Abstract
Effects of an alkylating anticancer drug, cyclophosphamide (Cp), on 23Na signal intensity (23Na SI) and water apparent diffusion coefficient (ADC) were examined in subcutaneously-implanted radiation-induced fibrosarcoma (RIF-1) tumors by in vivo 23Na and 1H magnetic resonance imaging (MRI). MRI experiments were performed on untreated control (n = 5) and Cp-treated (n = 6) C3H mice, once before Cp injection (300 mg/kg) then daily for 3 days after treatment. Tumor volumes were significantly lower in treated animals 2 and 3 days posttreatment. At the same time points, MRI experiments showed an increase in both 23Na SI and water ADC in treated tumors, whereas control tumors did not show any significant changes. The correlation between 23Na SI and water ADC changes was dramatically increased in the Cp-treated group, suggesting that the observed increases in 23Na SI and water ADC were caused by the same mechanism. Histologic sections showed decreased cell density in the regions of increased 23Na and water ADC SI. Destructive chemical analysis showed that Cp treatment increased the relative extracellular space and tumor [Na+]. We conclude that the changes in water ADC and 23Na SI were largely due to an increase in extracellular space. 23Na MRI and 1H water ADC measurements may provide valuable noninvasive techniques for monitoring chemotherapeutic responses.
Keywords: RIF-1, chemotherapy, MRI, sodium, diffusion
Introduction
An objective and accurate quantification of early treatment response in tumors is highly desirable. Measurements of parameters such as cellular energy status [1,2], tumor lactate [3] and choline [4] levels, glycolytic rates [3,5], tissue perfusion [6], and water diffusion coefficients [7,8] by magnetic resonance (MR) techniques have been suggested as noninvasive methods for monitoring response to therapy. Commonly used anticancer therapies damage and kill tumor cells, causing an increase in interstitial space due to cell shrinkage (apoptosis) or rupture (necrosis) [9]. It has been shown using isotope techniques that the alkylating anticancer drug, cyclophosphamide (Cp), significantly increases the fraction of extracellular water [10]. Damage to tissue microvasculature may also lead to vasogenic edema, increasing the volume of extracellular water. Intracellular water may also change because of alterations in membrane permeability and ion transport processes across the cell membrane. All of these changes may alter the mobility of water in damaged tissues. The diffusion of tissue water in vivo can be accurately and noninvasively estimated as an apparent diffusion coefficient (ADC) by using diffusion-weighed 1H nuclear magnetic resonance (NMR) [7,8,11,12]. Chenevert et al. [8] have shown that water ADC is correlated with, and highly sensitive to, changes in tumor cell density in histologic sections. Zhao et al. [7] showed a dose-dependent, reversible increase in water ADC in radiation-induced fibrosarcoma (RIF-1) tumors after Cp treatment, and the maximum water ADC increase was observed 4 days after treatment.
Monitoring and imaging tissue Na+ by MR techniques may also be useful for assessing response to therapy because of the biologic importance of sodium. Viable cells maintain a much lower intracellular Na+ concentration ([Na+]i) (10–30 mM) against a high extracellular Na+ concentration ([Na+]e) (∼150 mM). This transmembrane sodium gradient is maintained by the action of the Na+/K+ ATPase and is used to drive several vital cellular processes through the action of membrane-bound exchangers and cotransporters. For instance, intracellular pH (pHi) is regulated, to a large extent, by a Na+/H+ exchanger that transports excess H+ ions out of the cell by allowing Na+ ions into the cell. Changes in [Na+]i and pHi have also been suggested to be a part of the signaling mechanism that initiates cell division and proliferation [13–15].
Because of its high tissue concentration, 100% natural abundance, and short T1, 23Na is the second most sensitive MR nucleus in tissues, with only 1H being more sensitive. However, 23Na MR signal intensity is only 1/4000 that of 1H. This is largely because the water proton concentration in tissue is ∼100 M, whereas the [Na+] is only ∼50 mM. In addition, the relative NMR sensitivity of 23Na is 0.0925 compared to 1H [16]. Thus, the low signal-to-noise ratio (SNR) of 23Na, which leads to relatively long imaging times and/or poor spatial resolution, has restricted its use. Interest in 23Na magnetic resonance imaging (MRI) has been revitalized with the development of more effective data acquisition schemes and hardware improvements, which now allow quantitative imaging of tissue sodium in about 15 minutes [17–19].
23Na MRI signals from intracellular and extracellular compartments are isochronous because Na+ exists in only one chemical form in tissues. The tissue [Na+] is the volume-weighted mean of the [Na+] in the intracellular and extracellular spaces. Thus, an increase in extracellular space as a result of therapy-induced cell loss should increase tissue [Na+] ([Na+]tumor) and 23Na MRI signal intensity (23Na SI) because [Na+]e is ∼10 times greater than [Na+]i. The possible common mechanism for increases in water ADC and tissue 23Na SI suggests that these parameters may show similar changes during or after therapy. Therapy can also alter [Na+] because of changes in [Na+]i as a result of altered cellular physiology and metabolism before any cellular membrane destruction. Effective therapy could increase or decrease [Na+]i depending on the effects of therapy on cellular energy status and activity of membrane ion transport systems. Thus, 23Na MRI may provide additional information than that available from water ADC measurements alone.
In the present study, we used 23Na and 1H MRI to examine and correlate the changes in 23Na SI and water ADC in response to the chemotherapeutic drug Cp using the RIF-1 tumor model. We also investigated the mechanism of the observed changes in 23Na SI and water ADC through histology and destructive chemical analysis.
Materials and Methods
Tumor Model
All animal studies were approved by the Indiana University Institutional Animal Care and Use Committee. RIF-1 tumor cells were grown in monolayers using minimum essential medium (MEM; Mediatech, Herdon, VA) supplemented with 10% fetal bovine serum, 10 mM HEPES, and 1% penicillin under a 5% CO2 and 95% O2 atmosphere at 37°C. The tumor cells were passaged between in vitro and in vivo states according to the protocol of Twentyman et al. [20].
