Abstract
In this investigation, we examined the effects of different unsaturated fatty acid compositions of Saccharomyces cerevisiae on the growth-inhibiting effects of ethanol. The unsaturated fatty acid (UFA) composition of S. cerevisiae is relatively simple, consisting almost exclusively of the mono-UFAs palmitoleic acid (Δ9Z-C16:1) and oleic acid (Δ9Z-C18:1), with the former predominating. Both UFAs are formed in S. cerevisiae by the oxygen- and NADH-dependent desaturation of palmitic acid (C16:0) and stearic acid (C18:0), respectively, catalyzed by a single integral membrane desaturase encoded by the OLE1 gene. We systematically altered the UFA composition of yeast cells in a uniform genetic background (i) by genetic complementation of a desaturase-deficient ole1 knockout strain with cDNA expression constructs encoding insect desaturases with distinct regioselectivities (i.e., Δ9 and Δ11) and substrate chain-length preferences (i.e., C16:0 and C18:0); and, (ii) by supplementation of the same strain with synthetic mono-UFAs. Both experimental approaches demonstrated that oleic acid is the most efficacious UFA in overcoming the toxic effects of ethanol in growing yeast cells. Furthermore, the only other UFA tested that conferred a nominal degree of ethanol tolerance is cis-vaccenic acid (Δ11Z-C18:1), whereas neither Δ11Z-C16:1 nor palmitoleic acid (Δ9Z-C16:1) conferred any ethanol tolerance. We also showed that the most ethanol-tolerant transformant, which expresses the insect desaturase TniNPVE, produces twice as much oleic acid as palmitoleic acid in the absence of ethanol and undergoes a fourfold increase in the ratio of oleic acid to palmitoleic acid in response to exposure to 5% ethanol. These findings are consistent with the hypothesis that ethanol tolerance in yeast results from incorporation of oleic acid into lipid membranes, effecting a compensatory decrease in membrane fluidity that counteracts the fluidizing effects of ethanol.
Ethanol is well known as an inhibitor of growth of microorganisms. It has been reported to damage mitochondrial DNA in yeast cells (13) and to cause inactivation of some enzymes, such as hexokinase (2) and dehydrogenase (23). Nevertheless, some strains of the yeast Saccharomyces cerevisiae show tolerance and can adapt to high concentrations of ethanol (1, 9). Many studies have documented the alteration of cellular lipid composition in response to ethanol exposure. (4, 8, 14, 16, 21, 32). It has been found that S. cerevisiae cells grown in the presence of ethanol appear to increase the amount of monounsaturated fatty acids in cellular lipids (1, 4, 26). Since cell membranes have received extensive consideration as primary targets of ethanol stress, many reports have suggested a relationship between the fatty acid compositions of lipid membranes and ethanol stress tolerance (1, 4, 9, 21, 26). Although the correlation between ethanol tolerance and increased degree of fatty acid unsaturation of membrane lipids of S. cerevisiae is well documented, a causal relationship is not yet established.
Unlike in most other fungi (33), the predominant unsaturated fatty acids (UFAs) of S. cerevisiae are the mono-UFAs palmitoleic acid (Δ9Z-C16:1) and oleic acid (Δ9Z-C18:1), produced by the formation of a Z (cis) double bond between carbon atoms 9 and 10 of saturated 16- and 18-carbon fatty acids. These UFAs play an essential role in homeoviscous adaptation (11) and are synthesized in fungal and animal cells by acyl coenzyme A (CoA) Δ9Z-desaturases (3, 5, 30, 31), which have significant levels of conservation of amino acid sequence and inferred transmembrane structure (20, 31). Functional replacement of OLE1 with a cDNA encoding a rat desaturase indicates conservation of the functional interactions between desaturases and two essential electron transport components of the active desaturase complex, cytochrome b5 (a hemoprotein) and cytochrome b5 reductase (flavoprotein) (12, 22, 24, 27, 28). Recently, cDNAs encoding integral membrane desaturases of lepidopteran insects (moths) have also been shown to relieve the UFA auxotrophy of ole1 mutants of S. cerevisiae and to produce unique UFA profiles that reflect the distinctive substrate selectivities and regioselectivities of the expressed desaturases (17, 18, 20, 25). In this study, we have investigated the ethanol stress tolerance of these strains as well as the desaturase-deficient ole1 strain supplemented with specific Δ9 and Δ11 UFAs.
