Abstract
Transgenic tadpoles that express a dominant negative thyroid hormone (TH) receptor specifically in their skin undergo normal metamorphosis, with one exception: they retain a larval epidermis over the developing adult epithelium. TH-induced death of the tadpole epidermis is inhibited by the dominant negative TH receptor whereas the TH-induced response of the neighboring fibroblasts and the cells that form the adult skin occur normally. Therefore death of the tadpole skin is a direct and cell autonomous target of TH, and its protection has no detectable influence on TH-induced changes of other cell types.
The epidermis is one of many amphibian tadpole tissues that are remodeled at metamorphosis (reviewed in refs. 1 and 2). The tadpole epidermis consists of two to three cell layers in contact with an acellular collagen lamella. The premetamorphic dermis consists of the collagen lamella, an adjacent single layer of subepithelial fibroblasts, and melanophores (2). As thyroid hormone (TH) concentration rises in the tadpole during metamorphic climax the outer two cell layers of the epidermis are thought to die by apoptosis (3–6). The basal cells of the tadpole epidermis are progenitors for the adult frog epidermis (7–9). At metamorphosis the subepithelial fibroblasts secrete proteolytic enzymes such as collagenase-3 and invade the collagen lamella, the dermis thickens, and adult skin glands form (2, 10). A major difference between the tadpole and frog skin is that all cells in the tadpole epidermis replicate whereas only the basal cells replicate in frog skin (11). This latter “germinative” replication is characteristic of the epidermis of all adult vertebrate species whereas the self-replicating tadpole epidermis resembles the mammalian fetal skin called the periderm (12).
There are several molecular markers that distinguish gene expression in tadpole from frog skin. Subtractive hybridization of Xenopus laevis tails identified four genes that are down-regulated by TH (13). All four of these genes are expressed only in the apical or outer cell layer of the tadpole skin (14). A larval keratin is expressed from late embryogenesis throughout tadpole life in the apical and skein cells of the epidermis (15). An adult keratin has been identified that is activated at metamorphosis in the basal cells of the body epidermis (16). Recently, additional larval and adult members of the keratin gene family have been identified (9, 17, 18).
Although it is known that the larval epidermis dies during metamorphosis and that TH induces keratinization and detachment of epidermis cells from culture dishes (5, 6), we sought to determine whether this death occurs autonomously in vivo as a result of TH induction or indirectly by cell–cell interaction, perhaps as a result of the secreted proteases from the adjacent subepithelial fibroblasts. In addition, it has been proposed that products of the tadpole skin might play a role in the dissolution of other cell types in the tail (19, 20), and an epidermal collagenase has been reported to be up-regulated at metamorphosis in the bullfrog (21). Using the X. laevis promoter for larval keratin (15) we have targeted expression of a dominant negative TH receptor (TRDN) to the tadpole epidermis. Our results demonstrate that the larval epidermis is a direct target of TH. These transgenic tadpoles retain a larval epidermis even though they otherwise metamorphose normally and form an adult epidermis under the protected larval epidermal cells.
Materials and Methods
Plasmids and Transgenesis.
The X. laevis larval keratin promoter (Ker) was synthesized by amplifying 922 bp of upstream sequences from the X. laevis genomic DNA (GenBank accession no. XO4807; ref. 15) and cloned into a pCS2+-based vector (Ker:GFP), as described (22). The preparation of a TH receptor (TR)α cDNA truncated at the C terminus (amino acid 401) has been described (23). The C-terminal deletion removes the ability of the TR to bind TH and deletes a site for coactivator binding and therefore functions as a TRDN. The TR protein is known to reside in the nucleus and the effects of the TRDN are limited to the cell within which the protein lies. This is the basis for interpreting the effect of a TRDN as cell autonomous. The Ker:TRDN construct was made by replacing the GFP sequences from the Ker:GFP construct with the TRDN cDNA.
Transgenic animals were prepared by restriction enzyme-mediated integration into sperm nuclei (24), using minor modifications (25). Tadpoles transgenic for Ker:GFP were identified by their GFP fluorescence at day 4 of development by using a Leica MZ12 fluorescent stereo microscope. The Ker:TRDN construct was coinjected with a second construct, CRY1:GFP3, that expresses GFP in the eye lens under control of the X. laevis γ-crystallin promoter (a gift of Robert Grainger, University of Virginia, Charlottesville). Tadpoles transgenic for Ker:TRDN were screened by identifying GFP fluorescence in the eyes. Successful cotransgenesis occurs ≈80% of the time when two plasmids are injected together as a mixture (25). Transgenesis for Ker:TRDN was always confirmed by PCR.