Male C3H/HeN mice (Harlan, Indianapolis, IN), approximately 6 weeks old and weighing 18 to 20 g, were inoculated in the right or left flanks with a subcutaneous (sc) injection of ∼2 x 106 cells in 0.10 to 0.15 ml volume of Hank's balanced salt solution. Animals were anesthetized with an intraperitoneal (ip) injection of 50 mg/kg ketamine, 5 mg/kg acepromazine, and 0.25 mg/kg atropine. The tumors were allowed to grow for 2 to 3 weeks to a volume of 1.3 to 1.6 cm3 before performing the MRI experiments. Tumor growth was monitored by caliper measurement for planning the MRI experiments. Tumor volume was calculated from three orthogonal diameters (x, y, and z) using the formula (π/6)xyz. Ten tumor-bearing mice were treated with a single dose of Cp (300 mg/kg, ip; Sigma-Aldrich, St. Louis, MO), six of which were used for 1H and 23Na MRI experiments and four were used for 23Na relaxation time measurements. Nine animals served as untreated controls, five of which were used for imaging experiments and the remaining animals were used for 23Na relaxation time measurements. The MR experiments were performed prior to treatment with Cp and on days 1, 2, and 3 after treatment. After the 3-day posttherapy MR experiments, tumors were excised for histology or destructive chemical analysis.
In Vivo MRI Experiments
All MR experiments were performed on a 9.4-T, 31-cm horizontal bore system (Varian, Palo Alto, CA) equipped with a 12-cm-diameter shielded gradient set capable of up to 40 G/cm in three directions. A loop-gap resonator (inner diameter = 30 mm, depth = 25 mm) dual-tuned to 400 MHz for 1H and to 106 MHz for 23Na was used. The animals were anesthetized with 0.75% isoflurane delivered in medical air at 1 l/min using a mouse nose mask connected to a gas anesthesia machine (Vetland, Louisville, KY). The tumor and surrounding area were shaved to facilitate tumor measurement and coil placement. The animal was positioned on top of a custom-designed plastic cradle with the dual-tuned loop-gap resonator attached to it. The tumor was positioned inside the resonator, and the animal was held in place with tape. A detachable cylindrical phantom (6.5 mm diameter and 23 mm length) consisting of 154 mM NaCl was also placed inside the resonator to serve as a 23Na MRI signal intensity and water ADC standard. Warm air was blown through the magnet bore to maintain the temperature in the space surrounding the animal at 26 to 28°C, which was monitored with a fiber optic probe (FISO Technologies, Inc., Quebec, Canada). A rectal fiber optic temperature probe was used to monitor the animal core body temperature, which remained at 36 ± 1.4°C during the MRI experiments (1–1.5 hours). The magnet was shimmed to less than 100 Hz line width at half height of the 1H water signal.
23Na MRI
Three-dimensional transaxial 23Na MR images of the tumor were obtained using a gradient-echo imaging sequence. The following imaging parameters were used: 90 to 100 microseconds 90° excitation RF pulse, 50 milliseconds repetition time (TR), 10 milliseconds echo time (TE), and 64 x 32 x 8 data points over a 40 x 40 x 36 mm field of view (FOV). A relatively long TE was used to achieve short sweep width (3700 Hz) and to optimize the SNR in 23Na images. The noise in an image is directly proportional to the square root of the sweep width; thus, decreasing the sweep width should increase the image SNR. However, decreasing the sweep width increases the acquisition time and TE, resulting in signal loss due to T2* relaxation. The optimum TE and sweep width that give the maximum SNR were calculated as described by Vinitski et al. [21] from the relaxation characteristics of the tumor 23Na signal and other imaging parameters used in the study. In addition, the weighted signal summation (WSS) technique was employed in the two phase-encoding directions to further improve SNR [22]. In the WSS technique, the numbers of signal transients summed at different phase-encoding steps are varied such that WSS produces similar signal conditioning effects as produced by apodization with a Gaussian function. This method of data collection increases SNR by ∼50% compared to performing apodization after data collection [22]. A maximum of 128 and an average of 55 signal transients were collected for the phase-encoding steps. Total data collection time for the 3D 23Na image was 14 minutes. The time domain data were zero-filled once in both phase-encoding directions (giving a 64 x 64 x 16 data matrix) and Fourier-transformed.
1H MRI
Water ADC in the tumor was measured using a multi-slice diffusion-weighted imaging (DWI) sequence. The following imaging parameters were used: 1100 milliseconds TR, 60 milliseconds TE, 256 x 128 data points over a 40 x 40 FOV, 2.0 mm slice thickness, and 0.6 mm slice gap. DWI 1H images were collected using four interleaved b-factors (b = 0, 236, 945, and 1,679 sec/mm2). These b-values are similar to the values used in other publications [8,19,23]. Duration of diffusion gradient pulses was 10 milliseconds, and the delay between the gradient pulses was 40 milliseconds. The orientation of the diffusion gradients was in the read-out direction (y). Total imaging time was 19 minutes. 1H images and water ADC maps were reconstructed using the Image Browser software provided by Varian. The tumor volume and average water ADC were determined over a 3D volume of interest for each temporal measurement.
23Na Relaxation Time Measurements
Because the TE used in the 23Na MRI experiments was relatively long, 23Na relaxation times for control and Cp-treated tumors were measured. A 10-mm-diameter surface coil was used to measure T1 and T2 to avoid signal contamination from normal tissues surrounding the tumor. 23Na T1 was measured using a pulse-burst saturation recovery pulse sequence consisting of 50 saturation pulses followed by an incremental delay (15 values ranging from 1 to 256 milliseconds) and a 90° observed pulse and acquisition with Cyclops phase cycling. The instrument dead time was set to 10 microseconds for all relaxation experiments. 23Na T2f and T2s were measured using a Hahn spin-echo sequence consisting of a composite 180° pulse [24]. The TE was varied from 0.05 to 40 milliseconds. The instrument dead time of 10 microseconds was included as a part of the TE. One thousand twenty-four complex data points were collected over a sweep width of 3000 Hz, and either 128 or 256 transients were acquired at each relaxation delay for both T1 and T2 experiments. The relaxation times were computed by fitting the signal areas to both a monoexponential function and a biexponential function. The experimental conditions, including tumor volume, type of anesthesia, and drug treatment, were identical in both the 23Na MRI and relaxation time experiments.
