Abstract
We expressed the Arabidopsis thaliana gene for phytochelatin synthase (PCSAt) in Mesorhizobium huakuii subsp. rengei B3, a microsymbiont of Astragalus sinicus, a legume used as manure. The PCSAt gene was expressed under the control of the nifH promoter, which regulates the nodule-specific expression of the nifH gene. The expression of the PCSAt gene was demonstrated in free-living cells under low-oxygen conditions. Phytochelatin synthase (PCS) was expressed and catalyzed the synthesis of phytochelatins [(γ-Glu-Cys)n-Gly; PCs] in strain B3. A range of PCs, with values of n from 2 to 7, was synthesized by cells that expressed the PCSAt gene, whereas no PCs were found in control cells that harbored the empty plasmid. The presence of CdCl2 activated PCS and induced the synthesis of substantial amounts of PCs. Cells that contained PCs accumulated 36 nmol of Cd2+/mg (dry weight) of cells. The expression of the PCSAt gene in M. huakuii subsp. rengei B3 increased the ability of cells to bind Cd2+ approximately 9- to 19-fold. The PCS protein was detected by immunostaining bacteroids of mature nodules of A. sinicus containing the PCSAt gene. When recombinant M. huakuii subsp. rengei B3 established the symbiotic relationship with A. sinicus, the symbionts increased Cd2+ accumulation in nodules 1.5-fold.
Widespread pollution by heavy metals that are generated by various industries has serious adverse effects on human health and the environment (24). Decontamination of the soil and water around industrial plants presents major challenges for a long time. Genetic engineering suggests the possible use of specially designed microbial biosorbents with suitable selectivity and affinity for heavy metals. Overexpression of metal-binding peptides, such as metallothioneins (MTs), by bacterial cells results in enhanced accumulation of Cd2+ and offers a promising strategy for the development of microbe-based biosorbents for the remediation of metal-contaminated soil (16, 21, 28, 40).
Phytochelatins (PCs), which are naturally occurring metal-binding peptides, are an attractive alternative to MTs since they offer the potential for enhanced affinity and selectivity for heavy metals. PCs are short peptides composed of only three amino acids, namely, Glu, Cys, and Gly, with Glu and Cys residues linked through a γ-carboxylamide bond. The structure of such peptides can be represented by (γ-Glu-Cys)n-Gly, where n ranges from 2 to 11. PCs have been identified in a wide variety of plant species and in some microorganisms (4, 27, 41). Compared to MTs, PCs offer many advantages that are due to their unique structural characteristics, in particular, the continuously repeating γ-Glu-Cys units. For example, PCs have a higher metal-binding capacity (on a per-cysteine basis) than do MTs (18). In addition, PCs can incorporate high levels of inorganic sulfide, which results in very significant increases in the Cd2+-binding capacity of these peptides (19). Thus, PCs are attractive as metal-binding peptides for the development of microbe-based biosorbents for the remediation of metal-polluted soils.
Rhizobia are gram-negative bacteria that can establish a symbiotic relationship with leguminous plants. They grow slowly for long periods in soil, but if they infect a compatible legume, they can grow rapidly. Successful infection by a single bacterium can lead to the formation, on the root of a legume, of a nitrogen-fixing nodule that contains more than 108 bacterial progeny (6). The rhizobium-legume symbiosis is initiated when flavonoids and related plant compounds induce the bacterium to produce molecular signals that stimulate nodule organogenesis (8). Bacteria enter the developing nodule via infection threads (14) and are eventually taken up by the plant host cells by an endocytosis-like process. Each rhizobium undergoes differentiation into its endosymbiotic form, which is known as a bacteroid. Bacteroids can fix atmospheric nitrogen as ammonia, which is exported to and assimilated by the host plant (23).
Mesorhizobium huakuii subsp. rengei strain B3 (22, 25) is a bacterium that establishes a symbiotic relationship with Astragalus sinicus, a legume that has been used as green manure in rice fields in Japan and southern China, by eliciting the formation of nitrogen-fixing root nodules (3). A. sinicus is widely used as a natural fertilizer in rice fields during fallow periods. It would be of considerable interest if we could use this leguminous plant to increase fertilizer nitrogen and to remove heavy metals from soil at the same time.
