Abstract
The maspin gene functions as a tumor suppressor in human breasts, and its expression is frequently lost during breast cancer progression. In vitro models of human breast cancer indicate that the loss of maspin expression is closely linked to aberrant methylation of the maspin promoter. We conducted a study on 30 archival ductal carcinoma in situ (DCIS) specimens to determine if aberrant methylation of the maspin promoter occurred in vivo, and whether it occurred early in breast cancer evolution. Healthy tissue obtained from reduction mammoplasty was used as normal control. Results from immunohistochemical analysis indicate that maspin expression is lost in a substantial fraction of DCIS specimens (57%). Bisulfite sequencing of DNA isolated from laser capture-microdissected normal and neoplastic ducts showed that loss of maspin expression was often, but not always, linked to aberrant methylation of the maspin promoter, suggesting that other mechanisms, in addition to aberrant methylation, participate and/or cooperate to silence maspin gene expression. Taken together, these results indicate that aberrant methylation of the maspin promoter is an early event in human breast cancer.
Keywords: Methylation, breast cancer, tumor suppressor, maspin, laser capture, microdissection
Introduction
Complex changes in genomic methylation patterns are a hallmark of the cancer genome (for a review, see Refs. [1–3]). One of these changes is the aberrant methylation of tumor-suppressor gene promoters, which in turn is tightly linked to the inappropriate silencing of the associated tumorsuppressor gene [4]. Some tumor-suppressor genes are silenced by aberrant methylation in a variety of human tumors, whereas aberrant methylation of other tumor-suppressor genes occurs in a tumor-restricted fashion [5]. Examples of the former include p16 and ER, and examples of the latter include BRCA1 and Rb [6–10].
Loss of maspin gene expression is a common event in breast cancer in vivo [11–13] where maspin functions as a tumor-suppressor gene, which has been shown to inhibit the motility and invasive properties of breast cancer cells as well as their angiogenic and metastatic capabilities [11,14–17]. In vitro studies have demonstrated a tight link between the loss of maspin expression in breast cancer cells and the aberrant cytosine methylation and histone deacetylation of its promoter [18–20]. These data suggest that aberrant methylation of the maspin promoter may be an important mechanism underlying maspin gene silencing in human breast cancer.
We conducted a retrospective study on 30 archival ductal carcinoma in situ (DCIS) specimens and 2 normal healthy mammary specimens to determine if: 1) maspin expression is lost in early breast cancer as suggested by an earlier study [13]; 2) aberrant methylation of the maspin gene promoter occurs in vivo; and, if so, 3) whether or not this epigenetic change could be an early event in breast cancer evolution. The cytosine methylation status of the maspin promoter in ductal epithelial cells from carcinoma in situ was determined by sodium bisulfite sequencing, and correlated with maspin protein expression, as determined by immunohistochemistry. Because maspin shows cell type-specific patterns of methylation [21], it was imperative that pure tumor populations be analyzed. Laser capture microdissection (LCM) was used to obtain this selected material.
Although this is a relatively small study (30 DCIS and 2 normal controls), the results decisively demonstrate that the loss of maspin can be an early event in breast carcinogenesis and that aberrant methylation of the maspin promoter occurs in vivo at an early stage of cancer development. Importantly, although aberrant methylation occurred in a significant fraction of the cases analyzed, the loss of maspin expression was not solely associated with the aberrant methylation of its promoter, suggesting that multiple mechanisms likely participate in silencing maspin expression in breast cancer (e.g., p53 mutation; see Refs. [22–25]).
Materials and Methods
DCIS Specimens
Thirty archival paraffin-embedded DCIS specimens from patients who underwent lumpectomy or mastectomy were randomly obtained from patients who underwent surgery for DCIS between 1987 and 1999 at the University Medical Center (Tucson, AZ). All specimens were collected and maintained in accordance with institutional review board guidelines. From each tissue block, a series of 5-µm sections was cut. One of the sections from each patient sample was stained with hematoxylin and eosin (H&E) for pathologic evaluation and subsequent LCM. Other sections were used to assess maspin expression by immunohistochemistry.
Immunohistochemistry
Maspin immunohistochemistry was performed as previously described [26,27]. The antibody used to detect maspin protein expression was a mouse anti-human maspin antibody (dilution 1:200; Pharmingen, San Diego, CA). Formalinfixed, paraffin-embedded DCIS samples were sectioned at 5 µM and stained with H&E for routine histologic examination. Sections adjacent to the H&E-stained sections were used for immunohistochemical staining for maspin. Slides were deparaffinized using xylene, 100% ethanol, and 95% ethanol, followed by thorough deionized water wash. A waterbath antigen recovery technique, using citrate buffer, pH 6.0, was performed on all slides.
Immunohistochemical staining was performed on a Dako (Carpinteria, CA) autostainer with the RTU Vectastain Elite ABC Peroxidase Kit (Vector Laboratories, Burlingame, CA) to detect mouse anti-human maspin (dilution 1:200; Pharmingen). The antibody was diluted using an antibody diluent (Dako). After deparaffinization and antigen recovery, slides were washed in Tris-buffered saline with Tween 20 (TBST). Four blocking steps were applied: 0.03% hydrogen peroxide (Dako) for 15 minutes followed by a TBST wash after each step; finally, the serum-free protein block (Dako) was applied for 15 minutes. The primary antibody was applied to the slides and incubated for 60 minutes; slides were rinsed in TBST, followed by application of the RTU Vectastain secondary antibody kit for 20 minutes. Color for the RTU procedure was produced by adding DAB+ (brown) substrate for 3 to 5 minutes. Slides were then counterstained with Mayer's hematoxylin for 1 to 3 minutes. Images were obtained using a Zeiss Axioskop microscope (Carl Zeiss, Inc., Thornwood, NY), Spot 2 camera (Diagnostic Instruments, Inc., Sterling Heights, MI), and Zeiss Axiovision 2.0.5 software (Carl Zeiss, Inc.). Appropriate negative controls were included in each experiment, such as no primary antibody, as well as testing normal tissue known to be maspin-negative.
