Abstract
Human cytomegalovirus (HCMV) terminase is composed of subunits pUL56 (130 kDa) and pUL89 (∼75 kDa), encoded by the UL56 and UL89 genes. In a recent investigation, we demonstrated that the main ATPase activity is associated with the large terminase subunit pUL56. The protein has two putative ATP-binding sites, which were suggested to be composed of the sequence (amino acids 463–470) for ATP-binding site 1 and YNETFGKQ (amino acids 709–716) for the second site. We now demonstrate using a 1.5 kb fragment encoding the C-terminal half of pUL56 that ATP-binding site 1 is not critical for the function, whereas ATP-binding site 2 is required for the enzymatic activity. Mutation G714A in this protein reduced the ATPase activity to ∼65% and the double mutation G714A/K715N showed a reduction up to 75%. However, the substitution of E711A revoked the effect of the substitutions. The functional character of the ATP-binding site was demonstrated by transfer of YNETFGKQLSIACLR (709–723) to glutathione-S-transferase (GST). Interestingly, vanadate, an ATPase inhibitor, has the ability to block the ATPase activity of pUL56 as well as of Apyrase, while the antitumor ATP-mimetic agent geldanamycin, did not affect the ATP-binding of pUL56. Furthermore, in contrast to an inactive control compound, the specific HCMV terminase inhibitor BDCRB showed a partial inhibition of the pUL56-specific ATPase activity. Our results clearly demonstrated that (i) the enzymatic activity of the terminase subunit pUL56 could be inhibited by vanadate, (ii) only the ATP-binding site 2 is critical for the pUL56 function and (iii) glycine G714 is an invariant amino acid.
INTRODUCTION
Human cytomegalovirus (HCMV) DNA replication leads to the formation of concatemeric DNA, which must be resolved into genomic lengths during packaging. The packaging process requires the recognition of specific cis-acting elements, pac1 and pac2, by viral proteins, so-called terminases (1–3). Once the substrates, concatemers, are cleaved the unit-length genomes are nuclease resistant (4). Resulting unit-length genomes are encapsidated into preformed capsids and organized into a highly condensed, ordered structure (5). This condensation of DNA inside the capsid—up to 5000-fold concentrated compared with DNA in solution—reveals an accurate process. While different models of the DNA arrangement in the capsid have been proposed in the past, current evidence favors one model. Cerritelli et al. (6) and Zhang et al. (7) demonstrated by electron microscopy that the DNA is spooled across an axis in six coaxial shells. This packaging strategy enables the DNA to be as condensed as possible inside the viral heads.
Terminases were first described as specific enzymatic proteins for dsDNA bacteriophages and carry multiple activities, which are required for the whole packaging process (8–10). Bacteriophages that cut DNA at specific sites along the concatemers into unit-length genomes are λ, T3 and T7 (11–13). Terminases also drive DNA translocation to the capsids by providing ATPase activity. Studies by Guo et al. (14) and Shibata et al. (2) demonstrated that hydrolysis of one ATP provides energy to translocate 2 bp under in vitro conditions. In almost all bacteriophages the large terminase subunit catalyzes the ATP-dependent translocation of genomic DNA to the proheads (11,15–19). Although a number of models have been proposed, the mechanism of DNA translocation is not completely understood in any system.
In the case of HCMV, we recently demonstrated that the terminase consists of two proteins, the large subunit pUL56 (130 kDa) and the small subunit pUL89 (75 kDa). While the large subunit pUL56 is required for DNA-binding and capsid association, pUL89 is predominantly involved in the final cleavage of unit-length genomes (20,21). Both proteins are highly conserved throughout the herpesviruses; however, functional data are only available of the HSV-1 homolog of pUL56 (UL28). Adelman et al. (22) demonstrated that pUL28 is a sequence-specific DNA-binding protein and suggested it plays a role in viral DNA packaging. Furthermore, we showed that the subunit pUL56 has an abundant ATPase activity and could therefore provide energy for the translocation of the concatemers to the preformed capsids (23). In this study we generated a variety of pUL56 mutants carrying amino acid substitutions in the putative ATP-binding sites and analyzed their effect on ATP hydrolysis. Exclusively the ATP-binding site 2 is required for pUL56 function.
MATERIALS AND METHODS
Bacteria
Escherichia coli strain BL21 codon plus was used as the host strain for UL56-carrying pGEX-5X-1 constructs.
Plasmid construction and oligonucleotides
Plasmid GST–UL56C was generated by digestion of pRC/CMV-UL56 (24) and pGEX-5X-1 (Amersham Pharmacia Biotech, Freiburg, Germany) as described previously (24). The 1.5 kb fragment encoding the C-terminal half of HCMV pUL56 (amino acids 446–850) was ligated in-frame into pGEX-5X-1, yielding pGEX-UL56C.