The presence of 106 to 108 bacterial progeny of M. huakuii subsp. rengei B3 in each nodule on the roots of A. sinicus is advantageous for the expression of foreign genes that help to sequester heavy metals in contaminated soil. Once symbiosis is established, the heavy metals should accumulate in such nodules. In this report, we describe the introduction of the Arabidopsis thaliana gene for phytochelatin synthase (PCS; PCSAt) into M. huakuii subsp. rengei strain B3. The gene was expressed under the control of a bacteroid-specific promoter, namely, the promoter of the nifH gene (26, 30). The gene for PCS was expressed in free-living cells under microaerobic conditions when the promoter was activated by NifA (the regulatory protein for nif and fix promoters) (37). We investigated the ability of the recombinant cells to produce PCs and to accumulate Cd2+. Such cells might be useful for the development of a novel plant-bacterium remediation system for the removal of heavy metals from rice fields when genetically engineered M. huakuii subsp. rengei strain B3 establishes a symbiotic relationship with A. sinicus.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains used were Escherichia coli DH5αF′ [F′ Δ(lacZYA-argF) U169 deoR endA1 hsdR17(rR− mR+) supE44 thi-1 recA1 gyr96 relA1 (φ80dlacZ ΔM15)] and M. huakuii subsp. rengei strain B3, which was isolated from nodules of A. sinicus cv. Japan that had been grown in a rice field in Hiroshima, Japan (22, 25). The cloning vector pBluescriptKS was purchased from Stratagene (La Jolla, Calif.). The expression vectors pBBR1MCS-2 (provided by K. M. Peterson, Louisiana State University Medical Center, Shreveport) and pMP220 (35) were used for the expression of the PCSAt gene. The E. coli cells were grown at 37°C in Luria broth (31) or on agar (1.5%, wt/vol) plates supplemented with appropriate antibiotics. Strain B3 was grown in tryptone-yeast extract (TY) medium (2) and incubated at 30°C with shaking.
Gene constructs.
The 1.45-kb PCSAt gene (12) was amplified by PCR with primer PCF (5′-ACCATGGCTATGGCGAGTTTATATC-3′) and primer PCT(5′-CGGGATCCCTAATAGGCAGGAGCAGCGAGATCATCC-3′). Thetemplate was PCSAt in pUC18, and DNA was synthesized by using KOD polymerase (Toyobo, Osaka, Japan) and cloned into pBluescriptKS that had been digested with EcoRV, yielding the plasmid pBluescriptKSPCS. The sequence containing the promoter region of nifH, including the initiation codon and Shine-Dalgarno ribosome-binding site, was amplified with primers nifH1 (5′-CGGAATTCAGTCGCTATGCC-3′) and nifH2 (5′-ACCTGCCATGGTAGATTTCC-3′). The 240-bp amplified fragment, flanked by EcoRI and NcoI sites, was digested with EcoRI and NcoI and ligated into pBluescriptKSPCS that had been digested with EcoRI and NcoI. The resulting plasmid, pBluescriptKSnifHPCS, contained the PCSAt gene fused in frame downstream of the nifH promoter. A 1.7-kb KpnI-EcoRI fragment of pBluescriptKSPCS was ligated into KpnI- and EcoRI-digested pMP220. The resulting plasmid was designated pMPnifHPCS. For introduction of the nifH-PCS fusion gene into pBBR1MCS-2, a 1.7-kb EcoRI-SalI fragment of pBluescriptKSnifHPCS was ligated into EcoRI- and SalI-digested pBBR1MCS-2 to yield plasmid pBBRnifHPCS.
Heterologous expression of His-tagged PCSAt.
To detect the expression of PCS in bacteroids by immunostaining, the PCSAt was fused with a His tag at the C terminus. The resulting plasmid was designated pMPnifHPCSHis.
Introduction by electroporation of pMPnifHPCS and pBBRnifHPCS into strain B3.
The PCSAt gene in the expression vector pMPnifHPCS or pBBRnifHPCS was introduced into strain B3 by electroporation (13). Transformants were selected on TY medium plates (2) supplemented with tetracycline (20 μg/ml) and kanamycin (100 μg/ml) for pMPnifHPCS and pBBRnifHPCS, respectively. Several clones were isolated, and their sequences were checked by PCR and restriction digestion.
Analysis of gene expression by reverse transcription and PCR.
