Abstract
The Wnt/β-catenin/Tcf and IκB/NF-κB cascades are independent pathways involved in cell cycle control, cellular differentiation, and inflammation. Constitutive Wnt/β-catenin signaling occurs in certain cancers from mutation of components of the pathway and from activating growth factor receptors, including RON and MET. The resulting accumulation of cytoplasmic and nuclear β-catenin interacts with the Tcf/LEF transcription factors to induce target genes. The IκB kinase complex (IKK) that phosphorylates IκB contains IKKα, IKKβ, and IKKγ. Here we show that the cyclin D1 gene functions as a point of convergence between the Wnt/β-catenin and IκB pathways in mitogenic signaling. Mitogenic induction of G1-S phase progression and cyclin D1 expression was PI3K dependent, and cyclin D1−/− cells showed reduced PI3K-dependent S-phase entry. PI3K-dependent induction of cyclin D1 was blocked by inhibitors of PI3K/Akt/IκB/IKKα or β-catenin signaling. A single Tcf site in the cyclin D1 promoter was required for induction by PI3K or IKKα. In IKKα−/− cells, mitogen-induced DNA synthesis, and expression of Tcf-responsive genes was reduced. Reintroduction of IKKα restored normal mitogen induction of cyclin D1 through a Tcf site. In IKKα−/− cells, β-catenin phosphorylation was decreased and purified IKKα was sufficient for phosphorylation of β-catenin through its N-terminus in vitro. Because IKKα but not IKKβ induced cyclin D1 expression through Tcf activity, these studies indicate that the relative levels of IKKα and IKKβ may alter their substrate and signaling specificities to regulate mitogen-induced DNA synthesis through distinct mechanisms.
INTRODUCTION
The Wingless/Wnt pathway plays a crucial role in development and cell cycle control (Cadigan and Nusse, 1997; Huelsken and Behrens, 2000). Dysregulation of the Wingless/(Wnt)/β-catenin/Tcf pathway has been implicated in tumorigenesis of diverse types (Polakis, 2000a). Axin/Conductin, together with APC, promote β-catenin degradation through serine-threonine phosphorylation of the β-catenin N-terminus by GSK3β, which targets β-catenin for ubiquitination by a SCFβ-TRCP (β-transducin repeat-containing protein) ubiquitin ligase complex (Fuchs et al., 1999; Winston et al., 1999) and its degradation by the proteasome. On induction of Wnt signaling by extracellular ligands, the Frizzled receptors are activated. The activity of GSK3β and its effect on β-catenin is antagonized by Dishevelled, a downstream target of Frizzled, thus preventing the degradation of β-catenin by the proteasome. The resulting accumulation of β-catenin leads to its nuclear translocation and binding to Tcf/Lef transcription factors to induce target genes including cyclin D1 and c-Myc (He et al., 1998; Shtutman et al., 1999; Huelsken and Behrens, 2000).
In addition to components in the Wnt signaling pathway, several other pathways can regulate β-catenin/Tcf signaling and gene expression and confer aberrant cellular growth. The protein encoded by Gas6, a growth factor of the vitamin K–dependent family, which binds members of the Axl receptor tyrosine kinase family, stabilizes β-catenin, and induces Tcf signaling (Goruppi et al., 2001). Hepatocyte growth factor/scatter factor (Papkoff and Aikawa, 1998) and oncogenic mutations of RON and MET (Danilkovitch-Miagkova et al., 2001) can also increase cytosolic β-catenin and activate Lef/Tcf-responsive reporters. The Xenopus wnt target gene twin is induced by SMAD4 through the β-catenin/Tcf complex (Nishita et al., 2000). Conversely, genotoxic stress reduces β-catenin abundance in part through p53 signaling and a Siah1/Skp1/Ebi complex, which binds the β-catenin N-terminus independently of its GSK3β phosphorylation sites (Liu et al., 2001; Matsuzawa and Reed, 2001).
The c-myc and cyclin D1 genes that encode important regulators of cell proliferation have been identified as transcriptional targets of β-catenin (He et al., 1998; Shtutman et al., 1999; Tetsu and McCormick, 1999). Transcription of the cyclin D1 gene is induced through distinct DNA sequences in the promoter by diverse mitogenic and oncogenic signaling pathways including activating mutants of Ras, Src, Stat3, Stat5, and ErbB-2 (Albanese et al., 1995; Bromberg et al., 1999; Matsumura et al., 1999; Pestell et al., 1999; Lee et al., 2000). Distinct binding sites within the cyclin D1 promoter have been characterized for transcription factors including CREB and AP-1 proteins (Albanese et al., 1995; Watanabe et al., 1996a, 1996b; Brown et al., 1998), and a single site at −81 has shown to bind β-catenin/Tcf proteins (Shtutman et al., 1999). Although Tcf/Lef proteins can function as either enhancer or repressor elements (Bienz, 1998; Barker et al., 2000), the Tcf binding site of the cyclin D1 promoter at −81 functioned as an enhancer element that conveyed activation of the cyclin D1 promoter by components of the Wnt/β–catenin pathway (Shtutman et al., 1999; D'Amico et al., 2000; Lin et al., 2000; Sampson et al., 2001; Soriano et al., 2001). The cyclin D1 gene encodes a regulatory subunit of the holoenzyme that phosphorylates and inactivates the retinoblastoma (pRB) protein. Homozygous deletion of the cyclin D1 gene in mice demonstrated a requirement for cyclin D1 in normal mammary gland development during pregnancy and mouse embryo fibroblasts (MEFs) derived from the cyclin D1−/− animals have both defective induction of DNA synthesis and enhanced cellular apoptosis rates (Fantl et al., 1995; Sicinski et al., 1995; Albanese et al., 1999; Fantl et al., 1999). Cyclin D1 overexpression can enhance DNA synthesis, is required for transformation and contact-independent growth in several cell types and has been implicated in several human cancers including breast, colon, and prostate (Shtutman et al., 1999; Tetsu and McCormick, 1999; Lee et al., 2000). Thus, cyclin D1 plays an important role in tumorigenesis and cell cycle control.
The IκB/NF-κB pathway is another pathway involved in both cell cycle control and inflammation and has recently been implicated in cancer (Karin and Delhase, 2000; Yamamoto and Gaynor, 2001). The NF-κB transcriptional activity is normally inhibited by IκB proteins that sequester it in the cytoplasm (Karin and Delhase, 2000; Joyce et al., 2001). The IκB kinase complex (IKK) that phosphorylates IκB contains two functionally distinct kinases, IKKα and IKKβ. IKKβ plays a dominant role in NF-κB regulation by TNF-α and IL-1 (Delhase et al., 1999; Li et al., 1999a). In contrast, IKKα is required for murine skeletal and keratinocyte differentiation (Li et al., 1999a; Takeda et al., 1999; Hu et al., 2001). IKKα cannot compensate for the loss of IKKβ (Li et al., 1999a), suggesting that distinct targets are regulated by IKKα and IKKβ. Although the IκB/NF-κB and Wnt/β-catenin/Tcf pathways are independent signaling pathways, both IκB and β-catenin are regulated by phosphorylation at similar consensus N-terminal serines and are targeted for ubiquitination by a similar SCFβ-TrCP complex followed by proteasomal degradation. The consequences of this regulation are, however, very different (Fuchs et al., 1999; Winston et al., 1999). Thus, although the SCFβ-TrCP-mediated degradation of IκB leads to the induction of NF-κB activity, the SCFβ-TrCP-mediated degradation of β-catenin inhibits the activity of the Wnt pathway. In addition, although GSK3β contributes to the degradation of β-catenin and represses β-catenin/Tcf signaling, the activity of NF-κB is enhanced by GSK3β (Hoeflich et al., 2000; Polakis, 2000a).
The IKK complex is regulated by several IKK kinases including the NF-κB inducing kinase (NIK), TAK1, MEKK1, Cot/TPL2, and NAK, which coordinate physiological responses to distinct stimuli (Joyce et al., 2001). NF-κB activity is also enhanced by the serine threonine kinase Akt (Madrid et al., 2000; Romashkova and Makarov, 1999) that is known to induce cellular proliferation and survival (Datta et al., 1999) in response to PI3K activation (Franke et al., 1997; Klippel et al., 1998). Akt is recruited to IKKα by stimulation with growth factors, but not by TNF-α. Akt activation by PI3K is inhibited by the tumor suppressor PTEN, a D3 phosphoinositide phosphatase that induces G1 arrest in prostate cancer cells (Ramaswamy et al., 1999), consistent with both a role for PTEN as a prostate cancer cell tumor suppressor and a role of PI3K-Akt activation in cell cycle progression (Di Cristofano et al., 2001). The Gas6-dependent proliferation and activation of Tcf is also dependent on PI3K (Goruppi et al., 2001), suggesting a role for PI3K signaling in the regulation of β-catenin/Tcf signaling. The components of the cell cycle machinery that are regulated by IKKα and are required for PI3K-dependent cellular proliferation, however, remain to be determined.
Here we show a novel role for IKKα in mitogenic signaling through transcriptional induction of the cyclin D1 gene. We show that the serum induction of cyclin D1 and G1-S phase progression is PI3K-dependent and that cells lacking cyclin D1 show a reduction in PI3K-dependent S-phase entry. PI3K-dependent induction of cyclin D1 was blocked by an inhibitor of IKKα and activation of IKKα-induced cyclin D1. PI3K induction of cyclin D1 was inhibited by a dominant negative Tcf, and a single Tcf site in the cyclin D1 promoter was required for its induction by IKKα and PI3K. Mouse embryo fibroblasts derived from mice lacking IKKα showed reduced phosphorylation of β-catenin and reduced Tcf and cyclin D1 abundance and promoter activity. We had previously shown that IKKα exists in a complex with endogenous β-catenin (Lamberti et al., 2001). Herein we show that purified IKKα was sufficient for phosphorylation of β-catenin through its N-terminus in vitro, demonstrating that IKKα can function as a kinase independently of its heterodimeric partners. Because IKKα but not IKKβ induced cyclin D1 expresion and Tcf activity, these studies indicate that the relative levels of IKKα and IKKβ may alter their substrate and signaling specificities to regulate DNA synthesis through distinct mechanisms.
