Abstract
In the hippocampus at birth, most glutamatergic synapses are immature and functionally “silent” either because the neurotransmitter is released in insufficient amount to activate low-affinity α-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptors or because the appropriate receptor system is missing or nonfunctional. Here we show that, in the newborn rat, a brief application of nicotine at immature Schaffer collateral-CA1 connections strongly enhances neurotransmitter release and converts presynaptically silent synapses into conductive ones. This effect is persistent and can be mimicked by endogenous acetylcholine released from cholinergic fibers. Thus, during a critical period of postnatal development, activation of nicotinic acetylcholine receptors contributes to the maturation of functional synaptic contacts and the wiring of adult hippocampal circuitry.
Keywords: excitatory postsynaptic currents‖cholinergic stimulation‖Schaffer collateral-CA1 synapses‖presynaptic effect‖increased probability of glutamate release
A common feature of postnatal development, observed in a variety of different brain structures including the hippocampus, is the presence of immature synapses called “silent” because they do not transmit at rest. Conversion of silent synapses into functional ones constitutes an efficient mechanism for enhancing synaptic efficacy in the immature brain (1–4). It is commonly accepted that this conversion occurs mainly at a postsynaptic site with the insertion of new α-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA) receptors on the subsynaptic membrane (1, 2, 5–8). However, an unambiguous demonstration that silent synapses do not contain functional AMPA receptors is still lacking. An alternative, although nonexclusive, hypothesis is based on the involvement of presynaptic mechanisms such as an increased probability of transmitter release (3) and/or increased amount of transmitter released by a single quantum (4). Thus, an increased release of glutamate may contribute to the switching on of “presynaptically” silent synapses and to the persistent enhancement of synaptic efficacy (3, 4, 9, 10).
In the adult, enhancement of glutamate release from presynaptic nerve endings can be elicited by nicotinic receptors whose activation has been shown to exert a potent regulatory effect on the release of several neurotransmitters (11–14). The hippocampus, an important structure for learning and memory, expresses a variety of nicotinic acetylcholine receptors (nAChRs) localized on both presynaptic and postsynaptic membranes (15–19). Although the developmental expression of nAChR subunits has been extensively studied, their functional role in development is still unclear. An increasing body of evidence suggests that these receptors may contribute to the functional maturation of the brain (20–23). In the present experiments we have explored the possibility that activation of nAChRs in the newborn hippocampus produces a sustained increase in glutamate release, thus converting immature “presynaptically silent” synapses into functional ones.
Methods
Slice Preparation.
Transverse hippocampal slices (300–400 μm thick) from P1–P5 Wistar rats were prepared as described (3). Briefly, animals were decapitated after being anaesthetized with an i.p. injection of urethane (2 g/kg). The brain was quickly removed from the skull and placed in ice-cold artificial cerebrospinal fluid (ACSF) containing 130 mM NaCl, 3.5 mM KCl, 1.2 mM NaH2PO4, 25 mM NaHCO3, 1.3 mM MgCl2, 2 mM CaCl2, and 11 mM glucose, saturated with 95% O2 and 5% CO2 (pH 7.3–7.4). After 1 h, an individual slice was transferred to the recording chamber where it was continuously superfused with oxygenated ACSF at a rate of 2–3 ml/min.
Electrophysiological Recordings.
Miniature and evoked excitatory postsynaptic currents (EPSCs) were recorded from individual CA1 pyramidal neurons at 24°C and 33°C by using the patch-clamp technique in whole-cell configuration. Patch pipettes were filled with a solution containing 125 mM Cs-methanesulphonate, 10 mM CsCl, 10 mM Hepes, 0.6–2 mM EGTA, 2 mM MgATP, and 0.3 mM NaGTP (resistance 5 MΩ). In some experiments, bicuculline methiodide (10 μM) and tetrodotoxin (TTX, 10 nM) were added to the bath solution to block γ-aminobutyric acid type A receptors and reduce polysynaptic activity, respectively. Miniature EPSCs (mEPSCs) were recorded in the presence of TTX (1 μM). Schaffer collaterals were stimulated with bipolar twisted NiCr-insulated electrodes placed in stratum radiatum. Paired stimuli (50-ms interval, 100-μs duration) were applied at 0.25–0.5 Hz. The minimal intensity necessary to evoke a response at −60 mV was found and then slightly decreased to obtain only failures. Failures were usually estimated by visual discrimination. Synapses were considered presynaptically silent if they did not respond to the first stimulus in 100 consecutive trials but exhibited occasional responses to a second pulse (after the first one with 50-ms delay). These synapses have been shown to bear N-methyl-d-aspartate-mediated responses at positive potentials (3). A recent study (24) has shown that postsynaptically silent synapses are very rare during the first week of postnatal life. Therefore the present experiments have been focused on presynaptically silent synapses that at this developmental stage are quite frequent (3).