MATERIALS AND METHODS
Strains.
The S. cerevisiae desaturase-deficient ole1 knockout strain L8-14C (MATα ole1Δ::LEU2 leu2-3 leu2-112 trp1-1, ura3-52 his4), which is incapable of producing UFAs (31), was used in UFA supplementation experiments and as a host in transformation experiments to introduce YEpOLEX plasmids containing cDNAs encoding acyl-CoA Δ9 and Δ11Z-desaturases of Trichoplusia ni (17, 20) and Helicoverpa zea (25), and YEp352 containing the OLE1 gene encoding the Δ9Z desaturase of S. cerevisiae (31).
Desaturase expression plasmids and transformation procedures.
The construction of the plasmids used to direct the expression of the recombinant insect desaturases has been described previously (17, 20, 25). In brief, the open reading frames (ORFs) of desaturases from two moth species, Trichoplusia ni (17, 20) and Helicoverpa zea (25), were subcloned into a plasmid derivative of YEp352/OLE4.8 in a procedure similar to that used to construct a functional yeast/rat chimeric desaturase cDNA (17, 31). The final plasmids, designated YEpOLEX-HzeaKPSE, YEpOLEX-TniNPVE, YEpOLEX-HzeaLPAQ, and YEpOLEX-TniLPAQ, contain ORFs encoding Δ9 or Δ11Z-desaturases ligated via a four-codon linker in frame with and downstream from the 5′ end of the OLE1 ORF encoding the first 27 amino acids of the yeast desaturase, as in the original construct containing the functional yeast/rat desaturase gene (31). Sequences flanking the chimeric ORF consisted of the promoter and terminator elements of the OLE1 gene contained on the original YEp352/OLE4.8 plasmid.
L8-14C cells were transformed with the plasmid DNAs by a standard method (15), and URA+ transformants (reflecting complementation of the ura3-52 mutation of the host strain by the URA3 gene present in the YEpOLEX plasmid) were selected at 30°C on SD agar plates (1.7 g of Bio 101 yeast nitrogen base per liter, 5 g of ammonium sulfate per liter, and 20 g of Bio 101 Bacto agar per liter, made 20 g/liter in glucose after autoclaving) supplemented with histidine (20 mg/liter), leucine (40 mg/liter), tryptophan (60 mg/liter), oleic acid (0.5 mM), and palmitoleic acid (0.5 mM). To solubilize the UFAs, the medium was made 1% (vol/vol) in Tergitol (Sigma-Aldrich), and appropriate amounts of UFAs were added to the medium as 1 M ethanolic solutions. Individual URA+ transformant colonies were streaked onto the same medium, and individual colonies from the reselection were tested for complementation of the ole1 auxotrophy by plating onto YPD agar plates without any supplemental UFAs (YPD agar: 20 g of Bio 101 peptone Y per liter, 10 g of Bio 101 yeast extract Y per liter, and 20 g of Bio 101 Bacto agar per liter, made 20 g/liter in glucose after autoclaving).
Culture conditions.
To measure the growth of the transformant strains in the absence of ethanol, each culture was started by inoculating 250-ml Erlenmeyer flasks containing 50 ml of YPD liquid medium (20 g of Bio 101 peptone Y per liter, 10 g of yeast extract Y per liter) to a density of 2 × 104 cells per ml with cells from freshly grown late-log-phase (1 × 108 to 2 × 108 cells per ml) cultures and agitated at 300 rpm in a New Brunswick Scientific G25 orbital shaker at 30°C. Growth was monitored by optical density at 600 nm (OD600) and by hemocytometer counts.
For ethanol stress tolerance studies of the transformant strains, each culture was started by inoculating 50-ml Erlenmeyer flasks containing 10 ml of YPD liquid medium made 5% (vol/vol) in ethanol to a density of 106 cells per ml with cells from actively growing mid-log-phase (1 × 107 to 2 × 107 cells per ml) cultures and vigorously agitated at 30°C as above for 60 h. For studies of the UFA profiles of the TniNPVE transformant grown at different ethanol concentrations, cultures were established and grown as described above in the presence of 0, 1, 3, and 5% ethanol, and fatty acids were analyzed when the cell density reached 2 × 107 cells per ml.