To generate the construct GST–ATP containing the ATP-binding motif the plasmid pGEX-5X-1 was digested with EcoRI and XhoI prior to insertion of a fragment encod ing the amino acids 709–723 of HCMV pUL56. The respective fragment was obtained using the following annealed pair of synthetic oligonucleotides (restrictions sites are underlined): 5′-GGATTCCTATATAATGAAACTTTCG GGAAACAACTCTCGATCGCGTGCCTGCG-3′; 5′-AAC TCGAGCGCAGGCACGCGATCGAGAGTTGTTTCCCG AAAGTTTCATTATATAG-3′.
Oligonucleotide-directed mutagenesis of GST–UL56C (A and B) and GST–ATP (C). Construct (A) ATP-binding site 1: GST–UL56C G468A, GST–UL56C K469N, GST–Ul56C G468A/K469N, GST–UL56CΔGK; construct (B) ATP-binding site 2: GST–UL56C G714A, GST–UL56C K715N, GST–UL56C E711A, GST–UL56C G714A/K715N, GST–UL56C E711A/G114A, GST–UL56C E711A/K715N, GST–UL56C E711A/G714A/K715N, GST–UL56CΔGK; construct (C) ATP-binding motif: GST–ATP G714A, GST–ATP K715N and GST–ATP G714A/K715/N were generated using the QuickChangeTM site-directed mutagenesis kit (Stratagene) as recommended by the supplier and the following pairs of synthetic oligonucleotides as described in Table 1 (construct GST–UL56C or GST–ATP served as template). All mutations were verified by DNA sequencing.
Table 1. Oligonucleotide primers used in PCR.
Protein purification
A fresh overnight culture of Escherichia coli BL21 carrying the plasmid pGEX-UL56C or the plasmids with mutant UL56C was diluted 1:100 in LB medium (1 l) containing 50 µg/ml ampicillin and incubated at 37°C. After the cells reached an OD600 of 0.5 the GST-protein expression was induced by addition of isopropyl-1-thio-β-d-galactopyranoside to a final concentration of 0.1 mM and incubated for 2 h at 37°C. Sedimented cells were lysed in 40 ml binding buffer (20 mM sodium phosphate, 0.15 M NaCl, pH 7.4) containing 250 µl 1 M MgCl2, 25 µl 1 M MnCl, 40 µl DNase I (10 mg/ml) and 400 µl lysozyme (10 mg/ml) incubated on ice for 30 min and sonicated on ice. Undissolved material was sedimented at 5000 g at 4°C and passed through a 0.2 µm filter. The purification was performed with a 1 ml GSTrap™ column by using a Äkta™ Prime machine (Amersham Pharmacia Biotech) according to the instruction of the manufacturer. The column was washed with three bed volumes binding buffer prior to loading the proteins. Elution was performed with 10 bed volumes elution buffer (50 mM Tris–HCl, 10 mM glutathione, pH 8.0) and 10 fractions were collected. The fractions were analyzed by SDS–PAGE. Fractions containing the proteins were stored at –80°C.
ATPase activity
Purified GST-fusion proteins GST–UL56C or GST–UL56C mutants or the control proteins GST (each 0.3 µg) and Apyrase were incubated with 1 µCi of [γ-33P] ATP (specific activity 2500 Ci/mmol) in 30 µl of nuclease buffer [10 mM Tris–HCl (pH 7.5), 10 mM MgCl2, 1 mM DTT, 50 mM NaCl]. The samples were incubated at 37°C for 30 min. The reaction was terminated by adding EDTA up to a final concentration of 50 mM. A 1 µl aliquot of the reaction mixture was applied onto a 20 cm long PEI-F cellulose strip (Merck Eurolab, Darmstadt, Germany) and dried. The chromatogram was developed in 1 M formic acid and 0.5 M LiCl according to Guo et al. (14). The run was stopped when the solvent front reached 1 cm from the upper edge, dried and autoradiographed on Kodak BIOMAX MR. Radioactive signals were quantified using a BioImager and AIDA software (Raytest). The values of produced Pi were calculated by subtracting the GST-only value from each GST-fusion protein value. Non-radioactive nucleotides were used as standards. The spots were visualized under UV light and marked on the cellulose.
Inhibitors
Vanadate was obtained from Sigma and geldanamycin was a free sample from ICS Clinical Source GmbH (München, Germany). BDCRB [2-bromo-5,6-dichloro-1-(β-d-ribofuranosyl)benzimidazole] and CDMRB [2-chloro-5,6-dimethyl-1-(β-d-ribofuranosyl)benzimidazole] were synthesized in the laboratory of Professor L.B.Townsend (25,26, respectively).