In some experiments to test the effects of endogenous ACh on silent synapses, cholinergic fibers were stimulated with bipolar twisted NiCr-insulated electrodes positioned in stratum oriens (2-s trains of pulses, 100-μs duration each, applied at 25 Hz; see ref. 25).
Drugs used were: tetrodotoxin (Affinity Research Products, Exeter, U.K.); methyllycaconitine (MLA) and bicuculline methiodide (Tocris Cookson, Bristol, U.K.); nicotine, α-bungarotoxin (α-BGT), dihydro-β-erythtroidine (DHβE), and atropine (Sigma); and (-)-Spiro[1-azabicyclo[2.2.2]octane-3,5′-oxazolidin-2′-one] (AR-R17779, kindly provided by E. Sher, Eli Lilly). In some experiments 1,2-bis(2-aminophenoxy)ethane-N,N,N′, N′-tetraacetic acid (BAPTA) was added to the patch pipette at the concentration of 10 mM. In others, slices were preincubated and superfused with artificial cerebrospinal fluid containing the membrane-permeable calcium chelator BAPTA tetraacetoxymethylester (BAPTA-AM). BAPTA-AM was dissolved at a concentration of 50 μM in DMSO and used at a final concentration of 0.1%. Control records obtained in the presence of 0.1% DMSO were similar to those obtained in its absence.
If not otherwise stated, data are expressed as mean ± SEM. Statistical comparisons were made with a paired t test or Wilcoxon signed rank test (P < 0.05 was taken as significant).
Data Acquisition and Analysis.
Data acquisition was done by using the LTP114 software package for evoked responses (courtesy of W. W. Anderson, Bristol University, Bristol, U.K.) and PCLAMP 8 (Axon Instruments, Foster City, CA) for spontaneous minis. Current signals were transferred to a computer after digitization with an A/D converter (Digidata 1200, Axon Instruments, Foster City, CA). Data were sampled at 20 kHz and filtered with a cut-off frequency of 2 kHz. Evoked EPSCs were analyzed with the AXOGRAPH 4.6 program (Axon Instruments), which uses a detection algorithm based on a sliding template. Spontaneous EPSCs were analyzed with the minianalysis program (version 5.6.1, Synaptosoft, Decatur, GA). All events visually judged as EPSCs were manually indicated for additional analysis in the minianalysis program. The settings of the minianalysis program were: threshold amplitude >3 pA; area threshold >10 pA/msec; peak amplitude <20 ms of mEPSC onset; average baseline before onset >5 ms. In low probability cells, the paired-pulse ratio (PPR) was calculated as the ratio between the amplitude of the second and first EPSC over 100 consecutive trials in control and 20 min after treatment.
Results
Nicotine Causes the Appearance of Miniature AMPA-Mediated EPSCs in Silent Neurons.
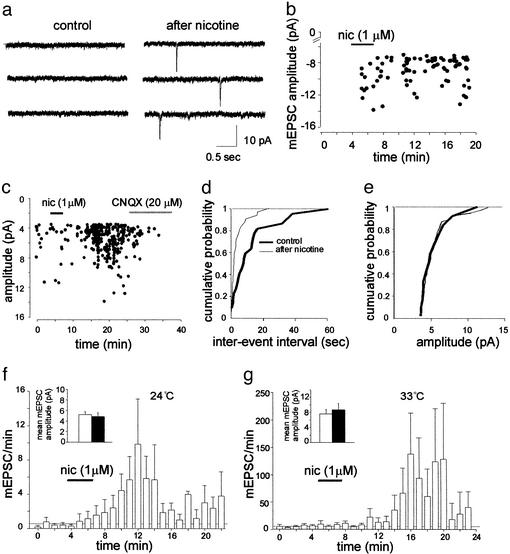
Spontaneous miniature AMPA-mediated EPSCs were recorded in the presence of tetrodotoxin (1 μM) from P2-P5 CA1 pyramidal cells at 24°C (n = 7) and 33°C (n = 7). In agreement with previous work (26, 27), in some neurons (6/14) spontaneous glutamatergic activity was not detected (four silent cells were recorded at 24°C and two at 33°C). Bath application of 1 μM nicotine, a concentration close to that present in the smoker's blood immediately after smoking a cigarette (28), caused the appearance of mEPSCs (Fig. 1 a and b). This effect lasted for at least 20 min and was prevented by bath application of 50 μM of DHβE, a competitive nAChR antagonist, known to block most nAChR subtypes in this concentration range (refs. 16, 29, and 30; n = 2; not shown). In silent neurons, the frequency of mEPSCs recorded at 24°C and 33°C caused by nicotine was 0.05 ± 0.02 Hz and 0.07 ± 0.05 Hz, respectively. Nicotine enhanced the frequency of mEPSCs also in nonsilent cells (Fig. 1 c and d; n = 8). Summary data for all cells (silent and nonsilent) recorded at 24°C and 33°C, respectively, are given in Fig. 1 f and g. At 24°C mEPSC frequency increased from 0.007 ± 0.001 Hz in control to 0.052 ± 0.010 Hz after nicotine and at 33°C from 0.093 ± 0.076 Hz to 0.836 ± 0.192 Hz. On average, nicotine did not significantly (P > 0.5, n = 8) modify mean EPSC amplitude that varied from 5.2 ± 0.6 pA to 4.9 ± 0.7 pA and from 6.52 ± 1.0 pA to 7.48 ± 1.5 pA before and after nicotine, at 24°C and at 33°C, respectively (see Fig. 1e). These data are consistent with the notion that nicotine exerts its action mainly at presynaptic sites.