For ethanol stress tolerance studies of the L8-14C (ole1) strain supplemented with specific UFAs, actively growing L8-14C cells (described below) were transferred to 10 ml of YPD liquid medium made 5% in ethanol and supplemented with the following UFA treatments: 1 mM Δ9Z-C18:1, 1 mM Δ9Z-C16:1, 1 mM cis-vaccenic acid (Δ11Z-C18:1), 0.33 mM Δ9Z-C16:1 plus 0.66 mM Δ9Z-C18:1, 0.33 mM Δ9Z-C16:1 plus 0.66 mM Δ11Z-C18:1, 0.66 mM Δ9Z-C16:1 plus 0.33 mM Δ9Z-C18:1, and 0.66 mM Δ9Z-C16:1 plus 0.33 mM Δ11Z-C18:1. UFAs (Sigma-Aldrich) were solubilized with Tergitol as described above for incorporation in SD agar plates. The inocula for the treatment groups were cells harvested from a mid-log-phase culture of the L8-14C strain started in a 250 ml Erlenmeyer flask containing 50 ml of YPD liquid medium supplemented with 0.5 mM palmitoleic and 0.5 mM oleic acid and grown as described above for growth in the absence of ethanol. When the culture reached a density of 2 × 107 cells/ml, the cells were pelleted, washed twice in water, and used immediately to inoculate each of the various 10-ml treatment groups to a density of 1 × 106 cells per ml.
Fatty acid analysis.
Cells were pelleted and washed in water twice, and total lipids were extracted with chloroform-methanol (2:1 [vol/vol]) and then methylated with 0.5 M KOH-methanol. The fatty acid methyl esters were analyzed by gas chromatography-mass spectroscopy with a Hewlett-Packard (HP) 5890 gas chromatograph (HP1 capillary column, inside diameter of 25 by 0.2 mm, film thickness of 0.5 μm) coupled to an HP 5970 series mass selective detector. The oven temperature was programmed at 125°C for 3 min and then at 5°C per min until 245°C and held for 32 min. The double-bond positions of UFA methyl esters were verified by mass spectral analysis of dimethyl disulfide adducts (6).
RESULTS
Fatty acid compositions of S. cerevisiae strains expressing insect desaturases.
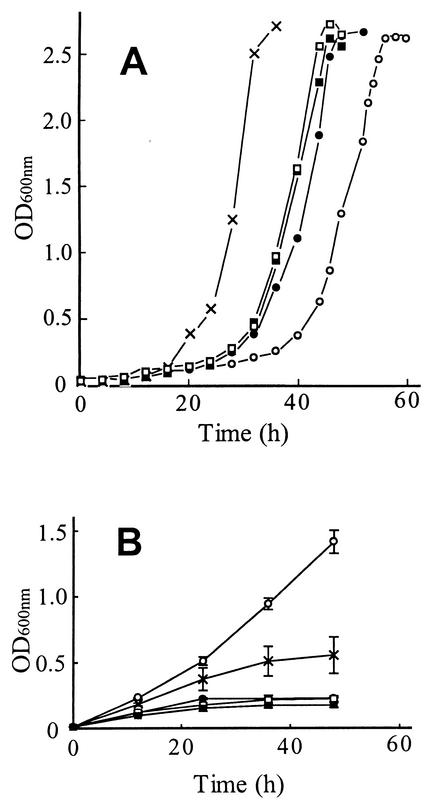
The proportions of 16- and 18-carbon saturated and unsaturated fatty acids produced in the S. cerevisiae L8-14C (ole1) strain transformed with YEpOLEX plasmids expressing acyl-CoA Δ9 or Δ11Z-desaturases of T. ni and H. zea are summarized in Table 1. The proportions of UFAs in mid-log-phase cells of the HzeaKPSE transformant were similar to those of the reconstituted OLE1 strain: i.e., Δ9Z-C16:1 and Δ9Z-C18:1 in about a 2:1 ratio, although the latter strain had a slightly higher ratio of UFAs to saturated fatty acids (SFAs). In contrast, the TniNPVE, HzeaLPAQ, and TniLPAQ transformants produced UFA compositions that are unusual for S. cerevisiae. In the TniNPVE transformant, the ratio of Δ9Z-C16:1 and Δ9Z-C18:1 was the opposite of that of the HzeaKPSE transformant, and the reconstituted OLE1 strain (i.e., Δ9Z-C18:1) predominated. The only UFA produced by the HzeaLPAQ transformant was Δ11Z-C16:1, whereas the TniLPAQ transformant produced about 2.5 times as much Δ11Z-C16:1 as Δ11Z-C18:1. All of the strains grew well in standard complete growth medium (YPD) lacking ethanol, although the reconstituted wild-type (OLE1) strain expressing the native yeast desaturase had a significantly shorter lag phase than the four recombinant strains expressing insect desaturases (Fig. 1A).