LCM/DNA Isolation
Microdissection of ductal epithelial cells from archival paraffin-embedded DCIS tissue biopsies was performed on an Arcturus PixCell II Laser Capture Microdissection microscope (Arcturus Bioscience, Inc. Mountain View, CA). Approximately 1000 normal or neoplastic epithelial cells were selectively isolated from the DCIS specimens. DNA from the microdissected tissue was extracted in a buffer containing 10 mmol of 0.04% proteinase K or Tris hydrochloride (pH 8.0), 1 mmol EDTA, and 1% Tween-20 at 37°C overnight, followed by heat inactivation at 95°C for 8 minutes. This DNA was used for bisulfite sequence analysis.
Bisulfite Sequencing of the Maspin Promoter
Genomic DNA from laser capture-microdissected specimens was modified with sodium bisulfite under the conditions described by Clark et al. [28]. Briefly, DNA was denatured with 0.3 M NaOH, reacted with 3.6 M sodium bisulfite (pH 5) at 55°C for 14 hours, desalted by using a Wizard Prep kit (Promega, Madison, WI), desulfonated with 0.3 M NaOH, and, finally, ethanol-precipitated in preparation for polymerase chain reaction (PCR). The maspin promoter [29] was amplified from the bisulfite-modified DNA by two rounds of PCR using nested primers specific for the bisulfite-modified sequence of the promoter. The first-round primers were: primer U2 (nt 673–nt 703), 5′-AAAAGAATGGAGATTAGAGTATTTTTTGTG-3′; primer D2 (nt 1114-nt 1141), 5′-CCTAAAATCACAATTATCCTAAAAAATA-3′. The second-round primers were: U4 (nt 827-nt 853) and primer D4 (nt 991-nt 1010). Both rounds of PCR were performed under the same parameters, with 1% of the first-round PCR product serving as the template in the second-round PCR. PCR amplification was performed under the following conditions: 95°C for 1 minute followed by 35 cycles of 92°C for 1 minute, 56°C for 3 minutes, and 72°C for 1 minute, and ending with a final extension of 72°C for 5 minutes and a quick chill to 4°C. One percent of the first-round PCR product was used for the second round of PCR using primers U4 and D4 under the same conditions. The resultant 184-bp PCR product was cloned into a TA vector according to the manufacturer's instructions (pGEM-T-Easy cloning kit; Promega). DNA from 10 positive recombinants were isolated using a Qiaprep Spin PlasmidMiniprep kit (Qiagen, Valencia, CA) and sequenced on an ABI automated DNA sequencer. The methylation status of individual CpG sites was determined by comparison of the sequence obtained with the known maspin sequence. The number of methylated CpGs at a specific site was divided by the number of clones (n = 10–21 in all cases) analyzed to yield a percent methylation for each site. Similarly, the overall number of methylated CpGs among all the clones from a given patient sample was divided by the total number of CpGs analyzed to yield an overall percent methylation (n = 70–147). We observed a clear-cut grouping of the overall methylation patterns among the samples, with one group displaying between 0% and 3% CpG methylation, and the other group showing >15% methylation. On this basis, we defined 15% as the threshold for aberrant methylation of the maspin promoter.
Results
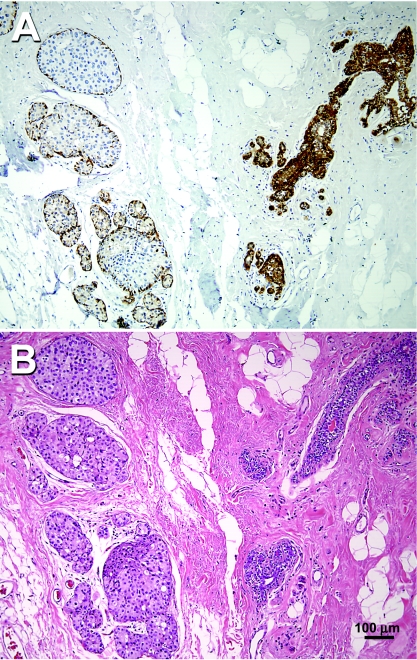
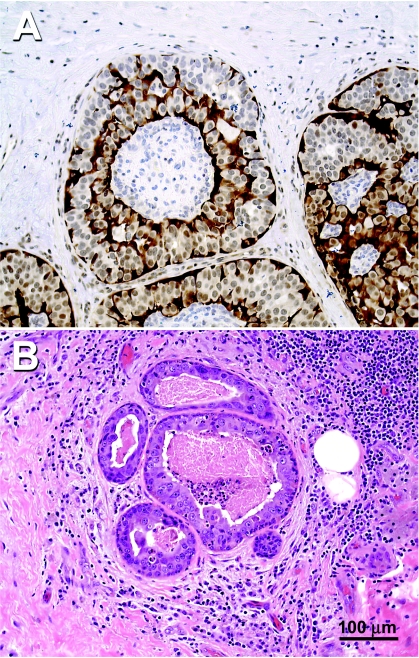
Maspin protein expression was assessed by immunohistochemistry in 30 DCIS specimens and 2 specimens obtained from healthy individuals who underwent reduction mammoplasty. As presented in Figure 1A, the normal mammary ducts from patient 5, seen in the right side of the photomicrograph, show maspin immunoreactivity as fine, granular, dark brown, diffuse precipitates in the cytoplasm of the basal and ductal epithelial cells as previously described [30]. In contrast, the mammary ducts with carcinoma in situ from patient 5, seen on the left side of the photomicrograph, have lost their maspin immunoreactivity in ductal epithelia, although the myoepithelial cells surrounding the transformed ductal cell remained positive for maspin immunoreactivity. The H&E staining of an adjacent section is shown in Figure 1B. Representative samples from the maspin immunohistochemical analysis are shown in Figure 2, in which cytoplasmic staining was localized in the mammary ductal epithelial cells obtained from healthy individuals (panels a and b), as well as normal tissue adjacent to neoplastic ducts (panels c and d). In contrast, the majority of DCIS specimens examined (17/30, 57%) of DCIS had completely lost maspin immunoreactivity (panels e–j).