Figure 1.
Nicotine induces the appearance of mEPSCs in silent neurons and enhances their frequency in active ones. (a) Representative traces from a P3 silent neuron before (Left) and after (Right) the application of nicotine (1 μM). (b) The amplitude of individual events (●, cell shown in a is plotted against time). (c) The amplitude of miniature events before (●), during (filled bar), and after nicotine application is plotted against time. Note complete suppression of miniature events with 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) (open bar). (d and e) Cumulative distributions of interevent interval (d) and amplitude (e) of mEPSCs shown in c before (thick line) and after (thin line) nicotine application. (f and g) Summary plot of mEPSCs frequency versus time for cells recorded at 24°C (n = 7; f) and 33°C (n = 7; g). Each column represents the number of miniatures per min. Dotted lines represent the mean mEPSC frequency in control conditions (also including silent cells). (Insets) mEPSCs amplitude (mean ± SEM of three cells in f and five in g) recorded before (open columns) and after (filled columns) nicotine application.
Nicotine Switches on Presynaptically Silent Synapses.
In a second set of experiments (at P1–P5), AMPA-mediated EPSCs were evoked at −60 mV by weak paired stimuli to the Schaffer collateral at 0.25–0.5 Hz. Whereas 40/75 cells exhibited few AMPA-mediated EPSCs to the first stimulus, the remaining 35 did not show any synaptic response over 100 consecutive trials. Silent neurons showed occasional EPSCs in response to a second stimulus, caused by paired-pulse facilitation, which largely depends on presynaptic increase in release probability (31). Therefore, these synapses were considered presynaptically silent because of low probability of glutamate release (3).
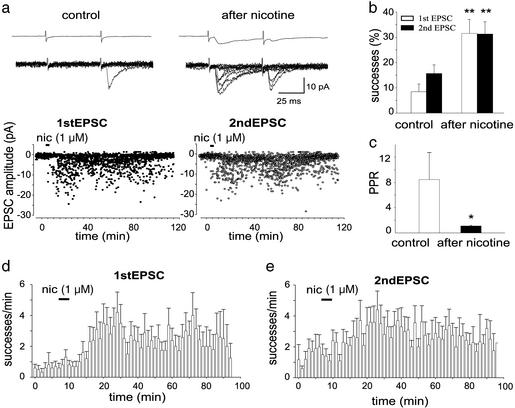
Application of nicotine (1 μM for 3 min) to silent synapses caused the appearance of responses to the first stimulus and increased the number of EPSCs to the second one (Fig. 2a, n = 6). In seven additional cells with low release probability (on average 14.5 ± 3.6%) nicotine produced a significant (P < 0.01) increase in the number of successes to both the first and second stimulus. In four of these neurons nicotine produced a biphasic effect consisting of an initial potentiation followed by a period (lasting 6 ± 1 min) with reduced probability and a late potentiation. Overall in 13 cells (silent + low probability ones) nicotine caused an increase of successes of 344 ± 70% and 198 ± 34% to the first and second EPSCs, respectively (Fig. 2b). In all cases the effect of nicotine was long lasting (up to 2 h) without decrement in EPSCs amplitude over time (Fig. 2 a, d, and e). No change in holding current or membrane input resistance was observed during or after nicotine application. In low probability synapses, the PPR changed from 8.4 ± 4.2 to 1.1 ± 0.1, in control and after nicotine, respectively (n = 7, Fig. 2c). Nicotine increased synaptic efficacy at 33°C. The mean change in the number of successes was 223 ± 40% and 132 ± 14% for the first and second EPSCs, respectively (n = 3). DHβE (50 μM) blocked the potentiating effect of nicotine and the associated change in PPR (Fig. 3 a and b). Application of nicotine to the same neuron (n = 4), 30–40 min after washing out DHβE, revealed a significant and persistent potentiation of the synaptic response, ruling out the possibility of EPSC rundown.