TABLE 1.
Compositions of major fatty acids of L8-14C yeast cells transformed with plasmids encoding integral membrane desaturasesa
| Transformant | Fatty acid content (%)b
|
|||
|---|---|---|---|---|
| Saturated
|
Monounsaturated
|
|||
| C16:0 | C18:0 | C16:1 | C18:1 | |
| Δ9Z | ||||
| OLE1 | 45.5 ± 2.2 | 4.7 ± 2.4 | 34.9 ± 0.8 | 14.9 ± 1.0 |
| HzeaKPSE | 49.5 ± 5.5 | 7.9 ± 2.2 | 31.7 ± 5.6 | 11.0 ± 2.0 |
| TniNPVE | 46.9 ± 4.0 | 8.6 ± 3.9 | 12.8 ± 1.9 | 31.7 ± 5.8 |
| Δ11Z | ||||
| HzeaLPAQ | 45.6 ± 3.6 | 11.9 ± 2.8 | 42.6 ± 6.3 | |
| TniLPAQ | 49.7 ± 4.8 | 12.5 ± 0.1 | 41.8 ± 11.8 | 11.2 ± 1.5 |
Analyzed at mid-log phase (2 × 107 to 3 × 107 cells per ml) in two separate experiments.
Determined from the peak areas of methyl esters.
FIG. 1.
Growth curves of ole1 yeast cells transformed with plasmids containing OLE1 (×), HzeaKPSE (•), HzeaLPAQ (▪), TniNPVE (○), and TniLPAQ (□) grown in YPD medium (A), or in YPD medium containing 5% ethanol (B). The data shown in panel B are the means of two separate experiments. The culture conditions were as described in Materials and Methods.
Ethanol stress tolerance of the transformant strains expressing insect desaturases.
The growth of the transformant strains under ethanol stress was investigated by adding freshly grown cells to YPD liquid medium containing 5% ethanol as described in Materials and Methods (Fig. 1B). The TniNPVE transformant was the most resistant to the growth-inhibiting effects of ethanol, followed by the reconstituted OLE1 strain. The transformants expressing the HzeaKPSE, HzeaLPAQ, and TniLPAQ desaturases showed no ethanol tolerance, even at ethanol concentrations as low as 2%, whereas the TniNPVE transformant was able to grow even in the presence of 7% ethanol (data not shown).
Ethanol-induced changes in the fatty acid composition of the TniNPVE transformant.
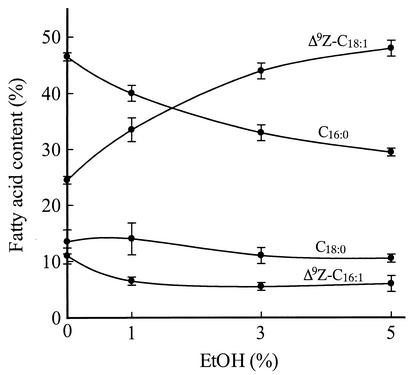
The fatty acid compositions of cells expressing the TniNPVE desaturase grown in the presence of ethanol concentrations from 0 to 5% were analyzed (Fig. 2). When ethanol was present in the medium, the relative amounts of C16:0, Δ9Z-C16:1, and Δ9Z-C18:1 in the TniNPVE transformant cells changed dramatically. In the presence of 5% ethanol, the proportion of Δ9Z-C18:1 doubled compared to that in cells grown in the absence of ethanol, whereas the levels of C16:0 and Δ9Z-C16:1 decreased by 38 and 50%, respectively. Thus, the proportion of Δ9Z-C18:1 to Δ9Z-C16:1 in TniNPVE cells grown in the presence of 5% ethanol increased approximately fourfold over the proportion in the same cells grown without ethanol.
FIG. 2.
Effect of ethanol concentration on the fatty acid composition of the TniNPVE transformant strain. The data shown are the means of two experiments performed in parallel. The culture conditions and fatty acid analysis were as described in Materials and Methods.