RESULTS
Modification of GST–UL56C
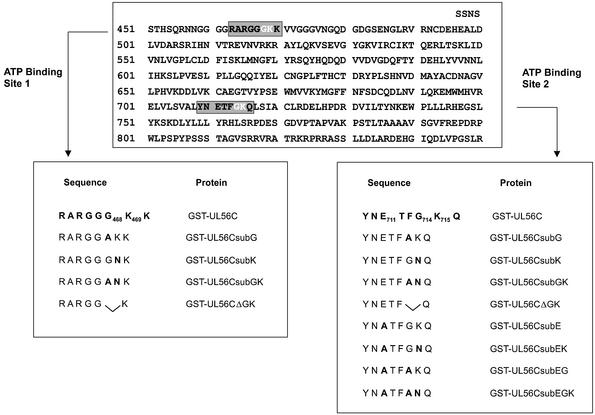
Based on the proposal by Walker et al. (27) glycine-rich motifs (G/AXXXXGKT/S) could constitute the minimal ATPase center. Sequence analysis of pUL56 revealed two possible regions, which fulfill the requirements for ATP-binding sites (Fig. 1). Both regions were located in the C-terminal half of the protein, the ATP-binding site 1 RARGGGKK (amino acids 462–470) and ATP-binding site 2 YNETFGKQ (amino acids 709–715). To identify critical amino acids several mutations were introduced by site-directed mutagenesis (Fig. 1). The expressed C-terminal portion of pUL56 is termed ‘GST–UL56C’ herein and mutations in the protein are named accordingly (Fig. 1).
Figure 1.
Schematic diagram of the C-terminal half of pUL56 and location of putative ATP-binding sites. Below the sequence are the amino acids of the wild-type and mutant proteins of each putative ATP-binding center.
The first ATP-binding site has no effect on UL56-associated ATPase activity
To analyze the role of the first ATP-binding site ATPase activity assays with mutations of glycine468 and lysine469 were performed. Hydrolysis of radiolabeled ATP of purified proteins was assayed following chromatographic separation of ATP and ADP + Pi on PEI-F cellulose. Reaction in the presence of wild-type GST–UL56C as well as Apyrase (EC 3.6.1.5) generated 33Pi (data not shown). Comparable observations were made by using the mutants G468A (GST–UL56CsubG), K469N (GST–UL56CsubK), G468A/K469N (GST–UL56Csub GK) and the protein with the deletion of GK (GST–UL56CΔGK). Thus demonstrating that the ATP-binding site 1 is not involved in ATP hydrolysis.
A single G714A mutation is responsible for the loss of ATPase activity
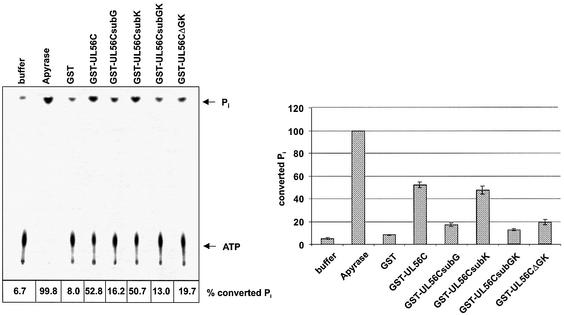
In order to determine the functions of the glycine714 and the lysine715 in the putative second ATP-binding domain the effect of substitutions were analyzed. ATP hydrolysis was verified by incubation with Apyrase (Fig. 2, Apyrase). While the substitution of K715N (Fig. 2, GST–UL56CsubK) had only a minimal effect, the G714A reduced the amount of ATP hydrolysis >65% (Fig. 2, GST–UL56CsubG). The same observation was obtained by deletion of the glycine/lysine residues (Fig. 2, GST–UL56CΔGK). The inhibitory effect was increased to ∼75% by the double substitution G714A/K715N (Fig. 2, GST–UL56CsubGK). GST as well as buffer were used as control reactions (Fig. 2). These experiments lead to the suggestion that glycine714 is required to constitute the ATPase center of the ATP-binding site 2.
Figure 2.
Analysis of the second ATP-binding site in mutant proteins. [γ-33P]ATP (1 µCi) was used as substrate. Lanes: 1, buffer control; 2, Apyrase; 3, GST, 4, GST–UL56C; 5, GST–UL56CsubG; 6, GST–UL56CsubK; 7, GST–UL56CsubGK; 8, GST–UL56CΔGK. The position of ATP and Pi are indicated by arrows. The relative amounts of hydrolyzed ATP are indicated by percent values and graphically depicted in the diagram. Error bars on the histogram are ± standard deviation from three different experiments.