Figure 1.
(A) Immunohistochemical analysis of maspin expression in human mammary tissue from patient 5. On the right side of the photomicrograph is a normal duct showing maspin-positive ductal epithelial cells. On the left side of the photomicrograph, a DCIS duct where the ductal epithelial cells have become maspinnegative can be seen. (B) H&E staining of an adjacent section from patient 5.
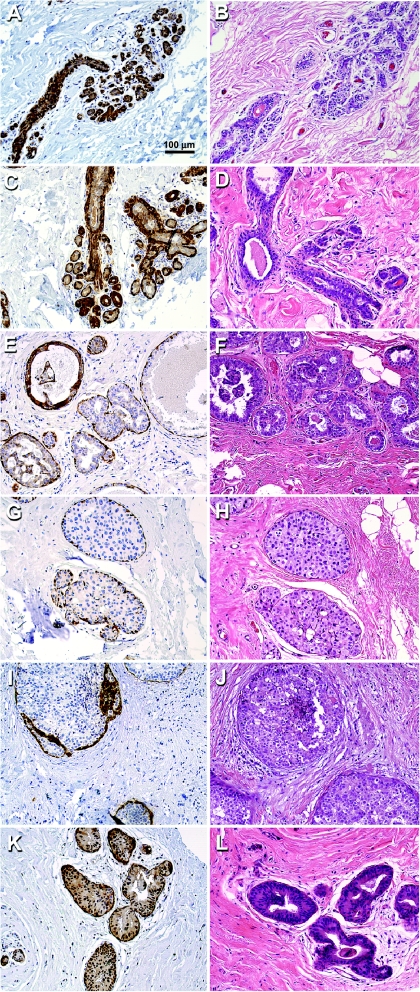
Figure 2.
Maspin immunohistochemistry and corresponding H&E staining for representative normal and DCIS specimens. (A and B) Maspin expression in normal breast tissue from a healthy individual (patient A). (C and D) Maspin expression in adjacent normal ducts taken from DCIS patient 12. (E–L) Maspin expression of neoplastic ducts in DCIS patients 2, 5, 9, and 19. Maspin promoter methylation patterns of these samples are shown in the histograms in Figure 4.
The remaining DCIS specimens were positive for maspin immunoreactivity; however, certain subtleties in maspin expression were observed. First, of the 13 DCIS specimens that were maspin-positive, 11 showed strong nuclear staining with or without cytoplasmic localization (panels k–l). These results are consistent with recent studies showing nuclear maspin staining [27,31,32], but the functional significance, if any, is unknown at present. Second, cell populations within the neoplastic ducts often showed mosaic patterns of maspin expression. Some cells were strongly positive for maspin expression only in the cytoplasm; some cells displayed maspin expression in both the cytoplasm and the nucleus; whereas other cells showed maspin expression only in the cytoplasm; and, finally, some cells were completely negative for maspin expression. In summary, the immunohistochemical analysis shows that maspin is expressed in normal mammary epithelial ductal cells; however, maspin expression is often lost or heterogeneously expressed in diseased mammary ducts.
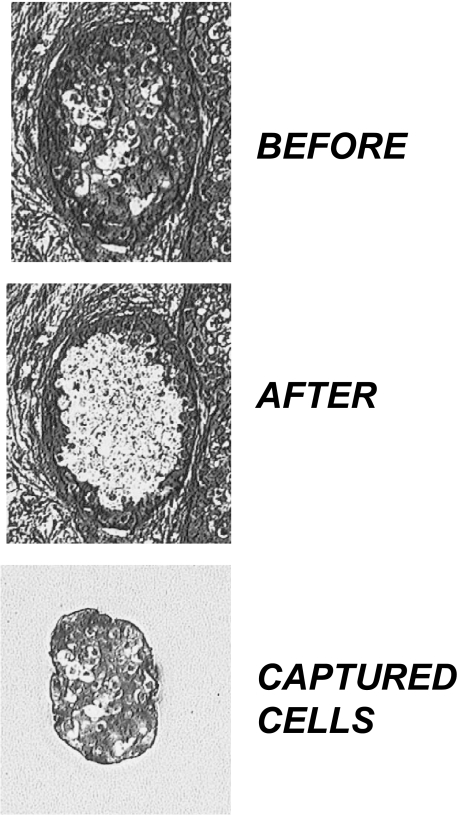
The methylation state of the maspin promoter is cell type-specific in normal cell types and is closely associated with its gene expression state [21]. As such, cytosine methylation analysis of the maspin promoter in human tissue required the use of LCM to carefully restrict the analysis to the defined target cells within the tissue. LCM proved to be an effective tool for specifically capturing ductal epithelial cells from within a DCIS lesion, as illustrated by the example shown in Figure 3. Of the 32 specimens (30 DCIS and 2 normal healthy specimens) analyzed by immunohistochemistry, adequate materials from 17 DCIS and both normal healthy samples were obtained for methylation analysis by bisulfite sequencing.
