Abstract
To further delineate ultraviolet A (UVA) signaling pathways in the human keratinocyte cell line HaCaT, we examined the potential role of mitogen-activated protein kinases (MAPKs) in UVA-induced activator protein-1 (AP-1) transactivation and c-Fos expression. UVA-induced phosphorylation of p38 and c-Jun N-terminal kinase (JNK) proteins was detected immediately after irradiation and disappeared after approximately 2 hours. Conversely, phosphorylation of extracellular signal-regulated kinase was significantly inhibited for up to 1 hour post-UVA irradiation. To examine the role of p38 and JNK MAPKs in UVA-induced AP-1 and c-fos transactivations, the selective pharmacologic MAPK inhibitors, SB202190 (p38 inhibitor) and SP600125 (JNK inhibitor), were used to independently treat stably transfected HaCaT cells in luciferase reporter assays. Both SB202190 and SP600125 dose-dependently inhibited UVA-induced AP-1 and c-fos transactivations. SB202190 (0.25–0.5 µM) and SP600125 (62–125 nM) treatments also primarily inhibited UVA-induced c-Fos expression. These results demonstrated that activation of both JNK and p38 play critical role in UVA-mediated AP-1 transactivation and c-Fos expression in these human keratinocyte cells. Targeted inhibition of these MAPKs with their selective pharmacologic inhibitors may be effective chemopreventive strategies for UVA-induced nonmelanoma skin cancer.
Keywords: UVA, AP-1, MAPK, c-Fos, HaCaT
Introduction
Exposure to sunlight is the major risk factor for the development of nonmelanoma skin cancer [1]. Because stratospheric ozone completely absorbs short-wavelength ultraviolet C (<280 nm), the relevant carcinogenic components of sunlight that reach the Earth's surface are ultraviolet B (UVB) (280–320 nm) and ultraviolet A (UVA) (320–400 nm) [2,3]. Although UVA is magnitudes less carcinogenic than UVB [4,5], chronic UVA exposure has been shown to induce photoaging [6] and skin tumors (papillomas and squamous cell carcinomas) [5,7,8] in experimental animals.
The signaling pathways involved in UVA-induced photo-aging and skin tumorigenesis are subjects of intense interest and have not been fully elucidated. UVA has been shown to alter the expression of numerous mammalian genes, such as heme oxygenase-1 [9], intercellular adhesion molecule-1 (ICAM-1) [10], and matrix metalloproteinase-1 [11], through the generation of reactive oxygen intermediates, specifically singlet O2 [12]. UVA irradiation has also been reported to activate several transcription factors, including activator protein-1 (AP-1) [13–15], activator protein-2 (AP-2) [10], nuclear factor kappa-B (NFκB) [16,17], and signal transducer and activator of transcription (STAT) 1 and 3 [18,19]. Both UVA-induced AP-1 and AP-2 activations were mediated through singlet oxygen [10,14].
AP-1 is an important regulatory protein involved in cell growth, differentiation, transformation, and apoptosis, and may also contribute to inflammatory and immune responses [20,21]. It can be induced by growth factors, cytokines, 12-O-tetradecanoylphorbol-13-acetate (TPA), UV radiation, and transforming oncoproteins [20]. The AP-1 complex consists of heterodimers of Fos (c-Fos, Fra-1, Fra-2, and FosB) and Jun (c-Jun, JunB, and JunD) family members, or homodimers and heterodimers of Jun family members that bind to TPA response elements (TREs) in AP-1-inducible gene promoters, contributing to transcriptional activity or repression of these genes [20].
Deregulated expression of the AP-1 complex has been shown to play a prominent role in skin tumor promotion. Loss of AP-1 DNA-binding activity resulted in loss of proliferative potential and induction of differentiation in human keratinocytes [22]. Dong et al. [23] demonstrated that anchorage-independent growth of JB6 cells required the transactivation of AP-1.
Deregulated expression of individual AP-1 complex components has also been shown to induce malignant transformation in vivo. Transfection of v-fos into murine papilloma cell lines expressing an activated Ha-ras oncogene resulted in the malignant conversion of these cells [24]. Similarly, the development of malignant skin tumors in v-Ha-ras transgenic mice following TPA treatment was inhibited in c-fos -/- mice [25]. Young et al. [26] also reported that TPA-mediated promotion in a two-stage skin carcinogenesis mouse model was suppressed by the stable expression of an epidermis-targeted dominant negative c-jun transgene. Similarly, Thompson et al. [27] demonstrated that the expression of the same epidermis-targeted dominant negative c-jun transgene inhibited okadaic acid-mediated skin tumor promotion.