MATERIALS AND METHODS
Construction of Reporter Genes and Expression Vectors
The human cyclin D1 promoter fragments linked to the luciferase reporter gene in the pA3LUC vector promoters of the c-fos gene (c-fosLUC), TOP-FLASH, FOP-FLASH, cyclinELUC, cyclinALUC, c-MycLUC, Engrailed 2 promoter (EngrLUC), 3xRelLUC, and pGL3LUC (Promega, Madison, WI) were previously described (He et al., 1998; Joyce et al., 1999; McGrew et al., 1999; D'Amico et al., 2000; Lee et al., 2000). The expression vectors for p110-K227E, p110-CAAX (Matsumura et al., 1999), the p110-kinase dead, the p85α, p85ΔiSH2-N, 85ΔiSH2-C, p85ΔbBCR were kind gifts from Dr. J. Downward (Rodriguez-Viciana et al., 1997); pCMV-c-Akt wt, Akt-K179 M, Akt-T308A, were from Dr. A. Bellacosa; and CMV-IκB (Super-repressor) [CMV-IκBα (Sr)] was a gift from Dr. D. Ballard (Brockman et al., 1995). Mammalian expression vectors for IKKα (S176/180E and A) and IKKβ (S177/181E and A) mutants were provided by Dr. F. Mercurio and for IKKα (K54 M) and IKKβ (K44A) were provided by Tularik Inc (South San Francisco, CA).
Reporter Assays, Cell Culture, and Chemicals
Cell culture and DNA transfection were performed exactly as previously described (Lipofectamine Plus; Life Technologies BRL, Rockville, MD; DiDonato et al., 1997; Zandi et al., 1997). Transfections were normalized using RSV-β-gal unless otherwise indicated (DiDonato et al., 1997; Zandi et al., 1997). The effect of an expression vector was compared with the effect of an equal amount of vector cassette. The DU145 cells were maintained in DMEM with 10% (vol/vol) calf serum and 1% penicillin/streptomycin. SW480 colon cancer cells and Cos-7 kidney cells were grown in DMEM (5% fetal bovine serum). The IKKα−/− mouse embryo fibroblasts (MEFS) and 3T3 cells were a generous gift from Dr. M. Karin. Cells were plated at ∼100,000 cells/well in 12-well plates. After 24 h, cells were transfected with the indicated DNA and a Renilla luciferase reporter as an internal control for transfection efficiency. All transfections were done at least in triplicate and were repeated at least three times. Treatments with the PI 3-kinase inhibitor LY294002, the MEK inhibitor PD098059 (10–20 μM), the p38 MAP kinase inhibitor SB203580 (10–20 μM), wortmannin (2, 5, 10 μM) were performed for 24 h, and results were compared with vehicle treatment. Luciferase assays were performed at room temperature using an AutoLumat LB 953 (EG&G Berthold, Natick, MA). Luciferase content was measured by calculating the light emitted during the initial 10 s of the reaction, and the values are expressed in arbitrary light units. Statistical analyses were performed using the Mann Whitney U test with significant differences established as p < 0.05. To select transfected cells, cotransfection experiments were conducted using magnetic separation of transfected cells using CD4 as the marker and the magnetic-activated cell separation system (MACS; Ashton et al., 1999).
Western Blots and Cell Cycle Analysis
Western blotting was performed with antibodies directed to cyclin D1 (DCS-6; NeoMarkers, Fremont, CA), TFIIB (Transduction Laboratories, Lexington, KY), IKKα (mAb was from PharMingen, San Diego, CA), IKKα, (polyclonal SC7182, Santa Cruz Biotechnology, Santa Cruz, CA) IKKβ, (polyclonal SC7607, Santa Cruz Biotechnology), β-catenin (Transduction Laboratories), phospho-β-catenin (Cell Signaling, Beverly, MA), Flag, (M2, Sigma Chemical Co., St. Louis, MO) and HA (12CA5, Sigma). Cell homogenates (50 μg) were electrophoresed in an SDS-12% polyacrylamide gel and transferred electrophoretically to a nitrocellulose membrane (Micron Separations Inc., Westborough, MA). After transfer, the gel was stained with Coomassie blue as a control for blotting efficiency. The blotting membrane was incubated for 2 h at 25°C in T-PBS buffer supplemented with 5% (wt/vol) dry milk to block nonspecific binding sites. After a 6-h incubation with primary antibody at a 1:1000 dilution (cyclin D1) or 1:2500 (α-tubulin) in T-PBS buffer containing 0.05% (vol/vol) Tween 20, the membrane was washed with the same buffer. For detection of cyclin D1 the membrane was incubated with goat anti-mouse horseradish peroxidase second antibody (Santa Cruz Biotechnology) and washed again. Immunoreactive proteins were visualized by the enhanced chemiluminescence system (Kirkegaard and Perry Laboratories, Gaithersburg, MD). Annexin V staining for apoptosis (Albanese et al., 1999) and cell cycle analysis were performed by flow cytometric analyses using a fluorescence-activated cell sorter (FACStar plus; Becton Dickinson & Co., Lincoln Park, NJ).
In Vitro Kinase Assays
Kinase assays were performed as described (Yamamoto et al., 2000). The baculovirus-produced IKKα protein was purified by nickel-agarose chromatography and then immunoprecipitated with 12CA5 mAb (Yamamoto et al., 2000). IKKα was added to kinase buffer containing 10 μCi of [γ-32P], 1 mM ATP, 1 mM dithiothreitol, 5 mM MgCl2, 100 mM NaCl, 50 mM Tris-HCl, pH 8.0, and then 1 μg of each of the substrates including GST-IκBα (1–54) or GST-β-cat constructs (Lamberti et al., 2001) was incubated for 15 min at 30°C. Reactions were incubated at 30°C for 30 min and stopped by the addition of protein loading buffer and heating to 90°C and SDS-PAGE and autoradiography.
RESULTS
PI3K-induction of Cyclin D1 Requires the Tcf Binding Site
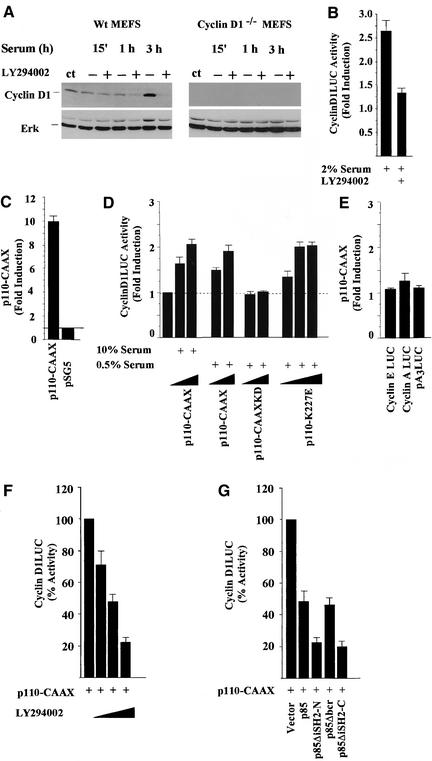
Activation of phosphatidyl inositol 3′-kinase (PI3K) mediates signaling induced by a number of growth factors and tumor promoters and is required for mitogenic stimulation by specific growth factors during the G1-S phase of the cell cycle (Klippel et al., 1998; Vanhaesebroeck and Waterfield, 1999). The role of PI3K in serum-induced cyclin D1 expression was examined in mouse embryo fibroblasts (MEFs). In wild-type (wt) MEFs, cyclin D1 protein levels were elevated by 3 h after serum stimulation, and the PI3K inhibitor LY294002 abrogated the induction (Figure 1A). Total ERK levels were unchanged under these conditions in both wt and Cyclin D1−/− MEFs (Figure 1A). Activity of the full-length human cyclin D1 promoter linked to a luciferase reporter gene was induced 2.5-fold by serum addition. The PI3K inhibitor reduced serum-induced activation of the cyclin D1 promoter by 80% (Figure 1B). Activation of PI3K and Akt plays a key role in DNA synthesis in prostate cancer cells (Ramaswamy et al., 1999; Di Cristofano et al., 2001). We therefore examined the role of PI3K in the PTEN containing prostate cancer cell line DU145. Because PI3K plays a role in signaling by diverse growth factors, including Gas6 in density-arrested cells (Goruppi et al., 2001), we examined the regulation of cyclin D1 by PI3K in density-arrested cells. The cyclin D1 promoter (−1745 CD1LUC) was induced 10-fold by p110α-CAAX compared with the empty vector (Figure 1C). In low-confluence cells the cyclin D1 promoter was induced significantly by p110α-CAAX in either high (Figure 1C) or low serum conditions (2.3-fold ± 0.18, n = 11, p < 0.01; Figure 1D). The kinase dead mutant (p110α-CAAX KD) did not affect cyclin D1 promoter activity, and the constitutively active p110α-K227E mutant induced cyclin D1 2.2-fold (Figure 1D). In contrast with the cyclin D1 promoter, the cyclin E and cyclin A promoters were not induced by p110α-CAAX (Figure 1E), suggesting that the induction of cyclin D1 is not an indirect effect of PI3K activity on DNA synthesis and the effect of p110α-CAAX is promoter specific. Because cryptic activation sequences, including AP-1, have been identified in several expression vectors, we examined the empty luciferase reporter pA3LUC in which the cyclin D1 promoter was cloned and found that pA3LUC was not induced (Figure 1E) in contrast with pGL3LUC, which was induced threefold by p110α-CAAX (Amanatullah et al., 2001). Cyclin D1 promoter activation by PI3K was reduced by the chemical inhibitor LY294002 (Figure 1F) or Wortmannin (our unpublished results). Type 1 PI3K is a heterodimeric holoenzyme, consisting of a regulatory (p85) and a catalytic (p110) subunit, which was initially identified through its role in Src-mediated transformation. p110α-CAAX induction of cyclin D1 promoter activity was reduced by the previously described dominant inhibitory mutants of the PI3K regulatory subunit (Rodriguez-Viciana et al., 1997; Figure 1G).