Total RNA was isolated from 3 ml of a microaerobic culture of B3(pMPnifHPCS) (36) with RNAwiz (Ambion, Austin, Tex.) according to the manufacturer's instructions. Then cDNA was synthesized from 0.25 μg of total RNA with antisense primer 5′-CAGGACCTTTGATGCATTTC-3′ and a SuperScriptII RT kit (Invitrogen, Carlsbad, Calif.), according to the instructions from Invitrogen. Independent PCRs with PCSAt-specific primers (5′-AGACAGTCTGACTTATGCTG-3′ and 5′-CAGGACCTTTGATGCATTTC-3′) were performed. Each reaction mixture contained 1.5 mM Mg2+, a 0.2 mM concentration of each deoxynucleoside triphosphate (dNTP), a 1 μM concentration of each primer, and 0.25 U of ExTaq polymerase (Takara, Osaka, Japan)
Identification of PCs and intermediates in their biosynthesis.
Five hundred milliliters of microaerobically conditioned culture cells in the presence of 30 μM CdCl2 (final concentration) was harvested. The samples were analyzed for reduced glutathione (GSH) and PCs by high-performance liquid chromatography (HPLC) and subsequent reaction with Ellman's reagent in combination with an ion pair method as described elsewhere (7, 11, 15).
Microaerobic culture and quantitation of Cd2+.
CdCl2 was added to the microaerobic culture cells to give final concentrations of 1 to 50 μM, and the cells were grown at 30°C with gentle shaking for 40 h. To determine the concentration of Cd2+, bacterial cells were pelleted, washed twice with 0.85% NaCl in 5 mM HEPES (pH 7.1), dried at 65°C for 5 h, and treated overnight with 70% nitric acid (29). The concentration of Cd2+ was measured directly in the soluble fraction with an atomic absorption spectrophotometer (model SAS7500A; Seiko, Tokyo, Japan).
Nodule formation and measurement of Cd2+ concentration.
Nodule formation on A. sinicus cv. Japan (Takayama Seed Co., Kyoto, Japan) infected with strain B3 was determined as described previously (22). Nodules from 6-week-old plants cultivated hydroponically in nitrogen-free modified medium (22) supplemented with 50 μM CdCl2 were harvested, washed, dried, and solubilized with 70% nitric acid (29). Cd2+concentrations were measured directly in the soluble fraction by determining atomic absorption with a spectrophotometer (29, 36).
Immunostaining of paraffin sections.
The paraffin sections of the nodules were prepared as described previously (36). Deparaffinized and rehydrated sections were incubated with a 1:200 dilution of anti-His antibody (Amersham Pharmacia Biotech, Buckinghamshire, England) in washing buffer (Tris-buffered saline containing 0.1% [wt/vol] bovine serum albumin and 0.1% [vol/vol] Tween 20). An alkaline phosphate-conjugated goat anti-mouse immunoglobulin G was used as a secondary antibody (Promega, Tokyo, Japan). The signal was detected by using nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate solution for 30 min to 10 h in the dark.
RESULTS AND DISCUSSION
Expression of PCSAt under control of the nifH promoter.
The plasmid with the PCSAt gene fused in frame downstream of the nifH promoter was subcloned into the broad-host-range expression vectors pBBR1MCS-2 and pMP220. The resulting plasmids were designated pBBRnifHPCS and pMPnifHPCS (Fig. 1). For the expression of genes under control of the nifH promoter in free-living cells, a low level of oxygen is necessary, since the fixation of nitrogen in nodules occurs under anaerobic conditions. A low concentration of oxygen induces expression of the nifA gene, and in a cascade, NifA activates the transcription of other nif genes (5). Thus, we analyzed the expression of PCSAt under the microaerobic conditions by reverse transcription and PCR. A single 120-bp fragment, corresponding to the predicted cDNA product, was observed when we used primers specific for PCSAt. The PCSAt-specific product was obtained from B3(pMPnifHPCS) cells in the presence of reverse transcriptase. There was no difference in PCSAt-specific product between B3(pMPnifHPCS) cells that had been exposed to CdCl2 and those that had not been exposed. The negative control (without reverse transcriptase) produced no PCSAt-specific product. No PCSAt-specific product was observed in the case of strain B3 that harbored the pMP220 empty plasmid (data not shown).
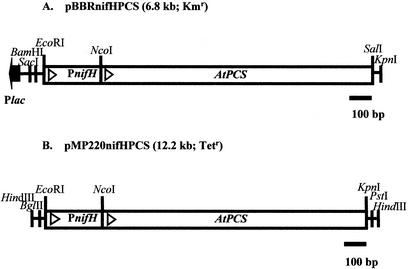
FIG. 1.