3,5,3′ Triiodothyronine (T3) Treatment, Immunohistochemistry, and in Situ Hybridization.
Staging of tadpoles was by the method of Nieuwkoop and Faber (NF) (26). Tadpoles at different stages were fixed in paraformaldehyde. The dorsal body skin and the underlying connective tissue (region between the hindlimbs and forelimbs) was peeled off, embedded, cryosectioned, and processed for hematoxylin and eosin histochemistry and in situ hybridization using digoxigenin-labeled antisense probes against gene 19, larval keratin (a gift of Thomas Sargent, National Institutes of Health, Bethesda), collagenase-3, and adult keratin as described (10).
To verify that the larval keratin promoter drives specifically in the tadpole epidermis, NF stage 54 tadpoles transgenic for Ker:GFP (n = 6) were fixed in paraformaldehyde, cryosectioned, and processed for immunohistochemistry as described (23). Larval keratin and GFP were detected in cryosections by using rabbit polyclonal primary antibodies against larval keratin (1/1,000 dilution, gift of Thomas Sargent) and GFP (1/1,000 dilution, Torrey Pines Biolabs, San Diego), and Alexa Fluor 488-conjugated anti-rabbit secondary antibody (Molecular Probes).
NF stage 54 tadpoles transgenic for Ker:TRDN (n = 6) were induced for 48 h with 30 nM of T3 diluted in 0.1× MMR (10 mM NaCl/0.2 mM KCl/0.1 mM MgCl2/0.2 mM CaCl2/0.5 mM Hepes, pH 7.5). The induced tadpoles, as well as a group of uninduced transgenic and control WT tadpoles (n = 3), were then fixed in paraformaldehyde. Their tails were embedded, cryosectioned, and processed for hematoxylin and eosin histochemistry and in situ hybridization as described above. To observe apoptosis in the epithelium, dorsal skin and the underlying connective tissue were peeled off and immunostained in whole mount with a primary antibody against an active form of caspase-3 (PharMingen, rabbit monoclonal, 1:250 dilution) and Alexa Fluor 488-conjugated anti-rabbit secondary Ab (1:400 dilution, Molecular Probes), as described (27). NF stage 64 tadpoles transgenic for Ker:TRDN (n = 10) undergoing spontaneous metamorphosis were also fixed in paraformaldehyde, and their dorsal body skin and tails were removed for cytology.
Results
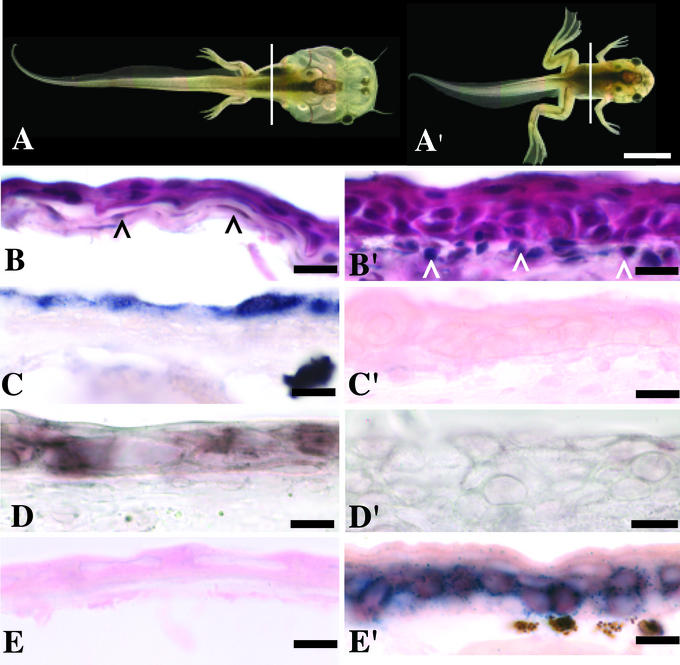
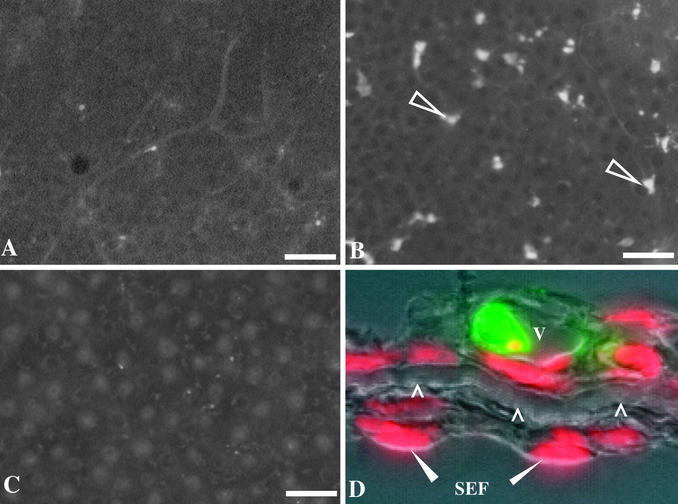