The E711A mutation increases ATP hydrolysis
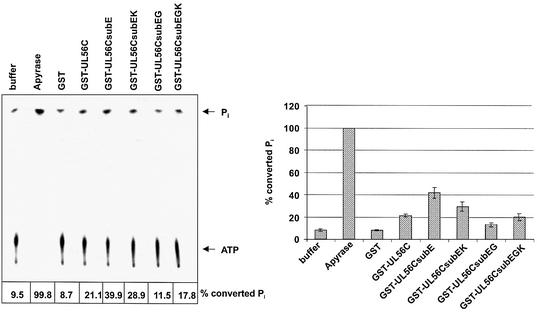
To further characterize the ATPase center the glutamic acid711 was mutated. Interestingly, the effect of E711A (Fig. 3, GST–UL56CsubE) was contrary to the G714A substitution; the enzymatic activity increased compared with the wild-type GST–UL56C. To determine the efficiency of ATP hydrolysis the reaction must be in a linear range. Therefore, the concentrations of the proteins were reduced 10-fold. The amount of increased ATPase activity was reduced in proteins with double mutations. The mutant E711A/K715N reduced the effect of the mutant E711A to 55% (Fig. 3, GST–UL56CsubEK) and E711A/G714A restored the inhibitory effect (Fig. 3, GST–UL56CsubEG). However, the triple mutant E711A/G714A/K715N had only a minor inhibitory effect (24%) compared with the mutant E711/G714 (56%; Fig. 3, Gst–UL56Csub EGK). Hydrolysis of radiolabeled ATP into ADP and Pi was measured by incubation with Apyrase (Fig. 3). GST, as well as the buffer, were used as control reactions (Fig. 3). Our results demonstrated that the substitution of glutamic acid711 increase the amount of ATP hydrolysis, indicating that the enzymatic pocket has a better accessibility.
Figure 3.
Influence of E711 mutants on the ATP hydrolysis. [γ-33P]ATP (1 µCi) was used as the substrate. Lanes; 1, buffer; 2, Apyrase; 3, GST; 4, GST–UL56C; 5, GST–UL56CsubE; 6, GST–UL56CsubEK; 7, GST–UL56CsubEG; 8, GST–UL56CsubEGK. The position of ATP and Pi are indicated by arrows. The amount of hydrolyzed Pi is shown on the bottom and in the graphic diagram. Error bars on the histogram are ± standard deviation from three different experiments.
Amino acid residues 709–723 of HCMV pUL56 are sufficient for ATPase activity
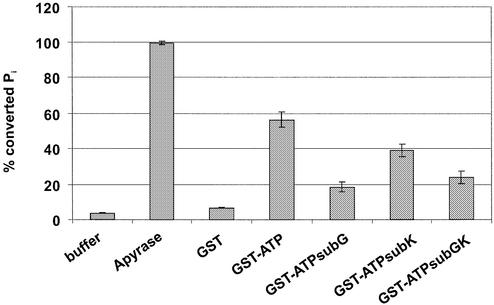
To further determine the functional character of the ATP-binding site, the pUL56 amino acids 709–723 sequence was fused to GST, and was termed GST–ATP. Interestingly, in the linear range the short protein had ATPase activity comparable with the C-terminal part of pUL56 (Fig. 4, GST–ATP). However, the mutation in GST–ATPsubG714A had the highest inhibitory effect (72%; Fig. 4, GST–ATPsubG) compared with the mutant GST–ATPsubK715N (38%; Fig. 4, GST–ATPsubK). The mutation in GST–ATPG714A/K715N had a minor inhibitory effect (48%) compared with the mutant GST–ATPsubE711A/G714A (54%; Fig. 4, GST– ATPsubEG). These results demonstrated that the short stretch of amino acids YNETFGKQLSIACLR is sufficient for ATPase activity, and indicated that it represents the ATP-binding site with the invariant amino acid glycine 714.
Figure 4.
Analysis of a short stretch of ATP-binding site. [γ-33P]ATP (1 µCi) was used as substrate. Lanes: 1, buffer control; 2, Apyrase; 3, GST, 4, GST–ATP; 5, GST–ATPsubG; 6, GST–ATPsubK; 7, GST–ATPsubEG; 8, GST–ATPΔGK. The position of ATP and Pi are indicated by arrows. The relative amounts of hydrolyzed ATP are indicated by percent values in the diagram. Error bars on the histogram are ± standard deviation from three different experiments.
Inhibition of the ATPase activity by vanadate
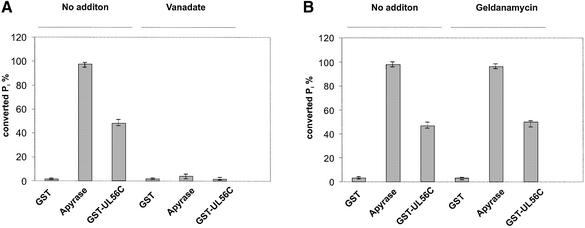
To investigate the effects of vanadate and geldanamycin, ATPase activity assays were performed in the presence of the inhibitors. The proteins were pre-incubated in the presence of 90 µM geldanamycin or 0.5 mM vanadate for 15 min at room temperature before the reaction was started by addition of ATP. Nearly 95% inhibition of Apyrase was obtained in the presence of vanadate (Fig. 5A). A similar observation was made by incubation with GST–UL56C, where 0.5 mM vanadate inhibited 96% of the activity (Fig. 6A). The control GST protein did not show an effect (Fig. 5A). Geldanamycin, the inhibitor of Hsp90, had neither an effect on Apyrase nor on GST–UL56C (Fig. 5B) thus demonstrating that vanadate could specifically inhibit the ATPase activity of GST–UL56C.
