Abstract
The EMBRYONIC FLOWER (EMF) genes EMF1 and EMF2 are required to maintain vegetative development and repress flower development. EMF1 encodes a putative transcriptional regulator, and EMF2 encodes a Polycomb group protein homolog. We examined expression profiles of emf mutants using GeneChip technology. The high degree of overlap in expression changes from the wild type among the emf1 and emf2 mutants was consistent with the functional similarity between the two genes. Expression profiles of emf seedlings before flower development were similar to that of Arabidopsis flowers, indicating the commitment of germinating emf seedlings to the reproductive fate. The germinating emf seedlings ectopically expressed flower organ genes, suggesting that vegetative development in wild-type plants results from EMF repression of the flower program, directly or indirectly. In addition, the seed development program is derepressed in the emf1 mutants. Gene expression analysis showed no clear regulation of CONSTANS (CO), FLOWERING LOCUS T (FT), LEAFY (LFY), and SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 by EMF1. Consistent with epistasis results that co, lfy, or ft cannot rescue rosette development in emf mutants, these data show that the mechanism of EMF-mediated repression of flower organ genes is independent of these flowering genes. Based on these findings, a new mechanism of EMF-mediated floral repression is proposed.
INTRODUCTION
Higher plants must flower to undergo sexual reproduction. To increase flower and progeny production, plants delay flowering until they have built up body size through vegetative growth. Flowering time can be finely regulated by floral inducers and floral repressors, as indicated by the isolation of late- and early-flowering mutants (Koornneef et al., 1998; Araki, 2001). The floral induction pathway in Arabidopsis has been under intense investigation, and multiple floral signal transduction pathways have been identified (Blazquez, 2000; Mouradov et al., 2002; Simpson and Dean, 2002). For example, daylength is perceived by phytochromes and cryptochromes and transduced via the late-flowering genes GIGANTEA (GI) and CONSTANS (CO) (Koornneef et al., 1991) to genes such as FLOWERING LOCUS T (FT) and SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 (SOC1) (AGAMOUS-LIKE20 [AGL20]) (Samach et al., 2000), which activate the flower meristem identity gene APETALA1 (AP1). The vernalization signal is mediated by FLOWERING LOCUS C (FLC) (Michaels and Amasino, 1999), which represses flowering by negatively regulating the expression of the floral promotion genes SOC1 and FT (Samach et al., 2000; Hepworth et al., 2002). LEAFY (LFY) is a key link between the flowering signals and flower development. The large increase in LFY RNA level in the flower meristem activates the expression of the downstream flower organ identity genes AP1, AP3, PISTILLATA (PI), and AGAMOUS (AG) to initiate flower organ development (Krizek and Meyerowitz, 1996; Busch et al., 1999; Wagner et al., 1999; Blazquez and Weigel, 2000).
Less is known about the mechanism that prevents flower development. Approximately a dozen early-flowering genes have been reported in Arabidopsis (Zagotta et al., 1992; Sung et al., 2003b). Some of these genes encode proteins that sense light or daylength, such as EARLY FLOWERING3 (ELF3) and ELF4 (Hicks et al., 2001; Doyle et al., 2002). Other genes, such as FLC, TERMINAL FLOWER2 (TFL2), and EARLY BOLTING IN SHORT DAYS (EBS) (Larsson et al., 1998; Michaels and Amasino, 1999; Gomez-Mena et al., 2001), inhibit the action of a key floral integrator, FT (Blazquez et al., 2001). The TFL1 gene encodes a possible membrane-associated protein that can bind hydrophobic ligands, and it acts antagonistically to LFY and AP1 and vice versa (Bradley et al., 1997; Liljegren et al., 1999).
The EMF genes are required for vegetative development. Plants defective in the EMF1 or EMF2 gene skip vegetative growth and flower upon germination (Sung et al., 1992). Four emf1 mutants and nine emf2 mutants have been identified (Sung et al., 2003a). The weak emf1 mutant emf1-1 and all nine emf2 mutants germinate directly into a small inflorescence that produces a few sterile flowers. In the strong emf1 mutants, all lateral organs, including the cotyledons, differentiate into carpelloid structures (Chen et al., 1997). EMF1 encodes a novel protein of 121 kD, which is predicted to be a transcriptional regulator (Aubert et al., 2001). EMF2 encodes a 71-kD Polycomb group (PcG) protein containing a zinc finger motif and a VEFS domain conserved among other PcG proteins, such as VERNALIZATION2 (Gendall et al., 2001), FERTILIZATION- INDEPENDENT SEED2, and Suppressor of zeste12 (Birve et al., 2001; Yoshida et al., 2001).
The EMF genes have been shown to regulate flowering time because modulation of EMF activities generates transgenic plants that flower at different times (Aubert et al., 2001; Yoshida et al., 2001). Consistent with previous findings based on genetic studies (Chen et al., 1997), transgenic studies confirm the role of the EMF genes in regulating inflorescence development. Some of the novel inflorescence architecture seen in transgenic plants with modulated EMF activities resembles that of transgenic plants with altered flower meristem identity gene activities (Liljegren et al., 1999). Both the nature of the EMF proteins and the phenotypes of the transgenic plants support the notion that the EMF-mediated floral repression mechanism interacts with the floral induction mechanism mediated by the late-flowering and floral meristem identity genes. However, the early-flowering phenotype of emf mutants cannot be rescued by any flowering time, flower meristem, or flower organ identity mutants studied (Yang et al., 1995; Chen et al., 1997; Haung and Yang, 1998), indicating that these genes do not act downstream from EMF to mediate flowering time. Thus, the nature of the EMF-mediated floral repression is not well understood.
To gain insights into the mechanism of EMF-regulated floral repression, we used Affymetrix GeneChip technology to examine the expression profiles of emf mutants. Mutant expression profiles were compared with wild-type developmental stage–specific expression profiles. The expression patterns of the flower organ identity genes and some flowering genes were verified by reverse transcription (RT) PCR analysis. An EMF-mediated repression mechanism based on chromatin remodeling is proposed to explain the global repression of the flower and seed programs during vegetative development.
RESULTS
Global Gene Expression Patterns in emf Mutants
GeneChip technology (Zhu et al., 2001) was used to profile global gene expression patterns in emf1-1, emf1-2, emf2-1, and the wild type. Total RNA was isolated from whole plants grown on agar plates (Figure 1) under short-day (SD) conditions as described in Methods. Each GeneChip contains ∼8900 probe sets representing 8300 unique Arabidopsis genes (Zhu and Wang, 2000; Zhu et al., 2001). Each probe set includes 16 probe pairs of perfect match probes and mismatch probes for each gene. The expression level of each gene was determined by the difference in the intensity of the hybridization signal between perfect match and mismatch probes (Zhu et al., 2001). Duplicate experiments for 15-day-old wild-type seedlings showed a very high correlation coefficient (0.997) among all 8735 probe sets, excluding Affymetrix GeneChip controls, demonstrating the reproducibility of the experiments (data not shown).
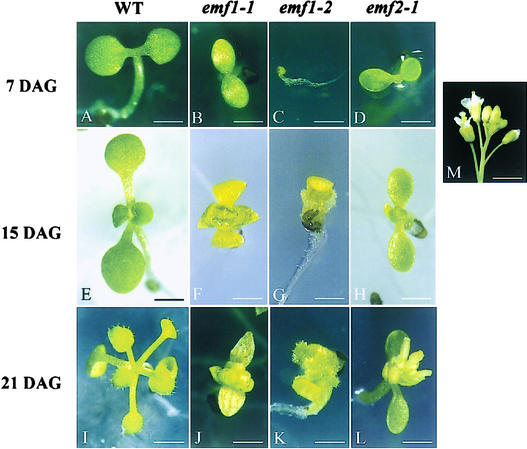
Figure 1.
Phenotypes of Wild-Type and emf Mutants.
Seven-day-old wild-type (WT) Columbia ecotype (A), emf1-1 (B), emf1-2 (C), and emf2-1 (D), 15-day-old wild-type Columbia (E), emf1-1 (F), emf1-2 (G), and emf2-1 (H), and 21-day-old wild-type Columbia (I), emf1-1 (J), emf1-2 (K), and emf2-1 (L). A Columbia ecotype flower cluster is shown in (M). Bars = 1 mm in (A) to (D) and (F) to (H), 1.4 mm in (E), 1.5 mm in (I), 1.1 mm in (J) to (L), and 3.8 mm in (M).