Construction of the PCSAt expression vectors. (A) Structure of the pBBRnifHPCS plasmid that contained the PCSAt gene fused, in frame, to the nifH promoter. A 1.7-kb EcoRI-SalI fragment containing PnifH-PCSAt was inserted into pBBR1MCS-2. (B) Structure of the pMPnifHPCS plasmid that contained the PCSAt gene fused, in frame, to the nifH promoter. A 1.7-kb EcoRI-KpnI fragment containing PnifH-PCSAt was inserted into pMP220. The orientations of nifH and the PCSAt gene are indicated by open triangles, and the orientation of the lac promoter (plac) is indicated by an arrow.
Identification of PCs and intermediates in their biosynthesis in free-living cells.
We performed an analysis by HPLC to examine the synthesis of PCs in strain B3 that harbored the PCSAt gene. PCs were detected in strain B3 that contained the PCSAt gene and that had been treated with 30 μM CdCl2 for 40 h (Fig. 2A). However, no PCs were detected in untreated cells (Fig. 2B) or in cells that harbored the pMP220 empty plasmid (data not shown). The production of PCs was dependent on the presence of a heavy metal, namely Cd2+, and our results indicated that the PCSAt gene was transcribed and translated under the control of the nifH promoter. Moreover, the enzymatic activity, as detected by the synthesis of PCs, was enhanced by CdCl2. This observation reflects the production of PCs in Rauvolfia serpentina, Silene cucubalus, and A. thaliana, which contain no or almost no PCs when grown without metal ions but produce PCs upon addition of a variety of metal ions (10, 12, 17).
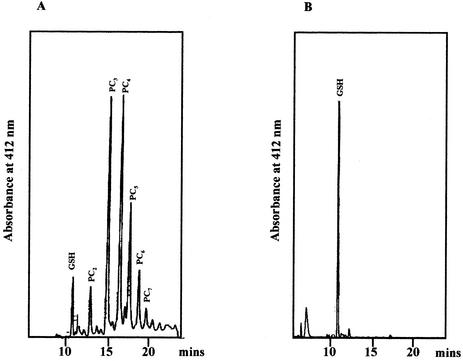
FIG. 2.
Accumulation of PCs by M. huakuii subsp. rengei B3 cells that expressed PCSAt. After 40 h of growth under microaerobic conditions in the presence (A) or absence (B) of 30 μM CdCl2, extracts of cells that harbored pMPnifHPCS (A and B) were analyzed by HPLC as described in the text. Peaks that represent GSH, PC2, PC3, PC4, PC5, PC6, and PC7 are identified.
The profiles of PCs from Cd2+-treated B3(pMPnifHPCS) cells after HPLC revealed the presence of PC2 [(γ-Glu-Cys)2-Gly] through PC7 [(γ-Glu-Cys)7-Gly]. Levels of PC3 and PC4 were higher than those of other PCs. We also examined PCs by HPLC for the presence of sulfhydryl groups. At 30 μM CdCl2, we detected 5.44 ± 2.08 nmol/mg (dry weight) of cells (mean ± standard deviation of results from three independent experiments [n = 3]) in terms of SH equivalents. The GSH content of CdCl2-treated cells decreased to 0.27 ± 0.09 nmol/mg (dry weight) of cells (n = 3), whereas untreated cells contained a GSH level of 1.0 ± 0.49 nmol/mg (dry weight) of cells (n = 3). The GSH content was high in cells of M. huakuii subsp. rengei B3(pMPnifHPCS) that had been grown in the absence of CdCl2, but the level of GSH decreased in cells grown in the presence of CdCl2. Thus, it is likely that GSH is a substrate for the production of PCs and that the expression of the PCSAt gene under control of the nifH promoter in bacteroids, which contain large amounts of GSH (20), might allow production of large amounts of PCs. The amount of PCs, in terms of SH equivalence, was similar to that observed in extracts of seedlings of A. thaliana exposed to 100 μM CdCl2 (7 nmol/mg [dry weight] of cells) (33). This similarity indicates that the level of expression and the folding or stability of the protein were appropriate in strain B3.
Accumulation of Cd2+ in free-living cells.