Figure 3.
LCM of neoplastic ductal epithelial tissue. Top panel shows a DCIS from patient 9 before LCM of neoplastic cells, whereas the middle panel shows the same tissue after LCM of the neoplastic cells. The bottom pane shows the captured cells that were analyzed for maspin promoter methylation using bisulfite sequencing.
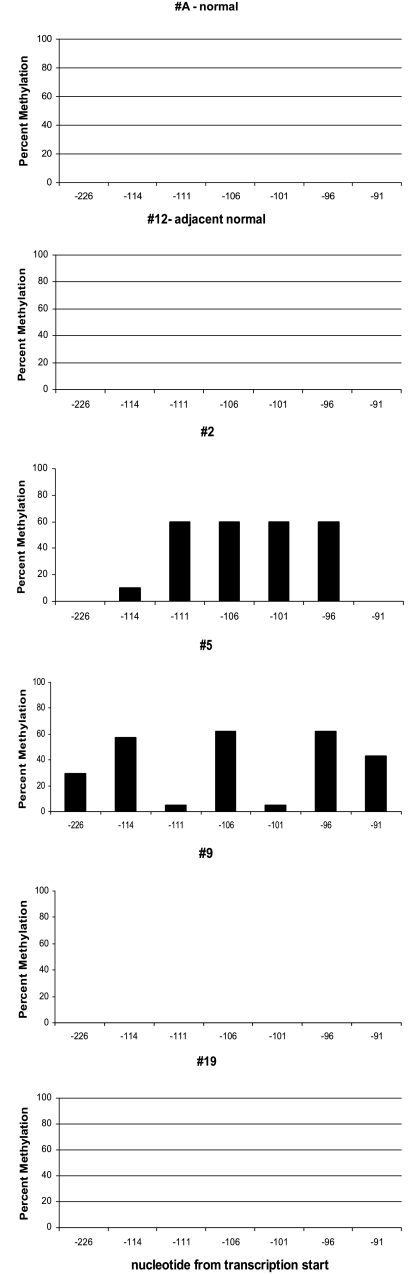
Using the laser-captured DCIS cells, as well as normal ductal epithelial cells, we sought to determine: 1) if aberrant cytosine methylation of the maspin promoter occurs in vivo; 2) if this methylation is an early event during breast carcinogenesis; and 3) if the aberrant methylation was associated with the loss of maspin expression. Using sodium bisulfite sequencing analysis, we analyzed the levels of 5-methylcytosine in seven CpG sites immediately 5′ of the transcriptional start site that have been consistently shown to be associated with the expression state of the maspin gene [20,21,33–35]. These results are shown in histogram format in Figure 4; the position of the CpG sites is shown relative to transcriptional start as obtained from the RefSeq in the UCSC genome database (-226 to -91) (http://genome.ucsc.edu), which corresponds to -103 to +32 of the maspin promoter as originally reported by Zhang et al. [29].
Figure 4.
Cytosine methylation status of the maspin promoter of the respective DCIS specimens shown in Figure 2. The methylation status of individual CpG sites was determined by comparison of the bisulfite sequence obtained with the known maspin sequence. The y-axis represents the percent methylation at the seven CpG sites in the region analyzed; the x-axis represents the nucleotide position relative to the maspin transcription start site, based on RefSeq data. Percent methylation of each site was determined by dividing the number of methylated CpG sites at a specific site by the total number of clones analyzed.
Normal human mammary ductal epithelial cells captured from sections of reduction mammoplasty specimens, or from normal ducts adjacent to tumor tissue were used as normal controls. In each case, the maspin promoter was completely unmethylated in the laser-captured maspin-positive normal breast epithelial cells (Figure 4); 0% of CpG sites were methylated in the 70 CpG sites analyzed by bisulfite sequencing. These results are consistent with previous observations that demonstrated a close link between a maspin-positive phenotype and an unmethylated maspin promoter in normal tissue [21]. In contrast, of the 11 tumors that were maspin-negative and for which adequate DNA for bisulfite sequencing was obtained, six of them displayed aberrant methylation of the maspin promoter, which we defined as >15% of CpG sites analyzed being methylated. This level of aberrant methylation was from five times to infinitely greater when compared with the levels seen in diseased mammary ducts scored as negative and to normal healthy mammary ducts (Table 1). The remaining five maspin-negative DCIS samples analyzed were scored as unmethylated, with levels of CpG methylation ranging from 0% to 3%. In summary, these results indicate that aberrant methylation of the maspin promoter is an epigenetic change that occurs early in the transformation of breast epithelial cells. Second, these results also indicate that aberrant methylation of the maspin promoter is associated with maspin gene silencing in vivo, similar to the situation observed in vitro, although it appears likely that other mechanisms participate and/or cooperate with aberrant methylation in maspin gene silencing.
Table 1.