A High Percentage of Overlap in Gene Expression Change among the Three emf Mutants
To compare the gene expression profiles in mutants of the weak and strong phenotypes and of the two different EMF genes, we chose three mutants: two weak mutants (emf1-1 and emf2-1) and a strong mutant (emf1-2). Based on preliminary analysis, a hybridization signal of <50 was considered background on this set of GeneChips (Zhu et al., 2001); only genes with hybridization signals of >50 in at least one of the four plant types (wild type and the three mutants) were included for further analysis. From the 7-, 15-, and 21-day-old samples, 3872, 3659, and 3820 genes corresponding to 4213, 4009, and 4222 probe sets, respectively, met our stringency criterion (Table 1).
Table 1.
Changes in Gene Expression in emf Mutants
| 7 DAG (3872)a
|
15 DAG (3659)
|
21 DAG (3820)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Mutant | Upb | Down | Total | Up | Down | Total | Up | Down | Total |
| emf1-1 | 831 (21.5) | 456 (11.8) | 1287 (33.3) | 448 (12.2) | 459 (12.5) | 907 (24.7) | 830 (21.7) | 1048 (27.4) | 1878 (49.1) |
| emf1-2 | 847 (21.9) | 601 (15.5) | 1448 (37.4) | 575 (15.7) | 659 (18.0) | 1234 (33.7) | 981 (25.7) | 1317 (34.5) | 2298 (60.2) |
| emf2-1 | 484 (12.5) | 408 (10.5) | 892 (23.0) | – | – | – | 732 (19.2) | 1012 (26.5) | 1744 (45.7) |
Number of genes showing intensity of >50 in at least one of the four samples, the wild type, and the three mutants.
Number of genes (percentage) upregulated or downregulated more than twofold in the mutant(s).
Gene expression changes were determined by the ratio of the hybridization signal of the mutant to the wild type. Genes showing a greater than twofold difference in hybridization signals between the emf mutant and wild-type samples were identified as genes upregulated or downregulated in the mutant (Table 1). Of the 3872 genes identified in the 7-day-old samples, 1287 (33.3%), 1448 (37.4%), and 892 (23.0%) showed differential expression in emf1-1, emf1-2, and emf2-1, respectively. The 21-day-old samples showed greater differences between the mutants and the wild type; of 3820 genes identified, 1878 (49.1%), 2298 (60.2%), and 1744 (45.7%) showed differential expression in emf1-1, emf1-2, and emf2-1, respectively (Table 1).
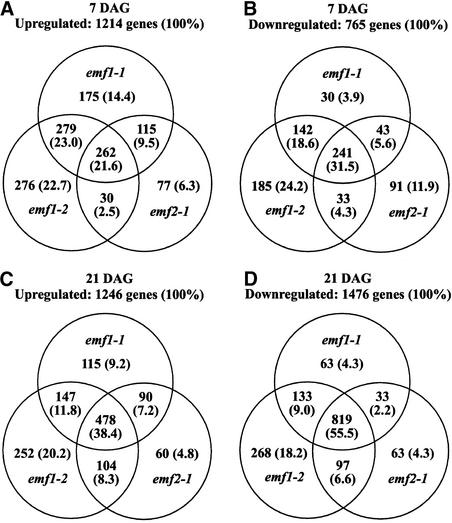
We then determined whether these genes were upregulated or downregulated similarly across the three mutants and found a high percentage of overlap in the genes that showed expression changes. Among the 3872 genes selected from the 7-day-old plants, 1214 were upregulated and 765 were downregulated in at least one of the 7-day-old emf mutants. A total of 262 of 1214 genes (21.6%) were upregulated and 241 of 765 genes (31.5%) were downregulated in all three mutants (Figures 2A and 2B). Higher overlap in expression change was observed among the three mutants at 21 days after germination (DAG). Among the 3820 genes selected, 478 of 1246 (38.4%) were upregulated and 819 of 1476 (55.5%) were downregulated in all three 21-day-old mutants (Figures 2C and 2D). The high percentage of genes with similar expression changes in emf1 and emf2 mutants indicates that these mutations affect similar gene programs and that the two EMF genes are related functionally.
Figure 2.
Genes Upregulated or Downregulated in 7- and 21-Day-Old emf1-1, emf1-2, and emf2-1 Mutants Relative to the Wild Type.
From the GeneChip data, 3872 and 3820 genes with a hybridization signal of >50 in 7- and 21-day-old plants, respectively, were selected and analyzed. Numbers and percentages (in parentheses) of genes upregulated at 7 (A) and 21 (C) DAG and downregulated at 7 (B) and 21 (D) DAG in a mutant with greater than twofold difference are shown.
Gene Expression Pattern Is More Similar between the Two emf1 Mutants Than between the Two Weak Mutants
The percentage of genes that showed similar expression change was compared among the three mutants. First, we found that the expression profiles of the two emf1 mutants were very similar. At 7 DAG, 44.6% of the genes were upregulated similarly and 50.1% of the genes were downregulated similarly (Figures 2A and 2B). In the 21-day-old samples, similar upregulation and downregulation were observed in more genes, 50.2 and 64.5%, respectively (Figures 2C and 2D), even though the two emf1 mutants had developed into distinctly different forms at 21 days (Figure 1). Second, we found that the overlap in expression profiles between the two weak mutants (emf2-1 and emf1-1) was less than that between the two emf1 mutants. In 7-day-old plants, 31.1 and 37.1% of the genes were upregulated and downregulated similarly in the two weak mutants (Figures 2A and 2B). In 21-day-old plants, 45.6 and 57.7% of the genes were upregulated and downregulated similarly (Figures 2C and 2D). Despite the phenotypic similarity between emf1-1 and emf2-1, which are morphologically distinct from emf1-2 (Figure 1), a greater molecular difference was found between emf2-1 and emf1-1 than between the two emf1 mutants. These results suggest differences in the gene programs regulated by the two EMF genes.
A High Degree of Overlap in Gene Expression Change between emf Seedlings and Wild-Type Floral Buds
Because emf mutants are expected to be committed to a reproductive fate (Sung et al., 1992), we compared the expression patterns of the mutants and wild-type floral buds and found a high degree of overlap. For example, 193 of 262 (73.7%) upregulated genes in all three emf mutants at 7 DAG also were upregulated in wild-type floral buds relative to wild-type seedlings. A total of 384 of 478 (80.3%) of the genes upregulated in all three emf mutants at 21 DAG were upregulated in the floral buds. An even higher percentage of overlap was observed among the downregulated genes. Ninety and 83.4% of the downregulated genes in the 7- and 21-day-old emf samples also were downregulated in wild-type floral buds (data not shown). Because 7-day-old emf mutants have not produced floral buds or floral organ primordia (Bai and Sung, 1995), activation of the flower program appears to have occurred before flower development in the emf mutants.
Functional Classification of the Genes Upregulated and Downregulated in emf Mutants
Functionally related genes were categorized to investigate the possible function of the EMF genes (Table 2). The gene names were based on the improved annotations for probe sets of the Affymetrix GeneChip (http://wwwbiology.ucsd.edu/labs/schroeder/downloads.html and http://genetics.mgh.harvard.edu/sheenweb/search_affy.html). Flowering genes, including flowering time, flower meristem identity, and flower organ identity genes, and seed maturation genes, including late embryogenesis–abundant (LEA) genes and seed storage protein genes, were upregulated in emf mutants. However, indoleacetic acid (IAA)–inducible and IAA transport genes, expansin genes, and genes related to photosynthesis light reaction were downregulated in emf mutants (Table 2).
Table 2.