Ethanol tolerance of desaturase-deficient ole1 cells supplemented with Δ9 and Δ11 UFAs.
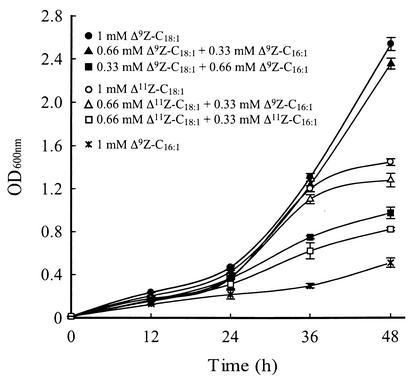
The L8-14C (ole1) strain was grown in medium containing 5% ethanol supplemented with UFAs as described in Materials and Methods. Figure 3 shows that Δ9Z-C18:1 conferred the greatest tolerance to the growth-inhibiting effects of ethanol, whereas Δ9Z-C16:1 conferred the least and Δ11Z-C18:1 conferred an intermediate level of ethanol tolerance.
FIG. 3.
Growth of ole1 cells in YPD medium containing 5% ethanol and supplemented with UFAs. Data shown are the means of two experiments performed in parallel. The culture conditions were as described in Materials and Methods.
DISCUSSION
We have transformed a desaturase-deficient strain of the yeast S. cerevisiae with plasmids expressing moth desaturases representing three distinct gene lineages that were established early in the evolution of the class Insecta (18). Because of the unique biochemical properties of the expressed desaturases, each transformant strain has a characteristic UFA composition that differs from the natural state (Table 1), thus permitting an examination of the effects of specific UFAs (e.g., Δ9Z-C16:1, Δ11Z-C16:1, or Δ9Z-C18:1) on ethanol tolerance in a uniform genetic background.
In the absence of ethanol, cultures of two of the transformant strains had lag phases that were significantly different from those of the others when started with inoculations of stationary-phase cells (Fig. 1A). The shorter lag phase of the reconstituted OLE1 strain compared to those of the other transformant strains could reflect more efficient assembly or catalysis of the active OLE1 desaturase complex, since its functional components, i.e., the desaturase and the electron transport proteins, have coevolved. In this regard, a significant difference between the active desaturase complex of the OLE1 strain and the strains expressing heterologous desaturases is that the OLE1 gene encodes a modular protein that includes a functional cytochrome b5 domain at the carboxyl terminal of the desaturase, whereas the cytochrome b5 component of all of the heterologous desaturase functional complexes of this study is an amphipathic mobile membrane protein encoded by a genetically discrete locus (22). The lag phase of the TniNPVE transformant, which produces about twice as much Δ9Z-C18:1 as Δ9Z-C16:1, is significantly longer than the transformants that produce more 16-carbon than 18-carbon UFAs, like the OLE1 strain. The latter observation and the predominance of Δ9Z-C16:1 in wild-type yeast cells suggest that, in the absence of ethanol, a minimal level of Δ9Z-C16:1 is essential to achieve optimal aerobic growth in S. cerevisiae.
Interestingly, the TniNPVE transformant showed exceptional tolerance to the growth-inhibiting effects of ethanol compared to those of both the reconstituted OLE1 strain and the other transformant strains expressing insect desaturases, which produce predominantly 16-carbon UFAs (Fig. 1B). This result is consistent with previous studies in S. cerevisiae (4, 8) and Schizosaccharomyces pombe (19), which implicate elevated Δ9Z-C18:1 in ethanol stress tolerance in yeasts. A possible mechanism of this effect is suggested by a study of mitochondrial DNA loss in wild-type S. cerevisiae cells during ethanol stress (13), suggesting that the disruption of the mitochondrial membrane was the primary cause of ethanol-induced respiratory deficiency rather than direct damage to DNA. A primary direct effect of ethanol on mitochondrial membranes is supported by a study of Chi and Arneborg (8), which showed that an ethanol-tolerant strain of S. cerevisiae had elevated levels of Δ9Z-C18:1 and a reduced frequency of ethanol-induced mutation to respiratory deficiency compared to an ethanol-sensitive strain. The authors suggested that increased proportions of Δ9Z-C18:1 counteract the membrane-fluidizing effects of ethanol and confer ethanol tolerance by maintaining functional mitochondria during ethanol stress. The conclusions drawn from these studies of ethanol tolerance in yeast cells are consistent with the essential role of UFAs in normal movement and inheritance of mitochondria in S. cerevisiae, demonstrated in an earlier genetic study by Stewart and Yaffe (29) of temperature-sensitive mitochondrial distribution and morphology (mdm) mutants, in which yeast cells with the mdm2 mutation (an allele of the OLE1 desaturase gene) exhibit severe temperature-dependent mitochondrial membrane defects, resulting in respiratory deficiency and growth inhibition.