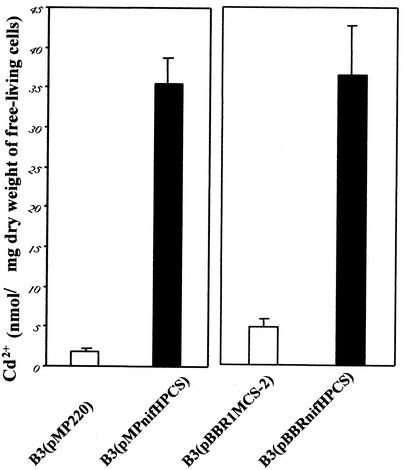
Cultures of strain B3 containing pBBRnifHPCS and pMPnifHPCS were supplemented with 30 μM CdCl2 under microaerobic conditions. Strain B3 containing PCs accumulated 9- to 19-fold more Cd2+ than did cells without the PCSAt gene (cells transfected with pBBR1MCS-2 or the pMP220 empty vector). The levels of Cd2+ were 35.52 ± 3.16 and 36.41 ± 4.03 nmol/mg (dry weight) of cells (n = 3) for B3(pMPnifHPCS) and B3(pBBRnifHPCS) cells, respectively (Fig. 3). For cells of strain B3 without PCs, namely, B3(pMP220) and B3(pBBR1MCS-2), we found only 1.84 ± 0.45 and 4.09 ± 1.05 nmol of Cd2+/mg (dry weight) of cells, respectively. The accumulation of Cd2+ in strain B3 that expressed PCSAt was higher than that in genetically engineered E. coli cells that expressed a eukaryotic MT as a fusion protein with a membrane or membrane-associated protein, namely, LamB or peptidoglycan-associated lipoprotein (34, 38, 39). E. coli cells that expressed the PCS gene from Arabidopsis or its analogs had strong PC synthase activity and accumulated heavy metals in cells (1, 12). The ability of M. huakuii subsp. rengei B3 that expressed PCSAt to accumulate Cd2+ was 12-fold higher than that of strain B3 that expressed MTL4, the gene for tetrameric human metallothionein (MTL4) (36). The increased accumulation of Cd2+ by cells that contained PCs might have been due to the fact that the ratio of Cd2+ to sulfhydryl groups for PCs is higher than that for vertebrate MT, namely, 1:2 and 1:3, respectively (11). Moreover, long-chain PCs might affect the accumulation of Cd2+. It has been reported that the longer PCs have a higher relative complexing affinity for heavy metals than do shorter ones (17, 32). Therefore, the features of the synthesis of PCs in B3(pMPnifHPCS) cells, which contained longer PCs, such as PC4 through PC7, should be advantageous for the removal of Cd2+ from soil when B3(pMPnifHPCS) cells establish a symbiotic relationship with A. sinicus.
FIG. 3.
Accumulation of Cd2+ by M. huakuii subsp. rengei B3 cells that expressed PCSAt. The amount of Cd2+ that accumulated in cells was determined by atomic absorption spectrometry and is indicated as nanomoles of Cd2+ per milligram (dry weight) of cells The values shown are means (± standard deviations) of the results for three independent experiments in each case. (Left) Accumulation of Cd2+ by free-living strain B3 that harbored the empty vector, namely, B3(pMP220) cells, and strain B3 that harbored the PCSAt gene, namely, B3(pMPnifHPCS) cells; (right) accumulation of Cd2+ by free-living strain B3 that harbored the empty vector pBBR1-MCS2, namely, B3(pBBR1-MCS2) cells, and strain B3 that harbored the PCSAt gene, namely, B3(pBBRnifHPCS) cells. Bacteria were grown under microaerobic conditions in TY medium plus 30 μM CdCl2 for 40 h.
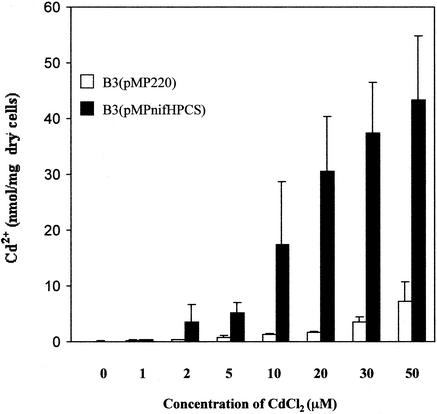
Increased concentrations of CdCl2 increased the accumulation of Cd2+ in strain B3 (Fig. 4). In strain B3(pMPnifHPCS), the level was 43.4 ± 11.43 nmol of Cd2+/mg (dry weight) of cells (n = 3) after treatment of the cells with 50 μM CdCl2. These observations indicated that higher concentrations of CdCl2 increased the activity of PCS and the synthesis of PCs, with subsequent increased accumulation of Cd2+ by the cells. It is possible that Cd2+ ions activated PCS and prolonged the synthesis of PCs until all Cd2+ ions had been chelated by PCs. Moreover, it has been reported that the presence of metal ions leads to the transpeptidation of the γ-Glu-Cys moieties of shorter PCs to longer PCs (9). Thus, prolonged exposure to CdCl2 results in production of longer PCs, which have a high relative complexing affinity for heavy metals (17, 32).