Number of Genes Involved in Different Functional Groups Upregulated or Downregulated at Least Twofold in emf Mutants
| Total No. of Genes Investigated |
Change in Gene Expression |
emf1-1
|
emf1-2
|
emf2-1
|
Names of Representative Upregulated or Downregulated Genesb |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Category | 7a | 15 | 21 | 7 | 15 | 21 | 7 | 21 | |||
| Flowering | 35 | Up | 9 | 13 | 16 | 12 | 10 | 16 | 6 | 18 | See Table 3 |
| Down | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | See Table 3 | ||
| Seed maturation | 32 | Up | 3 | 15 | 20 | 16 | 13 | 27 | 0 | 6 | See Table 4 |
| Down | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | See Table 4 | ||
| Photoreceptors | 12 | Up | 2 | 2 | 7 | 4 | 0 | 6 | 1 | 4 | PHYC/D, PHY-associated protein 2, PHYA suppressor, COP9 |
| Down | 4 | 2 | 2 | 4 | 3 | 2 | 4 | 2 | PHYA, PHY-associated protein 3, NPH1, flavin-type blue-light photoreceptor |
||
| Photosynthesis light reaction | 29 | Up | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | – |
| Down | 29 | 4 | 19 | 29 | 26 | 23 | 1 | 13 | CAB, PSI subunit precursors, PSII OEC | ||
| Auxin-related | 25 | Up | 1 | 0 | 2 | 1 | 0 | 2 | 0 | 3 | IAA12/21 |
| Down | 7 | 8 | 8 | 7 | 10 | 5 | 9 | 8 | IAA2/3/7/17/19, PIN3/4/7 | ||
| Gibberellin-related | 13 | Up | 0 | 0 | 2 | 0 | 1 | 3 | 0 | 0 | GA 20 oxidase, GA-regulated gene |
| Down | 3 | 1 | 2 | 3 | 2 | 2 | 1 | 1 | GASA4, GAST1-like | ||
| Ethylene-related | 9 | Up | 0 | 3 | 3 | 1 | 5 | 4 | 0 | 3 | EREBF4/5, ACC synthase |
| Down | 2 | 1 | 2 | 3 | 1 | 2 | 2 | 3 | ACC oxidase | ||
| Expansin | 5 | Up | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | – |
| Down | 4 | 2 | 3 | 3 | 3 | 4 | 4 | 4 | AtEXP1/6, putative expansins | ||
Data shown are days after germination.
See supplemental data online.
Flowering Genes, Particularly the Flower Organ Genes, Are Upregulated in emf Mutants
Twenty-one of 35 flowering genes investigated showed an increase in transcript level in at least one of the three mutants (Tables 2 and 3). Flower organ–specific genes, including the flower homeotic or MADS box genes, such as AP1, AP3, PI, AG, SHATTERPROOF1 (SHP1) (AGL1), SEPALLATA2 (SEP2) (AGL4), SEP3 (AGL9), AGL11, CRABS CLAW, and f-AtMBP, showed ectopic expression in all three emf mutants (Tables 2 and 3). These genes are expressed ectopically as early as 7 DAG, and the transcript levels of many of these genes continued to increase during the 3-week growth period (Table 3, Figure 3), probably because of the increase in FT, LFY, or SOC1 RNA in the last 2 weeks (Table 3).
Table 3.
Expression Levels of Flowering Genes Upregulated or Downregulated in emf Mutants
| Wild Type
|
emf1-1
|
emf1-2
|
emf2-1
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genea | Probe Set | Protein IDb | 7c | 15 | 21 | 7 | 15 | 21 | 7 | 15 | 21 | 7 | 21 | Floral Bud |
| GI | 17580 | CAB56039 | 779 | 759 | 676 | 554 | 370 | 596 | 477 | 654 | 1535 | 541 | 1565 | 523 |
| FT | 16113 | BAA77838 | −1 | 16 | −4 | 6 | 173 | 146 | 4 | 12 | 58 | 1 | 202 | 28 |
| SOC1 | 14123 | AAC06175 | 0 | 12 | 20 | 3 | 17 | 35 | 0 | 10 | 50 | −1 | 120 | 22 |
| FLF | 17501 | AAD21249 | 10 | 5 | 20 | 31 | 33 | 10 | 54 | 12 | 48 | 32 | 31 | 12 |
| AGL8 | 12876 | AAA97403 | 3 | 8 | 2 | 5 | 209 | 372 | 6 | 11 | 22 | −1 | 113 | 265 |
| CAL | 19456 | AAC67513 | 3 | 0 | 8 | 3 | 29 | 97 | 42 | 46 | 105 | 6 | 91 | 44 |
| AP1 | 12882 | CAA78909 | −11 | −13 | −14 | 11 | 172 | 104 | 105 | 8 | 75 | −7 | 37 | 319 |
| PI | 16648 | BAA87000 | 0 | 12 | 8 | 247 | 294 | 564 | 482 | 840 | 1320 | 819 | 1222 | 625 |
| AP3 | 19527 | AAD51900 | 6 | 5 | 3 | 410 | 318 | 149 | 561 | 521 | 209 | 247 | 127 | 216 |
| AG | 12864 | CAA37642 | 6 | 5 | 3 | 280 | 240 | 134 | 388 | 264 | 163 | 224 | 169 | 232 |
| SEP2 (AGL4) | 12873 | AAF02125 | −9 | −9 | −16 | 128 | 380 | 628 | 278 | 403 | 1164 | 79 | 808 | 314 |
| SEP3 (AGL9) | 16614 | AAC00586 | −4 | 50 | 17 | 235 | 504 | 547 | 362 | 286 | 583 | 262 | 782 | 630 |
| SHP1 (AGL1) | 12865 | AAA32730 | 1 | 12 | 5 | 129 | 61 | 345 | 159 | 51 | 597 | 47 | 370 | 179 |
| SHP2 (AGL5) | 12874 | AAA32735 | −3 | 5 | 3 | 24 | 23 | 61 | 23 | 80 | 107 | 3 | 22 | 14 |
| CRABS CLAW | 17538 | AAD30526 | 63 | 47 | 80 | 168 | 661 | 1462 | 1162 | 1394 | 3214 | 126 | 708 | 669 |
| SPT | 19740 | CAB16798 | 13 | 20 | 12 | 28 | 33 | 52 | 60 | 97 | 116 | 5 | 67 | 25 |
| AGL6 | 12875 | AAC06173 | 16 | 14 | 26 | 27 | 24 | 22 | 19 | 49 | 343 | 18 | 296 | 169 |
| AGL11 | 17131 | AAC49080 | 9 | 12 | 8 | 112 | 108 | 168 | 370 | 391 | 1225 | 84 | 96 | 104 |
| BELL1 | 16162 | AAB05099 | 13 | 48 | 19 | 42 | 36 | 41 | 49 | 71 | 45 | 32 | 78 | 31 |
| ANR1 | 19609 | AAD25638 | 3 | −1 | −3 | 10 | 60 | 216 | 50 | 44 | 232 | 44 | 129 | 0 |
| f-AtMBP | 20227 | BAA82151 | 5 | 19 | −3 | 96 | 401 | 1047 | 193 | 16 | 48 | 21 | 1228 | 1834 |
Genes selected based on intensity level of >50 in at least one of the four samples, the wild type and the three emf mutants.
Protein accession number in GenBank.
Data shown are days after germination.
Figure 3.
Temporal Expression Patterns of the Flowering Genes and Seed Maturation Genes in the Wild Type and emf Mutants.
Horizontal and vertical axes represent days after germination and intensities of the hybridization signal, respectively. See Tables 3 and 4 for the intensity of each gene's hybridization signal. WT, wild type.
The expression pattern of the flowering time genes and floral pathway integrators was examined. FT transcripts tended to increase in the three mutants relative to the wild type by 21 DAG, and GI transcripts were high in all samples. However, CO, LFY, and SOC1 levels were very low on the GeneChip in all four samples (Table 3; see also supplemental data online).
The Seed Maturation Program Is Upregulated in emf1 Mutants
Twenty-eight of 32 seed maturation genes, including the LEA genes and the seed storage protein genes, were upregulated in emf mutants by 21 DAG (Tables 2 and 4). During the 3-week period, the numbers and RNA levels of upregulated seed maturation genes increased (Tables 2 and 4). The strong mutant, emf1-2, had the highest transcript level at the earliest time point, whereas the emf2-1 mutant showed the least change from the wild type. At 7 DAG, 16 of the 32 seed maturation genes were upregulated in emf1-2 mutants, 3 were upregulated in emf1-1, and none was upregulated in emf2-1 (Table 2). At 21 DAG, 20 and 27 seed maturation genes were upregulated in the two emf1 mutants, whereas only 6 were upregulated in emf2-1, with much lower expression levels than in the two emf1 mutants (Tables 2 and 4).
Table 4.