We also demonstrate an ethanol dose-dependent compensatory change in the fatty acid composition of TniNPVE cells, with the proportion of Δ9Z-C18:1 increasing significantly, accompanied by decreases in the proportions of both C16:0 and Δ9Z-C16:1, in response to increasing ethanol concentration (Fig. 2). These changes are analogous to those found in the cell membranes of rat brain after chronic ethanol exposure, in which an increase in Δ9Z-C18:1 is accompanied by decreases in poly-UFAs as well as C16:0 (10). The postulated primary effect of Δ9Z-C18:1 on the fluid properties of lipid membranes exposed to ethanol is the opposite of the mechanistically related homeoviscous adaptation response to cold (11), in which cells alter their fatty acid composition to achieve a compensatory increase in membrane fluidity. Although no experimental evidence bears directly on how TniNPVE cells alter the proportions of fatty acids in response to ethanol, we speculate that physical changes in the endoplasmic reticulum could cause a conformational change in the desaturase that accentuates its substrate bias (i.e., towards C18:0); alternatively, the response could result from less direct mechanisms, such as ethanol-induced changes in the metabolic flux of saturated fatty acid precursors.
The results of experiments in which we supplemented desaturase-deficient L8-14C (ole1) cells with UFAs and monitored growth in the presence of 5% ethanol are complementary to our experiments with the transformant strains. Figure 3 clearly demonstrates that Δ9Z-C18:1 is implicated in conferring ethanol tolerance. Furthermore, Δ11Z-C18:1 was found to confer an intermediate level of ethanol tolerance compared to Δ9Z-C16:1. Although supplementation with Δ11Z-C16:1 was not done because this compound was not readily available to us at the time of the study, Fig. 1B shows that the HzeaLPAQ transformant, which produces Δ11Z-C16:1 as its sole UFA, and the TniLPAQ transformant, which produces Δ11Z-C16:1 and Δ11Z-C18:1 in about a 2:1 ratio, were unable to grow in the presence of 5% ethanol. Thus, neither Δ11Z-C16:1 nor Δ9Z-C16:1 confers ethanol tolerance in S. cerevisiae. Taken together, the results shown in Fig. 1B and 3 provide compelling evidence that elevated proportions of 18-carbon mono-UFAs, especially Δ9Z-C18:1, confer ethanol stress tolerance in S. cerevisiae.
Other microorganisms with different fatty acid compositions have adaptive responses to ethanol exposure that may be based on similar compensatory changes in the fluid properties of lipid membranes. For example, in Escherichia coli, ethanol stress results in an increase in Δ11Z-C18:1 and a decrease in Δ9Z-C16:1 (14). In the ethanol-tolerant microorganism Zymomonas mobilis, Δ11Z-C18:1 is the most abundant UFA, and there are only minor amounts of Δ9Z-C16:1 and C16:0 (7). These findings suggest that microorganisms that do not produce Δ9Z-C18:1 could be using Δ11Z-C18:1 as an alternative to Δ9Z-C18:1 for ethanol stress tolerance. They furthermore suggest that UFA composition could be used as a criterion in evaluating the potential ethanol tolerance of other microorganisms.
In conclusion, we report that UFA composition is a significant determinant of ethanol tolerance in S. cerevisiae and that Δ9Z-C18:1 is the most efficacious UFA in overcoming the toxic effects of ethanol in growing yeast cells. Our findings are consistent with the notion that ethanol tolerance in yeast results from the incorporation of Δ9Z-C18:1 in lipid membranes, effecting a compensatory decrease in membrane fluidity. Furthermore, the genetically based alterations of UFA composition reported here, which result from the functional replacement of the S. cerevisiae OLE1 desaturase with acyl-CoA desaturases from other sources, may be of practical value in increasing the environmental stress tolerance of commercially used yeasts.
Acknowledgments
We thank Patricia Marsella-Herrick for her technical assistance in this study.