Histology
Following the last 1H and 23Na MRI experiments 3 days after Cp injection, the animals were sacrificed by an over-dose of ketamine injected intraperitoneally. The anterior part of the tumor was marked with a permanent marker. The tumor and surrounding skin were detached from the animal body, fixed in 25% zinc-formalin solution (Anatech, Battle Creek, MI), and then embedded in paraffin. The histologic sections of the tumor were cut perpendicular to the body wall along the same plane as the MR images. Tissue sections were obtained at 5 mm thickness and stained with hematoxylin and eosin (H&E) to identify regions with different cell densities. Three histology slices, from the anterior, middle, and posterior regions of each tumor, were compared with water ADC maps and 23Na MRI. During the MRI experiments, special care was taken in positioning the tumor exactly in the middle of the magnet with the help of a read-out gradient along the z-axis. Thus, we assume that the hitologic slices from the middle region correspond to the middle slice in the MR images. There may be a slight mismatch in orientation between the two images, but it should not affect the comparison drastically because the 1H and 23Na MRI slices were relatively thick (∼2 mm) compared to the histologic slices (5 µm). The histologic regions with low cell density that contain relatively few intact nuclei and liquefied/caseous materials were designated as “necrotic” [25,26]. These regions can be differentiated from regions of higher cell density (“viable” regions) on lower-resolution images. Digital micrographs were obtained with a Nicon Coolpix 4500 (Nicon, Inc., Torrance, CA) and histologic slices were analyzed with Microstar IV (IMEB, Inc., Columbus, OH).
Destructive Chemical Analysis
The effects of Cp treatment on relative extracellular space (rECS) and [Na+]tumor were measured by destructive chemical analysis following the last 23Na relaxation time measurement 3 days after Cp injection. The animals were kept under isoflurane gas anesthesia and an 80 mM solution of cobalt ethylenediaminetetraacetate (CoEDTA-; Sigma-Aldrich), an extracellular space marker, was infused through a catheter in the tail vein at 0.2 ml/hr (6 min), 0.4 ml/hr (6 min), 0.6 ml/hr (6 min), 0.8 ml/hr (6 min), and 0.5 ml/hr (60 min). As shown earlier [27,28], a similar infusion protocol allowed equilibration of CoEDTA, throughout all extracellular space in rat tissues, including the tumor. A blood sample (∼0.5 ml) was withdrawn from the heart/chest area. The tumor was then quickly excised and all skin and muscles surrounding the tumor were removed. The tumor was immediately freeze-clamped using aluminum tongs precooled in liquid nitrogen, weighed, dried overnight at 60°C, and reweighed to establish the relative dry-to-wet weight ratio (rDW). The blood and tumor samples were prepared for analysis of Co3+ and Na+ by inductively coupled plasma (ICP) spectrometry using standard procedure [27]. The dried tissue samples were digested in 2 ml of concentrated nitric acid overnight in a water-heating bath held at 50°C, and blood samples were centrifuged to remove the erythrocytes from the plasma. The samples were then diluted in deionized water, and [Na+] and [Co3+] were measured by ICP at 330.232 and 228.616 nm, respectively. The rECS was determined by the equation:
| 1 |
where [Co3+]tumor and [Co3+]plasma are the concentrations of Co3+ in the tumor and blood plasma, respectively. This method assumes that the [Co3+] in the extracellular space of the tumor is equal to [Co3+]plasma.
Statistical Analysis
All data are presented as the mean ± SEM. Statistical analyses of the data were performed by ANOVA (Statistica/v. 5.1 program). P ≤ .05 was used to define statistical significance.
Results
Effect of Cp on Tumor Growth
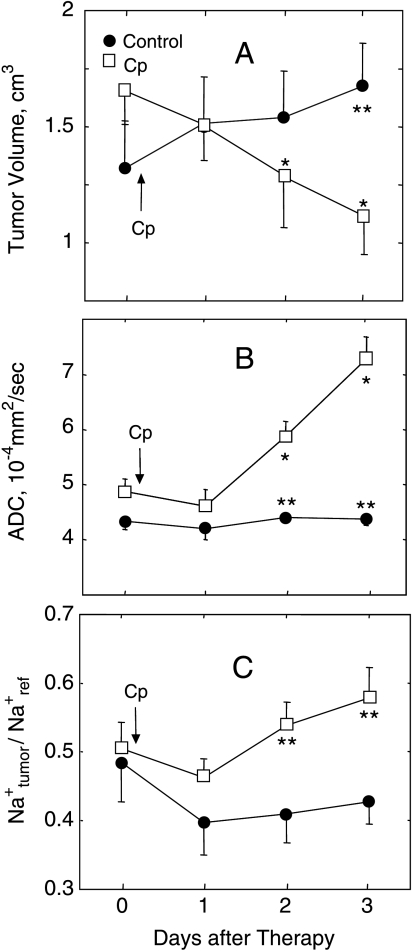
The mean tumor volumes of control and treated mice measured from 1H MRI are shown in Figure 1A. Before treatment, both groups had similar tumor volumes (1.3 ± 0.2 cm3 for control group, 1.7 ± 0.2 cm3 for treated group; P = .18). In Cp-treated animals, the mean tumor volume decreased to 1.3 ± 0.2 cm3 (P ≤ .05 vs pretreatment) on day 2 and to 1.1 ± 0.2 cm3 (P ≤ .05 vs pretreatment) on day 3 after treatment. The mean tumor volume in control animals (1.7 ± 0.2 cm3) was greater (P ≤ .05) than the baseline volume 3 days after treatment. The mean tumor volume in treated animals was significantly lower than in control animals 3 days after treatment (P ≤ .01).
Figure 1.