Expression Levels of Seed Maturation Genes, Including Seed Storage Protein and LEA Genes, Upregulated or Downregulated in emf Mutants
| Wild Type
|
emf1-1
|
emf1-2
|
emf2-1
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genea | Probe Set | Protein IDb | 7c | 15 | 21 | 7 | 15 | 21 | 7 | 15 | 21 | 7 | 21 | Floral Bud |
| 2S albumin 1 precursor | 15983 | CAB38844 | −11 | 49 | −3 | −5 | 242 | 483 | 12 | 12 | 806 | −14 | 6 | −9 |
| 2S albumin 3 precursor | 13194 | CAB38846 | −10 | 92 | −8 | −7 | 617 | 2832 | 161 | 140 | 4259 | −6 | 127 | −8 |
| 2S albumin 4 precursor | 15984 | AAA32746 | −3 | 28 | 8 | 1 | 244 | 3723 | 20 | 92 | 1660 | 3 | 30 | 0 |
| 12S | 13195 | CAA32493 | −1 | 70 | −2 | −2 | 295 | 2052 | 170 | 90 | 3967 | −1 | 85 | −2 |
| 12S cruciferin | 13200 | AAM13004 | −25 | −16 | −31 | −4 | 176 | 868 | 22 | 21 | 367 | −5 | −17 | −3 |
| 12S cruciferin | 16025 | AAB17379 | −17 | 80 | −14 | −18 | 662 | 1658 | 103 | 228 | 1722 | −29 | 11 | −9 |
| 12S CRA1 | 16425 | AAA32777 | −2 | 139 | −2 | 2 | 849 | 2024 | 362 | 266 | 2637 | 14 | 53 | 13 |
| Oleosin | 18714 | CAA44225 | 1 | −2 | −24 | −11 | 12 | 118 | 19 | 29 | 531 | −2 | −3 | −28 |
| Oleosin | 19003 | AAC42242 | −3 | −2 | 1 | 6 | 42 | 45 | 74 | 105 | 323 | 0 | 8 | 46 |
| Oleosin | 16575 | AAA87295 | 6 | 68 | 7 | 7 | 258 | 509 | 287 | 231 | 1804 | 16 | 21 | 13 |
| Oleosin type 4 | 18991 | CAA63011 | 3 | 24 | −12 | 5 | 105 | 336 | 31 | 24 | 645 | 10 | 1 | 19 |
| Oleosin 18.5K | 20412 | CAB36756 | 65 | 102 | 69 | 44 | 293 | 986 | 159 | 128 | 1780 | 59 | 76 | 49 |
| Vicilin-like | 20535 | AAD21484 | 4 | 11 | 10 | 1 | 47 | 558 | 76 | 41 | 1897 | 4 | 22 | 9 |
| LEA | 16023 | AAC23428 | 185 | 235 | 250 | 443 | 247 | 325 | 505 | 560 | 382 | 307 | 301 | 249 |
| LEA | 20004 | AAC61808 | −2 | 0 | 1 | 20 | 103 | 41 | 367 | 484 | 853 | 13 | 9 | 9 |
| LEA | 19181 | AAM64340 | 1901 | 976 | 780 | 1362 | 840 | 1381 | 2305 | 2356 | 2948 | 1924 | 1622 | 427 |
| LEA in group 5 | 15597 | BAA11016 | 1 | 3 | 7 | 2 | −1 | 2 | 2 | −33 | 174 | 1 | −1 | 0 |
| LEA M10 | 19355 | AAC78544 | −1 | −2 | 3 | 3 | 7 | 38 | −3 | −1 | 63 | −1 | 1 | 0 |
| LEA M17 | 16896 | AAC78545 | −3 | −8 | −9 | 3 | 363 | 412 | 85 | 30 | 464 | −10 | 26 | −6 |
| LEA76 homolog type 1 | 19918 | CAA63006 | −6 | −4 | −5 | 3 | 81 | 217 | 177 | 113 | 1287 | 2 | −1 | −3 |
| LEA76 homolog type 2 | 20641 | CAA63012 | 0 | 17 | 1 | 50 | 108 | 93 | 135 | 85 | 790 | 22 | 23 | 31 |
| LEA D113 homolog | 19152 | CAA63008 | 3 | 7 | −6 | 90 | 57 | 146 | 133 | 181 | 602 | 44 | 25 | 16 |
| LEA Le25 homolog | 14439 | CAA61676 | 4 | 5 | 6 | 1 | 41 | 75 | 81 | 298 | 697 | 7 | 8 | −6 |
| LEA-like | 18872 | CAA10352 | −3 | 2 | 2 | 5 | 19 | 66 | 38 | 57 | 685 | 3 | 1 | 1 |
| Embryo abundant | 14083 | AAC78535 | 55 | 214 | 56 | 223 | 140 | 163 | 216 | 92 | 323 | 110 | 232 | 18 |
| Embryo-specific ATS1 | 20681 | CAB36520 | 4 | 4 | −3 | 1 | 16 | 363 | 6 | 11 | 1496 | 0 | 12 | 2 |
| AtEm1 | 17282 | CAA77509 | −1 | −1 | 1 | 0 | 16 | 17 | 41 | 144 | 155 | 0 | −1 | −3 |
| AX110P-like | 20025 | AAD39613 | 29 | 24 | 27 | 31 | 22 | 31 | 45 | 31 | 71 | 29 | 61 | 22 |
Genes selected based on intensity level of >50 in at least one of the four samples, the wild type and the three emf mutants.
Protein accession number in GenBank.
Data shown are days after germination.
The temporal expression patterns of the seed maturation genes in the emf mutants are plotted in Figure 3, which demonstrates the continuous increase in the transcript levels in the emf1 mutants during the 3-week period. This result indicates that despite their ectopic expression, the expression of the seed maturation genes in emf1 plants still was developmentally controlled. Moreover, the progressive derepression of these seed-specific genes during emf1 development shows that their ectopic expression does not result from the residual expression of the seed maturation program after germination. Because strong emf1-2 mutants do not have carpelloid organs at 7 DAG and none of the emf1 mutants can produce seeds, seed-specific gene expression (Gaubier et al., 1993; Conceicao Ada and Krebbers, 1994) in these mutants does not result from seed development. Unlike the emf1 mutants, the seed maturation genes did not show obvious upregulation in the emf2-1 mutants, suggesting functional differences between EMF1 and EMF2 genes in the repression of the seed maturation genes.
Other Functional Categories of Genes That Show Differential Expression in the Mutants
Table 2 shows that auxin-inducible genes (IAA2, IAA3, IAA7, IAA17, and IAA19) and auxin transport genes (PIN3, PIN4, and PIN7) were downregulated in all three emf mutants. Compared with 7-, 15-, and 21-day-old wild-type genes, the auxin-inducible genes and auxin transport genes also were downregulated in floral buds (see supplemental data online). Although the role of these genes in floral buds is not clear, they serve as molecular markers confirming the floral state of the emf mutants.
Photosynthesis light reaction–related genes were downregulated in all three emf mutants (Table 2; see also supplemental data online). However, differences were detected between the two emf1 mutants and the emf2-1 mutant. For example, at 7 DAG, all 29 genes investigated were downregulated in both emf1-1 and emf1-2, but only one gene was slightly downregulated in emf2-1 (Table 2; see also supplemental data online). This finding again shows a functional difference between the EMF1 and EMF2 genes.
RT-PCR Results Confirm the Ectopic Expression of Flower Organ Identity Genes
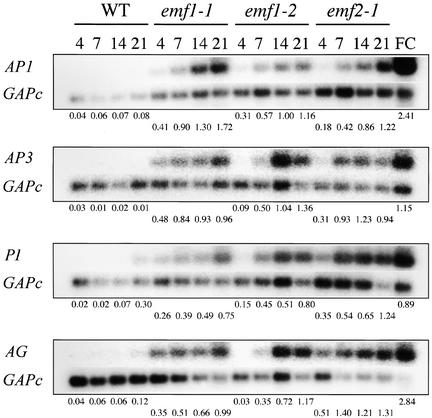
To confirm the GeneChip results on the ectopic expression of flower organ identity genes in emf mutants, we analyzed the temporal expression patterns of the four flower organ identity genes (AP1, AP3, PI, and AG) in the mutants and the wild type grown under SD conditions by RT-PCR. Flower clusters, consisting of inflorescence meristem and floral buds of various stages, were used as a positive control. Relative amounts of the flower organ identity gene transcripts were standardized against glyceraldehyde 3-phosphate dehydrogenase c (GAPc) transcripts; the ratio of the hybridization intensity of the flower organ identity gene to that of GAPc was determined as described in Methods.