Before metamorphic climax (NF 57) the tadpole skin consists of two to three cell layers, supported by an acellular collagen lamella and a single cell layer of subepithelial fibroblasts (Fig. 1B). By late metamorphic climax (NF 62) the skin has remodeled. The epithelium has become three to five cells thick, and the subepithelial fibroblasts have invaded the collagen lamella (Fig. 1B′). The larval skin-specific gene down-regulated at metamorphosis (gene 19) is expressed only in the outer most apical cell layer of the epidermis (Fig. 1C). A larval keratin has been identified that is expressed in the tadpole skin (14) (Fig. 1D). Expression of these genes is greatly reduced by NF 60 (not shown) and absent by NF 62 (Fig. 1 C′ and D′). An adult keratin is not expressed in tadpoles (Fig. 1E) but is strongly expressed by NF 62 (Fig. 1E′), in basal cells that are the progenitors of adult skin. Active caspase-3 immunoreactivity (a marker of apoptosis) is rarely present in the skin cells during premetamorphosis (NF 57) (Fig. 2A) but is widespread in the epithelium at the start of climax (NF 60) (Fig. 2B), a stage coinciding with the loss of larval-specific epidermal gene expression. Active caspase-3 immunoreactivity generally is restricted to the first two layers of tadpole epidermal cells. These cells are often highly vacuolated, a characteristic of autophagy (Fig. 2D). By late climax (NF 62) the scarcity of active caspase-3 immunoreactive cells is similar to that found in premetamorphic skin (Fig. 2C).
Figure 1.
Larval and adult-specific epidermal markers. Tadpoles and cross sections taken from dorsal skin (vertical lines) of premetamorphic stage NF 57 (A–E) and metamorphic climax stage NF 62 (A′–E′). Sections were stained with hematoxylin and eosin (B and B′), in situ hybridizations with antisense RNA probes (dark blue/purple) against gene 19 (C and C′), larval keratin (D and D′), and adult keratin (E and E′). Eosin stains the epithelial layers bright pink; black arrowheads (B) denote subepithelial fibroblasts, white arrowheads (B′) denote adult dermis. [Scale bars: 500 μM (A and A′) and 5 μM (B–E′).]
Figure 2.
Larval epithelial cell death at metamorphic climax coincides with expression of active caspase-3. Active caspase-3 immunoreactivity detected in whole-mount processed skin from the body is rarely present in larval skin before metamorphosis (NF 57) (A), strongly present at the start of metamorphic climax (NF 60; arrows) (B), and rarely present at the peak of climax (NF 62) after the larval skin has been replaced by adult skin (C). Adult skin glands present at NF 62 are weakly autofluorescent. (D) Active caspase-3 immunoreactivity (green) in cross sections of NF 60 skin is confined to a vacuolated cell (v) in the larval epithelium; red, 4′,6-diamidino-2-phenylindole (DAPI) nuclear counterstain; arrowheads, collagen lamella; solid arrowheads, subepithelial fibroblasts (SEF). [Scale bars: 100 μM (A–C) and 5 μM (D).]