Figure 1.
PI3K-induction of cyclin D1. (A) Western blot analysis of MEFs for cyclin D1 derived from wild-type (cyclin D1 wt) or cyclin D1−/− mice and treated with serum either with or without the PI3K inhibitor LY294002. The Western blot was probed for cyclin D1 and total ERK. (B) The serum-induced activity of the cyclin D1 promoter in the presence or absence of the PI3 kinases inhibitor LY294002 (20 μM). (C) DU145 cells at either >90% or (D) 30% confluence were transfected with a cyclin D1 promoter luciferase reporter plasmid (−1745CD1LUC) and either the p110α-CAAX, or (D) the p110α kinase dead mutant (p110α-CAAX-KD) or the constitutively active p110α-K227E mutant expression plasmid in the presence of either 10% or 0.5% serum. The fold induction of the luciferase reporter activity is shown for nine separate experiments as mean ± SEM throughout. (E) The effect of p110-CAAX on reporter plasmids for cyclin A and the cyclin E promoter, and the luciferase reporter pA3LUC. (F) The p110α-CAAX induction of the cyclin D1 promoter activity was inhibited by LY294002 (using 2, 20, and 100 μM). (G) The cyclin D1 promoter activity in the presence of p110α-CAAX is shown as 100% and is compared with the effect of cotransfected dominant negative inhibitors of PI3K including p85α, p85ΔiSH2-N, 85ΔiSH2-C, or p85ΔBCR (Rodriguez-Viciana et al., 1997). The results are shown compared with equal amounts of empty control vector for each expression vector plasmid.
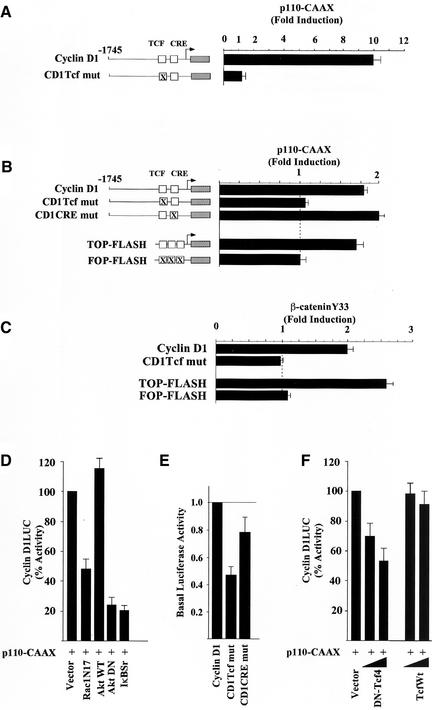
Oncogenic forms of p110α and p85 have been identified, and expression of a constitutively active PI3K was shown to trigger DNA synthesis through activation of several distinct signaling pathways (Chang et al., 1997; Klippel et al., 1998). The cyclin D1 promoter contains several distinct transcription factors binding sites targeted by different signaling pathways (reviewed in Pestell et al., 1999). Using a series of 5′ cyclin D1 promoter deletion constructions, the minimal p110α-CAAX responsive region was identified within the proximal 163 base pairs, which includes a Tcf site at −81 (our unpublished results). Point mutation of this sequence in the context of the −1745-base pair promoter fragment abolished induction at either high confluence (Figure 2A) or at low confluence (Figure 2B). p110α-CAAX induced the Tcf response element (TOP-FLASH) but had no effect on a reporter construct in which the Tcf site fails to bind Tcf/β-catenin (FOP-FLASH; Figure 2B). A constitutively active stable mutant of β-catenin (β-catenin Y33), found in colon cancer and the SW48 colon cancer cell line, activates β-catenin signaling when transfected into cultured cells. The sequence of the cyclin D1 promoter Tcf site is identical to the canonical sequence of the TOP-FLASH reporter. Consistent with the identification of a single Tcf site in the cyclin D1 promoter required for regulation by β-catenin/Tcf signaling in several studies (Shtutman et al., 1999; Lin et al., 2000; Soriano et al., 2001), the cyclin D1 promoter was induced twofold by β-catenin Y33 in DU145 (p < 0.01, n = 8) and point mutation of the cyclin D1 Tcf site at −81 abolished induction by both β-catenin Y33 and by p110α-CAAX (Figure 2, B and C). The twofold induction of −1745CD1LUC by β-catenin Y33 in DU145 is consistent with the threefold induction of cyclin D1 promoter activity described in Hela cells (Tetsu and McCormick, 1999).
Figure 2.
PI3K-induction of cyclin D1 requires the Tcf site and is dependent upon IκB. (A) The effects of the p110α-CAAX expression plasmid on the activity of the cyclin D1 promoter (−1745 CD1LUC) or of a point mutant of the Tcf site at −81 (−1745 Tcf mut) in DU145 cells grown to either > 90% or (B) 30% confluence. Regulation of the cyclin D1 and Tcf-responsive (TOPFLASH) and mutant (FOPFLASH) reporter constructs by either the p110-CAAX plasmid or (C) the activated β-catenin point mutant (Y33). The fold induction of the luciferase reporter activity is shown for at least nine separate experiments as mean ± SEM throughout. (D) The cyclin D1 promoter activity in the presence of p110α-CAAX is shown as 100% and is compared with the effect of cotransfected dominant negative inhibitors of PI3K including RacN17, Akt wt, AktDN (K179 M), or IκBαSr. The results are shown compared with equal amounts of empty control vector for each expression vector plasmid. (E) Point mutations of the cyclin D1 promoter Tcf or CRE site were compared with the basal promoter activity of −1745 CD1LUC. The activity of the wild-type promoter construction was set as 1.0. The data are mean ± SEM of five separate transfections. (F) The cyclin D1 promoter activity in the presence of p110α-CAAX (100%) is compared with the effect of dominant negative or wild-type Tcf.
To investigate the signaling pathways by which PI3K induced cyclin D1, we used previously well-characterized dominant negative mutant expression vectors. In agreement with a previous study in which expression of Rac1-N17 blocked PI3K-induced activity (Rodriguez-Viciana et al., 1997), p110-CAAX–induced cyclin D1 promoter activity was reduced 50% by Rac1-N17 (Figure 2D). Because PI3K activates Akt (Franke et al., 1995; King et al., 1997), we examined the role of Akt in PI3K induction of cyclin D1. A kinase-inactive dominant negative Akt (Akt K179 M), but not wild-type Akt, inhibited p110α-CAAX–induced activation of cyclin D1 (Figure 2D). Because Akt regulates several distinct pathways including NF-κB activity (Kane et al., 1999; Romashkova and Makarov, 1999), we examined the possibility that IKK activity may play a role in PI3K induction of cyclin D1. The dominant IκB inhibitor, CMV-IκBαSr, inhibited p110α-CAAX–induced activation of cyclin D1 (Figure 2D) but did not inhibit c-fos LUC activity (our unpublished results). The p38 MAPK inhibitor SB203580, the ERK inhibitor PD98059, and rapamycin had no effect on p110α-induced D1 activity (our unpublished results). Tcfs may serve as either activators or repressors of gene transcription through the Tcf site (Bienz, 1998; Barker et al., 2000). In DU145 cells we found that mutation of the cyclin D1 Tcf site reduced the basal promoter activity to 55%, consistent with previous studies suggesting the cyclin D1 Tcf site functions as a basal enhancer element in several cell types (Shtutman et al., 1999; D'Amico et al., 2000; Soriano et al., 2001; Figure 2E). Coexpression of a DN-Tcf, but not wild-type Tcf, inhibited p110-CAAX induced cyclin D1 promoter activity (Figure 2F). Together these studies suggest p110-CAAX induction of cyclin D1 promoter activity involves a Tcf signaling pathway.