As expected, the four flower organ identity genes were highly expressed in the wild-type flower clusters but not in wild-type seedlings or rosette shoots, except for the low levels of PI and AG transcripts in 21-day-old samples (Figure 4). On the other hand, all four genes were expressed ectopically in the three mutants. The AP1 and PI RNA levels were higher in all three mutants than in the wild type as early as 4 DAG. The ectopic expression of AP3 and AG was detected as early as 4 DAG in the two weak mutants and at 7 DAG in the strong mutant (Figure 4). The ratio of hybridization intensities showed a temporal increase in the transcript levels after the initial ectopic expression in the mutants. The RT-PCR results were consistent with the GeneChip results in that the flower organ identity genes were expressed ectopically in emf mutants and there was a progressive increase in transcript levels during mutant development.
Figure 4.
Expression of AP1, AP3, PI, and AG in emf Mutants.
Autoradiograph of semiquantitative RT-PCR analysis of AP1, AP3, PI, AG, and GAPc RNA levels in 4-, 7-, 14-, and 21-day-old wild-type (WT) Columbia, emf1-1, emf1-2, and emf2-1 plants grown under SD conditions. GAPc was used as a RT-PCR control. RT-PCR products amplified were hybridized with a probe of each gene. The relative amount of each gene after standardization using the GAPc signal as a reference is presented below each gel. FC, flower clusters consisting of inflorescence meristems and floral buds.
The two weak mutants have an inflorescence consisting of a few floral buds and several sessile leaves at 14 DAG and open flowers at 21 DAG (Chen et al., 1997) (Figure 1). Although the strong emf1 mutants do not seem to have an inflorescence and lack most floral organs, its lateral organs became carpelloid at 21 DAG (Figures 1G and 1K). Thus, the expression of the flower organ identity genes in 2- and 3-week-old emf mutants could result from flower and flower organ development. However, because 4- and 7-day-old emf mutants do not contain flower organ primordia, expression of the flower organ identity genes in the first week precedes, and thus does not result from, flower development.
Temporal Expression Patterns of CO, FT, LFY, and SOC1 in emf Mutants
During wild-type development, the expression of flower organ identity genes is activated by the floral pathway integrators FT, SOC1, and LFY (Mouradov et al., 2002; Simpson and Dean, 2002). To confirm the GeneChip results on FT and to investigate whether upregulation of the flower organ identity genes is caused by the upregulation of floral pathway integrators, we studied the expression of LFY, FT, SOC1, and a late-flowering gene, CO, by RT-PCR. RNA levels of these genes in 4-, 7-, 14-, and 21-day-old wild-type, emf1-1, emf1-2, and emf2-1 plants grown under SD conditions were investigated (Figure 5). RNA extracted from wild-type flower clusters consisting of inflorescence meristems and floral buds was used as a positive control. As expected, these genes were highly expressed in the flower clusters (Figure 5). In general, there was a temporal increase in transcript levels of the four genes in both mutants and the wild type. The temporal patterns of FT expression detected among the four plants were consistent with those determined by the GeneChip analysis.
Figure 5.
Expression of CO, FT, SOC1, and LFY in emf Mutants.
Autoradiograph of semiquantitative RT-PCR analysis of CO, FT, SOC1, LFY, and GAPc RNA levels in 4-, 7-, 14-, and 21-day-old wild-type (WT) Columbia, emf1-1, emf1-2, and emf2-1 plants grown under SD conditions. GAPc was used as a RT-PCR control. RT-PCR products amplified were hybridized with a probe of each gene. The relative amount of each gene after standardization using the GAPc signal as a reference is presented below each gel. FC, flower clusters consisting of inflorescence meristems and floral buds.
CO and FT RNA levels appeared to be higher in the two weak mutants than in the wild type as early as 4 DAG and definitely by 7 DAG. But these two genes did not show precocious expression in the strong mutants (Figure 5). Similar data were obtained with samples grown under long-day conditions (data not shown). SOC1 RNA levels were comparable between the two weak mutants and the wild type, whereas they were lower in the strong mutant. LFY RNA levels did not show upregulation in any of the three mutants relative to that in the wild type in the first week after germination. By the second week, the two weak mutants contained more LFY RNA than the wild type but the strong mutant did not (Figure 5).
In summary, the weak mutants expressed CO, FT, and LFY at an earlier time than the wild type. However, the strong emf1 mutant, emf1-2, did not show upregulation in any of the genes investigated, suggesting that precocious expression of these four genes is not responsible for the ectopic expression of the flower organ identity genes in emf1 mutants. Because there is no strong emf2 mutant available for study, it is difficult to determine the roles of CO and FT in the ectopic expression of the flower organ identity genes in the emf2 mutant.
Temporal and Spatial LFY::β-Glucuronidase Expression Pattern in emf Mutants
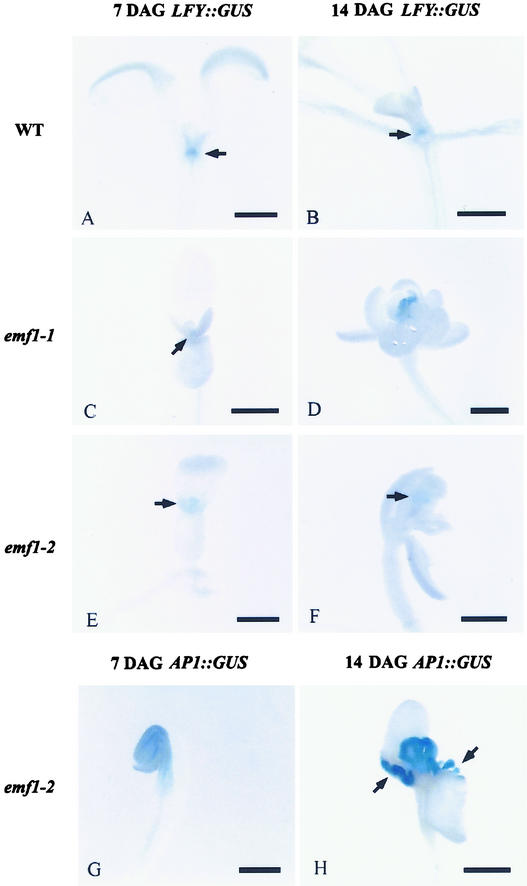
It has been reported that a high level of LFY expression activates the transcription of flower organ identity genes in wild-type plants (Parcy et al., 1998). Because LFY expression is restricted spatially to the shoot apex, an in situ assay would more accurately depict the temporal and spatial patterns of LFY expression than GeneChip or RT-PCR assays. We reported previously the ectopic AP1::β-glucuronidase (GUS) expression in emf mutants (Chen et al., 1997). In this study, we asked whether ectopic LFY::GUS expression in emf mutants could be responsible for the ectopic AP1 expression.
LFY::GUS activity was weak but detectable at the shoot apex of 4-day-old wild-type plants, as reported previously (Bai et al., 2000), and the activity was detected readily at the shoot apex of 7- and 14-day-old wild-type plants (Figures 6A and 6B). In emf1-1 and emf1-2, the LFY::GUS activity pattern was similar to that of the wild type, except that the activity in 14-day-old emf1-2 began to disappear, showing GUS activity on patches of carpelloid tissues in only a few plants (Figures 6C to 6F). At 4 and 7 DAG, no ectopic GUS activity was detected in emf1 mutants compared with the wild type, and at all ages investigated, no GUS activity was found outside of the mutant shoot apex.
Figure 6.
Histochemical Localization of GUS Activity in 7- and 14-Day-Old Wild-Type and emf1 Seedlings Harboring the LFY::GUS or AP1:: GUS Transgene.
GUS activity is indicated by blue color. Bars = 0.5 mm.
(A) LFY::GUS wild type (WT) at 7 DAG, showing GUS activity at the shoot tip (arrow).
(B) LFY::GUS wild type at 14 DAG, showing GUS activity at the meristematic region of the shoot tip (arrow).
(C) LFY::GUS emf1-1 at 7 DAG, showing GUS activity at the shoot tip (arrow).
(D) LFY::GUS emf1-1 at 14 DAG, showing GUS activity at the shoot tip.
(E) LFY::GUS emf1-2 at 7 DAG, showing GUS activity at the shoot tip (arrow).
(F) LFY::GUS emf1-2 at 14 DAG, showing GUS activity in a patch of the carpelloid tissue (arrow).
(G) AP1::GUS emf1-2 at 7 DAG, showing GUS activity in the shoot tip, cotyledons, and hypocotyl.