Figure 5.
Effect of vanadate and geldanamycin on the ATPase activity of pUL56. Hydrolysis of [γ-33P]ATP by GST, Apyrase or GST–UL56C were carried out for 45 min prior to termination of the reaction by adding 50 mM EDTA. Reactions in the presence of inhibitors (90 µM geldanamycin, 0.5 mM vanadate) were pre-incubated for 15 min at room temperature before addition of [γ-33P]ATP. The relative amounts of hydrolyzed ATP are indicated by percent values in the diagram. Error bars on the histogram are ± standard deviation from three different experiments.
Figure 6.
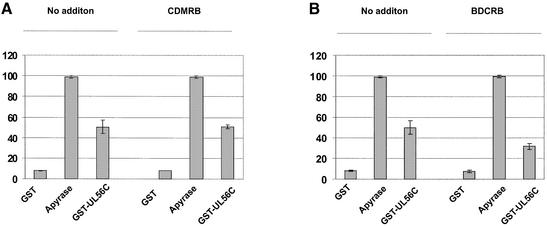
Influence of BDCRB on the ATPase activity of pUL56. [γ-33P]ATP (1 µCi) was used as substrate. The reactions of GST, Apyrase or GST–UL56C were terminated after 45 min by addition of 50 mM EDTA. Reactions in the presence of 60 µM BDCRB or 60 µM CDMRB were pre-incubated for 20 min at room temperature before addition of [γ-33P]ATP. The relative amounts of hydrolyzed ATP are indicated by percent values in the diagram. Error bars on the histogram are ± standard deviation from three different experiments.
Influence of BDCRB on the pUL56-associated ATPase activity
Experiments in the presence of BDCRB were carried out to reveal whether this anti-HCMV drug that targets pUL56 and pUL89 (28,29) had an effect on the ATPase activity. The results demonstrated that BDCRB had no effect on the enzyme activity of Apyrase (Fig. 6B). However, enzymatic activity of pUL56 in the presence of 60 µM BDCRB was reduced to at least 40% (Fig. 6B). Even in high concentrations the related control compound CDMRB—that is not active against HCMV—did not affect the enzyme reaction (Fig. 6A). These results show that a high concentration of BDCRB could reduce the pUL56-associated ATPase activity.
DISCUSSION
ATP plays multiple roles in viral DNA packaging catalyzed by terminases. ATPase activity of dsDNA bacteriophages terminases is required for strand separation after cleavage and translocation of genomic DNA into procapsids (11,14–16,30,31). It also has been reported that ATP binding, but not hydrolysis, is necessary for the accuracy of the endonucleolytic cleavage (30). Rao and Mitchell (32) demonstrated that the first ATP-binding site of the large terminase subunit gp17 of phage T4 is required for DNA packaging. Since different reactions require ATP it is tempting to speculate that different ATP-reactive sites in terminases exist.
Recently, we demonstrated that the HCMV large terminase subunit pUL56 exhibits a high ATPase activity (23). In this study we generated a number of mutants for identification and characterization of the ATP-binding site of pUL56. The classical ATPase catalytic centers have so-called phosphate-binding loops (P loop), which consist of a glycine-rich sequence that often has a lysine residue near the C-terminus (14,33,34). Based on the deduced primary amino acid sequence, pUL56 has two putative ATP-binding sites in the C-terminal half of the protein (Fig. 1, amino acids 463–470 and 709–716).
We investigated whether the ATP-binding site 1 (RARGGGKK) is involved in the pUL56-associated ATP hydrolysis. By site-directed mutagenesis it was shown that neither the glycine (G468) nor the lysine residue (K469) within the putative ATP-binding site 1 are critical for the pUL56 function. However, substitutions of amino acids in the second proposed ATP-binding site (YNETFGKQ) indicated that amino acid residues at positions 714 and 715 (G and K at amino acid positions 714 and 715 of pUL56) were essential for hydrolysis of ATP. By changing the lysine K715 into asparagine the ATPase activity was only slightly reduced (∼10%), however, a mutation changing the glycine G714 into alanine has a more severe effect; it reduced the hydrolysis by ∼70%. Interestingly, in bacteriophage λ the lysine K35 is an invariant amino acid of the P loop. Changing K35 to alanine or glutamic acid had a dramatic effect, because it altered the packaging activity and altered the affinity to DNA (35). However, the mutant has a similar ATPase activity to the wild type. In E.coli the lysine mutant K177Q of the RecBDC enzyme resulted in a dramatic reduction of ATP hydrolysis (36). A comparable observation was reported by Haber and Walker (37) of the change of lysine of the Salmonella MutS protein. Although the lysine is reported to be a linker to ATP by interaction of its ε-amino group with the β-, γ-phosphates of ATP (38), the mutation K715N had only a minimal effect on the ATP hydrolysis mediated by pUL56. Together with the observation from Yu and Weller (39) that a mutation from GKT to AKT in HSV-1 UL15 was unable to complement a UL15 mutant virus, it is tempting to speculate that in herpes viruses the conserved glycine and not the lysine of the P loop is critical for binding ATP in the catalytic center.