Figure 2.
Nicotine switches on presynaptically silent synapses. (a) The amplitudes of the first EPSC (Left, ●) and second EPSC (Right, ○) evoked by pair of stimuli before, during (bar), and after nicotine application are plotted against time. (Upper) Averages of 100 individual responses (successes and failures) obtained before (control) and after nicotine application. (Lower) Ten individual responses evoked in control and 20 min after washing out nicotine. (b) Mean percentage of successes to the first and second stimulus for all P1–P5 CA1 pyramidal neurons examined (n = 13) before (control) and 20 min after nicotine application. (c) PPR calculated in seven low probability cells before (control) and after nicotine application. (d and e) Summary plot of mean number of successes versus time in response to the first and second stimulus for 10 cells (including those presynaptically silent). Each column represents the number of successes per min. *, P < 0.05; **, P < 0.01.
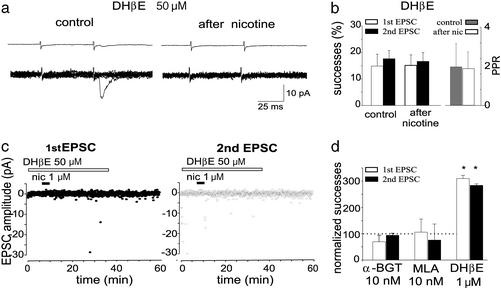
Figure 3.
Nicotine effect is mediated by α7 receptors. (a) The amplitudes of the first EPSC (Left, ●) and second EPSC (Right, ○) evoked by a pair of stimuli before, during, and after nicotine application in the presence of DHβE are plotted against time. (Upper) Averages of 100 individual responses (successes and failures) obtained before (control) and 20 min after nicotine application. (Lower) Ten individual responses evoked in control and after washing out nicotine. Note the lack of potentiation when nicotine was applied in the presence of the antagonist. This cell is the same shown in Fig. 2 (after washing out DHβE). (b) Mean percentage of successes (n = 13; Left) and PPR (n = 7; Right) obtained before and after nicotine application in the presence of DHβE. (c) Number of successes to the first (open column) and second (filled column) stimulus observed 20 min after application of nicotine in the presence of different nAChR antagonists. Successes are normalized to those present in control (dotted line). Note that DHβE (1 μM) did not block nicotine-induced potentiation (*, P < 0.05). Number of cells for α-BGT, MLA, and DHβE are five, six, and six, respectively.
In the hippocampus, nAChRs are distributed on both pyramidal neurons and interneurons (15–19). Nicotine might thus exert its effect indirectly through excitation of γ-aminobutyric acid (GABA)-ergic interneurons, which early in postnatal life have been shown to depolarize and excite principal cells (32). To test whether GABAergic interneurons were involved in nicotine action, the agonist was applied in the presence of bicuculline (3–10 μM). Nicotine still caused the appearance of EPSCs (the percentages of successes to the first and second pulse were 0 and 5.1 ± 4.4% or 17 ± 15.5% and 12 ± 7.6% before or 20 min after nicotine, respectively, n = 3; data not shown), indicating that GABA type A receptors are not involved in nicotine-induced potentiation.
The Potentiating Effect of Nicotine Involves α7 nAChRs.
Among various nAChR subunits, the α7 subtype is highly expressed in the hippocampus where it forms homopentamers that regulate transmitter release in both adult (33–35) and neonates (36). In an attempt to clarify whether this receptor was involved in regulation of glutamate release, we used the ACh analogue AR-17779, shown to selectively activate α7 receptors (37). Bath application of AR-17779 (100 μM for 3 min) converted silent synapses into conductive ones (n = 3). The number of successes to EPSC1 and EPSC2 varied from 0 and 4.6 ± 3.2% to 23 ± 14.6% and 21.3 ± 10.1% before and 20 min after AR-17779 application (data not shown).
