leviel V. Acute stimulatory effect of estradiol on striatal dopamine synthesis. J Neurochem 1995;65:1651-7. [DOI] [PubMed]
- 140.Miranda R, Sohrabji F, Singh M, Toran-Allerand D. Nerve growth factor (NGF) regulation of estrogen receptors in explant cultures of the developing forebrain. J Neurobiol 1996;31:77-87. [DOI] [PubMed]
- 141.Son JH, Chun HS, Joh TH, Cho S, Conti B, Lee JW. Neuroprotection and neuronal differentiation studies using substantia nigra dopaminergic cells derived from transgenic mouse embryos. J Neurosci 1999;19:10-20. [DOI] [PMC free article] [PubMed]
- 142.Offen D, Beart PM, Cheung NS, Pascoe CJ, Hochman A, Gorodin S, et al. Transgenic mice expressing human Bcl-2 in their neurons are resistant to 6-hydroxydopamine and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity. Proc Natl Acad Sci U S A 1998;95:5789-94. [DOI] [PMC free article] [PubMed]
- 143.Yang L, Matthews RT, Schulz JB, Klockgether T, Liao AW, Martinou JC, et al. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity is attenuated in mice overexpressing Bcl-2. J Neurosci 1998;18:8145-52. [DOI] [PMC free article] [PubMed]
- 144.Garcia-Segura LM, Cardona-Gomez P, Naftolin F, Chowen JA. Estradiol upregulates Bcl-2 expression in adult brain neurons. Neuroreport 1998;9:593-7. [DOI] [PubMed]
- 145.Laflamme N, Nappi RE, Drolet G, Labrie C, Rivest S. Expression and neuropeptidergic characterization of estrogen receptors (ERalpha and ERbeta) throughout the rat brain: anatomical evidence of distinct roles of each subtype. J Neurobiol 1998;36:357-78. [DOI] [PubMed]
- 146.Kritzer MF, Kohama SG. Ovarian hormones differentially influence immunoreactivity for dopamine beta-hydroxylase, choline acetyltransferase, and serotonin in the dorsolateral prefrontal cortex of adult rhesus monkeys. J Comp Neurol 1999;409:438-51. [DOI] [PubMed]
- 147.Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERalpha and ERbeta expressed in Chinese hamster ovary cells. Mol Endocrinol 1999;13:307-19. [DOI] [PubMed]
- 148.Raab H, Pilgrim C, Reisert I. Effects of sex and estrogen on tyrosine hydroxylase mRNA in cultured embryonic rat mesencephalon. Brain Res Mol Brain Res 1995;33:157-64. [DOI] [PubMed]
- 149.Beyer C, Karolczak M. Estrogenic stimulation of neurite growth in midbrain dopaminergic neurons depends on cAMP/protein kinase A signalling. J Neurosci Res 2000;59:107-16. [PubMed]
- 150.Behl C, Skutella T, Lezoualc'h F, Post A, Widmann M, Newton CJ, et al. Neuroprotection against oxidative stress by estrogens: structure-activity relationship. Mol Pharmacol 1997;51:535-41. [PubMed]
- 151.Green PS, Bishop J, Simpkins JW. 17 alpha-estradiol exerts neuroprotective effects on SK-N-SH cells. J Neurosci 1997;17:511-5. [DOI] [PMC free article] [PubMed]
- 152.Sawada H, Ibi M, Kihara T, Urushitani M, Akaike A, Shimohama S. Estradiol protects mesencephalic dopaminergic neurons from oxidative stress-induced neuronal death. J Neurosci Res 1998;54:707-19. [DOI] [PubMed]
- 153.Sawada H, Ibi M, Kihara T, Urushitani M, Honda K, Nakanishi M, et al. Mechanisms of antiapoptotic effects of estrogens in nigral dopaminergic neurons. FASEB J 2000;14:1202-14. [DOI] [PubMed]
- 154.Shoulson I. Where do we stand on neuroprotection? Where do we go from here? Mov Disord 1998;13:46-8. [PubMed]
- 155.Disshon KA, Boja JW, Dluzen DE. Inhibition of striatal dopamine transporter activity by 17beta-estradiol. Eur J Pharmacol 1998;345:207-11. [DOI] [PubMed]
- 156.Disshon KA, Dluzen DE. Estrogen as a neuromodulator of MPTP-induced neurotoxicity: effects upon striatal dopamine release. Brain Res 1997;764:9-16. [DOI] [PubMed]