Effects of Cp therapy (300 mg/kg, ip) on tumor volume (A), water ADC (B), and 23Na SI from the tumor relative to the reference (Na+tumor/Na+ref) (C) in sc implanted RIF-1 tumors. Tumor volumes were measured from 1H MRI. Water ADC and relative 23Na SI changes are the mean from the whole tumor. Cp treatment caused a significant decrease in tumor volume and significant increases in water ADC and 23Na SI 2 and 3 days posttherapy. Significance: P ≤ .05 (* - versus before treatment), P ≤ .01 (** - control versus Cp-treated). Data are presented as mean ± SEM.
ADC of Water
The mean water ADC values in control and treated tumors prior to and after Cp injection are shown in Figure 1B. Water ADC was similar in control and treated groups (4.4 ± 0.2 x 10-4 and 4.9 ± 0.2 x 10-4 mm2/sec, respectively) prior to treatment. In the control group, the mean water ADC did not change significantly over the 3 days, whereas in the treated group, water ADC increased significantly 2 and 3 days after Cp injection (5.8 ± 0.3 x 10-4 and 7.3 ± 0.4 x 10-4 mm2/sec, respectively) compared to baseline (P ≤ .05) and control tumors (P ≤ .01) for both time points.
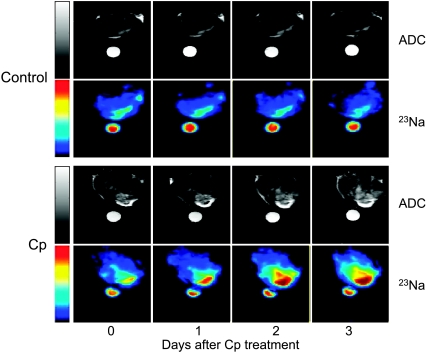
Examples of a pixel-by-pixel water ADC map for a control tumor and a treated tumor before and 1, 2, and 3 days after Cp injection are shown in Figure 2. The water ADC values before Cp treatment (day 0) were 4.9 x 10-4 and 5.4 x 10-4 mm2/sec in the control and treated tumors, respectively. After Cp injection, water ADC increased progressively to 5.9 x 10-4 , 6.5 x 10-4 , and 7.6 x 10-4 mm2/sec on days 1, 2, and 3, respectively. The water ADC increase was observed not only in tumor regions with low cell density (bright region in the lower right quadrant of the tumor in Figure 2) but throughout the whole tumor. In the untreated tumor, water ADC decreased slightly and was 4.5 x 10-4 mm2/sec on day 3.
Figure 2.
Water ADC maps and 23Na MR images of representative control and Cp-treated RIF-1 tumors before and 1, 2, and 3 days after Cp treatment. Water ADC and 23Na signal intensity increased with time after Cp treatment. A vial filled with a 154-mM NaCl solution was placed near the tumor as a reference.
23Na MRI and Magnetic Resonance Spectroscopy (MRS)
Figure 2 also shows 23Na MR images of the same control and treated tumors before and 1, 2, and 3 days after Cp injection. Similar to the water ADC maps, 23Na MRI showed heterogeneous signal intensity in both tumors, reflecting the inherent heterogeneity in the tumor microenvironment. In the control tumor, the 23Na signal was relatively stable and even slightly decreased by day 3. However, in the treated tumor, the 23Na SI increased steadily (44% by day 3 after Cp injection). The most dramatic increase in 23Na signal was observed in the region with low cell density, but an increase in signal intensity was detected throughout the tumor. Similar increases in 23Na SI were observed in all the treated tumors.
The changes in tumor 23Na SI with respect to the reference signal intensity for control and treated groups prior to and after Cp injection are shown in Figure 1C. The mean of tumor to reference 23Na SI ratio was similar for the control and Cp-treated groups at baseline: 0.48 ± 0.06 and 0.51 ± 0.04, respectively. The ratio did not change significantly for the control group. The treated group showed a progressive increase in tumor 23Na SI. The tumor to reference 23Na SI ratio was 0.54 ± 0.03 on day 2 and 0.58 ± 0.04 on day 3 after Cp injection (P ≤ .05 compared to the same time points for control).
The values of T1, T2s, and T2f, and the relative contribution of T2s to the observed 23Na signal for control and Cp-treated tumors are listed in Table 1. There were no significant differences in the relaxation times or the relative contribution of the fast and slow relaxation components between control and treated tumors at any time point (P ≥ .1). These relaxation parameters were measured using a separate group of animals than that used in the MRI experiments for two reasons. First, the imaging experiments took over an hour and we did not want to keep the animals under anesthesia and strained conditions any longer. Second, a surface coil was used for the relaxation time measurements to avoid contamination from healthy tissues near the tumor. The experimental conditions, including tumor volume, type of anesthesia, and drug treatment, were identical in both the 23Na MRI and relaxation time experiments.
Table 1.
Experimental Relaxation Times of 23Na Signal from Control and Cp-Treated Tumors Before and After Therapy.
| Day 0 | Day 1 | Day 2 | Day 3 | |
| T1 (msec) | ||||
| Control | 43.1 ± 3.6 | 40.6 ± 1.4 | 42.8 ± 3.5 | 43.6 ± 1.1 |
| Cp-treated | 42.0 ± 1.8 | 38.9 ± 0.4 | 43.1 ± 0.7 | 44.7 ± 1.7 |
| T2s (msec) | ||||
| Control | 22.7 ± 0.4 | 22.5 ± 0.6 | 22.0 ± 1.1 | 21.1 ± 0.6 |
| Cp-treated | 25.4 ± 1.7 | 21.6 ± 1.1 | 24.8 ± 0.5 | 26.1 ± 0.6 |
| T2f (msec) | ||||
| Control | 3.1 ± 0.2 | 3.2 ± 0.3 | 2.8 ± 0.1 | 2.8 ± 0.1 |
| Cp-treated | 3.7 ± 0.7 | 2.8 ± 0.2 | 3.5 ± 0.3 | 3.3 ± 0.2 |
| Relative contribution from T2f | ||||
| Control | 0.52 ± 0.01 | 0.50 ± 0.01 | 0.53 ± 0.01 | 0.56 ± 0.03 |
| Cp-treated | 0.43 ± 0.05 | 0.47 ± 0.02 | 0.49 ± 0.01 | 0.48 ± 0.01 |
Values are reported as mean ± SEM (n = 4 for each group). No significant differences were found between control and Cp-treated tumors at any time point studied.