Moreover, selective α7 nAChR antagonists such as MLA (10 nM, n = 6) and α-BGT (100 nM, n = 5) were able to block the potentiating effects of nicotine (Fig. 3c). In contrast, low concentrations of DHβE (1 μM), known to selectively block β2-containing nAChRs (16), were ineffective in preventing nicotine action. In the presence of 1 μM DHβE, nicotine-elicited increase in successes was 310.0 ± 12.3% and 284.0 ± 5.9 for the first and second EPSC, respectively (n = 6; Fig. 3c).
Intracellular Calcium Rise Through Postsynaptic nAChRs Does Not Contribute to Nicotine Effects.
It is known that α7 nAChRs are highly permeable to calcium (29, 30, 38). To see whether a rise of calcium in the postsynaptic cell via α7 nAChRs contributes to nicotine-induced persistent changes in synaptic efficacy, nicotine was applied to cells loaded with the calcium chelator BAPTA (10 mM, n = 9). In these neurons the agonist caused a potentiation of synaptic currents (data not shown) similar to that produced in control (without BAPTA) that was associated with a reduction in PPR (from 2.4 ± 0.2 to 1.1 ± 0.1, n = 3). The percentage of successes to the first pulse was 2.8 ± 1.9% and 16.8 ± 5.7% before and after nicotine, respectively. In slices incubated with the membrane-permeable calcium chelator BAPTA tetraacetoxymethylester (50 μM), nicotine was unable to change synaptic efficacy in the majority of cells tested (n = 5/8). All together these results are consistent with the notion that an increase in presynaptic calcium possibly via α7 nAChRs is essential for nicotine-induced persistent increase in synaptic efficacy.
Activation of nAChRs by Endogenously Released ACh Makes Silent Synapses Functional.
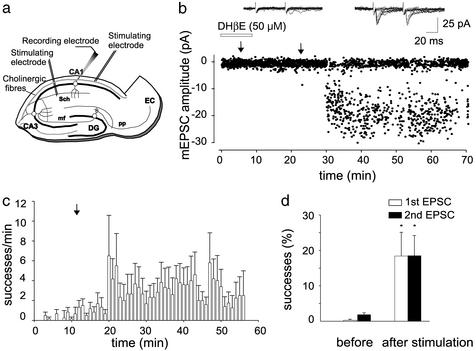
The hippocampus receives a large cholinergic innervation from the septo-hippocampal pathway (39) that is already functional early in postnatal life (25). To investigate whether endogenously released ACh is able to switch on presynaptically silent synapses, cholinergic fibers were stimulated in the presence of 1 μM atropine to block ACh muscarinic receptors (n = 7; Fig. 4a). In five cells silent to the first stimulus but showing only sporadic responses to the second, cholinergic stimulation caused the appearance of EPSC1 and potentiated EPSC2, an effect that was prevented by DHβE (50 μM; Fig. 4b). In the remaining two cells exhibiting only few responses to the first stimulus, cholinergic stimulation caused a persistent potentiation of EPSC1 and EPSC2. Overall, in seven neurons, the percentages of successes to the first and second stimuli were 0.3 ± 0.3% and 1.8 ± 0.5% or 18.4 ± 6.7% and 18.5 ± 5.7%, before and 20 min after stimulation of cholinergic fibers, respectively (Fig. 4 c and d). This effect was associated with a decrease in PPR (from 1.9 ± 1.2 to 1.4 ± 0.5). It should be stressed that in five cases cholinergic activation produced a biphasic effect resulting in activation of EPSC1 immediately after the train followed by a period (8 ± 1 min) of reduced probability and a late persistent potentiation (Fig. 4c).
Figure 4.
Activation of nicotinic receptors by endogenously released ACh converts presynaptically silent synapses into conductive ones and strongly enhances synaptic efficacy. (a) Schematic diagram showing localization of stimulating and recording electrodes. Cholinergic fibers were stimulated in the presence of atropine (1 μM). Sch, Schaffer collaterals; mf, mossy fiber; pp, perforant path; DG, dendate gyrus; EC, entorhinal cortex. (b) Amplitudes of the first response obtained before and after stimulation of cholinergic fibers (arrows) are plotted against time. Note the lack of EPSC potentiation when cholinergic fibers were stimulated in the presence of DHβE. (Insets) Ten superimposed individual responses evoked by pair of stimuli delivered to the Schaffer collateral 15 min after repetitive stimulation of the cholinergic pathway in the presence (Left) or absence (Right) of DHβE. (c) Summary plot of the mean number of successes per min versus time for six cells (silent and nonsilent). Repetitive stimulation of the cholinergic fibers is marked by an arrow. (d) Mean percentage of successes to the first and second stimulus for all P3-P5 CA1 pyramidal neurons examined (n = 7) before and 20 min after stimulation of cholinergic fibers. *, P < 0.05.