Cyclin D1 Is Required for PI3K-dependent S-Phase Entry in Primary Cells
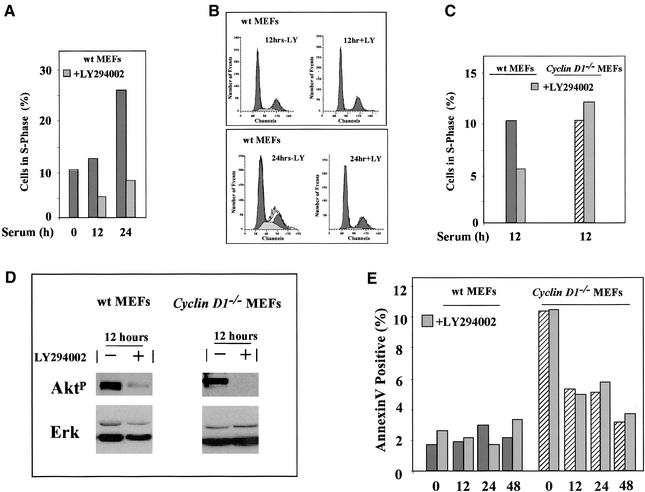
The current studies suggest cyclin D1 is a distal target of PI3K in serum-induced DNA synthesis. Cyclin D1 is known to play a role in the entry of cells into the DNA synthetic (S) phase induced by several growth factors and mitogens. The role of PI3K in serum-induced DNA synthesis through cyclin D1 is not known and was therefore further examined. In wt MEFs, serum-induced entry into S phase, increased from 10 to 26% (Figure 3A). LY294002 reduced the S-phase proportion from 26 to 7% at 24 h, indicating that serum-induced DNA synthesis is substantially PI3K dependent in MEFs (Figure 3, A and B). LY294002 treatment reduced serum-induced DNA synthesis by a mean of 39% at 12 h after serum addition but did not affect the serum-induced entry into the S-phase fraction in the cyclin D1−/− MEFs (Figure 3C, mean for n = 4 separate experiments). To confirm that LY294002 was effective at inhibiting signaling downstream of PI3K in both the cyclin D1 wt and cyclin D1−/− MEFs, western blotting was performed for phosphorylated Akt using a specific antibody, and the membrane was probed for total ERK as a control (Figure 3D). Serum-induced phosphorylation of Akt was reduced by LY294002 in both cell types (Figure 3D). Similar analyses of serum-induced DNA synthesis were performed in 3T3 cells derived from the cyclin D1+/+ and cyclin D1−/− MEFs with similar results (our unpublished results). To determine the role of PI3K in apoptosis mediated by serum deprivation, annexin V staining and sub G1 analysis was performed on the MEFs. Cyclin D1−/− MEFs exhibited a fivefold greater level of annexin V staining compared with wt MEFs, indicating increased basal apoptosis as previously shown (Albanese et al., 1999) that was rescued by serum (Figure 3E). LY294002 did not affect the level of apoptosis in either wt or cyclin D1−/− MEFs as determined by either annexin V staining (Figure 3E) or sub G1 analysis (our unpublished results). These studies suggest that a substantial component of serum-induced expression of cyclin D1 is PI3K dependent and that MEFs derived from animals deleted of the cyclin D1 gene show reduced PI3K-dependent induction of DNA synthesis.
Figure 3.
Involvement of cyclin D1 in PI3K-dependent S-phase entry in primary cells. (A) MEFs were treated either with vehicle (DMSO) or LY294002 (20 μM) and DNA synthesis assessed by FACS. (B) FACS analysis of wt MEFs in the presence and absence of LY294002 (LY; 20 μM) 12 and 24 h after serum stimulation. (C) The effects of LY294002 on S-phase wt and cyclin D1−/− MEFs are shown after serum stimulation as mean of four separate experiments. (D) Western blotting for phosphorylated Akt or total ERK of MEFs treated with serum and either vehicle or LY294002. (E) The level of apoptosis was determined by Annexin V staining in serum-starved–stimulated cyclin D1wt and cyclin D1−/− MEFs.
IKKα, but not IKKβ Induces Cyclin D1 through β-Catenin/Tcf
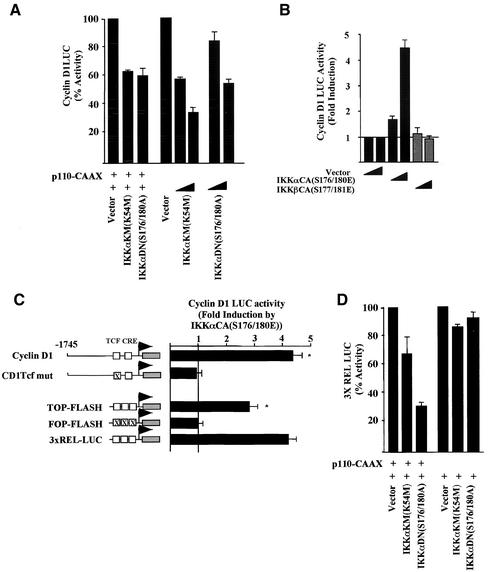
The studies described above indicate that the PI3K activation of cyclin D1 involves Akt and IκB (Ozes et al., 1999). As IKKs regulate IκB activity, we assessed the role of IKKs in PI3K-dependent activation of cyclin D1 using previously characterized dominant negative IKK mutants (Delhase et al., 1999). These expression vectors behaved as previously described in cultured cells (below). We found that both the dominant negative and kinase dead IKKα constructs reduced PI3K-induced cyclin D1 promoter activity and the basal promoter activity in a dose-dependent manner (Figure 4A). The constitutively active mutant IKKαCA(S176/180E) induced the cyclin D1 promoter 4.2-fold (Figure 4B). The IKKαCA expression vector was previously well characterized and was shown to integrate in the IKK kinase using the identical transfection approach (DiDonato et al., 1997; Zandi et al., 1997). In contrast with IKKα, the constitutively active IKKβ mutant (IKKβCA) decreased the cyclin D1 promoter activity (see below). Using a series of 5′ promoter deletion constructions the IKKα responsiveness was confined to the proximal −163 base pairs (our unpublished results). Mutation of the Tcf site in the context of the −1745-base pair fragment abolished induction of cyclin D1 by IKKαCA (Figure 4C). IKKαCA induced TOP-FLASH threefold but did not induce a reporter with mutations of the Tcf site (FOP-LUC; Figure 4C). IKKαCA also activated the canonical NF-κB–responsive sequences (3xRelLUC) to the same extent (Figure 4C). Consistent with previous studies, in which PI3K and Akt induced NFκB activity in response to IL-1 (Madrid et al., 2000, 2001), the IKKα kinase dead and dominant negative mutants reduced the activity of the NFκB-responsive reporter gene 3XRelLUC in the presence of p110α-CAAX (Figure 4D).
Figure 4.
IKKα induces the cyclin D1 gene through the β-catenin/Tcf site. (A) DU145 cells were transfected with the cyclin D1 promoter luciferase reporter plasmid (−1745 CD1LUC) and the p110α-CAAX, with the dominant inhibitors (IKKαKM(K54 M), IKKαDN(S176/180A)) or (B) constitutively active plasmid (IKKαCA(S176/180E) or IKKβ(S177/181E)). (C) Identification of the IKKα-responsive sequences in the cyclin D1 promoter. The IKKαCA(S176/180E) expression plasmid was coexpressed with the luciferase reporters shown and fold induction determined compared with equal amounts of empty expression vector cassette. (D) Effect of IKKα kinase dead (IKKαK54 M) and dominant negative mutants (IKKα(S176/180A)) on p110α-CAAX induced NF-κB activity assessed using the 3XREL LUC reporter.
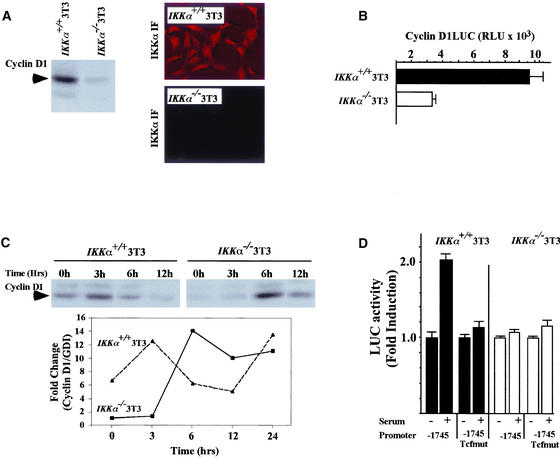
To provide genetic evidence for the involvement of IKKα activity in regulating cyclin D1, MEFs from IKKα−/− mice were selected by the 3T3 protocol. Cells were serum starved for 24 h and western blotting was performed to determine cyclin D1 levels. Immunostaining for IKKα showed the presence of IKKα in the wt 3T3 and the absence of staining in the IKKα−/− 3T3 cells (Figure 5A). We found that cyclin D1 abundance was reduced by 85% in the IKKα−/− cells (Figure 5A), and the activity of the cyclin D1 promoter in the IKKα−/− cells was lower by 67% compared with IKKα+/+ cells (Figure 5B). Serum treatment induced cyclin D1 abundance in wt MEFs by two- to threefold after 3 h, whereas in the IKKα−/− cells, induction was delayed until 6 h after serum stimulation (Figure 5C), suggesting a role for IKKα in both the basal level of cyclin D1 expression and in serum-induced cyclin D1 abundance. Because IKKα induced cyclin D1 through the Tcf site and serum-induction of cyclin D1 protein abundance was defective in the IKKα−/− cells, we assessed the role of the cyclin D1 promoter Tcf site in serum-induced activity. In wt 3T3 cells, serum-induced activation of the cyclin D1 promoter was reduced more than 90% by mutation of the Tcf site (Figure 5D). Furthermore, serum-induced activation of the cyclin D1 promoter was defective in the IKKα−/− cells (Figure 5D).
Figure 5.
Reduced mitogen-induced cyclin D1 expression in IKKα−/− cells involves Tcf signaling. (A) 3T3 cells from either wt or IKKα−/− mice were examined by Western blotting for cyclin D1, using equal amounts of total protein. Immunostaining for IKKα in the wt or IKKα−/− 3T3 cells. (B) The cyclin D1 promoter luciferase reporter plasmid (−1745 CD1LUC) was transfected into either wt or IKKα−/− 3T3 cells along with the β-galactosidase control reporter. Relative cyclin D1 promoter activity is shown as mean ± SEM for n = 3. (C) Western blotting for cyclin D1 of wt or IKKα−/− 3T3 cells treated with serum for the time points indicated. The fold change in cyclin D1 protein levels is shown normalized to GDI as a loading control. The data is representative of three separate experiments. (D) The cyclin D1 promoter (−1745CD1LUC) or the point mutant of the Tcf site (−1745Tcfmut) were compared for relative activity in wt or IKKα−/−3T3 cells. The data are mean ± SEM, n = 9.