Correlation between Water ADC and 23Na MRI
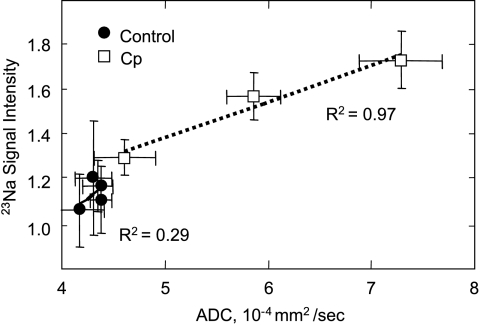
Spatial correlation between water ADC and 23Na SI for the control and treated tumors is apparent in the images shown in Figure 2. The regions with high water ADC are also hyperintense in 23Na MRI. Figure 3 shows a plot of mean water ADC and mean 23Na SI for control and treated tumors. Each data point represents all the tumors at a specific day. Although the correlation between water ADC and [Na+]tumor was poor in the control tumors, the correlation was significant (R2 = 0.97) in Cp-treated tumors.
Figure 3.
Correlation between mean water ADC and 23Na SI in untreated (●) and Cp-treated (□) RIF-1 tumors. Each data point represents all the tumors at a specific day. The R2 coefficient for Cp-treated tumors was more than three times higher than for the untreated tumors.
Histology
H&E-stained sections (low and high resolution, 20x) of a control and a Cp-treated tumor and the corresponding water ADC maps and 23Na images are shown in Figure 4. MRI maps and histologic segments have a slice thickness of 2 mm and 5 µm, respectively. Viable tissue is visible in the low-resolution histologic segments as the darker purple color, and necrotic areas are visible as the brighter pink areas. There is reasonable agreement between the MR images and the histologic sections in both tumors. For example, in the Cp-treated tumor, the region with the highest Na+ signal intensity corresponded to the histologic region with the lowest cell density (histologic section D), and the region with the lowest Na+ signal intensity corresponded to the most viable histologic section (C). Comparison of histologic sections of control and Cp-treated tumors shows that both high and low cell density regions of treated tumors (C and D) contain fewer cells than control tumors (A and B). The necrotic regions with low cell density of both control and Cp-treated tumors also contained high extracellular collagen, a characteristic of fibrosarcoma.
Figure 4.
H&E-stained histologic slices, water ADC maps, and 23Na MRI images of representative control and Cp-treated RIF-1 tumors 3 days after treatment. Histologic and MRI slices from the middle part of the tumors are presented. Arrows point to the regions identified as “viable” (A and C) and “necrotic” (B and D) in both tumors. These regions are presented as high-resolution (original magnification, x20) histologic images. The regions of tumors with higher 23Na signal and water ADC mostly correspond to the histologic regions with fewer cells.
Destructive Chemical Analysis
The ICP data for tissue compartmentalization and Na+ content for control and Cp-treated tumors 3 days after treatment are shown in Table 2. In Cp-treated tumors, rDW was significantly lower (P ≤ .05) and rECS was significantly higher (P ≤ .05) compared to the untreated tumors. The [Na+]tumor was 29.4% higher in Cp-treated tumors than in control tumors (58 ± 10 and 45 ± 7 mM, respectively; P ≤ .05).
Table 2.
Tissue Compartmentalization and Na+ Content of Control and Cp-Treated RIF-1 Tumors 3 Days After Therapy as Measured by Destructive Chemical Analysis.
| rDW | rECS | [Na+]tumor (mM) | |
| Control | 0.21 ± 0.01 | 0.26 ± 0.04 | 45 ± 7 |
| Cp-treated | 0.16 ± 0.01* | 0.46 ± 0.08* | 58 ± 10* |
Values are reported as mean ± SEM (n = 7 for control and n = 5 for Cp-treated group).
Significance: P ≤ .05 (control vs. Cp-treated).
Discussion
In this study, we examined the effects of Cp treatment on 23Na MRI as a noninvasive marker of tumor response to cancer chemotherapy and compared these Na+ signal changes with water ADC measured by diffusion-weighted 1H MRI. The Cp injection caused a significant reduction in tumor volume 2 and 3 days posttreatment. Poptani et al. [5] and Zhao et al. [7] have shown similar changes in RIF-1 tumor volumes after Cp treatment. Cp itself is a prodrug, which is oxidized in the liver to 4-hydroxycyclophosphamide and subsequently converted to nitrogen mustard and other metabolites. In RIF-1 tumors, Cp metabolites do not directly disrupt cell metabolism, but rather these metabolites alkylate DNA and proteins. It has been shown [29,30] that for RIF-1 and other animal tumors, Cp causes cell death by stimulating apoptosis, as evidenced by the induction of plasma membrane blebbing, DNA fragmentation, and cleavage of the caspase 3 and caspase 7 substrate poly(ADP-ribose) polymerase. These genetic and cellular transformations lead to some metabolic effects of Cp, such as a decrease in mitotic activity [5,31], an increase in water diffusion, and an increase in aerobic metabolism that decreases the glycolytic rate in RIF-1 cells [5].
We have shown that treatment of RIF-1 tumors with 300 mg/kg Cp significantly increased water ADC 2 and 3 days posttreatment. Braunschweiger [10] and Makin [30] have shown that Cp-induced changes in tissue microvasculature and apoptotic/necrotic damage of tumor cells lead to large reductions in cell volume and increases in the volume of extracellular water. These changes may increase the mobility of water in the damaged tissue and lead to an increase in water ADC in Cp-treated tumors, as we have shown. Our ICP and histologic results also support this hypothesis. The ICP data show a significant increase in rECS, whereas the histologic data show a decrease in the number of cells and an increase in extracellular space (Figure 3). It has been shown previously that the increase in water ADC correlates with both the increase in tumor necrotic fraction in RIF-1 tumors [25] and the decrease in tumor cell density in 9L glioma [8].