FIG. 4.
Effects of CdCl2 on the synthesis of PCs in M. huakuii subsp. rengei B3. Determinations were made by atomic absorption spectrometry of the amount of Cd2+ accumulated in strain B3 that harbored the empty vector, namely, B3(pMP220) cells, and in B3 cells that contained the PCSAt gene, namely, B3(pMPnifHPCS) cells. The amount of Cd2+ bound to bacterial cells is indicated in nanomoles per milligram (dry weight) of cells. The values shown are means (± standard deviations) of the results for three independent experiments in each case. Bacteria were grown under microaerobic conditions in the presence of various concentrations of CdCl2 (1 to 50 μM) as indicated for 40 h.
Expression of PCSAt in bacteroids.
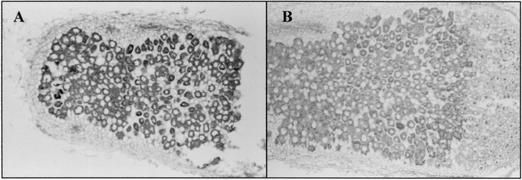
To assess whether the PCS was expressed in bacteroids within nodules, we performed immunostaining using the anti-His antibody to detect the presence of PCS-His tag in bacteroids. Bacteroids containing pMPnifHPCSHis showed a strong signal of immunostaining (Fig. 5). There was no signal of immunostaining in bacteroids containing pMP220. This result indicated that the PCSAt gene was expressed under the control of the nifH promoter within the bacteroids.
FIG. 5.
Immunostaining for detection of PCS protein in the mature nodules of A. sinicus containing B3(pMPnifHPCSHis) and B3(pMP220). The accumulation of PCS in bacteroids was detected by using anti-His antibody, followed by alkaline phosphatase-conjugated anti-mouse antibody. (A) The purple color that developed was detected in bacteroids containing PCSAt. (B) The purple color was not detected in bacteroids containing pMP220 empty vector.
The accumulation of Cd2+ in nodules has been investigated. The content of Cd2+ in the nodules containing bacteroids from strain B3(pMPnifHPCS) increased 1.5-fold compared with that of nodules containing bacteroids from strain B3(pMP220). In a previous work (36), the same symbionts expressed tetrameric MT protein to increase Cd2+ accumulation in nodules 1.7- to 2.0-fold. The limitation of Cd2+ accumulation by bacteroids is currently being investigated. However, a symbiotic relationship between genetically engineered M. huakuii subsp. rengei B3 and A. sinicus might help in the removal of Cd2+ from contaminated rice fields.
Acknowledgments
We thank M. Tansengco and N. Tsuji for valuable discussions.
This work was supported by a grant-in-aid for scientific research from the Ministry of Culture, Education, Science and Technology of Japan (Kaken Houga no. 12875155). R.S. is supported by a scholarship from MonbuKagakusho, Tokyo, Japan.