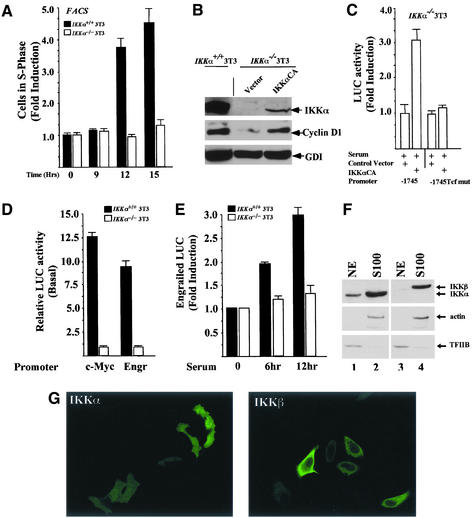
Consistent with the reduced abundance of cyclin D1 in the IKKα−/− 3T3 cells and the ability of cyclin D1 overexpression to promote DNA synthesis in fibroblasts (Pagano et al., 1994), serum-induced DNA synthesis was reduced in IKKα−/− 3T3 cells (Figure 6A). To determine whether the reduction in IKKα abundance was important in the reduced levels of cyclin D1, the IKKα−/− 3T3 cells were transfected with the IKKαCA expression vector and MACS-sorted, and the cell extracts were subjected to Western blotting. IKKα protein levels were increased in the IKKαCA-transfected IKKα−/− 3T3 cells (Figure 6B, lane 3). Although the relative levels of IKKα in the IKKα−/− 3T3 cells transfected with the IKKαCA expression vector were substantially less than the wt 3T3 cells, cyclin D1 levels were increased threefold compared with the IKKα−/− 3T3 cells, demonstrating a key role for IKKα in inducing cyclin D1 levels. Activity of the cyclin D1 promoter was also increased threefold in IKKα−/− 3T3 cells transfected with the IKKαCA expression vector. Furthermore, the induction of cyclin D1 by IKKαCA required the Tcf site (Figure 6C).
Figure 6.
IKKα regulates mitogen-induced DNA synthesis and is required for Tcf signaling to natural Tcf-responsive genes. (A) The serum-induced S-phase fraction of wt or IKKα−/− 3T3 cells was compared. The data are mean ± SEM, N = 7. (B) Western blotting of wt or IKKα−/− 3T3 cells transfected with an expression vector for IKKαCA or empty expression vector. IKKα and cyclin D1 immunoblotting is shown with GDI as an internal control for loading. (C) Relative activity of the cyclin D1 promoter or the corresponding Tcf point mutant in IKKα−/− 3T3 cells transfected with either IKKα expression vector or the control vector. The data are mean ± SEM, n = 8. (D) Relative activity of the c-Myc and Engr promoters (n = 6) in randomly cycling wt or IKKα−/− 3T3 cells. The activity of the promoter is set as 1 in the IKKα−/− 3T3 cells. (E) Activity of the Engr promoter in the presence of serum stimulation. The data is mean ± SEM of n = 12 separate experiments. (F) Nuclear and cytosolic fractions of Cos-7 cells were analyzed by Western blotting for the abundance of IKKβ or IKKα in the nuclear (NE) and cytosolic (S100) fractions. Internal controls for (nuclear; TFIIB) and cytoplasmic (actin) markers are shown. Substantially more IKKα than IKKβ was found in the nuclear extracts (NE) of Cos-7 cells. (G) Immunostaining for IKKα and IKKβ. IKKβ is predominantly extranuclear, whereas IKKα was found in both nuclear and cytoplasmic compartments.
To determine whether the activity of other known β-catenin responsive promoters were regulated by IKKα, the relative activity of the c-Myc (He et al., 1998) and Engrailed (McGrew et al., 1999) promoters were compared in the wt and IKKα−/− 3T3 cells, with relative activity normalized to an internal control of renilla luciferase activity. The relative activity of the Engr and c-Myc promoter activity was reduced 10- to 12-fold in the IKKα−/− 3T3 cells (Figure 6D). Furthermore, as with the cyclin D1 promoter, the serum-induced activity of the Engr promoter was substantially reduced in the IKKα3T3 cells (Figure 6D). Together, these studies demonstrate that the activity of both heterologous and natural Tcf responsive genes is dependent on IKKα in vivo. Furthermore, these studies demonstrate an important role for IKKα in regulating the kinetics of serum-induced expression of β-catenin/Tcf-responsive genes.
IKKα Associates with and Phosphorylates β-Catenin and Increases β-Catenin Abundance
In addition to the differences in Tcf-mediated activation of gene promoters, several lines of evidence suggest that IKKα and IKKβ fulfill distinct cellular functions. Thus, homozygous deletion of the IKKα and IKKβ genes results in distinct phenotypes (Hu et al., 1999, 2001; Li et al., 1999a; Takeda et al., 1999), and IKKβ acts more potently on IκB proteins and plays a more significant role in the NF-κB pathway in response to activation with TNF-α and IL-1 than IKKα (Delhase et al., 1999; Li et al., 1999a, 1999b). To investigate further the basis for these diverse functions, we determined the subcellular localization of IKKα and IKKβ and their cell-type expression patterns. Western blot analysis of nuclear and cytoplasmic extracts showed a differential localization of IKKα and IKKβ in Cos-7 cells with IKKα present in both the nuclear and cytoplasmic fractions (marked by TFIIB and actin, respectively), whereas IKKβ was predominantly cytoplasmic (Figure 6A), consistent with the immunohistochemical analysis (Figure 6B).
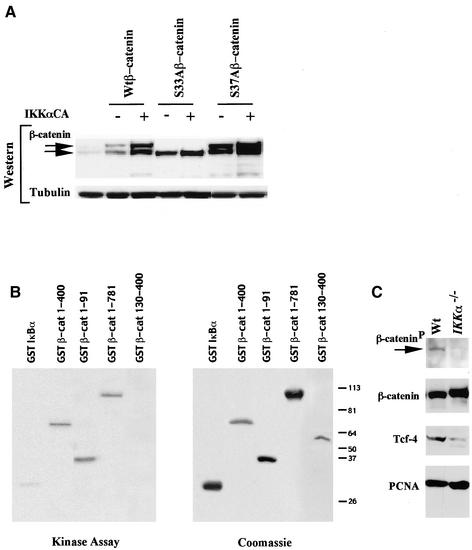
Consistent with a role for IKKα in regulating β-catenin phosphorylation and/or abundance, the total level of wt β-catenin and of a higher molecular weight form of β-catenin were increased in cells coexpressing IKKαCA and β-catenin expression vectors (Figure 7A). Point mutation of β-catenin at Ser33 to alanine abrogated the induction of the higher molecular weight form of β-catenin (Yost et al., 1996). The abundance of the β-catenin S37A mutant and the higher molecular weight form were also increased in cells transfected with IKKαCA, suggesting a dominant role for S33 in the generation of the high molecular weight form. Because IKKαCA induced cyclin D1 and Tcf reporter activity, we hypothesized that IKKα may regulate β-catenin abundance and/or phosphorylation. In our previous studies, IKK immunoprecipitation on fractionated Cos-7 cell extracts cotransfected with HA-tagged β-catenin and FLAG-tagged IKKα showed that β-catenin is present in IKKα immunoprecipitates and IKKα was also present in β-catenin immunoprecipitates (Lamberti et al., 2001). We had also demonstrated an association between endogenous β-catenin and IKKα by reciprocal IP-Western blotting of SW480 cell extracts (Lamberti et al., 2001). Consistent with these findings in cultured cells, we found that GST-β–catenin fusion proteins were efficient substrates for phosphorylation by IKKα in vitro in which IKKα was immunoprecipitated from cultured cells and used as the enzyme source (our unpublished results). The minimal region of β-catenin sufficient for phosphorylation by immunoprecipitated IKKα included the N-terminal portion of the molecule between aa 30 and 55 (our unpublished results). IKKα bound to and phosphorylated β-catenin in vitro with an efficiency that was similar to that of IκB as recently shown (Lamberti et al., 2001).
Figure 7.
IKKα phosphorylates β-catenin and increases β-catenin abundance. (A) The IKKαCA(S/E) expression plasmid was coexpressed in cells transfected with either wild-type or mutants (S33A, S37A) of β-catenin. Western blotting analysis showed an increase the total amount of β-catenin, including a higher molecular weight form (upper arrow). The S33Aβ-catenin shows no increase in the amount of the high molecular weight form. (B) IKKα kinase assays conducted using baculovirus expressed purified IKKα and the GST-β-catenin constructs as shown. Kinase activity (left panel) and the Coomassie stained gel for the substrate are shown. (C) Western blot analysis of IKKα−/− or IKKwt 3T3 cells with antibodies to phosphospecific β-catenin, total β-catenin, Tcf-4, and PCNA.
Because the IKKα immunoprecipitation may coprecipitate other components of the IKK complex to phosphorylate β-catenin, IKKα was produced in baculovirus, purified, and used as the enzyme source in IKKα kinase assays with β-catenin as substrate (Figure 7B). Purified IKKα was sufficient for phosphorylation of GST-β-catenin 1–400. Deletion of the N-terminus of β-catenin (130–400) abolished phosphorylation by IKKα, and the N-terminus from 1–91 was sufficient for phosphorylation by IKKα (Figure 7B). To determine if the endogenous IKKα is involved in the phosphorylation of β-catenin, equal amounts of proteins from IKKα−/− and wt MEFs were compared using an antiphospho-β-catenin antibody. The results shown in Figure 7C demonstrated that phosphorylated β-catenin exists in wt MEFs but with a significantly reduced abundance in the IKKα−/− cells. Interestingly, the levels of the nuclear effector of β-catenin, Tcf were also lower in the IKKα−/− cells. The abundance of the nuclear protein PCNA was similar between the IKKα−/− and wt MEFs.