Furthermore, after changing the glutamic acid E711 into alanine the enzymatic activity increased up to 89% compared with the wild-type pUL56. However, the double mutant E711A/G714A reduced the ATP hydrolysis comparable with the single G714A mutant. Amino acids like asparagine, asparagine acid, histidine and serine are the catalytic nucleophile of hydrolases (40,41). The P loop of pUL56 contains an asparagine residue at amino acid 710 and two serine residues at amino acids 705 and 718. Therefore, one could speculate that substitution of E711A thereby facilitates the access of these amino acids, which could result in an increased ATP hydrolysis. The putative roles of the asparagine (N710), serine (S705) and serine (S718) residues remain to be determined.
To further underline the specificity of the ATPase activity of HCMV pUL56 we used the inhibitors vanadate and geldanamycin. Vanadate has been used as an inhibitor of (Na-K) ATPase or other ATPases since Josepheson and Cantley (42) demonstrated this property. Geldanamycin, an ansamycin antibiotic, is known to inhibit the inherent ATPase activity of heat-shock protein Hsp90 (43). Geldanamycin was used as a control for contamination with chaperons. We have detected an inhibitory effect of vanadate on the ATP hydrolysis by pUL56 as well as by Apyrase of nearly 95%, whereas the enzymatic activity could not be inhibited by the antitumor antibiotic geldanamycin. Thus, confirming that pUL56 is the HCMV large terminase subunit providing energy for the translocation of the DNA into the procapsids.
In addition we used a specific anti-HCMV agent, BDCRB (25), that is known to inhibit HCMV DNA replication via the involvement of the terminase subunits pUL89 (28) and pUL56 (29). We investigated the ability of BDCRB to inhibit ATP hydrolysis. Interestingly BDCRB reduced the pUL56-associated ATPase activity to ∼40%. Recently we demonstrated that UL89-associated nuclease activity is reduced in the presence of high concentration of BDCRB (21). Together with our former observation, one could speculate that the effect of BDCRB in the infected cell might be a combination of the inhibition of the nuclease and ATPase activity. Both effects together should be sufficient to account for the potent inhibition of HCMV replication.
In conclusion, in this study we demonstrated that: (i) the large terminase subunit pUL56 contains two putative ATP-binding sites, but only the ATP-binding site 2 is involved in pUL56-associated ATP hydrolysis; (ii) glycine G714 is an invariant amino acid of the P loop; and (iii) pUL56-associated ATPase could be inhibited by vanadate and reduced with the specific terminase inhibitor BDCRB. Further studies will need to be undertaken aimed at a deepening of our understanding of the translocating process during DNA packaging.
Acknowledgments
ACKNOWLEDGEMENTS
E.B. thanks B. Fleckenstein for kind support. This study was supported by the Deutsche Forschungsgemeinschaft (Bo 1214/5-1) and the Johannes and Frieda Marohn Foundation.
REFERENCES
- 1.Chou J. and Roizman,B. (1985) The isomerization of the herpes simplex virus 1 genome: identification of the cis-acting and recombination sites within the domain of the a sequence. Cell, 41, 803–811. [DOI] [PubMed] [Google Scholar]
- 2.Shibata H., Fujisawa,H. and Minagawa,T. (1987) Characterization of the bacteriophage T3 DNA packaging reaction in vitro in a defined system. J. Mol. Biol., 196, 845–851. [DOI] [PubMed] [Google Scholar]
- 3.Spaete R.R. and Mocarski,E.S. (1985) The a sequence of the cytomegalovirus genome functions as a cleavage/packaging signal for herpes simplex virus defective genomes. J. Virol., 54, 817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deiss L.P., Chou,J. and Frenkel,N. (1986) Functional domains within the a sequence involved in the cleavage-packaging of herpes simplex virus DNA. J. Virol., 59, 605–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Earnshaw W.C. and Casjens,S.R. (1980) DNA packaging by the double-stranded DNA bacteriophages. Cell, 21, 319–331. [DOI] [PubMed] [Google Scholar]
- 6.Cerritelli M.E., Cheng,N., Rosenberg,A.H., McPherson,C.F., Booy,F.P. and Steven,A.C. (1997) Encapsidated conformation of bacteriophage T7 DNA. Cell, 91, 271–280. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Z., Green,B., Thuman-Commike,P.A., Jakana,J., Prevelige,P.E.,Jr, King,J. and Chiu,W. (2000) Visualization of the maturation transition in bacteriophage P22 by electron cryomicroscopy. J. Mol. Biol., 297, 615–629. [DOI] [PubMed] [Google Scholar]
- 8.Black L.W. (1988) DNA packaging in dsDNA bacteriophages. In Calendar,R. (ed), The Bacteriophages. Plenum, New York, USA, pp. 321–378.