The mitogen-activated protein kinase (MAPK) family of proteins include p38, c-Jun N-terminal kinase (JNK), and extracellular signal-regulated kinase (ERK). MAPKs are proline-directed serine/threonine kinases that are activated by dual phosphorylation on threonine and tyrosine residues in response to a wide variety of extracellular stimuli [28]. They mediate signal transduction from the cell surface to the nucleus. Activation of ERK is primarily involved in growth factor- and phorbol ester-stimulated responses. Responses to proinflammatory cytokines, UV radiation, and other stresses are mostly dependent on JNK and p38 activation [21,29]. MAPK signaling pathways have been shown to affect AP-1 activity by direct phosphorylation of AP-1 proteins and by influence on the abundance of individual AP-1 components in a cell [29,30].
c-Jun is directly phosphorylated by JNK at N-terminal serines 63 and 73, resulting in increased stability and transactivation potential [31,32]. c-Jun is also phosphorylated by ERK1/2 on C-terminal inhibitory sites [33,34]. Although the in vivo relevance is still unclear, ERK1/2 can also phosphorylate c-Fos and ATF-2 [28,35].
MAPKs also increase the abundance of AP-1 complex components by transcriptionally activating their promoters. The ternary complex factors Elk-1 (a substrate of ERK, p38, and JNK) and serum response factor accessory protein (SAP) 1a and 2 (substrates of ERK and p38) form complexes with dimeric serum response factors at the serum response element (SRE) in the c-fos promoter [28,30]. Additionally, activation of STAT1 and STAT3 by JNK [18] and possibly ERK [30,36] at the cis-inducible element in the c-fos promoter may act in cooperation with the SRE to influence c-fos expression [30]. Induction of c-jun expression is predominantly mediated by two TREs that preferentially bind c-Jun and ATF-2 heterodimers. These proteins are activated by phosphorylation in their transactivation domains [29]. JNK and p38 phosphorylate and activate ATF-2, whereas JNK phosphorylates the c-Jun activation domain [37,38]. Additionally, ERK and p38 activation may contribute to c-jun expression through phosphorylation of MEF2 proteins—transcription factors that also bind to the c-jun promoter [39,40].
Although a few studies have addressed the potential role of MAPK activation in UVA signaling, results are not consistent. Klotz et al. [12] reported a rapid and transient induction of p38 and JNK activity, but not ERK activity, in human skin fibroblasts. In contrast, UVA irradiation stimulated the activation of all three MAPKs in the NCTC 2544 human keratinocyte cell line [41] and in the mouse epidermal JB6 promotion-sensitive Cl 41 cell line [42,43]. Additionally, Djavaheri-Mergny and Dubertret [14] provided evidence that UVA-induced AP-1 activation required the Raf/ERK pathway in NCTC 2544 keratinocytes.
Our previous results demonstrated that UVA irradiation of the human immortalized keratinocyte cell line HaCaT induced the expression of several AP-1 family members, including c-Fos and c-Jun, and potentiated the transactivation of the c-fos promoter and the AP-1-binding site in the collagenase-1 gene promoter [15]. To further delineate the UVA signaling pathway(s) in these keratinocytes, we examined the potential role of MAPKs in UVA-induced AP-1 transactivation and c-fos expression through the use of specific pharmacologic MAPK inhibitors. We report, for the first time, that p38 and JNK MAPKs contributed to UVA-induced AP-1 activation and UVA-induced c-fos transactivation as well as UVA-induced c-Fos protein expression. The use of SB202190 and SP600125 to selectively inhibit their respective UVA-induced stress-activated protein kinases may be a useful chemopreventive strategy for UVA-induced nonmelanoma skin cancer.
Materials and Methods
Cell Culture
The human keratinocyte cell line, HaCaT, was stably transfected with a sequence from the human collagenase-1 gene promoter (-73 to +63) containing one endogenous AP-1-binding site driving a luciferase reporter gene (HCL14 cells), as reported previously [44]. HaCaT cells were also independently stably transfected with a sequence from the human c-fos promoter (-404 to +41) driving a luciferase reporter gene (FL30 cells), as reported previously [44]. These cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum and 100 U/ml penicillin/streptomycin at 37°C and 5% CO2. Cells were grown to near confluence and then serum-starved for 24 to 28 hours prior to treatment. Treatment of cells with selective pharmacologic MAPK inhibitors involved a 1-hour preirradiation incubation and variable postirradiation incubations. Each compound was diluted in serum-free medium prior to use.