Our findings that IKKα phosphorylates β-catenin and that IKKαCA increases Tcf activity and β-catenin abundance suggests that β-catenin phosphorylation by IKKα may contribute to the regulation of β-catenin–mediated Tcf-dependent gene transcription. The consequent induction of cyclin D1 by PI3K-IKKα-Tcf signaling contributes to the induction of DNA synthesis.
DISCUSSION
This study demonstrates for the first time a requirement for IKKα in response to mitogens and DNA synthesis and the induction thereby of cyclin D1 abundance and promoter activity through a β-catenin/Tcf pathway. IKKα selectively and directly induced cyclin D1 but not cyclin E or cyclin A. Reintroduction of IKKα into IKKα-deficient cells restored cyclin D1 expression and promoter activity in a Tcf-dependent manner. Using a dominant negative mutant of Tcf activity we showed that IKKα induction of cyclin D1 requires β-catenin/Tcf activity. IKKα was shown to be a key genetic determinant of the activity of several other Tcf responsive genes (c-Myc, Engr, TcfLUC). IKKα-deficient cells demonstrated a delayed induction of serum-induced DNA synthesis and a delayed induction of serum-induced activity of the cyclin D1 and Engr promoters. Together these studies indicate a key role for IKKα in coordinating the kinetics of mitogen responsiveness to a subset of cellular targets. These studies are consistent with an evolving view that separate components of the IKK complex may subserve distinct functions to convey signal transduction specificity (Ghosh and Karin, 2002).
Serum induction of DNA synthesis and cyclin D1 expression was PI3K dependent, and cyclin D1 was required for the PI3K-dependent induction of DNA synthesis. PI3K-dependent, serum-induced DNA synthesis was substantially reduced in cyclin D1-deficient cells, indicating a key role for cyclin D1 in this signaling pathway. Although serum deprivation increased apoptosis in cyclin D1−/− MEFs, the inhibition of apoptosis by serum addition was not affected by PI3K inhibition, demonstrating distinct functions of cyclin D1 in PI3K-dependent proliferation versus apoptosis. Although the upstream effectors of IKKα that contribute to the induction of β-catenin remain to be identified, the current studies demonstrate that the PI3K-dependent induction of cyclin D1 involves IKKα. PI3K is involved in a PDGF-regulated pathway that activates Akt, leading to an association with and activation of IKKα in cultured cells (Romashkova and Makarov, 1999), which is consistent with a role for PI3K in activating a subset of IKKα functions. Although IκB-independent effects of Akt on NF-κB have been reported (Madrid et al., 2000; Reddy et al., 2000) and IKKα phosphorylation by Akt is not essential for IKK activation of NF-κB signaling (Delhase and Karin, 2000), increasing evidence suggests IKKα conveys important kinase-dependent and -independent functions. Because the dominant inhibitors of Akt, IKKα, and Tcf reduced the induction of cyclin D1 by constitutively active PI3K mutants, it appears that PI3K may be an important upstream inducer of IKKα in the context of β-catenin/Tcf signaling.
The current studies identify the cyclin D1 Tcf site as the common target of activated PI3K, IKKα, and β-catenin and establish, using dominant negative mutants, a colinearity of these components to regulate cyclin D1 expression in cultured cells. Wnt family ligands and Frizzled family receptors define one important mechanism that can induce β-catenin/Tcf signaling (Polakis, 2000a). Although until quite recently, the activity of the β-catenin/Tcf pathway was thought to be regulated only by Wnts, a substantial body of evidence suggests that important additional regulators of this pathway exist. The protein encoded by Gas6, a growth factor of the vitamin K–dependent family, which binds the Axl receptor of the tyrosine kinase family, stabilizes β-catenin and induces Tcf signaling (Goruppi et al., 2001). Hepatocyte growth factor/scatter factor (Papkoff and Aikawa, 1998) and oncogenic mutations of RON and MET (Danilkovitch-Miagkova et al., 2001) all increase cytosolic β-catenin and activate Lef/Tcf-responsive reporters. The Xenopus wnt target gene twin is induced by SMAD4 through the β-catenin/Tcf complex (Nishita et al., 2000). In addition, suppressor screens in Drosophila have identified Dpresenilin as a target of Armadillo (a homolog of β-catenin; Cox et al., 2000) and a cell-adhesion–dependent pathway involving the integrin-linked kinase (ILK) was also shown to regulate β-catenin levels and activity (Lin and Perrimon, 1999; Payre et al., 1999; Tsuda et al., 1999). The cyclin D1 gene, which plays a critical role in oncogenic signaling pathways, is regulated (via its Tcf site) by several components that can also regulate β-catenin/Tcf signaling. Previous studies have demonstrated a role for the β-catenin-Tcf signaling pathway in activation of the cyclin D1 gene by expressing mutants of β-catenin, Wnt-1, ILK and repression via presenilin 1 (PS1) or HBP1 (Shtutman et al., 1999; Barker et al., 2000; Lin et al., 2000; Soriano et al., 2001). Although the cyclin D1 gene is known to be regulated by Tcf signaling, the upstream activators of this pathway were not known. Through identifying the β-catenin/Tcf site of the cyclin D1 promoter as a target of PI3K and IKKα signaling the current studies provide important evidence for a new signaling pathway that regulates β-catenin signaling and cyclin D1 expression.
The reduction of cyclin D1 promoter activity and protein abundance in the IKKα−/− cells in the current studies, provides genetic evidence for IKKα as an inducer of cyclin D1 abundance. Recent studies also provided genetic evidence that this IKKα dominant negative mutant inhibits cyclin D1 expression (Cao et al., 2001). IKKαAA knockin mice, in which the IKKα catalytic subunit activation loop serines were substituted for alanines, exhibited reduced cyclin D1 abundance in the mammary gland (Cao et al., 2001). The IKKαAA knockin mice failed to develop normal lobular alveolar architecture during pregnancy, and the mammary gland phenotype resembled that of the cyclin D1−/− mice (Cao et al., 2001), providing further support for a genetic link between IKKα and cyclin D1. The current studies are important in identifying cyclin D1 as a direct, rather than an indirect, transcriptional target of IKKα and extend these observations by identifying the molecular mechanisms by which IKKα directly induces cyclin D1.
Several lines of evidence in the current studies demonstrate a key role for IKKα in activating β-catenin/Tcf signaling at the cyclin D1 promoter. First, the reduced nuclear Tcf abundance in the IKKα−/− cells provides genetic evidence supporting an important role for IKKα in activating β-catenin/Tcf signaling. Second, activating mutants of IKKα induced the cyclin D1 promoter significantly (4.7-fold) and induced Tcf activity assessed as an heterologous reporter linked to the luciferase reporter gene. Third, mutation of a single Tcf site at −81 abrogated induction of the cyclin D1 promoter by either IKKα or an activating mutation of β-catenin. Together, these studies demonstrate that IKKα induces Tcf signaling through this site. In recent studies, IKK immunopreciptiated from cultured cells phosphorylated β-catenin as a substrate in vitro (Lamberti et al., 2001). As IKK immunoprecipitation coprecipitates IKKα, IKKβ, and IKKγ (NEMO), these findings raised the important question of whether IKKα alone phosphorylated IKKα.
In the current studies both transfected and endogenous IKKα was found in association with β-catenin in a variety of cultured cells. A constitutively active form of IKKα increased both β-catenin abundance and phosphorylation and induced Tcf-dependent transcription. Phosphorylation of β-catenin by IKKα required the N-terminus of β-catenin, and its phosphorylation apparently contributed to the increase in β-catenin levels. This effect of IKKα on β-catenin was dependent, at least in part, on the S33 residue of β-catenin that has an important role in regulating β-catenin stability. The N-terminal domain of β-catenin containing these serine residues was sufficient for in vitro phosphorylation and is identical to the domain found in the N-terminus of IκBα that is phosphorylated by the IKK complex (Aberle et al., 1997, Orford et al. 1997). Phosphorylation of serine residues 33 and 37 of β-catenin has been implicated in regulating protein stability and signaling by GSK3β (Yost et al., 1996). Mutation of β-catenin at S33 abrogated the ability of IKKα to induce the higher molecular weight form of β-catenin and partially reduced the increase in β-catenin levels. Because the IKKα-dependent phosphorylation of β-catenin activated Tcf signaling, this effect most probably represents a different means by which β-catenin is regulated either by GSK3β-β-TrCP or Siah-1 (Polakis, 2000a).
Cyclin D1 and β-catenin overexpression correlates with poor prognosis in human breast cancer, suggesting a role for cyclin D1 in β-catenin/Tcf-mediated signaling and cell transformation (Shtutman et al., 1999; Barker et al., 2000; Lin et al., 2000). Aberrant activation of the Tcf pathway by mutations in components of the Wnt-signaling pathway is believed to contribute to the development of a variety of human cancers, including colon, breast, and prostate cancer (Polakis, 2000b). IKKα appears to provide an important link to the control of cyclin D1 gene expression through induction of Tcf signaling. Understanding the precise mechanism by which IKKα regulates β-catenin signaling and the factors specifying IKK activity on β-catenin compared with NF-κB remains pivotal in determining the signal transduction specificity regulated by these two important pathways.
ACKNOWLEDGMENTS
We thank Drs K. Akama, D. Ballard, M. Karin, F. McCormick, F. Mercurio, and B. Vogelstein for plasmids and cells. This work was supported by grants to R.G.P. from National Institutes of Health (RO1CA70896, RO1CA75503, RO1CA86072, RO1CA86071), the Breast Cancer Alliance Inc., and The Susan Komen Breast Cancer Foundation. R.G.P. is a recipient of the Weil Caulier Irma T. Hirschl Career Scientist award and was the Diane Belfer Faculty Scholar in Cancer Research. C.A. is a recipient of RO3 AG20337. S.B. is supported by the Susan G. Komen Breast Cancer Foundation and the Department of Defense.