This work was supported by grants from the U.S. Department of Agriculture (97-35302-4345 and 2001-35302-09926) and the Environmental Protection Agency/National Science Foundation (BES-9728367) to D.C.K.
REFERENCES
- 1.Alexandre, H., I. Rousseaux, and C. Charpentier. 1994. Ethanol adaptation mechanisms in Saccharomyces cerevisiae. Biotechnol. Appl. Biochem. 20:173-183. [PubMed] [Google Scholar]
- 2.Augustin, H. W., G. Kopperschlager, H. Steffen, and E. Hofmann. 1965. Hexokinase as limiting factor of anaerobic glucose consumption of Saccharomyces carlsbergensis NCYC74. Biochim. Biophys. Acta 110:437-439. [PubMed] [Google Scholar]
- 3.Baker, N., and F. Lynen. 1971. Factors involved in fatty acyl CoA desaturation by fungal microsomes. Eur. J. Biochem. 19:200-210. [DOI] [PubMed] [Google Scholar]
- 4.Beaven, M. J., C. Charpentier, and A. H. Rose. 1982. Production and tolerance of ethanol in relation to phospholipid fatty-acyl composition in Saccharomyces cerevisiae NCYC. J. Gen. Microbiol. 128:1447-1455. [Google Scholar]
- 5.Bloomfield, D. K., and K. Bloch. 1960. The formation of Δ-9 unsaturated fatty acids. J. Biol. Chem. 235:337-344. [PubMed] [Google Scholar]
- 6.Buser, H. R., H. Arn, P. Guerin, and S. Rauscher. 1983. Determination of double bond position in mono-unsaturated acetates by mass spectrometry of dimethyl disulfide adducts. Anal. Chem. 55:818-822. [Google Scholar]
- 7.Carey, V. C., and L. O. Ingram. 1983. Lipid composition of Zymomonas mobilis: effects of ethanol and glucose. J. Bacteriol. 154:1291-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chi, Z., and N. Arneborg. 1999. Relationship between lipid composition, frequency of ethanol-induced respiratory deficient mutants, and ethanol tolerance in Saccharomyces cerevisiae. J. Appl. Microbiol. 86:1047-1052. [DOI] [PubMed] [Google Scholar]
- 9.Ghareib, M., K. A. Youssef, and A. A. Khalil. 1988. Ethanol tolerance of Saccharomyces cerevisiae and its relationship to lipid content and composition. Folia Microbiol. 33:447-452. [DOI] [PubMed] [Google Scholar]
- 10.Gustavsson, L. 1990. Brain lipid changes after ethanol exposure. Upsala J. Med. Sci. Suppl. 48:245-266. [PubMed] [Google Scholar]
- 11.Hazel, J. R., and E. E. Williams. 1990. The role of alterations in membrane lipid composition in enabling physiological adaptation of organisms to their physical environment. Prog. Lipid Res. 29:167-227. [DOI] [PubMed] [Google Scholar]
- 12.Holloway, P. W. 1971. A requirement for three protein components in microsomal coenzyme A desaturation. Biochemistry 10:1556-1560. [DOI] [PubMed] [Google Scholar]
- 13.Ibeas, J. I., and J. Jimenez. 1997. Mitochodrial DNA loss caused by ethanol in Saccharomyces flor yeasts. Appl. Environ. Microbiol. 63:7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ingram, L. O. 1976. Adaptation of membrane lipids to alcohols. J. Bacteriol. 125:670-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito, H., Y. Fukuda, K. Murata, and Y. Kimura. 1983. Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 153:163-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kajiwara, S., A. Shirai, T. Fujii, T. Toguri, K. Nakamura, and K. Ohtaguchi. 1996. Polyunsaturated fatty acid biosynthesis in Saccharomyces cerevisiae: expression of ethanol tolerance and the FAD2 gene from Arabidopsis thaliana. Appl. Environ. Microbiol. 62:4309-4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knipple, D. C., C.-L. Rosenfield, S. J. Miller, W. Liu, J. Tang, P. W. K. Ma, and W. L. Roelofs. 1998. Cloning and functional expression of a cDNA encoding a pheromone gland-specific acyl-CoA Δ11-desaturase of the cabbage looper moth, Trichoplusia ni. Proc. Natl. Acad. Sci. USA 95:15287-15292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knipple, D. C., C.-L. Rosenfield, K. M. You, and S. E. Jeong. 2002. Evolution of the integral membrane desaturase gene family in moths and flies. Genetics 162:1737-1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koukou, A. I., D. Tsoukatos, and C. Drainas. 1990. Effect of ethanol on the phospholipid and fatty acid content of Schizosaccharomyces pombe membrane. J. Gen. Microbiol. 136:1271-1277. [DOI] [PubMed] [Google Scholar]