The b-values used in this work are similar to the values used in other publications [8,19,23]. We did not detect any nonexponential behavior with these b-values. Such behavior could be detected by using higher b-values and a larger number of data points. However, we did not attempt this because it would require long data collection times, especially at high b-values where SNR is low. We were simply interested in using water ADC as a therapy response parameter.
The increase in extracellular space following therapy can cause not only an increase in water ADC but also an increase in [Na+]tumor, as [Na+]e is 10 to 15 times higher than [Na+]i. We found that, on average, both 23Na SI and water ADC increased throughout the tumor after Cp treatment. The increase in 23Na SI after chemotherapy could be because of an increase in [Na+]tumor or a change in 23Na relaxation times. Our data showed that Cp treatment or untreated growth of RIF-1 tumors did not significantly change the T1, T2s, and T2f values, or the relative contributions of T2s and T2f (Table 1). These results suggest that the observed increase in 23Na MRI signal intensity after Cp treatment was due to increases in [Na+]tumor caused by Cp treatment. Our ICP data confirmed that in Cp-treated tumors, [Na+]tumor is significantly increased 3 days after treatment (45 ± 7 mM, control; 58 ± 10 mM, Cp-treated) (Table 2). The value of the Cp-induced increase in [Na+]tumor was comparable for both MRI (36.8%) and ICP (29.4%) methods.
Although the extracellular space increases after therapy, we believe that [Na+]e remains constant. [Na+]e can be maintained constantly by the transport of Na+ from the vascular and/or interstitial space of the nearby uncompromised tissue even in hypoxic or necrotic regions. Moreover, previous 1H MRI studies show that tumor perfusion is increased after therapy [5]. Thus, transport of Na+ from the vascular space can maintain [Na+]e, and an increase in extracellular space results in increased [Na+]tumor after therapy.
There was a good correlation between 23Na SI and water ADC in the Cp-treated tumors (Figure 3). One possible reason for this effect may be that [Na+]tumor increases with increased extracellular space because of cells lost through apoptosis and/or necrosis. Schepkin et al. [19] also showed that a large increase in 23Na MRI signal intensity occurred 7 to 9 days following treatment with 1,3-bis (2-chlorethyl)-1-nitrosourea (BCNU; another chemotherapeutic alkylating agent), which correlated to the period of the greatest chemotherapy-induced cellular necrosis based on water ADC changes and histopathology.
Changes in [Na+]tumor and water ADC may be related, but 23Na images may provide more functional information because therapy can also alter [Na+]i, which depends on the cellular energy status and activity of ion transport processes. Many reports show that radiotherapy and most chemotherapies (including treatment of RIF-1 tumors with Cp) cause increased ATP levels and decreased Pi in experimental tumors [1,32,33]. This effect is thought to be a result of increased perfusion and oxygenation of the tumor [3,5,34]. The improved cellular energy status should aid in maintaining low [Na+]i because of the high activity of the Na+ /K+ ATPase. However, a decrease in [Na+]i following effective therapy may not be a general phenomenon. An ischemia-like response of tumors to therapy (decreased ATP level) and/or partial destruction of the cellular membrane or membrane-embedded proteins can produce an increase in [Na+]i. For example, Ben-Yoseph and Ross [35] demonstrated that treatment of sc implanted 9L glioma with polyethylene glycol-stabilized glucose oxidase results in a 96% reduction in the ATP/Pi and a 0.72-U decline in pHi. In this case, [Na+]i should increase. Thus, because of the sensitivity of 23Na MRI to cellular function, it may provide additional information than that available from water ADC measurements alone.
Differences in tumor model and treatment can play important roles in tumor Na+ response. In contrast to Schepkin et al. [19] and the results presented here, Winter et al. [27] show that BCNU therapy of subcutaneously implanted 9L glioma results in decreased SQ and TQF 23Na SI compared to untreated control tumors. This difference in therapy response could be because of differences in rECS changes in the different tumor models. Winter et al. [27] did not observe any difference in rECS between treated and untreated tumors but showed improved cellular energetics and increased pHi in sc implanted 9L glioma after BCNU therapy. These metabolic changes could decrease intracellular and total tissue Na+. We have performed some initial 23Na MRI and water ADC measurements on subcutaneously implanted 9L glioma with BCNU therapy. Our preliminary data suggest that both tissue Na+ and water ADC are lower in BCNU-treated tumors compared to untreated controls.
Usually both water ADC maps and 23Na images had some verifiable heterogeneity in subcutaneously implanted RIF-1 tumors. In some regions, when 23Na SI was high, water ADC was not dramatically increased. A possible reason for this discrepancy may be that 23Na SI can also increase as a result of increase in [Na+]i. Movement of Na+ and water into the cells can cause a decrease in water ADC because of a high macromolecule concentration inside the cells. An increase in extracellular space increases both water ADC and [Na+]tumor, whereas cytotoxic edema decreases water ADC but increases [Na+]tumor. Thus, a combination of both increased extracellular space and cytotoxic edema can cause the observed discrepancy between water ADC and 23Na SI.
Conclusions
In vivo MRI experiments showed that both 23Na SI and water ADC increase 2 days after chemotherapy of subcutaneously implanted RIF-1 tumors with Cp. These effects were largely due to an increase in extracellular space as shown by histology and destructive chemical analysis. The increase in 23Na MRI signal intensity after Cp treatment observed in this study may prove useful for detecting early therapy response and may support the mechanism for water ADC changes.
Acknowledgements
The authors thank Paige Hopewell, Rebecca Kerkhoff, and Samuel G. Jennings for valuable comments and assistance in the preparation of the manuscript; and Yonghua Xu and Mandar Jagtap for help with analysis of histologic slices.