(H) AP1::GUS emf1-2 at 14 DAG, showing GUS activity in the carpelloid structure and the papillae tissue developed at the base of the cotyledons (arrows).
For comparison with the LFY::GUS result, we performed the AP1::GUS assay in parallel with the LFY::GUS assay. Precocious expression of AP1::GUS activity in emf seedlings was detected at 4, 7, and 14 DAG, as reported previously (Chen et al., 1997; Chou et al., 2001). emf1-2 mutants showed strong AP1::GUS activity in tissues other than the shoot apex, that is, in cotyledons and hypocotyls at 7 DAG (Figure 6G), and in the basal region of cotyledons containing carpelloid structures at 14 DAG (Figure 6H). The fact that LFY::GUS activity was confined strictly to the shoot apical region, and was never present in the cotyledons and hypocotyls, indicates that the ectopic AP1 expression in emf1-2 is not activated by LFY.
DISCUSSION
To study the molecular mechanism of EMF-mediated floral repression, we compared gene expression patterns in the emf mutants and the wild type. Our findings show that both EMF genes repress the flower program. There are differences in the gene expression patterns among the three emf mutants: some may be gene specific and others may reflect different developmental states of the mutants.
The transcript levels of FT, AP1, AP3, PI, and AG determined by GeneChip and RT-PCR analysis show similar trends and lead to similar conclusions (Table 3, Figures 4 and 5). The ectopic expression of the flower organ identity genes in emf1 seedlings in the GeneChip and RT-PCR results confirms the increased AG RNA and AP1::GUS activities in the mutants reported previously (Chen et al., 1997).
EMF Genes Maintain Vegetative Development by Repressing the Flower Program
The high degree of overlap in upregulated and downregulated genes among the three emf mutants suggests that the EMF genes regulate similar gene programs. Although 7-day-old emf mutants have the same organs as the wild-type seedlings (i.e., cotyledon, root, and hypocotyl), they show a similar gene expression pattern as the floral buds (Figure 1, Tables 1 and 3). Of particular interest are the flower organ identity genes (AP1, AP3, PI, and AG) and other flower organ–specific genes (CRABS CLAW, SEP2, SEP3, and AGL11), which are upregulated in all three emf mutants at all time points investigated (Table 3, Figure 3). Because 7-day-old emf mutants do not have flower organ primordia, the expression of these genes is not a result of flower development but rather a consequence of the lack of EMF repression. In wild-type plants, rosette shoots develop as a result of EMF repression of the flower program. In fact, constitutive activation of the flowering program can generate an emf-like phenotype (Kardailsky et al., 1999; Simpson and Dean, 2002). These phenomena demonstrate that vegetative development could result from the repression of reproductive development.
The Three Mutants Are Committed to Different Reproductive States
The emf mutants skip the vegetative shoot and germinate as reproductive shoots. The distinct morphological features of the strong and weak mutants must result from developmental differences, which are reflected by the differential expression patterns of the developmentally regulated genes.
The inflorescence state of the two weak mutants is reflected by their precocious expression of CO, FT, and LFY (Table 3, Figure 5). Similar results were reported by other investigators. For example, 14-day-old emf2-3 mutants under long days contain more FT transcripts than the wild type (Yoshida et al., 2001), and 10-day-old emf1-1 and emf2-1 mutants contain more LFY transcripts than the wild type (Chou et al., 2001). On the other hand, the strong mutant, emf1-2, showed no clear increase in CO, FT, SOC1, and LFY transcript levels relative to the wild type (Figure 5), consistent with the notion of the absence of an inflorescence stage in emf1-2.
The seed maturation gene expression pattern also indicates developmental differences among the three emf mutants (Table 4, Figure 3). Upregulation of seed maturation genes, including LEA and seed storage protein genes, was observed in 7-day-old emf1-2 plants but not as early in the weak mutants, emf1-1 and emf2-1 (Table 4).
Consistent with the mutant phenotypes, the differential gene expression pattern—normal or reduced expression of the floral pathway integrator genes in emf1-2, yet stronger expression of flower organ and seed maturation genes than in the two weak mutants—indicates that, at germination, emf1-2 resides at a more advanced reproductive state than the two weak mutants.
Possible Mechanism of EMF-Mediated Floral Repression
The EMF genes may regulate flowering time by modulating the activity of key floral pathway integrators, such as FT, LFY, and SOC1 (Mouradov et al., 2002; Simpson and Dean, 2002), as do the other floral repressors FLC and EBS (Blazquez et al., 2001; Gomez-Mena et al., 2001; Hepworth et al., 2002). However, genetic analysis shows that this is not the case. ft and lfy cannot delay flowering time in emf mutants, as do the other flowering time and flower organ identity mutants tested (Yang et al., 1995; Chen et al., 1997; Haung and Yang, 1998). Although ft can lengthen the inflorescence phase, it cannot rescue rosette development in emf mutants (Haung and Yang, 1998). Gene expression analysis based on temporal and spatial expression pattern is in agreement with genetic data in that LFY, FT, or SOC1 does not mediate EMF1 action on flowering time (Figures 5 and 6). Although FT is upregulated as early as the flower organ identity genes in the 4-day-old weak mutants, the absence of precocious expression of FT in the strong emf1 mutant and the inability of ft to rescue rosette development in both emf1 and emf2 mutants preclude the possibility of FT-mediated EMF action on flowering time. Thus, precocious FT expression probably is attributable to the inflorescence state of the weak emf seedling.
The predicted amino acid sequence suggests that EMF1 is a transcriptional regulator and that EMF2 has a C2H2 zinc finger domain. These two proteins could be involved in transcriptional regulation, resulting in the global repression of reproductive gene programs during vegetative development. Because EMF2 encodes a PcG protein homolog, it may be involved in transcriptional regulation at the chromatin level. In Drosophila, PcG proteins form protein complexes that restrict the access of transcriptional activators to the target genes (Lewin, 2000; Tie et al., 2001). The discovery that several other early-flowering genes encode chromatin-related proteins (Goodrich et al., 1997; Gaudin et al., 2001) suggests that these proteins may be involved in remodeling the chromatin, thereby restricting the access of the floral promoters (e.g., LFY) to the flower organ identity genes during vegetative development. As promoters increase in concentration in the reproductive shoot, they gain access to the target genes and potentially can activate gene transcription via the recruitment of chromatin-remodeling proteins (Wagner and Meyerowitz, 2002). The progressive increase in the expression of flower organ–specific genes in the emf mutants suggests that in the absence of EMF repression, the expression of the flower organ genes is subject to regulation by the floral promoters. Hence, we propose that to control flowering time, the floral promoters and EMF repressors converge on the same target genes, the flower organ–specific genes. Recently it was demonstrated that FERTILIZATION-INDEPENDENT ENDOSPERM, a PcG protein, is involved in floral repression as well as the repression of endosperm development (Kinoshita et al., 2001). It is possible that these proteins display overlapping functions through participation in common protein complexes that modulate chromatin structure.
METHODS
Plant Materials and Growth Conditions
Surface-sterilized Arabidopsis thaliana seeds were plated on agar plates containing half-strength Murashige and Skoog (1962) salts and vitamins, 1.5% Suc, and 0.8% agar. The plates were placed for 2 days in the cold, and then seedlings were grown under short-day conditions (8 h of light/16 h of dark) at 21°C. For plants grown on soil, 10- to 12-day-old normal plants grown under short-day conditions were transferred to soil and grown under long-day conditions (16 h of light/8 h of dark) at 21°C in the greenhouse.