Pharmacologic Inhibitors of MAPKs
SB202190 (Calbiochem, San Diego, CA) is a potent, cell-permeable, selective, and reversible inhibitor of p38α and β1 isoforms. Inhibition is competitive with ATP and requires substrates to accommodate the fluorophenyl ring structure of the pyridinyl imidazole in their ATP-binding pocket. Structures of other p38 isoforms, JNKs, and ERK1/2 do not allow inhibitor binding at equivalent positions [45].
SP600125 (Calbiochem) is a potent, cell-permeable, selective, and reversible inhibitor of JNK1, JNK2, and JNK3 isoforms. Inhibition is competitive with ATP and may involve the interaction of the nitrogen-containing ring system of the anthrapyrazolone with key residues in the kinase active site of substrates [46].
PD98059 (Alexis Biochemicals, Carlsbad, CA) is a selective, cell-permeable inhibitor of MEK1, MEK2, and MEK5 that does not directly inhibit kinase activity but rather prevents the activation of the kinase [45,47,48]. It has also been shown to directly inhibit cyclooxygenase-1 and cyclooxygenase-2 enzyme activities [49].
UVA Irradiation
Cells were irradiated with a bank of four F20T12/BL/HO UVA bulbs (National Biological, Twinsburg, OH) that were powered by an Advance electronic ballast REL-4P32-RH-TP (120 V, 60 Hz, 90 A) (Advance Transformer Co., Chicago, IL). Spectral emission was reported previously [15]. A UVX radiometer equipped with a UVX-36 sensor (UVP, Upland, CA) was used to measure radiation doses. Plate glass (4 mm) was used to filter wavelengths below 320 nm. Irradiation was performed in a sterile, well-ventilated laminar flow hood to eliminate thermal stimulation. Cells were irradiated in phosphate-buffered saline (PBS) supplemented with 0.01% MgCl2 and 0.01% CaCl2 at room temperature. Control cells were mock-irradiated under similar conditions. Cultures were continued in serum-free DMEM until harvest.
Luciferase Assay for AP-1 and c-fos Transactivations
Total cellular protein from stably transfected HaCaT cells was extracted in lysis buffer (15 mM MgSO4, 25 mM glycylglycine, 4 mM EGTA, 1% vol/vol Triton X-100, and 1 mM DTT) and was quantitated using the Bio-Rad Dc Protein Assay kit (Bio-Rad Laboratories, Hercules, CA). Luciferase activity of 20 to 30 µg of total cellular protein was measured using the TD-20/20 Luminometer (Turner Designs, Sunnyvale, CA).
Western Analysis
Total cellular protein was extracted in lysis buffer (150 mM NaCl, 20 mM Tris, pH 7.5, 1 mM EDTA, 1 mM EGTA, 2.5 mM Na4P2O7, 1 mM β-glycerol phosphate, 1 mM Na3VO4, 1 µg/ml leupeptin, and 1% Triton X-100) supplemented with additional phosphatase inhibitors (1 mM Na3VO4, 2 µg/ml leupeptin, and 10 µg/ml aprotinin) and was quantitated using the Bio-Rad Dc Protein Assay kit (Bio-Rad Laboratories). Lysates (40 µg) were resolved on 12.5% sodium dodecyl sulfate (SDS) polyacrylamide gels and were then transferred to Immobilon-P nylon membranes (Millipore, Bedford, MA). Membranes were blocked with 5% evaporated milk in 1 x TBS/0.05% Tween 20 (TBST) for 2 hours at room temperature. Primary antibodies against AP-1 family members (Santa Cruz Biotechnology, Santa Cruz, CA) were diluted at 1:1000 (c-Fos) or 1:500 (c-Jun), primary antibody against a-tubulin (Oncogene Research Products, Boston, MA) was diluted at 1:2500, and primary antibodies against p38, JNK, and ERK MAPKs (Cell Signaling, Beverly, MA) were diluted at 1:2000 in 5% evaporated milk/TBST and were incubated with membranes for 2 hours at room temperature. Primary antibodies against phospho-MAPKs (Cell Signaling) and phospho-c-Jun (Cell Signaling) were diluted at 1:800 (phospho-p38) or 1:1000 in 5% evaporated milk/TBST and were incubated with membranes overnight at 4°C. Anti-rabbit HRP-conjugated secondary antibodies (Cell Signaling) were diluted at 1:2000, whereas anti-mouse HRP-conjugated secondary antibodies (Santa Cruz Biotechnology) were diluted at 1:5000 (c-Fos) and 1:8000 (α-tubulin) in 5% evaporated milk/TBST and were incubated with membranes for 1 hour at room temperature. Membranes were washed three times for 10 minutes in TBST after each antibody incubation. Protein bands were visualized using the ECL kit (Amersham Pharmacia Biotech, Piscataway, NJ).