- 157.Dluzen DE, McDermott JL. Neuroprotective role of estrogen upon methamphetamine and related neurotoxins within the nigrostriatal dopaminergic system. Ann N Y Acad Sci 2000; 914: 112-26. [DOI] [PubMed]
- 158.Arvin M, Fedorkova L, Disshon KA, Dluzen DE, Leipheimer RE. Estrogen modulates responses of striatal dopamine neurons to MPP(+): evaluations using in vitro and in vivo techniques. Brain Res 2000;872:160-71. [DOI] [PubMed]
- 159.Stoof JC, Winogrodzka A, van Muiswinkel FL, Wolters EC, Voorn P, Groenewegen HJ, et al. Leads for the development of neuroprotective treatment in Parkinson's disease and brain imaging methods for estimating treatment efficacy. Eur J Pharmacol 1999;375:75-86. [DOI] [PubMed]
- 160.Hiemke C, Ghraf R. Interaction of non-steroidal antiestrogens with dopamine receptor binding. J Steroid Biochem 1984;21:663-7. [DOI] [PubMed]
- 161.Toney TW, Katzenellenbogen BS. An evaluation of the interactions of antiestrogens with pituitary and striatal dopamine receptors. J Recept Res 1987;7:695-712. [DOI] [PubMed]
- 162.Ferretti C, Blengio M, Ghi P, Racca S, Genazzani E, Portaleone P. Tamoxifen counteracts estradiol induced effects on striatal and hypophyseal dopamine receptors. Life Sci 1988;42:2457-65. [DOI] [PubMed]
- 163.McDermott JL, Liu B, Dluzen DE. Tamoxifen treatment of ovariectomized mice alters dopamine release from striatal tissue fragments superfused in vitro. Brain Res 1995;698:248-52. [DOI] [PubMed]
- 164.McDermott JL, Anderson LI, Dluzen DE. Interactive effects of tamoxifen and estrogen upon the nigrostriatal dopaminergic system. Neuroendocrinology 1997;66:181-7. [DOI] [PubMed]
- 165.McDermott JL, Anderson LI, Dluzen DE. Tamoxifen alters dopamine output through direct actions upon superfused corpus striatal tissue fragments. Neurochem Int 1998;32:299-307. [DOI] [PubMed]
- 166.McDermott JL, Anderson LI, Dluzen DE. Interactive effects of tamoxifen and oestrogen upon the nigrostriatal dopaminergic system: long-term treatments. J Neuroendocrinol 1999;11:801-3. [DOI] [PubMed]
- 167.Nicoletti F, Speciale C, Sortino MA, Panetta MS, Di Giorgio RM, Canonico PL. Estrogen effects on nigral glutamic acid decarboxylase activity: a possible role for catecholestrogen. Eur J Pharmacol 1985;115:297-300. [DOI] [PubMed]
- 168.Chaurasia CS, Chen CE, Rubin J, Dewey SL. Effects of tamoxifen on striatal dopamine and 5-hydroxytryptamine release in freely moving male rats: an in-vivo microdialysis investigation. J Pharm Pharmacol 1998;50:1377-85. [DOI] [PubMed]
- 169.Silva I, Mello LE, Freymuller E, Haidar MA, Baracat EC. Estrogen, progestogen and tamoxifen increase synaptic density of the hippocampus of ovariectomized rats. Neurosci Lett 2000; 291: 183-6. [DOI] [PubMed]
- 170.Murphy DD, Segal M. Regulation of dendritic spine density in cultured rat hippocampal neurons by steroid hormones. J Neurosci 1996;16:4059-68. [DOI] [PMC free article] [PubMed]
- 171.Nickelsen T, Lufkin EG, Riggs BL, Cox DA, Crook TH. Raloxifene hydrochloride, a selective estrogen receptor modulator: safety assessment of effects on cognitive function and mood in postmenopausal women. Psychoneuroendocrinology 1999; 24: 115-28. [DOI] [PubMed]
- 172.Wu X, Glinn MA, Ostrowski NL, Su Y, Ni B, Cole HW, et al. Raloxifene and estradiol benzoate both fully restore hippocampal choline acetyltransferase activity in ovariectomized rats. Brain Res 1999;847:98-104. [DOI] [PubMed]
- 173.McEwen BS, Tanapat P, Weiland NG. Inhibition of dendritic spine induction on hippocampal CA1 pyramidal neurons by a nonsteroidal estrogen antagonist in female rats. Endocrinology 1999; 140:1044-7. [DOI] [PubMed]