Footnotes
DOI: 10.1091/mbc.02–06–0101.
REFERENCES
- Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-Catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albanese C, et al. Activation of the cyclin D1 gene by the E1A-associated protein p300 through AP-1 inhibits cellular apoptosis. J Biol Chem. 1999;274:34186–34195. doi: 10.1074/jbc.274.48.34186. [DOI] [PubMed] [Google Scholar]
- Albanese C, Johnson J, Watanabe G, Eklund N, Vu D, Arnold A, Pestell RG. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J Biol Chem. 1995;270:23589–23597. doi: 10.1074/jbc.270.40.23589. [DOI] [PubMed] [Google Scholar]
- Amanatullah D, Zafonte B, Albanese C, Fu M-F, Messiers C, Hassell J, Pestell RG. Ras regulation of cyclin D1 promoter. Methods Enzymol. 2001;332:116–127. doi: 10.1016/s0076-6879(01)33050-1. [DOI] [PubMed] [Google Scholar]
- Ashton AW, Watanabe G, Albanese C, Harrington EO, Ware JA, Pestell RG. Protein kinase Cδ inhibition of S-phase transition in capillary endothelial cells involves the cyclin dependant kinase inhibitor p27Kip1. J Biol Chem. 1999;274:20805–20811. doi: 10.1074/jbc.274.30.20805. [DOI] [PubMed] [Google Scholar]
- Barker N, Morin PJ, Clevers H. The yin-yang of TCF/β-catenin signaling. Adv Cancer Res. 2000;77:1–4. doi: 10.1016/s0065-230x(08)60783-6. [DOI] [PubMed] [Google Scholar]
- Bienz M. TCF: transcriptional activator or repressor. Curr Opin Cell Biol. 1998;10:366–372. doi: 10.1016/s0955-0674(98)80013-6. [DOI] [PubMed] [Google Scholar]
- Brockman JA, Scherer DC, McKinsey TA, Hall SM, Qi X, Lee WY, Ballard DW. Coupling of a signal response domain in I kappa B alpha to multiple pathways for NF-kappa B activation. Mol Cell Biol. 1995;15:2809–2818. doi: 10.1128/mcb.15.5.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell JE. Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- Brown JR, Nigh E, Lee RJ, Ye H, Thompson MA, Saudou F, Pestell RG, Greenberg ME. Fos family members induce cell cycle entry by activating cyclin D1. Mol Cell Biol. 1998;18:5609–5619. doi: 10.1128/mcb.18.9.5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- Cao Y, Bonizzi G, Seagroves T, Greten FR, Johnson R, Schmidt EV, Karin M. IKKα provides an essential link between RANK signaling and cyclin D1 expression during mammary gland development. Cell. 2001;107:763–775. doi: 10.1016/s0092-8674(01)00599-2. [DOI] [PubMed] [Google Scholar]
- Chang HW, Aoki M, Fruman D, Auger KR, Bellacosa A, Tsichlis PN, Cantley LC, Roberts TM, Vogt PK. Transformation of chicken cells by the gene encoding the catalytic subunit of PI 3-kinase. Science. 1997;276:1848–1850. doi: 10.1126/science.276.5320.1848. [DOI] [PubMed] [Google Scholar]
- Cox RT, McEwen DG, Myster DL, Duronio RJ, Loureiro J, Peifer M. A screen for mutations that suppress the phenotype of Drosophila armadillo, the β-catenin homolog. Genetics. 2000;155:1725–1740. doi: 10.1093/genetics/155.4.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amico M, et al. The integrin-linked kinase regulates the cyclin D1 gene through glycogen synthase kinase 3β and CREB-dependent pathways. J Biol Chem. 2000;275:32649–32657. doi: 10.1074/jbc.M000643200. [DOI] [PubMed] [Google Scholar]
- Danilkovitch-Miagkova A, Miagkov A, Skeel A, Nakaigawa N, Zbar B, Leonard EJ. Oncogenic mutants of Ron, and Met receptor tyrosine kinases cause activation of the β-catenin pathway. Mol Cell Biol. 2001;21:5857–5868. doi: 10.1128/MCB.21.17.5857-5868.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- Delhase M, Hayakawa M, Chen Y, Karin M. Positive and negative regulation of IκB kinase activity through IKKβ subunit phosphorylation. Science. 1999;284:309–313. doi: 10.1126/science.284.5412.309. [DOI] [PubMed] [Google Scholar]
- Delhase M, Karin M. Kinase regulation in inflammatory response. Nature. 2000;406:367–368. doi: 10.1038/35019154. [DOI] [PubMed] [Google Scholar]
- Di Cristofano A, De Acetis M, Koff A, Cordon-Cardo C, Pandolfi PP. Pten and p27 KIP1 cooperate in prostate cancer tumor suppression in the mouse. Nat Genet. 2001;27:222–224. doi: 10.1038/84879. [DOI] [PubMed] [Google Scholar]
- DiDonato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin M. A cytokine-responsive IkappaB kinase that activates the transcription factor NF-kappaB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- Fantl V, Edwards AW, Steel JH, Vonderhaar BK, Dickson D. Impaired mammary gland development in Cyc−/− mice during pregnancy and lactation is epithelial cell autonomous. Development. 1999;212:1–11. doi: 10.1006/dbio.1999.9329. [DOI] [PubMed] [Google Scholar]
- Fantl V, Stamp G, Andrews A, Rosewell I, Dickson C. Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev. 1995;9:2364–2372. doi: 10.1101/gad.9.19.2364. [DOI] [PubMed] [Google Scholar]
- Franke T, Kaplan D, Cantley L, Toker A. Direct regulation of the Akt proto-oncogene product by phosphotidylinositol-3,4-bisphosphate. Science. 1997;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- Franke TF, Yang SI, Chan TO, Datta K, Kazlauskas A, Morrisin DK, Kaplan DR, Tsichlis PN. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- Fuchs SY, Chen A, Xiong Y, Pan ZQ, Ronai Z. HOS, a human homolog of Slimb, forms an SCF complex with Skp1 and Cullin1 and targets the phosphorylation-dependent degradation of IkappaB and beta-catenin. Oncogene. 1999;18:2039–2046. doi: 10.1038/sj.onc.1202760. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Karin M. Missing pieces in the NF-κB puzzle. Cell. 2002;109:S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- Goruppi S, Chiaruttini C, Ruaro ME, Varnum B, Schneide C. Gas6 induces growth, beta-catenin stabilization, and T-cell factor transcriptional activation in contact-inhibited C57 mammary cells. Mol Cell Biol. 2001;21:902–915. doi: 10.1128/MCB.21.3.902-915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- Hoeflich KP, Luo J, Rubie EA, Tsao M-S, Jin O, Woodgett JR. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature. 2000;406:86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- Hu Y, Baud V, Delhase M, Zhang P, Deerinck T, Ellisman M, Johnson R, Karin M. Abnormal morphogenesis but intact IKK activation in mice lacking the IKKalpha subunit of IkappaB kinase. Science. 1999;284:316–320. doi: 10.1126/science.284.5412.316. [DOI] [PubMed] [Google Scholar]
- Hu Y, Baud V, Oga T, Kim KI, Yoshida K, Karin M. IKKα controls formation of the epidermis independently of NF-kB. Nature. 2001;410:710–714. doi: 10.1038/35070605. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Behrens J. The Wnt signaling pathway. J Cell Sci. 2000;113:3545–3546. doi: 10.1242/jcs.113.20.3545. [DOI] [PubMed] [Google Scholar]
- Joyce D, Albanese C, Steer J, Fu M, Bouzahzah B, Pestell RG. NF-kB and cell-cycle regulation: the cyclin connection. Review Cytokine Growth Factor Rev. 2001;12:73–90. doi: 10.1016/s1359-6101(00)00018-6. [DOI] [PubMed] [Google Scholar]
- Joyce D, et al. Integration of Rac-dependent regulation of cyclin D1 transcription through an NF-κB-dependent pathway. J Biol Chem. 1999;274:25245–25249. doi: 10.1074/jbc.274.36.25245. [DOI] [PubMed] [Google Scholar]
- Kane LP, Shapiro VS, Stokoe D, Weiss A. Induction of NF-kB by the Akt/PKB kinase. Curr Biol. 1999;9:601–604. doi: 10.1016/s0960-9822(99)80265-6. [DOI] [PubMed] [Google Scholar]
- Karin M, Delhase M. The IκB kinase (IKK) and NF-κB: key elements of proinflammatory signaling. Semin Immunol. 2000;12:85–98. doi: 10.1006/smim.2000.0210. [DOI] [PubMed] [Google Scholar]
- King WG, Mattaliano MD, Chan TO, Tsichlis PN, Brugge JS. Phosphatidylinositol 3-kinase is required for integrin-stimulated AKT and Raf-1/mitogen-activated protein kinase pathway activation. Mol Cell Biol. 1997;17:4406–4418. doi: 10.1128/mcb.17.8.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klippel A, Escobedo MA, Wachowicz MS, Apell G, Brown TW, Giedlin MA, Kavanaugh WM, Williams LT. Activation of phosphatidylinositol 3-kinase is sufficient for cell cycle entry and promotes cellular changes characteristic of oncogenic transformation. Mol Cell Biol. 1998;18:5699–5711. doi: 10.1128/mcb.18.10.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberti C, Lin KM, Yamamoto Y, Verma U, Verma IM, Byers S, Gaynor RB. Regulation of beta-catenin function by the IkappaB kinases. J Biol Chem. 2001;276:42276–42286. doi: 10.1074/jbc.M104227200. [DOI] [PubMed] [Google Scholar]