Association between Maspin Expression and Promoter Methylation in Human DCIS.
| Code | Diagnosis | Comments | Maspin Immunoreactivity in Laser-Captured Cells* | Maspin Promoter Methylation† | % Methylation‡ |
| A | Healthy control | Reduction mammoplasty | + | - | 0 |
| B | Healthy control | Reduction mammoplasty | + | - | 0 |
| 1 | DCIS | Comedo with necrosis | - | + | 43 |
| 2 | DCIS | Comedo with necrosis | - | + | 35 |
| 3 | DCIS | Comedo with necrosis | - | + | 34 |
| 4 | DCIS | Noncomedo | - | + | 17 |
| 5 | DCIS | Comedo | - | + | 38 |
| 6 | DCIS | Comedo | - | + | 16 |
| 7 | DCIS | Comedo | - | - | 0 |
| 8 | DCIS | Comedo | - | - | 3 |
| 9 | DCIS | Comedo with necrosis | - | - | 0 |
| 10 | DCIS | Comedo | - | - | 0 |
| 11 | DCIS | Comedo with necrosis | - | - | 0 |
| 12 | DCIS | Noncomedo | - | nd | nd |
| 13 | DCIS | Comedo | - | nd | nd |
| 14 | DCIS | Comedo | - | nd | nd |
| 15 | DCIS | Noncomedo | - | nd | nd |
| 16 | DCIS | Comedo with necrosis | - | nd | nd |
| 17 | DCIS | Comedo | +/- | nd | nd |
| 18 | DCIS | Comedo | - | nd. | nd |
| 19 | DCIS | Noncomedo | +/- | - | 0 |
| 20 | DCIS | Comedo with invasion | + | nd | nd |
| 21 | DCIS | Comedo | + | nd | nd |
| 22 | DCIS | Noncomedo | + | nd | nd |
| 23 | DCIS | Noncomedo (secretory) | + | nd | nd |
| 24 | DCIS | Comedo | + | nd | nd |
| 25 | DCIS | Comedo with necrosis | + | nd | nd |
| 26 | DCIS | Comedo | + | + | 48 |
| 27 | DCIS | Comedo | +/- | + | 17 |
| 28 | DCIS | Comedo | +/- | + | 31 |
| 29 | DCIS | Comedo | +/- | + | 29 |
| 30 | DCIS | Comedo | +/- | - | 1 |
(+) Greater than 90% of epithelial cells within the DCIS lesion displayed a degree of maspin immunoreactivity at levels comparable to normal ductal epithelial cells (e.g., Figure 2A); (-) >90% of epithelial cells within the DCIS lesion displayed no maspin immunoreactivity; (+/-) DCIS lesion where maspin immunoreactivity was heterogeneous among the neoplastic epithelial cells analyzed.
Determined by bisulfite DNA sequencing: (+) >15% of CpG sites analyzed were methylated; (-) <3% of CpG sites analyzed were methylated; nd, not determined.
Percent methylation was determined by taking the number of methylated CpG sites detected by bisulfite sequencing divided by the number of CpG sites analyzed (>70 CpG sites analyzed per sample).
Based on the data tabulated in Table 1, it can be noted that a small set of DCIS specimens scored positive for maspin protein expression also displays methylation in the maspin promoter. This is likely due to the extreme heterogeneity of some of the tumors analyzed as discussed above. For example, in the immunohistochemical analysis of maspin in sample 3, neoplastic ducts displayed a remarkable diversity in their maspin expression as illustrated by the example shown in Figure 5. In this particular duct, it can be seen that a significant portion of cells shows a range of localization patterns, predominantly cytoplasmic staining, some with nuclear staining, whereas other cells were completely maspin-negative. Although not conclusive, we speculate that the methylated maspin alleles identified in such samples were derived from the maspin-negative cells.
Figure 5.
(A) Immunohistochemical analysis of maspin shows differential subcellular localization and intratumoral heterogeneity of maspin expression in DCIS patient 3. (B) H&E staining of an adjacent section from patient 3.
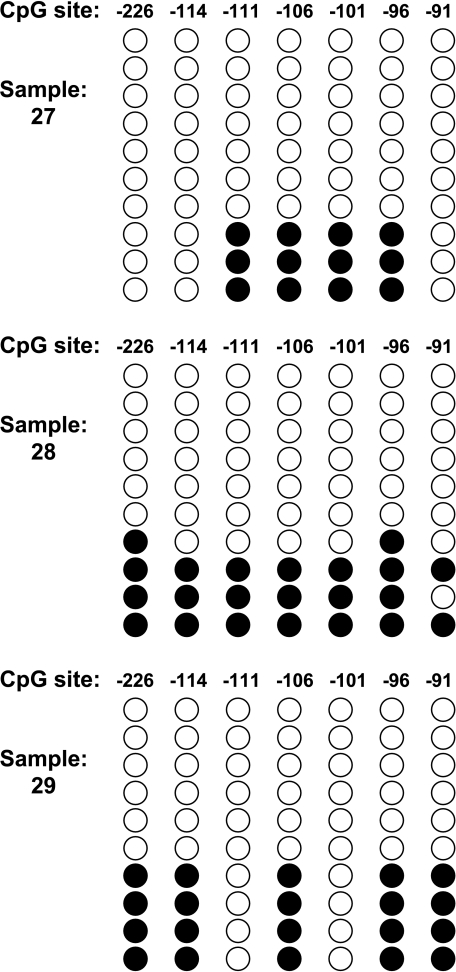
Support of this possibility is revealed in the analysis of the bisulfite sequences from samples 27, 28, and 29 (Figure 6). In this figure, the clonal analysis of the bisulfite sequencing is shown for these samples. A majority of the clones are totally devoid of methylation—the type of pattern that is seen in normal maspin-positive breast tissue. In addition, there is a minority of alleles where aberrant methylation is clearly detected. This presence of both completely unmethylated alleles and heavily methylated alleles is consistent with tumor cell heterogeneity where some cells express maspin and others do not, as seen in the immunohistochemical analysis.