- 174.Luine VN, McEwen BS. Effects of an estrogen antagonist on enzyme activities and [3H]estradiol nuclear binding in uterus, pituitary and brain. Endocrinology 1977;100:903-10. [DOI] [PubMed]
- 175.Genazzani AR, Bernardi F, Stomati M, Rubino S, Giardina L, Luisi S, et al. Raloxifene analog LY 117018 effects on central and peripheral beta-endorphin. Gynecol Endocrinol 1999;13:249-58. [DOI] [PubMed]
- 176.Genazzani AR, Bernardi F, Stomati M, Monteleone P, Luisi S, Rubino S, et al. Effects of estradiol and raloxifene analog on brain, adrenal and serum allopregnanolone content in fertile and ovariectomized female rats. Neuroendocrinology 2000;72:162-70. [DOI] [PubMed]
- 177.Cyr M, Landry M, Di Paolo T. Modulation by estrogen-receptor directed drugs of 5-hydroxytryptamine-2A receptors in rat brain. Neuropsychopharmacology 2000;23:69-78. [DOI] [PubMed]
- 178.Deutch AY, Cameron DS. Pharmacological characterization of dopamine systems in the nucleus accumbens core and shell. Neuroscience 1992;46:49-56. [DOI] [PubMed]
- 179.Sumner BE, Grant KE, Rosie R, Hegele-Hartung C, Fritzemeier K, Fink G. Effects of tamoxifen on serotonin transporter and 5-hydroxytryptamine(2A) receptor binding sites and mRNA levels in the brain of ovariectomized rats with or without acute estradiol replacement. Brain Res Mol Brain Res 1999;73:119-28. [DOI] [PubMed]
- 180.Cyr M, Thibault C, Morissette M, Landry M, Di Paolo T. Estrogen-like activity of tamoxifen and raloxifene on NMDA receptor binding and expression of its subunits in rat brain. Neuropsychopharmacology. In press. [DOI] [PubMed]
- 181.Cyr M, Morissette M, Landry M, Di Paolo T. Estrogenic activity of tamoxifen and raloxifene on rat brain AMPA receptors. Neuroreport 2001;12:535-9. [DOI] [PubMed]
- 182.Deutch AY. The regulation of subcortical dopamine systems by the prefrontal cortex: interactions of central dopamine systems and the pathogenesis of schizophrenia. J Neural Transm Suppl 1992;36:61-89. [DOI] [PubMed]
- 183.Singer CA, Rogers KL, Strickland TM, Dorsa DM. Estrogen protects primary cortical neurons from glutamate toxicity. Neurosci Lett 1996;212:13-6. [DOI] [PubMed]
- 184.Anderson LI, McDermott JL, Dluzen DE. Estrogen-tamoxifen interactions upon striatal dopaminergic neurotoxicity produced by MPTP. Soc Neurosci Abstr 1999:25(2):2088.
- 185.Gao X, Dluzen DE. Tamoxifen abolishes estrogen's neuroprotective effect upon methamphetamine neurotoxicity of the nigrostriatal dopaminergic system. Neuroscience 2001;103:385-94. [DOI] [PubMed]
- 186.Nilsen J, Mor G, Naftolin F. Raloxifene induces neurite outgrowth in estrogen receptor positive PC12 cells. Menopause 1998; 5:211-6. [PubMed]
- 187.Jones KP. Oral contraception: current use and attitudes. Contraception 1999;59:17S-20S. [DOI] [PubMed]
- 188.Carr BR. Uniqueness of oral contraceptive progestins. Contraception 1998;58:23S-7S;quiz 67S. [DOI] [PubMed]
- 189.Dluzen DE, Ramirez VD. Modulatory effects of progesterone upon dopamine release from the corpus striatum of ovariectomized estrogen-treated rats are stereo-specific. Brain Res 1991; 538:176-9. [DOI] [PubMed]
- 190.Gereau RW, Kedzie KA, Renner KJ. Effect of progesterone on serotonin turnover in rats primed with estrogen implants into the ventromedial hypothalamus. Brain Res Bull 1993;32:293-300. [DOI] [PubMed]
- 191.Renner KJ, Krey LC, Luine VN. Effect of progesterone on monoamine turnover in the brain of the estrogen-primed rat. Brain Res Bull 1987;19:195-202. [DOI] [PubMed]
- 192.Morissette M, Levesque D, Belanger A, Di Paolo T. A physiological dose of estradiol with progesterone affects striatum biogenic amines. Can J Physiol Pharmacol 1990;68:1520-6. [DOI] [PubMed]