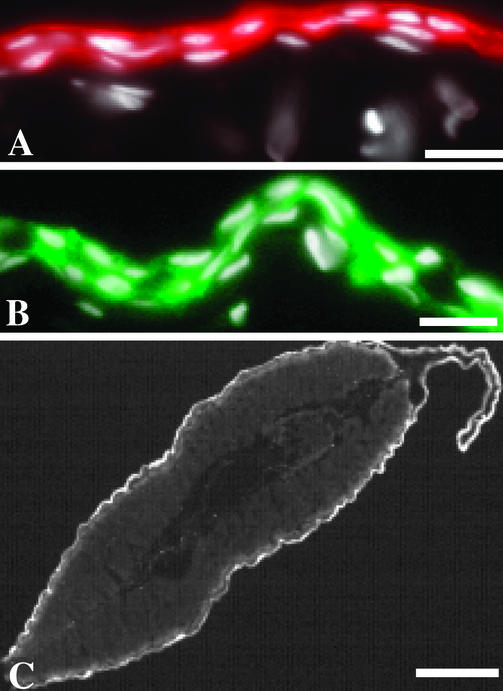
The fidelity of the Ker was assessed in tadpoles transgenic for Ker:GFP. Expression of the GFP colocalizes with larval keratin protein in the skin of the body and tail (Fig. 3), an observation seen in all six transgenic animals. GFP expression in transgenic tadpoles is absent by the start of metamorphic climax (NF 60) (not shown), suggesting that this cloned Ker has the same specificity as the endogenous promoter from which it was derived.
Figure 3.
Larval keratin colocalizes with GFP expression in tadpoles transgenic for Ker:GFP. (A) Larval keratin immunoreactivity (red) in the body skin of a premetamorphic tadpole (NF 54) is confined to the epithelial layer. (B) The larval keratin promoter drives GFP expression (green) specifically in the epithelial layers of skin from the body. (C) Cross section of the tail showing expression of Ker:GFP (white). A and B are counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (white). [Scale bars: 10 μM (A and B) and 100 μM (C).]
In all (n = 3) premetamorphic tadpoles (NF 54) transgenic for Ker:TRDN (and in nontransgenic controls) gene 19 is expressed in the apical cell layer of tail skin (Fig. 4 A and A′). After a 48-h induction of NF 54 tadpoles with 30 nM T3, gene 19 message was absent in nontransgenic tadpoles (n = 6), but retained in three of six transgenic tadpoles (Fig. 4 B and B′). Expression of collagenase-3 in the subepithelial fibroblasts was induced in all (n = 6) transgenic and nontransgenic tadpoles after 48 h of treatment with T3 (Fig. 4 C and C′), whereas expression was absent in all uninduced controls (n = 3) (not shown). Before induction with T3, active caspase-3 immunoreactivity is sparse in dorsal body skin of transgenic and nontransgenic tadpoles (n = 3) (Fig. 2A). After 48 h of T3 induction active caspase-3 immunoreactivity is widespread throughout the skin of all nontransgenic tadpoles (n = 6) (Fig. 4D). However, in the same transgenic individuals that retained gene 19 expression, active caspase-3 immunoreactivity (Fig. 4D′) was much lower and similar to levels observed before induction (Fig. 2A).
Figure 4.
Targeted expression of a TRDN to the larval epithelium inhibits down-regulation of gene 19 and up-regulation of active caspase-3 after induction of premetamorphic tadpoles (NF 54) with 30 nM T3 for 48 h. (A–D) WT tadpoles. (A′–D′) Tadpoles transgenic for Ker:TRDN. (A and A′) Antisense RNA probes hybridized in tail cross sections show the presence of gene 19 in both WT and transgenic tadpoles before induction. Gene 19 is down-regulated by TH in the WT skin (B) but continues to be expressed in transgenic skin after T3 induction (B′). Collagenase-3 is up-regulated by TH in subepithelial fibroblasts in WT (C) and transgenic (C′) skin. cl, collagen lamella. After T3 induction, active caspase-3 immunoreactivity (arrowheads) is broadly expressed in WT skin (D) but has much less expression in transgenic skin (D′). Black spots in whole-mounted skin (D and D′) are melanophores. [Scale bars: 5 μM (A–C′) and 200 μM (D and D′).]