Discussion
These findings provide evidence that a single exposure to nicotine causes persistent changes in synaptic efficacy in immature glutamatergic synapses. Mechanisms for such long-lasting modification entail conversion of presynaptically silent synapses into functional ones. Activation of nAChRs localized on presynaptic glutamatergic nerve endings (18, 33, 40) is likely to be involved in the persistent enhancement of synaptic transmission because: (i) nicotine caused the appearance of mEPSCs in silent neurons and increased the frequency but not the amplitude in those exhibiting spontaneous events; (ii) both nicotine and endogenously released ACh increased the probability of successes and reduced the number of transmitter failures; and (iii) in low probability neurons nicotine decreased the PPR. A short-term increase in mEPSC frequency by nicotine was observed in several CNS structures (33, 40, 41). A sustained enhancement of spontaneous glutamatergic transmission after brief repetitive pulses of ACh was described in cocultures of medial habenula and interpeduncular nucleus (42). In the present experiments, facilitation of synaptic transmission lasted for up to 2 h and could be elicited not only by nicotine but also by endogenously released ACh. It has been recently reported that in the CA1 region of the hippocampus persistent changes in synaptic strength can be elicited by stimulation of afferent fibers paired with nicotine-induced membrane depolarization (18). This type of mechanism present only in a minority of cells in the adult hippocampus (18) was commonly observed in neurones of the ventral tegmental area and was prevented by N-methyl-d receptor antagonists (41). In the present experiments potentiation occurred when cells were voltage-clamped at −60 mV, indicating that the persistent increase in glutamate release was independent of postsynaptic depolarization and N-methyl-d receptor activation but exclusively relied on presynaptic mechanisms. Moreover, the observation that nicotine increased synaptic efficacy in cells loaded with BAPTA but not exposed to extracellular BAPTA tetraacetoxymethylester indicates a presynaptic site of induction.
The present experiments clearly show that homomeric α7 nAChRs are the main target of nicotine. Indeed, in the hippocampus, α7 nAChRs have been shown to be present and functional at the early stage of postnatal development (36). The observation that AR-17779, a compound known to selectively activate α7 nAChRs (37), is able to mimic the potentiating effects of nicotine supports this view. Moreover, nicotine action was prevented by two selective α7 receptor antagonists, MLA and α-BGT, indicating that activation of α7 nAChRs is responsible for switching on silent synapses and strengthening synaptic efficacy. Activation of α7 nAChRs in the ventral tegmental area by nicotine has been shown to cause a persistent enhancement of glutamate release from presynaptic nerve endings (43). This property, together with desensitization of β2-containing receptors located on γ-aminobutyric acid-ergic neurons, may account for nicotine-stimulated persistent changes of glutamatergic transmission and dopamine level in the mesolimbic reward system (43). Interestingly, in the present experiments, EPSC potentiation was still observed in the presence of low concentrations of DHβE, known to selectively block β2-containing receptors (15, 16, 44), suggesting that these receptor subtypes do not significantly contribute to the nicotine effects. Several mechanisms may account for nicotine-stimulated changes in synaptic efficacy. It is likely that calcium entering into the terminals through α7 nAChRs (29, 30, 38) and through voltage-dependent calcium channels (45) produces downstream effects such as calcium-induced calcium release and activation of second messenger cascades, leading to modification of exocytotic processes and probability of glutamate release. These mechanisms may account for nicotine-induced appearance of mEPSCs in silent neurons. Moreover, the involvement of intracellular second messengers may underline the latency of nicotine action observed in a subset of neurons.
The observations that activation of nAChRs is able to convert silent synapses into conductive ones and to persistently enhance synaptic efficacy in the newborn are consistent with the general view that adequate activation of nAChRs is essential for the functional maturation of the brain (20–23, 46). Indeed, chronic exposure of nAChRs to nicotine either in utero through maternal smoking or in perinatal life leads to irreversible brain damage (47) and constitutes a major independent risk factor for sudden infant death syndrome (46, 48).
Acknowledgments
We thank Drs. Lena, Maskos, and Voronin for carefully reading the manuscript. L.M. was the recipient of a Novartis Pharma fellowship. C.L.M. was the recipient of a fellowship from the Ministère de la Recherche. This work was supported in part by the Ministero dell'Istruzione, dell'Universita' e della Ricerca (to E.C.), the Collège de France, the Centre National de la Recherche Scientifique, the Commission for the European Communities, the Association pour la Recherche Contre le Cancer, and the Association Française Contre les Myopathies (to J.-P.C.).