- 193.Di Paolo T, Levesque D, Daigle M. A physiological dose of progesterone affects rat striatum biogenic amine metabolism. Eur J Pharmacol 1986;125:11-6. [DOI] [PubMed]
- 194.Chaudhuri SK, Chattopadhyay RN, Maitra SK, Ray S, Chaudhuri S. Effects of progesterone on some brain neurotransmitters in intact rats. Indian J Physiol Pharmacol 1992;36:255-8. [PubMed]
- 195.Dluzen DE, Ramirez VD. Progesterone enhances L-dopa-stimulated dopamine release from the caudate nucleus of freely behaving ovariectomized-estrogen-primed rats. Brain Res 1989; 494:122-8. [DOI] [PubMed]
- 196.Attali G, Weizman A, Gil-Ad I, Rehavi M. Opposite modulatory effects of ovarian hormones on rat brain dopamine and serotonin transporters. Brain Res 1997;756:153-9. [DOI] [PubMed]
- 197.Gomez-Mancilla B, Bedard PJ. Effect of estrogen and progesterone on L-dopa induced dyskinesia in MPTP-treated monkeys. Neurosci Lett 1992;135:129-32. [DOI] [PubMed]
- 198.Hernandez ML, Fernandez-Ruiz JJ, de Miguel R, Ramos JA. Time-dependent effects of ovarian steroids on tyrosine hydroxylase activity in the limbic forebrain of female rats. J Neural Transm Gen Sect 1991;83:77-84. [DOI] [PubMed]
- 199.Fernandez-Ruiz JJ, de Miguel R, Hernandez ML, Ramos JA. Time-course of the effects of ovarian steroids on the activity of limbic and striatal dopaminergic neurons in female rat brain. Pharmacol Biochem Behav 1990;36:603-6. [DOI] [PubMed]
- 200.Archer JSM. Relationship between estrogen, serotonin, and depression. Menopause 1999;6:71-8. [PubMed]
- 201.Pearce J, Hawton K, Blake F. Psychological and sexual symptoms associated with the menopause and the effects of hormone replacement therapy. Br J Psychiatry 1995;167:163-73. [DOI] [PubMed]
- 202.Sherwin BB, Gelfand MM. A prospective one-year study of estrogen and progestin in postmenopausal women: effects on clinical symptoms and lipoprotein lipids. Obstet Gynecol 1989; 73: 759-66. [PubMed]
- 203.Holst J, Backstrom T, Hammarback S, von Schoultz B. Progestogen addition during oestrogen replacement therapy—effects on vasomotor symptoms and mood. Maturitas 1989; 11:13-20. [DOI] [PubMed]
- 204.Behl C. Alzheimer's disease and oxidative stress: implications for novel therapeutic approaches. Prog Neurobiol 1999;57:301-23. [DOI] [PubMed]
- 205.Behl C, Widmann M, Trapp T, Holsboer F. 17-beta estradiol protects neurons from oxidative stress-induced cell death in vitro. Biochem Biophys Res Commun 1995;216:473-82. [DOI] [PubMed]
- 206.Abolfathi Z, Di Paolo T. Modulation of dopamine receptor agonist binding sites by cations and estradiol in intact pituitary and 7315a tumors. Biochem Pharmacol 1991;42:2163-9. [DOI] [PubMed]
- 207.Levesque D, Di Paolo T. Modulation by estradiol and progesterone of the GTP effect on striatal D-2 dopamine receptors. Biochem Pharmacol 1993;45:723-33. [DOI] [PubMed]
- 208.Rado A, Crystle CD, Townsley JD. Concentration of estrogens in maternal peripheral plasma in late pregnancy, during labor and post partum. J Clin Endocrinol Metab 1970;30:497-503. [DOI] [PubMed]
- 209.Simpkins JW, Green PS, Gridley KE, Singh M, de Fiebre NC, Rajakumar G. Role of estrogen replacement therapy in memory enhancement and the prevention of neuronal loss associated with Alzheimer's disease. Am J Med 1997;103:19S-25S. [DOI] [PubMed]
- 210.Merchenthaler I, Shugrue PJ. Estrogen receptor-beta: a novel mediator of estrogen action in brain and reproductive tissues. Morphological considerations. J Endocrinol Invest 1999;22:10-2. [PubMed]
- 211.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Academic Press; 1982.