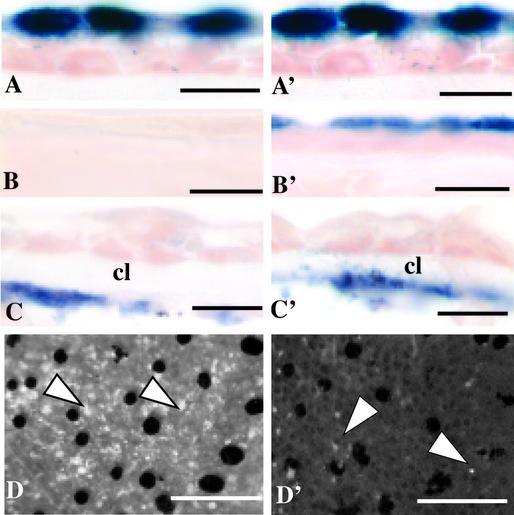
In spontaneously metamorphosing tadpoles, all transgenic tadpoles (n = 10) appeared similar to nontransgenic controls (n = 10) near the end of metamorphic climax (NF 64) and displayed normal tail resorption (Fig. 5 A and A′). In the dorsal body skin of all NF 64 controls gene 19 and larval keratin expression was absent (Fig. 5 B and C), and adult keratin was expressed in the basal cells of the epidermis (Fig. 5D). In all control tails gene 19 expression (Fig. 5F) and larval keratin expression (not shown) were absent, as was adult keratin expression (not shown). In contrast to controls, the dorsal body skin in 3/10 transgenic animals (presumedly those with the highest levels of TRDN expression) expressed gene 19 and larval keratin message in the outermost epithelial layer (Fig. 5 B′ and C′). In situ hybridization double-labeling with two different probes showed that the gene 19-expressing apical layer is separated from the adult keratin-expressing basal layer by a single layer of cells (Fig. 5D′). In contrast to controls the same three transgenic animals that retained expression of gene 19 in their body skin also retained gene 19 expression throughout their tail epithelium (Fig. 5F′). These animals also expressed larval keratin but not adult keratin in their tails (not shown).
Figure 5.
Froglets (NF 64) transgenic for Ker:TRDN retain larval epithelium at the end of metamorphosis. Vertical lines denote regions of skin sectioned from the body and tail of a WT (A) and Ker:TRDN (A′) froglet. (B) Gene 19 expression (dark blue/purple) visualized by in situ hybridization is absent in WT body and tail (F) skin but retained in transgenic body (B′) and tail (F′) skin (black dots are pigment clusters). * denotes adult skin glands. Larval keratin expression in body skin is absent in WT (C) but present in transgenic (C′) skin. Adult keratin is expressed in basal cells of both WT (D) and transgenic (D′) skin. Body skin labeled with both gene 19 and adult keratin show the retention of gene 19 expression in transgenic skin (E′), but not in control skin (E). Control (F) and transgenic (F′) tail tips hybridized with gene 19 probe identify retention of larval gene expression in the Ker:TRDN animal. [Scale bars: 500 μM (A and A′), 5 μM (B–E′), and 50 μM (F and F′).]
Discussion
The change of larval to adult epidermis in anurans has been documented extensively by histology (2, 28, 29). At the climax of metamorphosis larval skin cells become keratinized (6) and their nuclei become pycnotic (3, 4), suggestive of apoptosis. We have used active caspase-3 immunocytology to assay for apoptosis of larval epidermal cells (Figs. 2 and 4D). This useful marker reveals an abundance of dying larval epidermal cells starting at metamorphic climax (Fig. 2). In the body the basal progenitor cells that will give rise to the adult epidermis proliferate, the underlying collagen lamella is invaded by fibroblasts, and the dermis thickens and develops further. Only rare patches of adult skin cells ever form in the tail even at the peak of metamorphic climax when almost all of the body skin has become adult (30).
Our goal has been to determine the extent that TH-induced death of the larval epidermis is a direct target of the hormone, and whether the dying epidermis influences neighboring cell types such as the adult basal cells and subepithelial fibroblasts that will also change as a result of TH. This same question has been answered for muscle programs during metamorphosis (27). The fast muscle of the tail dies a cell autonomous death while the slow muscle cords die along with general tail resorption presumably digested by the proteolytic enzymes released by the fibroblasts.