Abbreviations
- ADC
apparent diffusion coefficient
- CoEDTA-
cobalt ethylenediaminetetraacetate
- Cp
cyclophosphamide
- DWI
diffusion-weighted images
- FOV
field of view
- H&E
hematoxylin and eosin
- ICP
inductively coupled plasma
- ip
intraperitoneal
- NMR
nuclear magnetic resonance
- 23Na SI
23Na signal intensity
- [Na+]e
extracellular sodium concentration
- [Na+]i
intracellular sodium concentration
- [Na+]total
total sodium concentration
- rDW
relative dry-to-wet weight ratio
- rECS
relative extracellular space
- MEM
minimum essential medium
- MRI
magnetic resonance imaging
- MRS
magnetic resonance spectroscopy
- RIF-1
radiation-induced fibrosarcoma
- SNR
signal-to-noise ratio
- sc
subcutaneous
- T1
longitudinal relaxation times
- T2f and T2s
fast and slow transverse relaxation times
- TE
echo time
- TR
repetition time
- WSS
weighted signal summation
Footnotes
This research was supported, in part, by National Institutes of Health grants HL54574, CA84434, and CA94040.
References
- 1.Li SJ, Wehrle JP, Rajan SS, Steen RG, Glickson JD, Hilton J. Response of radiation-induced fibrosarcoma-1 in mice to cyclophosphamide monitored by in vivo 31P nuclear magnetic resonance spectroscopy. Cancer Res. 1988;48:4736–4742. [PubMed] [Google Scholar]
- 2.Street JC, Mahmood U, Matei C, Koutcher JA. In vivo and in vitro studies of cyclophosphamide chemotherapy in a mouse mammary carcinoma by 31P NMR spectroscopy. NMR Biomed. 1995;8:149–158. doi: 10.1002/nbm.1940080403. [DOI] [PubMed] [Google Scholar]
- 3.Poptani H, Bansal N, Jenkins WT, Blessington D, Mancuso A, Nelson DS, Feldman M, Delikatny EJ, Chance B, Glickson JD. Cyclophosphamide treatment modifies tumor oxygenation and glycolytic rates of RIF-1 tumors: 13C magnetic resonance spectroscopy, Eppendorf electrode, and redox scanning. Cancer Res. 2003;63:8813–8820. [PubMed] [Google Scholar]
- 4.Poptani H, Pickup S, Delikatny EJ, Magnitsky S, Mancuso A, Nelson DS, Glickson JD. Increased diffusion coefficient with decreased choline in response to cyclophosphamide therapy of RIF-1 tumors. Proc Int Soc Magn Reson Med. 2004;11:2035. [Google Scholar]
- 5.Poptani H, Bansal N, Graham RA, Mancuso A, Nelson DS, Glickson JD. Detecting early response to cyclophosphamide treatment of RIF-1 tumors using selective multiple quantum spectroscopy (SelMQC) and dynamic contrast enhanced imaging. NMR Biomed. 2003;16:102–111. doi: 10.1002/nbm.816. [DOI] [PubMed] [Google Scholar]
- 6.Robinson SP, McIntyre DJ, Checkley D, Tessier JJ, Howe FA, Griffiths JR, Ashton SE, Ryan AJ, Blakey DC, Waterton JC. Tumour dose response to the antivascular agent ZD6126 assessed by magnetic resonance imaging. Br J Cancer. 2003;88:1592–1597. doi: 10.1038/sj.bjc.6600926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao M, Pipe JG, Bonnett J, Evelhoch JL. Early detection of treatment response by diffusion-weighted 1H-NMR spectroscopy in a murine tumour in vivo. Br J Cancer. 1996;73:61–64. doi: 10.1038/bjc.1996.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chenevert TL, Stegman LD, Taylor JM, Robertson PL, Greenberg HS, Rehemtulla A, Ross BD. Diffusion magnetic resonance imaging: an early surrogate marker of therapeutic efficacy in brain tumors. J Natl Cancer Inst. 2000;92:2029–2036. doi: 10.1093/jnci/92.24.2029. [DOI] [PubMed] [Google Scholar]
- 9.Latour LL, Svoboda K, Mitra PP, Sotak CH. Time-dependent diffusion of water in a biological model system. Proc Natl Acad Sci USA. 1994;91:1229–1233. doi: 10.1073/pnas.91.4.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braunschweiger PG. Effect of cyclophosphamide on the pathophysiology of RIF-1 solid tumors. Cancer Res. 1988;48:4206–4210. [PubMed] [Google Scholar]
- 11.Stegman LD, Rehemtulla A, Hamstra DA, Rice DJ, Jonas SJ, Stout KL, Chenevert TL, Ross BD. Diffusion MRI detects early events in the response of a glioma model to the yeast cytosine deaminase gene therapy strategy. Gene Ther. 2000;7:1005–1010. doi: 10.1038/sj.gt.3301199. [DOI] [PubMed] [Google Scholar]
- 12.Ross BD, Chenevert TL, Rehemtulla A. Magnetic resonance imaging in cancer research. Eur J Cancer. 2002;38:2147–2156. doi: 10.1016/s0959-8049(02)00387-8. [DOI] [PubMed] [Google Scholar]
- 13.Cameron IL, Smith NK, Pool TB, Sparks RL. Intracellular concentration of sodium and other elements as related to mitogenesis and oncogenesis in vivo. Cancer Res. 1980;40:1493–1500. [PubMed] [Google Scholar]
- 14.Koch KS, Leffert HL. Increased sodium ion influx is necessary to initiate rat hepatocyte proliferation. Cell. 1979;18:153–163. doi: 10.1016/0092-8674(79)90364-7. [DOI] [PubMed] [Google Scholar]
- 15.Kline RP, Wu EX, Petrylak DP, Szabolcs M, Alderson PO, Weisfeldt ML, Cannon P, Katz J. Rapid in vivo monitoring of chemotherapeutic response using weighted sodium magnetic resonance imaging. Clin Cancer Res. 2000;6:2146–2156. [PubMed] [Google Scholar]