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazole propionate
- ACh
acetylcholine
- nAChR
nicotinic ACh receptor
- EPSC
excitatory postsynaptic current
- mEPSC
miniature EPSC
- MLA
methyllycaconitine
- α-BGT
α-bungarotoxin
- DHβE
dihydro-β-erythtroidine
- BAPTA
1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
- PPR
paired-pulse ratio
References
- 1.Durand G M, Kovalchuk Y, Konnerth A. Nature. 1996;381:71–75. doi: 10.1038/381071a0. [DOI] [PubMed] [Google Scholar]
- 2.Liao D, Hessler N A, Malinow R. Nature. 1995;375:400–404. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- 3.Gasparini S, Saviane C, Voronin L, Cherubini E. Proc Natl Acad Sci USA. 2000;97:9741–9746. doi: 10.1073/pnas.170032297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi S, Klingauf J, Tsien R W. Nat Neurosci. 2000;3:330–336. doi: 10.1038/73895. [DOI] [PubMed] [Google Scholar]
- 5.Kullmann D M. Neuron. 1994;12:1111–1120. doi: 10.1016/0896-6273(94)90318-2. [DOI] [PubMed] [Google Scholar]
- 6.Isaac J T R, Nicoll R A, Malenka R C. Neuron. 1999;15:427–434. doi: 10.1016/0896-6273(95)90046-2. [DOI] [PubMed] [Google Scholar]
- 7.Malenka R C, Nicoll R A. Science. 1995;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- 8.Montgomery J M, Pavlidis P, Madison D V. Neuron. 2001;29:691–701. doi: 10.1016/s0896-6273(01)00244-6. [DOI] [PubMed] [Google Scholar]
- 9.Rengher J J, Egles C, Liu G A. Neuron. 2001;29:469–484. doi: 10.1016/s0896-6273(01)00219-7. [DOI] [PubMed] [Google Scholar]
- 10.Antonova I, Arancio O, Trillat A C, Wang H G, Zablow L, Udo H, Kandel E R, Hawkins R D. Science. 2001;294:1547–1550. doi: 10.1126/science.1066273. [DOI] [PubMed] [Google Scholar]
- 11.Vidal C, Changeux J-P. Neuroscience. 1993;56:23–32. doi: 10.1016/0306-4522(93)90558-w. [DOI] [PubMed] [Google Scholar]
- 12.McGehee D S, Role L W. Annu Rev Physiol. 1995;57:521–546. doi: 10.1146/annurev.ph.57.030195.002513. [DOI] [PubMed] [Google Scholar]
- 13.Role L W, Berg D K. Neuron. 1996;16:1077–1085. doi: 10.1016/s0896-6273(00)80134-8. [DOI] [PubMed] [Google Scholar]
- 14.Wonnacott S. Trends Neurosci. 1997;20:92–98. doi: 10.1016/s0166-2236(96)10073-4. [DOI] [PubMed] [Google Scholar]
- 15.Alkondon M, Albuquerque E X. J Pharmacol Exp Ther. 1993;265:1455–1473. [PubMed] [Google Scholar]
- 16.Zoli M, Lena C, Picciotto M R, Changeux J-P. J Neurosci. 1998;18:4461–4472. doi: 10.1523/JNEUROSCI.18-12-04461.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sudweeks S N, Yakel J L. J Physiol (London) 2000;527:515–528. doi: 10.1111/j.1469-7793.2000.00515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ji D, Lape R, Dani J A. Neuron. 2001;31:131–141. doi: 10.1016/s0896-6273(01)00332-4. [DOI] [PubMed] [Google Scholar]
- 19.Fabian-Fine R, Skehel P, Errington M L, Davies H A, Sher E, Stewart M G, Fine A. J Neurosci. 2001;21:7993–8003. doi: 10.1523/JNEUROSCI.21-20-07993.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang K T, Berg D K. J Neurosci. 1999;19:3701–3710. doi: 10.1523/JNEUROSCI.19-10-03701.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aramakis V B, Hsieh C Y, Leslie F M, Metherate R. J Neurosci. 2000;20:6106–6116. doi: 10.1523/JNEUROSCI.20-16-06106.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossi F M, Pizzorusso T, Porciatti V, Marubio L M, Maffei L, Changeux J-P. Proc Natl Acad Sci USA. 2001;98:6453–6458. doi: 10.1073/pnas.101120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawa K. J Physiol (London) 2002;538:87–102. doi: 10.1113/jphysiol.2001.012885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Groc L, Gustafsson B, Hanse E. J Neurosci. 2002;22:5552–5562. doi: 10.1523/JNEUROSCI.22-13-05552.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Avignone E, Cherubini E. J Physiol (London) 1999;518:97–107. doi: 10.1111/j.1469-7793.1999.0097r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hosokawa Y, Sciancalepore M, Stratta F, Martina M, Cherubini E. Eur J Neurosci. 2001;6:805–813. doi: 10.1111/j.1460-9568.1994.tb00991.x. [DOI] [PubMed] [Google Scholar]