Several genes have been identified that are expressed differentially in tadpole compared with frog skin. This list includes several keratins (9, 15–18, 31) and a set of TH-induced down-regulated genes that all are expressed in the apical cell layer of the tadpole skin (14). Our examination of cell autonomy of larval skin death has been made possible by the specificity of a larval keratin promoter (ref. 15; Fig. 3) and the availability of larval (14) and adult (31) skin markers. This larval keratin promoter drives GFP or the TRDN expression in the two outer cell layers of the tadpole epidermis, mirroring the expression pattern of larval keratin itself. The expression of larval keratin is down-regulated normally at the climax of metamorphosis just after the genes specific to the apical cells, including gene 19, are down-regulated by TH (14). The onset of adult keratin expression begins late in prometamorphosis and is always visualized in a deep cell layer of the skin, presumably the “basal” cells. There are no molecular markers that would help us identify the adult progenitor cells (basal cells) before they express adult keratin, and we therefore cannot test whether the larval keratin promoter is expressed in these cells. However, expression of the TRDN in the skin of transgenic tadpoles does not inhibit the TH-induced up-regulation of the adult keratin skin marker, suggesting that the transgene is not expressed in basal cells. In contrast, the TRDN inhibits the loss of larval epithelium. As with the adult basal cells, the neighboring subepithelial fibroblasts that up-regulate proteolytic enzymes as exemplified by collagenase-3 (Fig. 4 C and C′) are also unaffected by the inhibition of larval skin death and the tail resorbs normally. Remarkably, the transgenic tadpoles metamorphose normally except for one feature. They retain a larval epidermis over their developing adult skin (Fig. 5E′). These findings suggest that the larval skin neither induces epithelial-mesenchyme remodeling nor tail resorption in direct response to TH. This does not exclude the possibility that larval skin produces a tail resorption-inducing substance that is not controlled by TH, as suggested (19, 20). We presume that the protected larval epidermal cells on our transgenic tadpoles ultimately would be sloughed off as the generative adult epithelial cells move exteriorly.
Acknowledgments
We thank Dr. Deborah Berry, Prof. Katsutoshi Yoshizato, and our colleagues for their helpful comments on this article. This work was supported by a National Research Service Award fellowship (to A.M.S.) and grants from the National Institutes of Health and the G. Harold and Leila Y. Mathers Charitable Trust (to D.D.B.).
Abbreviations
- TH
thyroid hormone
- T3
3,5,3′ triiodothyronine
- TR
TH receptor
- TRDN
dominant negative THR
- Ker
Xenopus laevis larval keratin promoter
- NF
Nieuwkoop and Faber
References
- 1.Dodd M H I, Dodd J M. In: Physiology of the Amphibia. Lofts B, editor. New York: Academic; 1976. pp. 467–599. [Google Scholar]
- 2.Fox H. In: Metamorphosis: The Eighth Symposium of the British Society for Developmental Biology. Balls M, Bownes M, editors. Oxford: Clarendon; 1985. pp. 59–87. [Google Scholar]
- 3.Kerr J F R, Harmon B, Searle J. J Cell Sci. 1974;14:571–585. doi: 10.1242/jcs.14.3.571. [DOI] [PubMed] [Google Scholar]
- 4.Kinoshita T, Sasaki F, Watanabe K. J Morphol. 1985;185:269–275. doi: 10.1002/jmor.1051850211. [DOI] [PubMed] [Google Scholar]
- 5.Nishikawa A, Yoshizato K. J Exp Zool. 1986;237:221–230. doi: 10.1002/jez.1402370208. [DOI] [PubMed] [Google Scholar]
- 6.Nishikawa A, Kaiho M, Yoshizato K. Dev Biol. 1989;131:337–344. doi: 10.1016/s0012-1606(89)80007-7. [DOI] [PubMed] [Google Scholar]