- 9.Bhattacharyya S.P. and Rao,V.B. (1993) A novel terminase activity associated with the DNA-packaging protein gp17 of bacteriophage T4. Virology, 196, 34–44. [DOI] [PubMed] [Google Scholar]
- 10.Feiss M. and Becker,A. (1983) DNA packaging and cutting. In Hendrix,R.W., Roberts,J.W., Stahl,F.W. and Weisberg,R.A. (eds), Lambda II. Cold Spring Harbor, New York, USA, pp. 305–330.
- 11.Becker A. and Murialdo,H. (1990) Bacteriophage lambda DNA: the beginning of the end. J. Bacteriol., 172, 2819–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujisawa H. and Hearing,P. (1994) Structure, function and specificty of the DNA packaging signals in double-stranded DNA viruses. Semin. Virol., 5, 5–13. [Google Scholar]
- 13.Feiss M. (1986) Structure, function and specificity of the DNA packaging signals in double-stranded DNA viruses. Trends Genet., 2, 100–104. [Google Scholar]
- 14.Guo P., Peterson,C. and Anderson,D. (1987) Prohead and DNA-gp3-dependent ATPase activity of the DNA packaging protein gp16 of bacteriophage phi 29. J. Mol. Biol., 197, 229–236. [DOI] [PubMed] [Google Scholar]
- 15.Catalano C.E., Cue,D. and Feiss,M. (1995) Virus DNA packaging: the strategy used by phage lambda. Mol. Microbiol., 16, 1075–1086. [DOI] [PubMed] [Google Scholar]
- 16.Hang J.Q., Tack,B.F. and Feiss,M. (2000) ATPase center of bacteriophage lambda terminase involved in post-cleavage stages of DNA packaging: identification of ATP-interactive amino acids. J. Mol. Biol., 302, 777–795. [DOI] [PubMed] [Google Scholar]
- 17.Bhattacharyya S.P. and Rao,V.B. (1994) Structural analysis of DNA cleaved in vivo by bacteriophage T4 terminase. Gene, 146, 67–72. [DOI] [PubMed] [Google Scholar]
- 18.Morita M., Taska,M. and Fujisawa,H. (1993) DNA packaging ATPase of bacteriophage T3. Virology, 193, 748–752. [DOI] [PubMed] [Google Scholar]
- 19.Rao V.B. and Black,L.M. (1988) Cloning, overexpression and purification of the terminase proteins gp16 and gp17 of bacteriophage T4. Construction of a defined in-vitro DNA packaging system using purified terminase proteins. J. Mol. Biol., 200, 475–488. [DOI] [PubMed] [Google Scholar]
- 20.Bogner E., Radsak,K. and Stinski,M.F. (1998) The gene product of human cytomegalovirus open reading frame UL56 binds the pac motif and has specific nuclease activity. J. Virol., 72, 2259–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scheffczik H., Savva,C.G.W, Holzenburg,A., Kolesnikova,L. and Bogner,E. (2002) The terminase subunits pUL56 and pUL89 of human cytomegalovirus are DNA metabolizing proteins with toroidal structure. Nucleic Acids Res., 30, 1695–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adelman K., Salmon,B. and Baines,J.D. (2001) Herpes simplex virus DNA packaging sequences adopt novel structures that are specifically recognized by a component of the cleavage and packaging machinery. Proc. Natl Acad. Sci. USA, 98, 3086–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hwang J.-S. and Bogner,E. (2002) ATPase activity of the terminase subunit pUl56 of human cytomegalovirus. J. Biol. Chem., 277, 6943–6948. [DOI] [PubMed] [Google Scholar]
- 24.Giesen K., Radsak,K. and Bogner,E. (2000) The potential terminase subunit pUL56 of human cytomegalovirus is translocated into the nucleus by its own NLS and interacts with importin α. J. Gen. Virol., 81, 2231–2244. [DOI] [PubMed] [Google Scholar]
- 25.Townsend L.B., Devivar,R.V., Turk,S.R., Nassiri,M.R. and Drach,J.C. (1995) Design, synthesis and antiviral activity of certain 2,5,6-trihalo-1-(beta-d-ribofuranosyl) benzimidazoles. J. Med. Chem., 38, 4098–4150. [DOI] [PubMed] [Google Scholar]
- 26.Townsend L.B. and Revankar,G.R. (1970) Benzimidazole nucleosides, nucleotides and related derivatives. Chem. Rev., 70, 389–438. [DOI] [PubMed] [Google Scholar]