Semiquantitative Reverse Transcription–PCR and Hybridization
Total RNA was isolated using RNAWIZ (Ambion, Austin, TX) from whole seedlings harvested between 11 am and 4 pm. Using 2 μg of total RNA pretreated with RNase Free DNase I (Amersham Pharmacia Biotech, Piscataway, NJ) and reverse transcriptase from Moloney murine leukemia virus (Promega, Madison, WI), cDNA was synthesized. PCR amplification was performed using 2 μL of 50 μL of RT reaction and Promega Taq DNA polymerase according to the recommendations of the supplier. Twenty-eight to 30 cycles were used for CO and LFY, 26 to 28 cycles were used for FT and SOC1, 25 to 27 cycles were used for AP1, 24 to 26 cycles were used for AP3 and AG, 22 to 24 cycles were used for PI, and 21 to 23 cycles were used for GAPc. The number of PCR cycles chosen was shown to be in the linear range of the reaction in a separate experiment using different amounts of cDNA template. The control, GAPc, was amplified in the same tube as each gene studied. Primers used for the PCR reactions were as follows: 5′-GAACAAATTGAGCAGCTCAAG-3′ and 5′-GCAGCTTTAGAGTTTTGTTAC-3′ for SOC1; 5′-TGAAGGACG-AGGAGCTTGAAG-3′ and 5′-CATCTTTCCTTGACCTGCGTC-3′ for LFY; 5′-TTGAACGCTATGAGAGGTAC-3′ and 5′-TTTTCCCTCTCCTTGATC-TG-3′ for AP1; 5′-AGGAGATCGTAGATCTGTAC-3′ and 5′-TCCTCCATT-GTCTACTAGTC-3′ for AP3; 5′-ATCTTGGTGCTATGTTGGAC-3′ and 5′-CTCATCATCATTCCTCTTGC-3′ for PI; and 5′-GGTACAAGAAGGCAA-TATCG-3′ and 5′-TTGTTCCTCTCATTTTCAGC-3′ for AG. FT primers described by Kardailsky et al. (1999), CO primers described by Putterill et al. (1995), and GAPc primers described by Aubert et al. (2001) were used. The amplified fragments were separated on an agarose gel, blotted onto a membrane, and hybridized with radiolabeled probes. Radioactive signals were detected using a Typhoon 8600 imager (Molecular Dynamics, Sunnyvale, CA) and quantified using ImageQuant (version 5.2) software (Molecular Dynamics). The data shown are representative of the time points in at least two independent RT-PCR experiments.
GeneChip Experiments
For total RNA isolation, 800 to 1500 whole plants for each emf1-1, emf1-2, and emf2-1 mutant sample were pooled from different experiments to eliminate much of the variation in gene expression patterns caused by subtle differences in environmental conditions and among individuals. All samples were harvested between 11 am and 4 pm. Total RNA was isolated using RNeasy columns (Qiagen, Valencia, CA) according to the manufacturer's protocols. cDNA synthesis, biotinylated cRNA probe synthesis, and hybridization were performed as described by Tepperman et al. (2001) and Zhu et al. (2001). The probe array was scanned twice, and the intensities were averaged with a Hewlett-Packard GeneArray Scanner. GeneChip Suite 4.0 (Affymetrix, Santa Clara, CA) was used for image analysis. The average intensity of all probe sets was used for normalization and scaled to 100 in the absolute analysis for each probe array.
Genetic Crosses and β-Glucuronidase Activity Assays of the LFY::β-Glucuronidase and AP1::β-Glucuronidase Transgenic Plants
To introduce LFY::β-glucuronidase (GUS) into emf1 mutants, heterozygous emf1 plants were crossed with a line homozygous for LFY::GUS, which was kindly supplied by Detlef Weigel (Salk Institute, San Diego, CA). F1 plants were selfed. F2 plants homozygous or heterozygous for LFY::GUS and heterozygous for emf1 were grown to generate F3 plants, of which one-fourth or three-sixteenths were the desired strains. The AP1::GUS emf1 lines were described by Chen et al. (1997).
Four- to 14-day-old seedlings of LFY::GUS emf1-1, LFY::GUS emf1-2, AP1::GUS emf1-1, and AP1::GUS emf1-2 plants were prepared for GUS assays. GUS activity in these plants was detected histochemically using a protocol adapted from Jefferson et al. (1987) with slight modifications. Briefly, the tissue was incubated in 2 mM 5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid in 50 mM phosphate buffer, pH 7.0, containing 0.5 mM K3Fe(CN)6 and 0.5 mM K4Fe(CN)6 for 16 h at 37°C, rinsed with 50 mM phosphate buffer, fixed and cleared with ethanol (95%):acetic acid (9:1, v/v) for 2 to 4 h at room temperature, and observed and photographed using a Zeiss Stemi SV 11 stereomicroscope equipped with a DEI 450 digital video camera (Optronics, Goleta, CA). Images were captured using NIH Image software as modified by Scion Images (version 1.62c). Captured images were converted from 72 to 300 dots per inch with Adobe Photoshop version 5.5 (Adobe Systems, Mountain View, CA). All subsequent image manipulation and figure preparation were performed with Adobe Photoshop.
Upon request, all novel materials described in this article will be made available in a timely manner for noncommercial research purposes.
Supplementary Material
Acknowledgments
We thank Detlef Weigel for providing the LFY::GUS line, Martin Yanofsky for the AP1::GUS line, Jin-Chen Cheng and Linda Castle for genetic crosses, Hicham Zegzouti and Alex Fagnon for technical assistance, and Kvin Lertpiriyapong and Mary Alice Yund for critical reading of the manuscript. This work was supported by U.S. Department of Agriculture Competitive Grants 93-37301-8704 and 99-35301-7984 and by funding from the Torrey Mesa Research Institute, Syngenta Research and Technology, to Z.R.S.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.007831.
Footnotes
Online version contains Web-only data.
References
- Araki, T. (2001). Transition from vegetative to reproductive phase. Curr. Opin. Plant Biol. 4, 63–68. [DOI] [PubMed] [Google Scholar]
- Aubert, D., Chen, L., Moon, Y.-H., Martin, D., Castle, L.A., Yang, C.-H., and Sung, Z.R. (2001). EMF1, a novel protein involved in the control of shoot architecture and flowering in Arabidopsis. Plant Cell 13, 1865–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai, S., Chen, L., Yund, M.A., and Sung, Z.R. (2000). Mechanisms of plant embryo development. Curr. Top. Dev. Biol. 50, 61–88. [DOI] [PubMed] [Google Scholar]
- Bai, S., and Sung, Z.R. (1995). The role of EMF1 in regulating the vegetative and reproductive transition in Arabidopsis thaliana (Brassicaceae). Am. J. Bot. 82, 1095–1103. [Google Scholar]
- Birve, A., Sengupta, A.K., Beuchle, D., Larsson, J., Kennison, J.A., Rasmuson-Lestander, A., and Muller, J. (2001). Su(z)12, a novel Drosophila Polycomb group gene that is conserved in vertebrates and plants. Development 128, 3371–3379. [DOI] [PubMed] [Google Scholar]
- Blazquez, M. (2000). Flower development pathways. J. Cell Sci. 113, 3547–3548. [DOI] [PubMed] [Google Scholar]
- Blazquez, M., Koornneef, M., and Putterill, J. (2001). Flowering on time: Genes that regulate the floral transition. Workshop on the molecular basis of flowering time control. EMBO Rep. 2, 1078–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazquez, M.A., and Weigel, D. (2000). Integration of floral inductive signals in Arabidopsis. Nature 404, 889–892. [DOI] [PubMed] [Google Scholar]
- Bradley, D., Ratcliffe, O., Vincent, C., Carpenter, R., and Coen, E. (1997). Inflorescence commitment and architecture in Arabidopsis. Science 275, 80–83. [DOI] [PubMed] [Google Scholar]
- Busch, M.A., Bomblies, K., and Weigel, D. (1999). Activation of a floral homeotic gene in Arabidopsis. Science 285, 585–587. [DOI] [PubMed] [Google Scholar]
- Chen, L., Cheng, J.C., Castle, L., and Sung, Z.R. (1997). EMF genes regulate Arabidopsis inflorescence development. Plant Cell 9, 2011–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou, M.L., Haung, M.D., and Yang, C.H. (2001). EMF genes interact with late-flowering genes in regulating floral initiation genes during shoot development in Arabidopsis thaliana. Plant Cell Physiol. 42, 499–507. [DOI] [PubMed] [Google Scholar]
- Conceicao Ada, S., and Krebbers, E. (1994). A cotyledon regulatory region is responsible for the different spatial expression patterns of Arabidopsis 2S albumin genes. Plant J. 5, 493–505. [DOI] [PubMed] [Google Scholar]