- Lee RJ, et al. Cyclin D1 is required for transformation by activated Neu and is induced through an E2F-dependent signaling pathway. Mol Cell Biol. 2000;20:672–683. doi: 10.1128/mcb.20.2.672-683.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Lu Q, Hwang JY, Buscher D, Lee KF, Izpisua-Belmonte JC, Verma IM. IKK1-deficient mice exhibit abnormal development of skin and skeleton. Genes Dev. 1999a;13:1322–1328. doi: 10.1101/gad.13.10.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZW, Chu W, Hu Y, Delhase M, Deerinck T, Ellisman M, Johnson R, Karin M. The IKKbeta subunit of IkappaB kinase (IKK) is essential for nuclear factor kappaB activation and prevention of apoptosis. J Exp Med. 1999b;189:1839–1845. doi: 10.1084/jem.189.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S-Y, Xia W, Wang JC, Kwong KY, Spohn B, Wen Y, Pestell RG, Hung M-C. β-Catenin, a novel prognostic marker for breast cancer: its role in cyclin D1 expression and cancer progression. Proc Natl Acad Sci USA. 2000;97:4262–4266. doi: 10.1073/pnas.060025397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Perrimon N. Dally cooperates with Drosophila Frizzled 2 to transduce Wingless signaling. Nature. 1999;400:281–284. doi: 10.1038/22343. [DOI] [PubMed] [Google Scholar]
- Liu J, Stevens J, Rote CA, Yost HJ, Hu Y, Neufeld KL, White TL, Matsunami N. Siah-1 mediates a novel β-catenin degradation pathway linking p53 to the adenomatous polyposis coli protein. Mol Cell. 2001;7:927–936. doi: 10.1016/s1097-2765(01)00241-6. [DOI] [PubMed] [Google Scholar]
- Madrid LV, Mayo MW, Reuther JY, Baldwin AS. Akt stimulates the transactivation potential of the RelA/p65 Subunit of NF-kappa B through utilization of the Ikappa B kinase and activation of the mitogen-activated protein kinase p38. J Biol Chem. 2001;276:18934–40. doi: 10.1074/jbc.M101103200. [DOI] [PubMed] [Google Scholar]
- Madrid LV, Wang CY, Guttridge DC, Schottelius AJ, Baldwin ASJ, Mayo MW. Akt suppresses apoptosis by stimulating the transactivation potential of the RelA/p65 subunit of NF-kB. Mol Cell Biol. 2000;20:1626–1638. doi: 10.1128/mcb.20.5.1626-1638.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura I, Kitamura T, Wakao H, Tanaka H, Hashimoto K, Albanese C, Downward J, Pestell RG, Kanakura Y. Transcriptional regulation of cyclin D1 promoter by STAT5: its involvement in cytokine-dependent growth of hematopoietic cells. EMBO J. 1999;18:1367–1377. doi: 10.1093/emboj/18.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa S-i, Reed JC. Siak-1, SIP and Ebi collaborate in a novel pathway. Mol Cell. 2001;7:915–926. doi: 10.1016/s1097-2765(01)00242-8. [DOI] [PubMed] [Google Scholar]
- McGrew LL, Takemaru K, Bates R, Moon RT. Direct regulation of the Xenopus engrailed-2 promoter by the Wnt signaling pathway, and a molecular screen for Wnt-responsive genes, confirm a role for Wnt signaling during neural patterning in Xenopus. Mech Dev. 1999;87:21–32. doi: 10.1016/s0925-4773(99)00136-7. [DOI] [PubMed] [Google Scholar]
- Nishita M, Hashimoto MK, Ogata S, Laurent MN, Ueno N, Shibuya H, Cho KW. Interaction between Wnt and TGF-beta signaling pathways during formation of Spemann's organizer. Nature. 2000;403:781–785. doi: 10.1038/35001602. [DOI] [PubMed] [Google Scholar]
- Orford K, Crockett C, Weisman A, Byers SW. Serine phosphorylation-regulated ubiquitination and proteosomal degradation of β-catenin. J Biol Chem. 1997;272:24735–24738. doi: 10.1074/jbc.272.40.24735. [DOI] [PubMed] [Google Scholar]
- Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumor necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- Pagano M, Theodorus AM, Tam SW, Draetta GF. Cyclin D1-mediated inhibition of repair and replicative DNA synthesis in human fibroblasts. Genes Dev. 1994;8:1627–1639. doi: 10.1101/gad.8.14.1627. [DOI] [PubMed] [Google Scholar]
- Papkoff J, Aikawa M. WNT-1 and HGF regulate GSK3 beta activity and beta-catenin signaling in mammary epithelial cells. Biochem Biophys Res Commun. 1998;247:851–858. doi: 10.1006/bbrc.1998.8888. [DOI] [PubMed] [Google Scholar]
- Payre F, Vincent A, Carreno S. ovo/svb integrates Wingless and DER pathways to control epidermis differentiation. Nature. 1999;400:271–275. doi: 10.1038/22330. [DOI] [PubMed] [Google Scholar]
- Pestell RG, Albanese C, Reutens AT, Segall JE, Lee RJ, Arnold A. The cyclins and cyclin-dependent kinase inhibitors in hormonal regulation of proliferation and differentiation. Endocr Rev. 1999;20:501–534. doi: 10.1210/edrv.20.4.0373. [DOI] [PubMed] [Google Scholar]
- Polakis P. More than one way to skin a catenin. Cell. 2000a;105:563–565. doi: 10.1016/s0092-8674(01)00379-8. [DOI] [PubMed] [Google Scholar]
- Polakis P. Wnt signaling and cancer. Genes Dev. 2000b;14:1837–1851. [PubMed] [Google Scholar]
- Ramaswamy S, Nakamura N, Vazquez F, Batt DB, Perera S, Roberts TM, Sellers WR. Regulation of G1 progression by the PTEN tumor suppressor protein is linked to inhibition of the phosphatidylinositol 3-kinase/Akt pathway. Proc Natl Acad Sci USA. 1999;96:2110–2115. doi: 10.1073/pnas.96.5.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy SA, Huang JH, Liao WS. Phosphatidylinositol 3-kinase as a mediator of TNF-induced NF-kappa B activation. J Immunol. 2000;164:1355–1363. doi: 10.4049/jimmunol.164.3.1355. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Viciana P, Warne PH, Khwaja A, Marte BM, Pappin D, Das P, Waterfield MD, Ridley A, Downward J. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by ras. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- Romashkova JA, Makarov SS. NF-κB is a target of AKT in anti-apoptotic PDGF signaling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- Sampson EM, Haque ZK, Ku M-C, Tevosian SG, Albanese C, Pestell RG, Paulson KE, Yee AS. Negative regulation of the Wnt–β-catenin pathway by the transcriptional repressor HBP1. EMBO J. 2001;20:4500–4511. doi: 10.1093/emboj/20.16.4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, Ben-Ze'ev A. The cyclin D1 gene is a target of the β-catenin/LEF-1 pathway. Proc Natl Acad Sci USA. 1999;96:5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicinski P, et al. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell. 1995;82:621–630. doi: 10.1016/0092-8674(95)90034-9. [DOI] [PubMed] [Google Scholar]
- Soriano S, Kang DE, Fu M, Chevallier N, Pestell RG, Zheng H, Koo EH. Presenilin 1 modulates β-catenin/LEF signaling independently of APP and Notch processing. J Cell Biol. 2001;152:785–794. doi: 10.1083/jcb.152.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, et al. Limb and skin abnormalities in mice lacking IKKα. Science. 1999;284:313–316. doi: 10.1126/science.284.5412.313. [DOI] [PubMed] [Google Scholar]
- Tetsu O, McCormick F. β-Catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–424. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- Tsuda M, et al. The cell-surface proteoglycan Dally regulates Wingless signaling in Drosophila. Nature. 1999;400:276–280. doi: 10.1038/22336. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Waterfield MD. Signaling by distinct classes of phosphoinositide 3-kinases. Exp Cell Res. 1999;253:239–254. doi: 10.1006/excr.1999.4701. [DOI] [PubMed] [Google Scholar]
- Watanabe G, et al. Induction of cyclin D1 by simian virus 40 small tumor antigen. Proc Natl Acad Sci USA. 1996a;93:12861–12866. doi: 10.1073/pnas.93.23.12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe G, Lee RJ, Albanese C, Rainey WE, Batlle D, Pestell RG. Angiotensin II (AII) activation of cyclin D1-dependent kinase activity. J Biol Chem. 1996b;271:22570–22577. doi: 10.1074/jbc.271.37.22570. [DOI] [PubMed] [Google Scholar]
- Winston JT, Strack P, Beer-Romero P, Chu C, Elledge SJ, Harper JW. The SCF-βTRCP ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IκBβ and β-catenin and stimulates IκBα ubiquitination in vitro. Genes Dev. 1999;3:270–283. doi: 10.1101/gad.13.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Gaynor RB. Therapeutic potential of the NF-kB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001;107:135–142. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Yin MJ, Gaynor RB. IkappaB kinase alpha (IKKalpha) regulation of IKKbeta kinase activity. Mol Cell Biol. 2000;20:3655–66. doi: 10.1128/mcb.20.10.3655-3666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost C, Torres M, Miller JR, Huang E, Kimelman D, Moon RT. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–54. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- Zandi E, Rothwarf DM, Delhase M, Hayakawa M, Karin M. The IkappaB kinase complex (IKK) contains two kinase subunits, IKKalpha and IKKbeta, necessary for IkappaB phosphorylation and NF-kappaB activation. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]