Figure 6.
Methylation heterogeneity in phenotypically heterogeneous tumor samples. Location and methylation status of seven CpG sites in the maspin promoter are shown. Each circle represents a CpG site. Open circles are unmethylated CpG sites and filled circles are methylated CpG sites. Each row of circles represents the methylation results obtained from an individual clone. Each column indicates the location of the CpG site in the maspin promoter relative to transcription start.
Discussion
The maspin gene was cloned on the basis of its downregulation in breast cancer cells compared with normal cells, and subsequent functional studies as well as clinical studies have shown maspin to play a tumor-suppressor role in human breast cancer [11,13–19]. Surprisingly, there is a paradoxical activation of maspin gene expression in other tumor types, including ovarian, lung, and pancreatic cancer, and gastric intestinal metaplasia [27,31,33,34,36]. Similar to maspin gene silencing in breast cancer, inappropriate activation of maspin expression in other tumors is also linked to changes in maspin promoter methylation [33,34,37].
In this study, we analyzed 30 DCIS specimens as well as 2 normal breast specimens obtained from healthy females for maspin expression by immunohistochemistry, and 19 of these specimens were further evaluated for maspin promoter methylation by bisulfite sequencing. The data revealed that maspin expression was lost in the ductal epithelial cells in over 50% of the DCIS specimens, suggesting that loss of maspin expression can be a frequent and relatively early event in human breast carcinogenesis, which is in general agreement with an earlier study of DCIS with a smaller sample number [13]. With respect to the epigenetic statusof the maspin promoter, we found that normal ductal epithelial cells taken from healthy females, as well as normal ductal cells adjacent to neoplastic ducts, have unmethylated maspin promoters—a finding consistent with earlier studies [20]. In contrast, of the 17 DCIS specimens that were laser capture-microdissected and subsequently analyzed by bisulfite sequencing, nine displayed aberrant levels of maspin promoter methylation. In general, aberrant methylation of the maspin promoter was associated with loss of maspin immunoreactivity in the specimen; however, some samples that were scored as maspin-positive also showed an aberrant methylation of the maspin promoter. In these cases, analysis of maspin staining showed a mosaic pattern of maspin protein expression in the respective cell populations, such that some cells were maspin-positive and other cells were maspin-negative. We speculate that the aberrantly methylated maspin promoter sequences were derived from the maspin-negative cells in the population.
Finally, it should be pointed out that the region in the maspin promoter analyzed for aberrant methylation includes putative sites for a number of different transcription factors, and it remains possible that the aberrant methylation directly blocks a transcription factor from binding its cognate site. Of the transcription factors previously implicated in maspin gene expression [24,38], only the p53-binding sites and an AP1-binding site were noted in the region analyzed. As neither of these proteins has been demonstrated to be sensitive to methylation, it seems more likely that methylation indirectly blocks access of these transcription factors to their cognate sites by participating in the remodeling of chromatin to create a transcription factor-inaccessible state. Another possibility is that the loss of critical transcription factors, such as p53 or AP1, renders the region susceptible to inappropriate cytosine methylation.
In summary, although this is a relatively small study (30 DCIS and 2 normal controls), the results positively demonstrate that aberrant methylation of the maspin promoter occurs in vivo and can be an early event during human breast carcinogenesis. In addition, it appears that the aberrant methylation of the maspin promoter is linked to the loss of maspin expression in DCIS, although it is clear that aberrant promoter methylation is not the only mechanism by which maspin is silenced. Taken together, these data suggest that changes in maspin expression reflect a disruption of normal epigenetic control during neoplastic transformation, and indicate that loss of epigenetic stability is an early event in human breast carcinogenesis. In future studies, it will be worthwhile to determine if the frequent loss of maspin expression in advanced breast tumors is also associated with aberrant methylation of the maspin promoter. These important epigenetic signatures of tumorigenic progression may ultimately aid in the molecular prognostication of breast cancer.
Acknowledgements
The authors thank Nick Holtan, Adam Palazzo, and Matthew Fitzgerald for expert technical assistance.
Footnotes
We thank the Genetic Analysis and Technology Core of the University of Arizona, and the Experimental Pathology Core of the Southwest Environmental Health Sciences Center (NIEHS ES06694). M.M.O. is a recipient of a Yuma Friends of Arizona Health Sciences Award. This work was supported by ADCRC grant 6-046 and NIH grant CA65562 to B.W.F.; NIH CA75681 and The Marilyn Rozeboom Endowment from the Order of the Eastern Star to M.J.C.H.; and NIH CA73612 and CA66081 to F.E.D.