In Vivo p38 Activity Assay
The in vivo p38 activity assay was reported previously [50]. Briefly, total cellular protein was extracted in lysis buffer (150 mM NaCl, 20 mM Tris, pH 7.5, 1 mM EDTA, 1 mM EGTA, 2.5 mM Na4P2O7, 1 mM β-glycerol phosphate, 1 mM Na3VO4, 1 µg/ml leupeptin, and 1% Triton X-100) supplemented with additional phosphatase inhibitors (1 mM Na3VO4, 2 µg/ml leupeptin, and 10 µg/ml aprotinin) and was quantitated using the Bio-Rad Dc Protein Assay kit (Bio-Rad Laboratories). Lysates (10 µg) were resolved on 12.5% SDS polyacrylamide gels overnight at 4°C and were then transferred to Immobilon-P nylon membranes (Millipore). The membrane was blocked with 5% evaporated milk/1x TBS/0.1% Tween 20 for 1 hour at room temperature and was then washed three times for 5 minutes in 1x TBS/0.1% Tween 20. MAPK-activated protein kinase-2 (MAPKAPK2) primary antibody (Cell Signaling) was diluted at 1:1000 in 5% BSA/1x TBS/0.1% Tween 20 and was incubated with the membrane overnight at 4°C. HRP-conjugated secondary antibody (Cell Signaling) was diluted at 1:2000 in 5% evaporated milk/1x TBS/0.1% Tween 20 and was incubated with the membrane for 1 hour at room temperature. The membrane was washed three times for 5 minutes in 1x TBS/0.1% Tween 20 after each antibody incubation. Protein bands were visualized using the ECL kit (Amersham Pharmacia Biotech). Both phosphorylated and unphosphorylated bands were detected.
Results
Effects of UVA Irradiation on the Activation of MAPKs
As reported previously, maximal AP-1 transactivation in the stably transfected HaCaT cells was achieved with a dose of 250 kJ/m2 UVA. This dose of UVA increased AP-1 transactivation levels between 2 and 8 hours post-UVA [15]. Therefore, time courses for Western analyses to detect the UVA-induced activation of MAPKs were performed on these cells using 250 kJ/m2 UVA, beginning immediately after irradiation and spanning 8 hours post-UVA irradiation (Figure 1).
Figure 1.
Western time course for UVA-induced MAPK activation. HCL14 cells were mock-irradiated or irradiated with 250 kJ/m2 UVA and harvested at the appropriate time points postirradiation. Forty micrograms of total cell lysate was electrophoresed on 12.5% SDS polyacrylamide gels, transferred to Immobilon-P membranes, and immunodetected using optimal primary and secondary antibody concentrations for each MAPK. Western blot data are representative of at least two independent experiments. (A) p38; (B) JNK; and (C) ERK.
Phosphorylation of p38 was detected immediately after UVA irradiation and persisted for approximately 1 hour, with only very weak activation detected at 2, 4, and 8 hours postirradiation (Figure 1A). Total p38 levels were unaffected by UVA irradiation. Increased phosphorylation of JNK was detected immediately after irradiation and disappeared after approximately 2 hours, with maximal expression between 15 and 30 minutes postirradiation (Figure 1B). UVA irradiation did not affect basal levels of JNK. Interestingly, UVA irradiation transiently but consistently inhibited the activation of ERK. This inhibition was detected immediately after irradiation and persisted for up to 1 hour post-UVA (Figure 1C). Conversely, ERK was transiently activated in mock-treated cells. This activation may have been due to the environmental change experienced by the cells after irradiation, specifically the switch from supplemented PBS to serum-free medium. Despite this activation, ERK phosphorylation was consistently lower in irradiated lysates compared to controls. Basal levels of ERK expression were unaffected by UVA irradiation.