- 16.Seshan V, Bansal N. In: In vivo31P and 23Na NMR spectroscopy and imaging. NMR Spectroscopy Techniques. Bruch MD, editor. New York, NY: Marcel Dekker; 1996. pp. 557–607. [Google Scholar]
- 17.Ouwerkerk R, Bleich KB, Gillen JS, Pomper MG, Bottomley PA. Tissue sodium concentration in human brain tumors as measured with 23Na MR imaging. Radiology. 2003;227:529–537. doi: 10.1148/radiol.2272020483. [DOI] [PubMed] [Google Scholar]
- 18.Thulborn KR, Davis D, Adams H, Gindin T, Zhou J. Quantitative tissue sodium concentration mapping of the growth of focal cerebral tumors with sodium magnetic resonance imaging. Magn Reson Med. 1999;41:351–359. doi: 10.1002/(sici)1522-2594(199902)41:2<351::aid-mrm20>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 19.Schepkin VD, Ross BD, Chenevert TL, Rehemtulla A, Sharma S, Kumar M, Stojanovska J. Sodium magnetic resonance imaging of chemotherapeutic response in a rat glioma. Magn Reson Med. 2004;53:85–92. doi: 10.1002/mrm.20332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Twentyman PR, Brown JM, Gray JW, Franko AJ, Scoles MA, Kallman RF. A new mouse tumor model system (RIF-1) for comparison of end-point studies. J Natl Cancer Inst. 1980;64:595–604. [PubMed] [Google Scholar]
- 21.Vinitski S, Griffey R, Fuka M, Matwiyoff N, Prost R. Effect of the sampling rate on magnetic resonance imaging. Magn Reson Med. 1987;5:278–285. doi: 10.1002/mrm.1910050309. [DOI] [PubMed] [Google Scholar]
- 22.Bansal N, Seshan V. Three-dimensional triple quantum-filtered 23Na imaging of rabbit kidney with weighted signal averaging. J Magn Reson Imaging. 1995;5:761–767. doi: 10.1002/jmri.1880050624. [DOI] [PubMed] [Google Scholar]
- 23.Hall DE, Moffat BA, Stojanovska J, Johnson TD, Li Z, Hamstra DA, Rehemtulla A, Chenevert TL, Carter J, Pietronigro D, et al. Therapeutic efficacy of DTI-015 using diffusion magnetic resonance imaging as an early surrogate marker. Clin Cancer Res. 2004;10:7852–7859. doi: 10.1158/1078-0432.CCR-04-1218. [DOI] [PubMed] [Google Scholar]
- 24.Bansal N, Germann MJ, Seshan V, Shires GT, III, Malloy CR, Sherry AD. Thulium 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrakis(methylene phosphonate) as a 23Na shift reagent for the in vivo rat liver. Biochemistry. 1993;32:5638–5643. doi: 10.1021/bi00072a020. [DOI] [PubMed] [Google Scholar]
- 25.Helmer KG, Meiler MR, Sotak CH, Petruccelli JD. Comparison of the return-to-the-origin probability and the apparent diffusion coefficient of water as indicators of necrosis in RIF-1 tumors. Magn Reson Med. 2003;49:468–478. doi: 10.1002/mrm.10400. [DOI] [PubMed] [Google Scholar]
- 26.Carano RA, Ross AL, Ross J, Williams SP, Koeppen H, Schwall RH, van Bruggen N. Quantification of tumor tissue populations by multispectral analysis. Magn Reson Med. 2004;51:542–551. doi: 10.1002/mrm.10731. [DOI] [PubMed] [Google Scholar]
- 27.Winter PM, Poptani H, Bansal N. Effects of chemotherapy by 1,3-bis(2-chloroethyl)-1-nitrosourea on single-quantum- and triple-quantum-filtered 23Na and 31P nuclear magnetic resonance of the subcutaneously implanted 9L glioma. Cancer Res. 2001;61:2002–2007. [PubMed] [Google Scholar]
- 28.Makos JD, Malloy CR, Sherry AD. Distribution of TmDOTP 5- in rat tissues: TmDOTP 5- vs CoEDTA- as markers of extracellular tissue space. J Appl Physiol. 1998;85:1800–1805. doi: 10.1152/jappl.1998.85.5.1800. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz PS, Waxman DJ. Cyclophosphamide induces caspase 9-dependent apoptosis in 9L tumor cells. Mol Pharmacol. 2001;60:1268–1279. doi: 10.1124/mol.60.6.1268. [DOI] [PubMed] [Google Scholar]
- 30.Makin G. Targeting apoptosis in cancer chemotherapy. Expert Opin Ther Targets. 2002;6:73–84. doi: 10.1517/14728222.6.1.73. [DOI] [PubMed] [Google Scholar]
- 31.Duvvuri U, Poptani H, Feldman M, Nadal-Desbarats L, Gee MS, Lee WM, Reddy R, Leigh JS, Glickson JD. Quantitative T1rho magnetic resonance imaging of RIF-1 tumors in vivo: detection of early response to cyclophosphamide therapy. Cancer Res. 2001;61:7747–7753. [PubMed] [Google Scholar]
- 32.Rajan SS, Wehrle JP, Li SJ, Steen RG, Glickson JD. 31P NMR spectroscopic study of bioenergetic changes in radiation-induced fibrosarcoma-1 after radiation therapy. NMR Biomed. 1989;2:165–171. doi: 10.1002/nbm.1940020406. [DOI] [PubMed] [Google Scholar]
- 33.Li SJ, Wehrle JP, Glickson JD, Kumar N, Braunschweiger PG. Tumor bioenergetics and blood flow in RIF-1 murine tumors treated with 5-fluorouracil. Magn Reson Med. 1991;22:47–56. doi: 10.1002/mrm.1910220106. [DOI] [PubMed] [Google Scholar]
- 34.Steen RG. Response of solid tumors to chemotherapy monitored by in vivo 31P nuclear magnetic resonance spectroscopy: a review. Cancer Res. 1989;49:4075–4085. [PubMed] [Google Scholar]
- 35.Ben-Yoseph O, Ross BD. Oxidation therapy: the use of a reactive oxygen species-generating enzyme system for tumour treatment. Br J Cancer. 1994;70:1131–1135. doi: 10.1038/bjc.1994.460. [DOI] [PMC free article] [PubMed] [Google Scholar]