- 27.Walker J.E., Sarasate,M., Runswick,M.J. and Gay,N.J. (1982) Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J., 1, 945–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Underwood M.R., Harvey,R.J., Stanat,S.C., Hemphill,M.L., Miller,T., Drach,J.C., Townsend,L.B. and Biron,K.K. (1998) Inhibition of human cytomegalovirus DNA maturation by a benzimidazole ribonucleoside is mediated through the UL89 gene product. J. Virol., 72, 717–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krosky P.M., Underwood,M.R., Turk,S.R., Feng,K.W-H, Jain,R.K., Ptak,R.G., Westerman,A.C., Biron,K.K., Townsend,L.B. and Drach,J.C. (1998). Resistance of human cytomegalovirus to benzimidazole ribonucleosides maps to two open reading frames: UL89 and UL56. J. Virol., 72, 4721–4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higgins R.R. and Becker,A. (1994) The lambda terminase enzyme measures the point of its endonucleolytic attack 47 +/– 2 bp away from its site of specific DNA binding, the R site. EMBO J., 13, 6152–6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubinchik S., Parris,W. and Gold,M. (1994) The in vitro ATPases of bacteriophage lambda terminase and its large subunit, gene product A. The relationship with their DNA helicase and packaging activities. J. Biol. Chem., 269, 13586–13593. [PubMed] [Google Scholar]
- 32.Rao V.B. and Mitchell,M.S. (2001) The N-terminal ATPase site in the large terminase protein gp17 is critically required for DNA packaging in bacteriophage T4. J. Mol. Biol., 314, 401–411. [DOI] [PubMed] [Google Scholar]
- 33.Kuebler D. and Rao,V.B. (1998) Functional analysis of the DNA-packaging/terminase protein gp17 from bacteriophage T4. J. Mol. Biol., 281, 803–814. [DOI] [PubMed] [Google Scholar]
- 34.Saraste M., Sibbald,P.R. and Wittinghofer,A. (1990) The P-loop—a common motif in ATP- and GTP-binding proteins. Trends Biochem. Sci., 15, 430–434. [DOI] [PubMed] [Google Scholar]
- 35.Hwang Y. and Feiss,M. (1997) Mutations affecting lysine-35 of gpNu1, the small subunit of bacteriophage lambda terminase, alter the strength and specificity of holoterminase interactions with DNA. Virology, 231, 218–230. [DOI] [PubMed] [Google Scholar]
- 36.Korangy F. and Julin,D.A. (1992) Alteration by site-directed mutagenesis of the conserved lysine residue in the ATP-binding consensus sequence of the RecD subunit of them Escherichia coli RecBCD enzyme. J. Biol. Chem., 267, 1727–1732. [PubMed] [Google Scholar]
- 37.Haber L.T. and Walker,G.C. (1991) Altering the conserved nucleotide binding motif in the Salmonella typhimurium MutS mismatch repair protein affects both its ATPase and mismatch binding activities. EMBO J., 10, 2707–2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Story R.M. and Steitz,T.A. (1992) Structure of the recA protein-ADP complex. Nature, 355, 374–376. [DOI] [PubMed] [Google Scholar]
- 39.Yu D. and Weller,S.K. (1998) Herpes simplex virus type 1 cleavage and packaging proteins UL15 and UL28 are associated with B but not C capsids during packaging.Virology, 243, 32–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arand M., Wagner,H. and Oesch,F. (1996) Asp333, Asp495 and His523 form the catalytic triad of rat soluble epoxide hydrolase. J. Biol. Chem., 271, 4223–4229. [DOI] [PubMed] [Google Scholar]
- 41.Arand M., Muler,F., Mecky,A., Hinz,W., Urban,P., Pompon,D., Keller,R. and Oesch,F. (1999) Catalytic triad of microsomal epoxide hydrolase: replacement of Glu404 with Asp leads to a strongly increased turnover rate. Biochem. J., 337, 37–43. [PMC free article] [PubMed] [Google Scholar]
- 42.Josephson L. and Cantley,L.C.,Jr (1979) Isolation of a potent (Na-K)ATPase inhibitor from striated muscle. Biochemistry, 16, 4572–4578. [DOI] [PubMed] [Google Scholar]
- 43.Whitesell L., Mimnaugh,E.G., Decosta,B., Myers,C.E. and Neckers,L.M. (1994) Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc. Natl Acad. Sci. USA, 91, 8324–8328. [DOI] [PMC free article] [PubMed] [Google Scholar]