- 27.Tyzio R, Represa A, Jorquera I, Ben-Ari Y, Gozlan H, Aniksztejn L. J Neurosci. 1999;19:10372–10382. doi: 10.1523/JNEUROSCI.19-23-10372.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dani J A, Heinemann S. Neuron. 1996;16:905–908. doi: 10.1016/s0896-6273(00)80112-9. [DOI] [PubMed] [Google Scholar]
- 29.Bertrand D, Devillers-Thiery A, Revah F, Galzi J L, Hussy N, Mulle C, Bertrand S, Ballivet M, Changeux J-P. Proc Natl Acad Sci USA. 1992;89:1261–1265. doi: 10.1073/pnas.89.4.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bertrand D, Bertrand S, Ballivet M. Neurosci Lett. 1992;146:87–90. doi: 10.1016/0304-3940(92)90179-b. [DOI] [PubMed] [Google Scholar]
- 31.Zucker R S. Annu Rev Neurosci. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]
- 32.Cherubini E, Gaiarsa J-L, Ben Ari Y. Trends Neurosci. 1991;14:515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- 33.Gray R, Rajan A S, Radcliffe K A, Yakehiro M, Dani J A. Nature. 1996;383:713–716. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- 34.Radcliffe K A, Dani J A. J Neurosci. 1998;18:7075–7083. doi: 10.1523/JNEUROSCI.18-18-07075.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alkondon M, Pereira E F, Almeida L E, Eisenberg H M, Albuquerque E X. J Neurosci. 1999;19:2693–2705. doi: 10.1523/JNEUROSCI.19-07-02693.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maggi L, Sher E, Cherubini E. J Physiol (London) 2001;536:89–100. doi: 10.1111/j.1469-7793.2001.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mullen G, Napier J, Balestra M, DeCory T, Hale G, Macor J, Mack R, Loch J, III, Wu E, Kover A, et al. J Med Chem. 2000;43:4045–4050. doi: 10.1021/jm000249r. [DOI] [PubMed] [Google Scholar]
- 38.Tsuneki H, Klink R, Lena C, Korn H, Changeux J-P. Eur J Neurosci. 2000;12:2475–2485. doi: 10.1046/j.1460-9568.2000.00138.x. [DOI] [PubMed] [Google Scholar]
- 39.Kasa P. Progr Neurobiol. 1986;26:211–272. doi: 10.1016/0301-0082(86)90016-x. [DOI] [PubMed] [Google Scholar]
- 40.McGehee D S, Heath M J, Gelber S, Devay P, Role L W. Science. 1995;269:1692–1696. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]
- 41.Mansvelder H D, McGehee D S. Neuron. 2000;27:349–357. doi: 10.1016/s0896-6273(00)00042-8. [DOI] [PubMed] [Google Scholar]
- 42.Girod R, Role L W. J Neurosci. 2001;21:5182–5190. doi: 10.1523/JNEUROSCI.21-14-05182.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mansvelder H D, Keath J R, McGehee D S. Neuron. 2002;33:905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 44.Luetje C V, Patrick J, Seguela P. FASEB J. 1990;4:2753–2760. doi: 10.1096/fasebj.4.10.2197155. [DOI] [PubMed] [Google Scholar]
- 45.Kulak J M, McIntosh J M, Yoshikami D, Olivera B M. J Neurochem. 2001;77:1581–1589. doi: 10.1046/j.1471-4159.2001.00357.x. [DOI] [PubMed] [Google Scholar]
- 46.Cohen G, Han Z Y, Grailhe R, Gallego J, Gaultier C, Changeux J-P, Lagercrantz H. Proc Natl Acad Sci USA. 2002;99:13272–13277. doi: 10.1073/pnas.192463599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ernst M, Moolchan E T, Robinson M L. J Am Acad Child Adolesc Psychiatry. 2001;40:630–641. doi: 10.1097/00004583-200106000-00007. [DOI] [PubMed] [Google Scholar]
- 48.MacDorman M F, Cnattingius S, Hoffman H J, Kramer M S, Haglund B. Am J Epidemiol. 1997;146:249–257. doi: 10.1093/oxfordjournals.aje.a009260. [DOI] [PubMed] [Google Scholar]