- 7.Yoshizato K. Dev Growth Differ. 1992;34:607–612. doi: 10.1111/j.1440-169X.1992.tb00028.x. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki K, Utoh R, Kotani K, Obara M, Yoshizato K. Dev Growth Differ. 2002;44:225–238. doi: 10.1046/j.1440-169x.2002.00637.x. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe Y, Tanaka R, Kobayashi H, Utoh R, Suzuki K-I, Obara M, Yoshizato K. Dev Dyn. 2002;225:561–570. doi: 10.1002/dvdy.10196. [DOI] [PubMed] [Google Scholar]
- 10.Berry D L, Schwartzman R, Brown D D. Dev Biol. 1998;203:12–23. doi: 10.1006/dbio.1998.8974. [DOI] [PubMed] [Google Scholar]
- 11.Robinson D H, Heintzelman M B. Anat Rec. 1987;217:305–317. doi: 10.1002/ar.1092170310. [DOI] [PubMed] [Google Scholar]
- 12.Holbrook K A. In: Biochemistry and Physiology of the Skin. Goldsmith L A, editor. Oxford: Oxford Univ. Press; 1983. pp. 64–101. [Google Scholar]
- 13.Wang Z, Brown D D. J Biol Chem. 1993;268:16270–16278. [PubMed] [Google Scholar]
- 14.Furlow J D, Berry D L, Wang Z, Brown D D. Dev Biol. 1997;182:284–298. doi: 10.1006/dbio.1996.8478. [DOI] [PubMed] [Google Scholar]
- 15.Miyatani S, Winkles J A, Sargent T D, Dawid I B. J Cell Biol. 1986;103:1957–1965. doi: 10.1083/jcb.103.5.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.French R P, Warshawsky D, Tybor L, Mylniczenko N, Miller L. Dev Genet. 1994;15:356–365. doi: 10.1002/dvg.1020150407. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki K, Sato K, Katsu K, Hayashita H, Kristensen D B, Yoshizato K. Differentiation. 2001;68:44–54. doi: 10.1046/j.1432-0436.2001.068001044.x. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe Y, Kobayashi H, Suzuki K, Kotani K, Yoshizato K. Biochim Biophys Acta. 2001;1517:339–350. doi: 10.1016/s0167-4781(00)00281-5. [DOI] [PubMed] [Google Scholar]
- 19.Niki K, Namiki H, Kikuyama S, Yoshizato K. Dev Biol. 1982;94:116–120. doi: 10.1016/0012-1606(82)90074-4. [DOI] [PubMed] [Google Scholar]
- 20.Niki K, Yoshizato K. Dev Biol. 1986;118:306–308. doi: 10.1016/0012-1606(86)90097-7. [DOI] [PubMed] [Google Scholar]
- 21.Eisen A Z, Gross J. Dev Biol. 1965;12:408–418. doi: 10.1016/0012-1606(65)90006-0. [DOI] [PubMed] [Google Scholar]
- 22.Marsh-Armstrong N, Huang H, Berry D L, Brown D D. Proc Natl Acad Sci USA. 1999;96:14389–14393. doi: 10.1073/pnas.96.25.14389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schreiber A M, Das B, Huang H, Marsh-Armstrong N, Brown D D. Proc Natl Acad Sci USA. 2001;98:10739–10744. doi: 10.1073/pnas.191361698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kroll K L, Amaya E. Development (Cambridge, UK) 1996;122:3173–3183. doi: 10.1242/dev.122.10.3173. [DOI] [PubMed] [Google Scholar]
- 25.Huang H, Marsh-Armstrong N, Brown D D. Proc Natl Acad Sci USA. 1999;96:962–967. doi: 10.1073/pnas.96.3.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nieuwkoop P D, Faber J. Normal Table of Xenopus laevis (Daudin) Amsterdam: Elsevier; 1956. [Google Scholar]
- 27.Das B, Schreiber A M, Huang H, Brown D D. Proc Natl Acad Sci USA. 2002;99:12230–12235. doi: 10.1073/pnas.182430599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kemp N E. Dev Biol. 1963;7:244–254. doi: 10.1016/0012-1606(63)90121-0. [DOI] [PubMed] [Google Scholar]
- 29.Yoshizato K. In: Metamorphosis: Postembryonic Reprogramming of Gene Expression in Amphibian and Insect Cells. Gilbert L I, Tata J R, Atkinson B G, editors. New York: Academic; 1996. pp. 647–671. [Google Scholar]
- 30.Kinoshita T, Sasaki F. Histochemistry. 1994;101:397–404. doi: 10.1007/BF00269489. [DOI] [PubMed] [Google Scholar]
- 31.Mathiesen P M, Miller L. Genes Dev. 1987;1:1107–1117. doi: 10.1101/gad.1.10.1107. [DOI] [PubMed] [Google Scholar]