Target Specificity of SB202190
Concentrations of SB202190 as high as 15 µM have been used in HaCaT cells to assess the role of p38 α/β isoforms in UV-induced signaling pathways [50–53]. However, much lower doses of this compound (2 µM) have been shown to inhibit UVA-induced p38 activity [53]. In vivo p38 activity assays were performed using even lower doses of SB202190 (0.25 and 0.5 µM) in HCL14 cells to ensure that these doses were also inhibitory. At 30 minutes post-UVA irradiation, the p38 MAPK substrate MAPKAPK2 was primarily phosphorylated, whereas MAPKAPK2 in mock-treated cells was unphosphorylated. Treatment with a combination of UVA and SB202190 resulted in detection of both phosphorylated and unphosphorylated forms of MAPKAPK2, indicating that SB202190 reduced the capacity of p38 to phosphorylate and activate MAPKAPK2 (Figure 2A).
Figure 2.
Target specificity of SB202190. HCL14 cells were pretreated with SB202190 in serum-free medium for 1 hour, mock-irradiated or irradiated with 250 kJ/m2 UVA, and then continued in serum-free medium supplemented with SB202190 until each desired time point. Ten micrograms (MAPKAPK2) or 40 µg (phospho-JNK and phospho-ERK) of total cell lysate was electrophoresed on 12.5% SDS polyacrylamide gels, transferred to Immobilon-P membranes, and immunodetected using optimal primary and secondary antibody concentrations for the target proteins. Western blot data are representative of at least two independent experiments. (A) Effect of SB202190 on p38 activity through detection of changes in MAPKAPK2 phosphorylation; (B) effect of SB202190 on JNK activation; and (C) effect of SB202190 on ERK activation.
To determine the target specificity of SB202190, a time point was selected for each MAPK that would allow for the detection of inhibition of UVA-mediated forms of the kinases by the pharmacologic inhibitor. No effects on JNK phosphorylation were detected with 0.25 or 0.5 µM SB202190 at 30 minutes post-UVA irradiation (Figure 2B). Minimal effects of SB202190 on ERK phosphorylation were observed, with band intensity decreasing slightly in both unirradiated and irradiated groups at 15 minutes postirradiation (Figure 2C).
Target Specificity of SP600125
The anthrapyrazolone SP600125 has been recently marketed as a potent, selective, and reversible inhibitor of all three JNK isoforms. The inhibitory activity of SP600125 in Jurkat T cells and primary human monocytes required IC50 doses between 5 and 10 µM [46]. However, in HaCaT cells, much lower doses were sufficient to inhibit UVA-induced JNK activity and c-Jun expression. We previously showed that c-Jun was biphasically activated by UVA irradiation. Preexisting c-Jun protein was phosphorylated at early time points, followed by maximal c-Jun expression between 2 and 4 hours after UVA irradiation [15]. The JNK inhibitor SP600125 diminished UVA-induced JNK activity as well as c-Jun expression with a dose of 125 nM at 2 hours postirradiation (Figure 3A). The effect on total c-Jun levels might be a consequence of c-Jun autoregulation through the TRE-binding sites in the c-Jun promoter [54]. SP600125 treatment of these human keratinocytes in combination with UVA resulted in the appearance of a slower-migrating, unidentified band that may represent a modified form of c-Jun. These doses of SP600125 did not significantly affect p38 or ERK1/2 phosphorylation at 15 minutes postirradiation (Figure 3, B and C, respectively).
Figure 3.
Target specificity of SP600125. HCL14 cells were pretreated with a SP600125 in serum-free medium for 1 hour, mock-irradiated or irradiated with 250 kJ/m2 UVA, and then continued in serum-free medium supplemented with SP600125 until each desired time point. Forty micrograms of total cell lysate was electrophoresed on 12.5% SDS polyacrylamide gels, transferred to Immobilon-P membranes, and immunodetected using optimal primary and secondary antibody concentrations for the target proteins. Western blot data are representative of at least two independent experiments. (A) Effect of SP600125 on JNK activity as detected by changes in c-Jun phosphorylation and expression; (B) effect of SP600125 on p38 activation; and (C) effect of SP600125 on ERK activation.
Effects of SB202190 and SP600125 on UVA-Induced AP-1 Transactivation
As previously reported, maximal AP-1 transactivation was achieved with a dose of 250 kJ/m2 at 4 hours post-UVA irradiation [15]. Therefore, these experimental conditions were used to examine the downstream effects of p38 and JNK MAPK inhibition. Independent dose responses with SB202190 and SP600125 were performed to determine the potential involvement of p38 and/or JNK in UVA-induced AP-1 transactivation using HaCaT cells stably transfected with the collagenase promoter driving a luciferase reporter gene (HCL14 cells). As shown in Figure 4A, UVA-induced AP-1 transactivation was significantly and dose-dependently blocked with SB202190 treatment at 4 hours post-UVA irradiation. Doses as low as 0.25 µM inhibited UVA-induced AP-1 transactivation by as much as 64% compared to controls. SP600125 also significantly and dose-dependently blocked UVA-induced AP-1 transactivation at this time point (Figure 4B). A 78-nM dose of SP600125 resulted in an approximately 51% reduction in UVA-induced AP-1 transactivation compared to controls. The increased AP-1 activity in unirradiated cells between 62 and 109 nM SP600125 in this representative experiment was not consistently observed. Basal levels of AP-1 activity tended to bobble around control levels in additional experiments.