- Doyle, M.R., Davis, S.J., Bastow, R.M., McWatters, H.G., Kozma-Bognar, L., Nagy, F., Millar, A.J., and Amasino, R.M. (2002). The ELF4 controls circadian rhythms and flowering time in Arabidopsis thaliana. Nature 419, 74–77. [DOI] [PubMed] [Google Scholar]
- Gaubier, P., Raynal, M., Hull, G., Huestis, G.M., Grellet, F., Arenas, C., Pages, M., and Delseny, M. (1993). Two different Em-like genes are expressed in Arabidopsis thaliana seeds during maturation. Mol. Gen. Genet. 238, 409–418. [DOI] [PubMed] [Google Scholar]
- Gaudin, V., Libault, M., Pouteau, S., Juul, T., Zhao, G., Lefebvre, D., and Grandjean, O. (2001). Mutations in LIKE HETEROCHROMATIN PROTEIN 1 affect flowering time and plant architecture in Arabidopsis. Development 128, 4847–4858. [DOI] [PubMed] [Google Scholar]
- Gendall, A.R., Levy, Y.Y., Wilson, A., and Dean, C. (2001). The VERNALIZATION 2 gene mediates the epigenetic regulation of vernalization in Arabidopsis. Cell 107, 525–535. [DOI] [PubMed] [Google Scholar]
- Gomez-Mena, C., Pineiro, M., Franco-Zorrilla, J.M., Salinas, J., Coupland, G., and Martinez-Zapater, J.M. (2001). early bolting in short days: An Arabidopsis mutation that causes early flowering and partially suppresses the floral phenotype of leafy. Plant Cell 13, 1011–1024. [PMC free article] [PubMed] [Google Scholar]
- Goodrich, J., Puangsomlee, P., Martin, M., Long, D., Meyerowitz, E.M., and Coupland, G. (1997). A Polycomb-group gene regulates homeotic gene expression in Arabidopsis. Nature 386, 44–51. [DOI] [PubMed] [Google Scholar]
- Haung, M.D., and Yang, C.H. (1998). EMF genes interact with late-flowering genes to regulate Arabidopsis shoot development. Plant Cell Physiol. 39, 382–393. [DOI] [PubMed] [Google Scholar]
- Hepworth, S.R., Valverde, F., Ravenscroft, D., Mouradov, A., and Coupland, G. (2002). Antagonistic regulation of flowering-time gene SOC1 by CONSTANS and FLC via separate promoter motifs. EMBO J. 21, 4327–4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks, K.A., Albertson, T.M., and Wagner, D.R. (2001). EARLY FLOWERING3 encodes a novel protein that regulates circadian clock function and flowering in Arabidopsis. Plant Cell 13, 1281–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson, R.A., Kavanagh, T.A., and Bevan, M.W. (1987). GUS fusions: Beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6, 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardailsky, I., Shukla, V.K., Ahn, J.H., Dagenais, N., Christensen, S.K., Nguyen, J.T., Chory, J., Harrison, M.J., and Weigel, D. (1999). Activation tagging of the floral inducer FT. Science 286, 1962–1965. [DOI] [PubMed] [Google Scholar]
- Kinoshita, T., Harada, J.J., Goldberg, R.B., and Fischer, R.L. (2001). Polycomb repression of flowering during early plant development. Proc. Natl. Acad. Sci. USA 98, 14156–14161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef, M., Alonso-Blanco, C., Blankestijn-de Vries, H., Hanhart, C.J., and Peeters, A.J. (1998). Genetic interactions among late-flowering mutants of Arabidopsis. Genetics 148, 885–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef, M., Hanhart, C.J., and van der Veen, J.H. (1991). A genetic and physiological analysis of late flowering mutants in Arabidopsis thaliana. Mol. Gen. Genet. 229, 57–66. [DOI] [PubMed] [Google Scholar]
- Krizek, B.A., and Meyerowitz, E.M. (1996). The Arabidopsis homeotic genes APETALA3 and PISTILLATA are sufficient to provide the B class organ identity function. Development 122, 11–22. [DOI] [PubMed] [Google Scholar]
- Larsson, A.S., Landberg, K., and Meeks-Wagner, D. (1998). The TERMINAL FLOWER2 (TFL2) gene controls the reproductive transition and meristem identity in Arabidopsis thaliana. Genetics 149, 597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin, B. (2000). Genes VII. (Oxford, UK: Oxford University Press).
- Liljegren, S.J., Gustafson-Brown, C., Pinyopich, A., Ditta, G.S., and Yanofsky, M.F. (1999). Interactions among APETALA1, LEAFY, and TERMINAL FLOWER1 specify meristem fate. Plant Cell 11, 1007–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels, S.D., and Amasino, R.M. (1999). FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 11, 949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouradov, A., Cremer, F., and Coupland, G. (2002). Control of flowering time: Interacting pathways as a basis for diversity. Plant Cell 14 (suppl.), S111.–S130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15, 473–497. [Google Scholar]
- Parcy, F., Nilsson, O., Busch, M.A., Lee, I., and Weigel, D. (1998). A genetic framework for floral patterning. Nature 395, 561–566. [DOI] [PubMed] [Google Scholar]
- Putterill, J., Robson, F., Lee, K., Simon, R., and Coupland, G. (1995). The CONSTANS gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell 80, 847–857. [DOI] [PubMed] [Google Scholar]
- Samach, A., Onouchi, H., Gold, S.E., Ditta, G.S., Schwarz-Sommer, Z., Yanofsky, M.F., and Coupland, G. (2000). Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis. Science 288, 1613–1616. [DOI] [PubMed] [Google Scholar]
- Simpson, G.G., and Dean, C. (2002). Arabidopsis, the Rosetta stone of flowering time? Science 296, 285–289. [DOI] [PubMed] [Google Scholar]
- Sung, Z.R., Belachew, A., Shunong, B., and Bertrand-Garcia, R. (1992). EMF, an Arabidopsis gene required for vegetative shoot development. Science 258, 1645–1647. [DOI] [PubMed] [Google Scholar]
- Sung, Z.R., Chen, L., Moon, Y.-H., and Yoshida, N. (2003. a). Molecular mechanism of shoot determinacy and flowering in Arabidopsis. HortScience, in press.
- Sung, Z.R., Chen, L.J., Moon, Y.H., and Lertpiriyapong, K. (2003. b). Mechanisms of floral repression in Arabidopsis. Curr. Opin. Plant Biol. 6, 29–35. [DOI] [PubMed] [Google Scholar]
- Tepperman, J.M., Zhu, T., Chang, H.S., Wang, X., and Quail, P.H. (2001). Multiple transcription-factor genes are early targets of phytochrome A signaling. Proc. Natl. Acad. Sci. USA 98, 9437–9442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tie, F., Furuyama, T., Prasad-Sinha, J., Jane, E., and Harte, P.J. (2001). The Drosophila Polycomb group proteins ESC and E(Z) are present in a complex containing the histone-binding protein p55 and the histone deacetylase RPD3. Development 128, 275–286. [DOI] [PubMed] [Google Scholar]
- Wagner, D., and Meyerowitz, E.M. (2002). SPLAYED, a novel SWI/SNF ATPase homolog, controls reproductive development in Arabidopsis. Curr. Biol. 12, 85–94. [DOI] [PubMed] [Google Scholar]
- Wagner, D., Sablowski, R.W., and Meyerowitz, E.M. (1999). Transcriptional activation of APETALA1 by LEAFY. Science 285, 582–584. [DOI] [PubMed] [Google Scholar]
- Yang, C.H., Chen, L.J., and Sung, Z.R. (1995). Genetic regulation of shoot development in Arabidopsis: Role of the EMF genes. Dev. Biol. 169, 421–435. [DOI] [PubMed] [Google Scholar]
- Yoshida, N., Yanai, Y., Chen, L., Kato, Y., Hiratsuka, J., Miwa, T., Sung, Z.R., and Takahashi, S. (2001). EMBRYONIC FLOWER2, a novel Polycomb group protein homolog, mediates shoot development and flowering in Arabidopsis. Plant Cell 13, 2471–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagotta, M.T., Shannon, S., Jacobs, C.I., and Meeks-Wagner, D.R. (1992). Early-flowering mutants of Arabidopsis thaliana. Aust. J. Plant Physiol. 19, 411–418. [Google Scholar]
- Zhu, T., Budworth, P., Han, B., Brown, D., Chang, H.-S., Zou, G., and Wang, X. (2001). Toward elucidating the global gene expression patterns of developing Arabidopsis: Parallel analysis of 8300 genes by a high-density oligonucleotide probe array. Plant Physiol. Biochem. 39, 221–242. [Google Scholar]
- Zhu, T., and Wang, X. (2000). Large-scale profiling of the Arabidopsis transcriptome. Plant Physiol. 124, 1472–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.