- 20.Liu, W., P. W. K. Ma, P. Marsella-Herrick, C.-L. Rosenfield, D. C. Knipple, and W. L. Roelofs. 1999. Cloning and functional expression of a cDNA encoding a metabolic acyl-CoA Δ9-desaturase of the cabbage looper moth, Trichoplusia ni. Insect Biochem. Mol. Biol. 29:435-443. [DOI] [PubMed] [Google Scholar]
- 21.Mishra, P., and R. Prasad. 1989. Relationship between ethanol tolerance and fatty acyl composition of Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 30:294-298. [Google Scholar]
- 22.Mitchell, A. G., and C. E. Martin. 1995. A novel cytochrome b5-like domain is linked to the carboxyl terminus of the Saccharomyces cerevisiae Δ-9 fatty acid desaturase. J. Biol. Chem. 270:29766-29772. [DOI] [PubMed] [Google Scholar]
- 23.Nagodawithana, T. W., and K. H. Steinkraus. 1976. Influence of the rate of ethanol production and accumulation on the viability of Saccharomyces cerevisiae in rapid fermentation. Appl. Environ. Microbiol. 31:158-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rogers, M. J., and P. Strittmatter. 1973. Lipid-protein interactions in the reconstitution of the microsomal reduced nicotinamide adenine dinucleotide-cytochrome b5 reductase system. J. Biol. Chem. 248:800-806. [PubMed] [Google Scholar]
- 25.Rosenfield, C.-L., K. M. You, P. Marsella-Herrick, W. L. Roelofs, and D. C. Knipple. 2001. Structural and functional conservation and divergence among acyl-CoA desaturases of two noctuid species, the corn earworm moth, Helicoverpa zea, the cabbage looper, Trichoplusia ni. Insect Biochem. Mol. Biol. 31:949-964. [DOI] [PubMed] [Google Scholar]
- 26.Sajbidor, J., Z. Ciesarova, and D. Smogrovicova. 1995. Influence of ethanol on the lipid content and fatty acid composition of Saccharomyces cerevisiae. Folia Microbiol. 40:508-510. [DOI] [PubMed] [Google Scholar]
- 27.Shimakata, T., K. Mihara, and R. Sato. 1972. Reconstitution of hepatic microsomal stearoyl-CoA desaturase system from solubilized components. J. Biochem. 72:1163-1174. [DOI] [PubMed] [Google Scholar]
- 28.Spatz, L., and P. Strittmatter. 1971. A form of reduced nicotinamide adenine dinucleotide-cytochrome b5 reductase containing both the catalytic site and an additional hydrophobic membrane-binding segment. J. Biol. Chem. 248:793-799. [PubMed] [Google Scholar]
- 29.Stewart, L. C., and M. P. Yaffe. 1991. A role for unsaturated fatty acids in mitochondrial movement and inheritance. J. Cell Biol. 115:1249-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strittmatter, P., L. Spatz, D. Corcoran, M. J. Rogers, B. Setlow, and R. Redline. 1974. Purification and properties of rat liver microsomal stearyl coenzyme A desaturase. Proc. Natl. Acad. Sci. USA 71:4565-4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stukey, J. E., V. M. McDonough, and C. E. Martin. 1990. The OLE1 gene of Saccharomyces cerevisiae encodes the Δ9 fatty acid desaturase and can be functionally replaced by the rat stearoyl-CoA desaturase gene. J. Biol. Chem. 265:20144-20149. [PubMed] [Google Scholar]
- 32.Swan, T. M., and K. Watson. 1999. Stress tolerance in a yeast lipid mutant: membrane lipids influence tolerance to heat and ethanol independently of heat shock proteins and trehalose. Can. J. Microbiol. 45:472-479. [DOI] [PubMed] [Google Scholar]
- 33.Weete, J. D. 1974. Fungal lipid biochemistry: distribution and metabolism. Plenum Publishing Corp., New York, N.Y.