References
- 1.Baylin SB, Herman JG. DNA hypermethylation in tumorigenesis: epigenetics joins genetics. Trends Genet. 2000;16:168–174. doi: 10.1016/s0168-9525(99)01971-x. [DOI] [PubMed] [Google Scholar]
- 2.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3:415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 3.Feinberg AP, Cui H, Ohlsson R. DNA methylation and genomic imprinting: insights from cancer into epigenetic mechanisms. Semin Cancer Biol. 2002;12:389–398. doi: 10.1016/s1044-579x(02)00059-7. [DOI] [PubMed] [Google Scholar]
- 4.Herman JG. Hypermethylation of tumor suppressor genes in cancer. Semin Cancer Biol. 1999;9:359–367. doi: 10.1006/scbi.1999.0138. [DOI] [PubMed] [Google Scholar]
- 5.Costello JF, Fruhwald MC, Smiraglia DJ, Rush LJ, Robertson GP, Gao X, Wright FA, Feramisco JD, Peltomaki P, Lang JC, Schuller DE, Yu L, Bloomfield CD, Caligiuri MA, Yates A, Nishikawa R, SuHuang H, Petrelli NJ, Zhang X, O'Dorisio MS, Held WA, Cavenee WK, Plass C. Aberrant CpG-island methylation has non-random and tumour-type-specific patterns [see comments] Nat Genet. 2000;24:132–138. doi: 10.1038/72785. [DOI] [PubMed] [Google Scholar]
- 6.Melki JR, Vincent PC, Clark SJ. Concurrent DNA hypermethylation of multiple genes in acute myeloid leukemia. Cancer Res. 1999;59:3730–3740. [PubMed] [Google Scholar]
- 7.Stirzaker C, Millar DS, Paul CL, Warnecke PM, Harrison J, Vincent PC, Frommer M, Clark SJ. Extensive DNA methylation spanning the Rb promoter in retinoblastoma tumors. Cancer Res. 1997;57:2229–2237. [PubMed] [Google Scholar]
- 8.Bianco T, Chenevix-Trench G, Walsh DC, Cooper JE, Dobrovic A. Tumour-specific distribution of BRCA1 promoter region methylation supports a pathogenetic role in breast and ovarian cancer. Carcinogenesis. 2000;21:147–151. doi: 10.1093/carcin/21.2.147. [DOI] [PubMed] [Google Scholar]
- 9.Herman JG, Merlo A, Mao L, Lapidus RG, Issa JP, Davidson NE, Sidransky D, Baylin SB. Inactivation of the CDKN2/p16/MTS1 gene is frequently associated with aberrant DNA methylation in all common human cancers. Cancer Res. 1995;55:4525–4530. [PubMed] [Google Scholar]
- 10.Rice JC, Ozcelik H, Maxeiner P, Andrulis I, Futscher BW. Methylation of the BRCA1 promoter is associated with decreased BRCA1 mRNA levels in clinical breast cancer specimens. Carcinogenesis. 2000;21:1761–1765. doi: 10.1093/carcin/21.9.1761. [DOI] [PubMed] [Google Scholar]
- 11.Streuli CH. Maspin is a tumour suppressor that inhibits breast cancer tumour metastasis in vivo. Breast Cancer Res. 2002;4:137–140. doi: 10.1186/bcr437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maass N, Hojo T, Rosel F, Ikeda T, Jonat W, Nagasaki K. Down regulation of the tumor suppressor gene maspin in breast carcinoma is associated with a higher risk of distant metastasis. Clin Biochem. 2001;34:303–307. doi: 10.1016/s0009-9120(01)00220-x. [DOI] [PubMed] [Google Scholar]
- 13.Maass N, Teffner M, Rosel F, Pawaresch R, Jonat W, Nagasaki K, Rudolph P. Decline in the expression of the serine proteinase inhibitor maspin is associated with tumour progression in ductal carcinomas of the breast. J Pathol. 2001;195:321–326. doi: 10.1002/path.948. [DOI] [PubMed] [Google Scholar]
- 14.Zou Z, Anisowicz A, Hendrix MJ, Thor A, Neveu M, Sheng S, Rafidi K, Seftor E, Sager R. Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science. 1994;263:526–529. doi: 10.1126/science.8290962. [DOI] [PubMed] [Google Scholar]
- 15.Zhang M, Volpert O, Shi YH, Bouck N. Maspin is an angiogenesis inhibitor. Nat Med. 2000;6:196–199. doi: 10.1038/72303. [DOI] [PubMed] [Google Scholar]
- 16.Sheng S, Carey J, Seftor EA, Dias L, Hendrix MJ, Sager R. Maspin acts at the cell membrane to inhibit invasion and motility of mammary and prostatic cancer cells. Proc Natl Acad Sci USA. 1996;93:11669–11674. doi: 10.1073/pnas.93.21.11669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seftor RE, Seftor EA, Sheng S, Pemberton PA, Sager R, Hendrix MJ. Maspin suppresses the invasive phenotype of human breast carcinoma. Cancer Res. 1998;58:5681–5685. [PubMed] [Google Scholar]
- 18.Maass N, Biallek M, Rosel F, Schem C, Ohike N, Zhang M, Jonat W, Nagasaki K. Hypermethylation and histone deacetylation lead to silencing of the maspin gene in human breast cancer. Biochem Biophys Res Commun. 2002;297:125–128. doi: 10.1016/s0006-291x(02)02136-8. [DOI] [PubMed] [Google Scholar]
- 19.Primeau M, Gagnon J, Momparler RL. Synergistic antineoplastic action of DNA methylation inhibitor 5-AZA-2′-deoxycytidine and histone deacetylase inhibitor depsipeptide on human breast carcinoma cells. Int J Cancer. 2003;103:177–184. doi: 10.1002/ijc.10789. [DOI] [PubMed] [Google Scholar]