REFERENCES
- 1.Bae, W., W. Chen, A. Mulchandani, and R. K. Mehra. 2000. Enhanced bioaccumulation of heavy metals by bacterial cells displaying synthetic phytochelatins. Biotechnol. Bioeng. 70:518-523. [DOI] [PubMed] [Google Scholar]
- 2.Beringer, J. E. 1974. R factor transfer in Rhizobium leguminosarum. J. Gen. Microbiol. 24:967-980. [DOI] [PubMed] [Google Scholar]
- 3.Chen, W. X., G. S. Li, Y. L. Qi, E. T. Wang, H. L. Yuan, and J. L. Li. 1991. Rhizobium huakuii sp. nov. isolated from the root nodules of Astragalus sinicus. Int. J. Syst. Bacteriol. 41:275-280. [Google Scholar]
- 4.Cobbett, C. S. 2000. Phytochelatins and their roles in heavy metal detoxification. Plant Physiol. 123:825-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ditta, G., E. Virts, A. Palomares, and C.-H. Kim. 1987. The nifA gene of Rhizobium meliloti is oxygen regulated. J. Bacteriol. 169:3217-3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Downie, A. 1997. Fixing a symbiotic circle. Nature 387:352-353. [DOI] [PubMed] [Google Scholar]
- 7.Ellman, G. L. 1959. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 82:70-77. [DOI] [PubMed] [Google Scholar]
- 8.Fisher, F. T., and S. R. Long. 1992. Rhizobium-plant signal exchange. Nature 357:655-660. [DOI] [PubMed] [Google Scholar]
- 9.Grill, E., S. Loeffler, E. L. Winnacker, and M. H. Zenk. 1989. Phytochelatins, the heavy-metal-binding peptides of plants, are synthesized from glutathione by a specific γ-glutamylcysteine dipeptidyl transpeptidase (phytochelatin synthase). Proc. Natl. Acad. Sci. USA 86:6838-6842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grill, E., E. L. Winnacker, and M. H. Zenk. 1987. Phytochelatins, a class of heavy-metal-binding peptides from plants, are functionally analogous to metallothioneins. Proc. Natl. Acad. Sci. USA 84:439-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grill, E., E. L. Winnacker, and M. H. Zenk. 1985. Phytochelatins: the principal heavy-metal complexing peptides of higher plants. Science 230:674-676. [DOI] [PubMed] [Google Scholar]
- 12.Ha, S. B., A. P. Smith, R. Howden, W. M. Dietrich, S. Bugg, M. J. O'Connell, P. B. Goldsbrough, and C. S. Cobbett. 1999. Phytochelatin synthase genes from Arabidopsis and the yeast Schizosaccharomyces pombe. Plant Cell 11:1153-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayashi, M., Y. Maeda, Y. Hashimoto, and Y. Murooka. 2000. Efficient transformation of Mesorhizobium huakuii subsp. rengei and Rhizobium species. J. Biosci. Bioeng. 89:550-553. [DOI] [PubMed] [Google Scholar]
- 14.Higashi, S., K. Koshiyama, and M. Abe. 1986. Electron microscopic observation of infection threads in driselase-treated nodules of Astragalus sinicus. Can. J. Microbiol. 32:947-952. [Google Scholar]
- 15.Hirata, K., Y. Tsujimoto, T. Namba, T. Ohta, N. Hirayanagi, H. Miyasaka, M. H. Zenk, and K. Miyamoto. 2001. Strong induction of phytochelatin synthesis by zinc in marine green alga, Dunaliella tertiolecta. J. Biosci. Bioeng. 92:24-29. [DOI] [PubMed] [Google Scholar]
- 16.Kille, P., D. R. Winge, J. L. Harwood, and J. Kay. 1991. A plant metallothionein produced in Escherichia coli. FEBS Lett. 295:171-175. [DOI] [PubMed] [Google Scholar]
- 17.Loeffler, S., A. Hochberger, E. Grill, E. L. Winnacker, and M. H. Zenk. 1989. Termination of the phytochelatin synthase reaction through sequestration of heavy metals by the reaction product. FEBS Lett. 258:42-46. [Google Scholar]
- 18.Mehra, R. K., and P. Mulchandani. 1995. Glutathione-mediated transfer of Cu(I) into phytochelatins. Biochem. J. 307:697-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehra, R. K., P. Mulchandani, and T. C. Hunter. 1994. Role of CdS quantum crystallites in cadmium resistance in Candida glabrata. Biochem. Biophys. Res. Commun. 200:1193-1200. [DOI] [PubMed] [Google Scholar]
- 20.Moran, J. F., I. Iturbe-Ormaetxe, M. A. Matamoros, M. C. Rubio, M. R. Clemente, N. J. Brewin, and M. Becana. 2000. Glutathione and homoglutathione synthetases of legume nodules. Cloning, expression, and subcellular localization. Plant Physiol. 124:1381-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murooka, Y., and T. Nagaoka. 1987. Expression of cloned monkey metallothionein in Escherichia coli. Appl. Environ. Microbiol. 53:204-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murooka, Y., Y. Xu, H. Sanda, M. Araki, T. Morinaga, and A. Yokota. 1993. Formation of root nodules by Rhizobium huakuii biovar renge bv. nov. on Astragalus sinicus cv. Japan. J. Ferment. Bioeng. 76:38-44. [Google Scholar]