- 20.Domann FE, Rice JC, Hendrix MJ, Futscher BW. Epigenetic silencing of maspin gene expression in human breast cancers. Int J Cancer. 2000;85:805–810. doi: 10.1002/(sici)1097-0215(20000315)85:6<805::aid-ijc12>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 21.Futscher BW, Oshiro MM, Wozniak RJ, Holtan N, Hanigan CL, Duan H, Domann FE. Role for DNA methylation in the control of cell type specific maspin expression. Nat Genet. 2002;31:175–179. doi: 10.1038/ng886. [DOI] [PubMed] [Google Scholar]
- 22.Oshiro MM, Watts GS, Wozniak RJ, Junk DJ, Munoz-Rodriguez JL, Domann FE, Futscher BW. Mutant p53 and aberrant cytosine methylation cooperate to silence gene expression. Oncogene. 2003;22:3624–3634. doi: 10.1038/sj.onc.1206545. [DOI] [PubMed] [Google Scholar]
- 23.Zhang W, Zhang M. Tissue microarray analysis of maspin expression and its reverse correlation with mutant p53 in various tumors. Int J Oncol. 2002;20:1145–1150. [PubMed] [Google Scholar]
- 24.Zou Z, Gao C, Nagaich AK, Connell T, Saito S, Moul JW, Seth P, Appella E, Srivastava S. p53 regulates the expression of the tumor suppressor gene maspin. J Biol Chem. 2000;275:6051–6054. doi: 10.1074/jbc.275.9.6051. [DOI] [PubMed] [Google Scholar]
- 25.Boltze C, Schneider-Stock R, Meyer F, Peters B, Quednow C, Roessner A, Roessner A. Maspin in thyroid cancer: its relationship with p53 and clinical outcome. Oncol Rep. 2003;10:1783–1787. [PubMed] [Google Scholar]
- 26.Dokras A, Gardner LM, Kirschmann DA, Seftor EA, Hendrix MJ. The tumour suppressor gene maspin is differentially regulated in cytotrophoblasts during human placental development. Placenta. 2002;23:274–280. doi: 10.1053/plac.2001.0784. [DOI] [PubMed] [Google Scholar]
- 27.Sood AK, Fletcher MS, Gruman LM, Coffin JE, Jabbari S, Khalkhali-Ellis Z, Arbour N, Seftor EA, Hendrix MJ. The paradoxical expression of maspin in ovarian carcinoma. Clin Cancer Res. 2002;8:2924–2932. [PubMed] [Google Scholar]
- 28.Clark SJ, Harrison J, Paul CL, Frommer M. High sensitivity mapping of methylated cytosines. Nucleic Acids Res. 1994;22:2990–2997. doi: 10.1093/nar/22.15.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang M, Magit D, Sager R. Expression of maspin in prostate cells is regulated by a positive ets element and a negative hormonal responsive element site recognized by androgen receptor. Proc Natl Acad Sci USA. 1997;94:5673–5678. doi: 10.1073/pnas.94.11.5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pemberton PA, Tipton AR, Pavloff N, Smith J, Erickson JR, Mouchabeck ZM, Kiefer MC. Maspin is an intracellular serpin that partitions into secretory vesicles and is present at the cell surface. J Histochem Cytochem. 1997;45:1697–1706. doi: 10.1177/002215549704501213. [DOI] [PubMed] [Google Scholar]
- 31.Smith SL, Watson SG, Ratschiller D, Gugger M, Betticher DC, Heighway J. Maspin—the most commonly-expressed gene of the 18q21.3 serpin cluster in lung cancer—is strongly expressed in preneoplastic bronchial lesions. Oncogene. 2003;22:8677–8687. doi: 10.1038/sj.onc.1207127. [DOI] [PubMed] [Google Scholar]
- 32.Mohsin SK, Zhang M, Clark GM, CraigAllred D. Maspin expression in invasive breast cancer: association with other prognostic factors. J Pathol. 2003;199:432–435. doi: 10.1002/path.1319. [DOI] [PubMed] [Google Scholar]
- 33.Akiyama Y, Maesawa C, Ogasawara S, Terashima M, Masuda T. Cell-type-specific repression of the maspin gene is disrupted frequently by demethylation at the promoter region in gastric intestinal metaplasia and cancer cells. Am J Pathol. 2003;163:1911–1919. doi: 10.1016/S0002-9440(10)63549-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fitzgerald M, Oshiro M, Holtan N, Krager K, Cullen JJ, Futscher BW, Domann FE. Human pancreatic carcinoma cells activate maspin expression through loss of epigenetic control. Neoplasia. 2003;5:427–436. doi: 10.1016/s1476-5586(03)80045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boltze C, Schneider-Stock R, Quednow C, Hinze R, Mawrin C, Hribaschek A, Roessner A, Hoang-Vu C. Silencing of the maspin gene by promoter hypermethylation in thyroid cancer. Int J Mol Med. 2003;12:479–484. [PubMed] [Google Scholar]
- 36.Maass N, Hojo T, Ueding M, Luttges J, Kloppel G, Jonat W, Nagasaki K. Expression of the tumor suppressor gene maspin in human pancreatic cancers. Clin Cancer Res. 2001;7:812–817. [PubMed] [Google Scholar]
- 37.Ohike N, Maass N, Mundhenke C, Biallek M, Zhang M, Jonat W, Luttges J, Morohoshi T, Kloppel G, Nagasaki K. Clinicopathological significance and molecular regulation of maspin expression in ductal adenocarcinoma of the pancreas. Cancer Lett. 2003;199:193–200. doi: 10.1016/s0304-3835(03)00390-2. [DOI] [PubMed] [Google Scholar]
- 38.Zhang M, Maass N, Magit D, Sager R. Transactivation through Ets and AP1 transcription sites determines the expression of the tumor-suppressing gene maspin. Cell Growth Differ. 1997;8:179–186. [PubMed] [Google Scholar]