- 23.Mylona, P., K. Powlowski, and T. Bisseling. 1995. Symbiotic nitrogen fixation. Plant Cell 7:869-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nriagu, J. O., and J. M. Pacyna. 1988. Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature 333:134-139. [DOI] [PubMed] [Google Scholar]
- 25.Nuswantara, S., M. Fujie, T. Yamada, W. Malek, M. Inaba, Y. Kaneko, and Y. Murooka. 1999. Phylogenic position of Mesorhizobium huakuii subsp. rengei, a symbiont of Astragalus sinicus cv. Japan. J. Biosci. Bioeng. 87:49-55. [DOI] [PubMed] [Google Scholar]
- 26.Perret, X., C. Freiberg, A. Rosenthal, W. J. Broughton, and R. Fellay. 1999. High-resolution transcriptional analysis of the symbiotic plasmid of Rhizobium sp. NGR234. Mol. Microbiol. 32:415-425. [DOI] [PubMed] [Google Scholar]
- 27.Rauser, W. E. 1995. Phytochelatins and related peptides. Structure, biosynthesis, and function. Plant Physiol. 109:1141-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romeyer, F. M., F. A. Jacobs, and R. Brousseau. 1990. Expression of a Neurospora crassa metallothionein and its variants in Escherichia coli. Appl. Environ. Microbiol. 56:2748-2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romeyer, F. M., F. A. Jacobs, L. Masson, Z. Hana, and R. Brousseau. 1988. Bioaccumulation of heavy metals in Escherichia coli expressing an inducible synthetic human metallothionein gene. J. Biotechnol. 8:202-207. [Google Scholar]
- 30.Ruvkun, G. B., V. Sundaresan, and F. M. Ausubel. 1982. Directed transposon Tn5 mutagenesis and complementation analysis of Rhizobium meliloti symbiotic nitrogen fixation genes. Cell 29:551-559. [DOI] [PubMed] [Google Scholar]
- 31.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 32.Satofuka, H., S. Amano, H. Atomi, M. Takagi, K. Hirata, K. Miyamoto, and T. Imanaka. 1999. Rapid method for detection and detoxification of heavy metal ions in water environments using phytochelatins. J. Ferment. Bioeng. 88:287-292. [DOI] [PubMed] [Google Scholar]
- 33.Schmoger, M. E. V., M. Oven, and E. Grill. 2000. Detoxification of arsenic by phytochelatins in plants. Plant Physiol. 122:793-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sousa, C., P. Kotrba, T. Ruml, A. Cebolla, and V. De Lorenzo. 1998. Metalloadsorption by Escherichia coli cells displaying yeast and mammalian metallothioneins anchored to the outer membrane protein LamB. J. Bacteriol. 180:2280-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spaink, H. P., R. J. H. Okker, C. A. Wijffelman, E. Pees, and B. J. J. Lugtenberg. 1987. Promoters in the nodulation region of the Rhizobium leguminosarum Sym plasmid pRL1Jl. Plant Mol. Biol. 9:27-39. [DOI] [PubMed] [Google Scholar]
- 36.Sriprang, R., M. Hayashi, M. Yamashita, H. Ono, K. Saeki, and Y. Murooka. 2002. A novel bioremediation system for heavy metals using the symbiosis between leguminous plant and genetically engineered rhizobia. J. Biotechnol. 99:279-293. [DOI] [PubMed] [Google Scholar]
- 37.Valderrama, B., A. Dávalos, L. Girard, E. Morett, and J. Mora. 1996. Regulatory proteins and cis-acting elements involved in the transcriptional control of Rhizobium etli reiterated nifH genes. J. Bacteriol. 178:3119-3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valls, M., R. Gonzalez-Duarte, S. Atrian, and V. De Lorenzo. 1998. Bioaccumulation of heavy metals with protein fusions of metallothionein to bacterial OMPs. Biochimie 80:855-861. [DOI] [PubMed] [Google Scholar]
- 39.Valls, M., S. Atrian, V. de Lorenzo, and L. A. Fernandez. 2000. Engineering a mouse metallothionein on the cell surface of Ralstonia eutropha CH34 for immobilization of heavy metals in soil. Nat. Biotechnol. 18:661-665. [DOI] [PubMed] [Google Scholar]
- 40.Yoshida, N., T. Kato, T. Yoshida, K. Ogawa, M. Yamashita, and Y. Murooka. 2002. Bacterium-based heavy metal biosorbents: enhanced uptake of cadmium by Escherichia coli expressing a metallothionein fused to beta-galactosidase. BioTechniques 32:551-556. [DOI] [PubMed] [Google Scholar]
- 41.Zenk, M. H. 1996. Heavy metal detoxification in higher plants: a review. Gene 179:21-30. [DOI] [PubMed] [Google Scholar]