Figure 4.
Effects of SB202190 and SP600125 on UVA-induced AP-1 transactivation. HCL14 cells were pretreated with a MAPK inhibitor in serum-free medium for 1 hour, mock-irradiated or irradiated with 250 kJ/m2 UVA, and then continued in serum-free medium supplemented with the respective doses of inhibitor for 4 hours postirradiation. Thirty micrograms of total cell lysate was analyzed for each luciferase reporter assay. Fold inductions with respect to mock-treated controls are listed above their respective bars for each dose. Each bar represents the mean±SD of triplicate samples. Data are representative of at least two independent experiments. (A) SB202190; and (B) SP600125.
Effects of SB202190 and SP600125 on UVA-Induced c-fos Transactivation
To determine whether p38 and JNK were involved in UVA-induced c-fos transactivation, HaCaT cells that were stably transfected with the human c-fos promoter driving a luciferase reporter gene (FL30 cells) were treated with varying doses of their pharmacologic inhibitors in combination with 250 kJ/m2 UVA. As shown in Figure 5A, UVA-induced c-fos transactivation was significantly and dose-dependently blocked with SB202190 at 4 hours post-UVA irradiation. The effects of drug combined with UVA irradiation paralleled the effects observed with this combination treatment on AP-1 transactivation, with a dose of 0.25 µM SB202190 reducing UVA-induced c-fos transactivation by 61% compared to controls. SP600125 also significantly and dose-dependently blocked UVA-induced c-fos transactivation (Figure 5B). Although SP600125 dose-dependently inhibited both AP-1 and c-fos transactivations, 62 nM SP600125 consistently blocked UVA-induced c-fos transactivation (approximately 39% of control values) but did not effectively block UVA-induced AP-1 transactivation. In fact, in some experiments, this dose of SP600125 appeared to slightly enhance AP-1 transactivation.
Figure 5.
Effects of SB202190 and SP600125 on UVA-induced c-fos transactivation. FL30 cells were pretreated with a MAPK inhibitor in serum-free medium for 1 hour, mock-irradiated or irradiated with 250 kJ/m2 UVA, and then continued in serum-free medium supplemented with the respective doses of inhibitor for 4 hours postirradiation. Thirty micrograms of total cell lysate was analyzed for each luciferase reporter assay. Fold inductions with respect to mock-treated controls are listed above their respective bars for each dose. Each bar represents the mean±SD of triplicate samples. Data are representative of at least two independent experiments. (A) SB202190; and (B) SP600125.
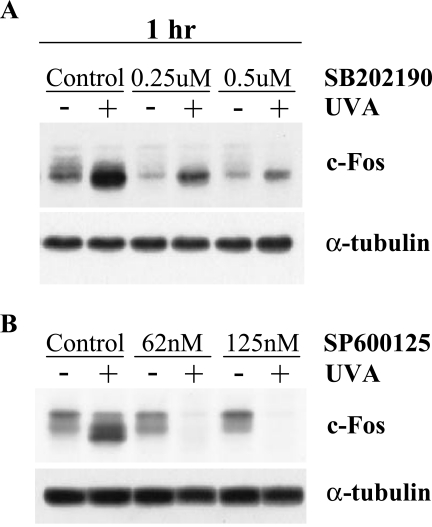
Effects of SB202190 and SP600125 on c-Fos Expression
To further explore the downstream effects of p38 and JNK inhibition in irradiated and unirradiated keratinocytes, changes in c-Fos expression were examined. At 1 hour postirradiation, the dose of SB202190 that blocked both AP-1 and c-fos transactivations also significantly inhibited UVA-induced c-Fos expression and only marginally affected basal c-Fos expression (Figure 6A). Doses of SP600125 that reduced UVA-induced c-fos transactivation were also able to block UVA-induced c-Fos expression, whereas basal c-Fos expression was not affected by drug treatment (Figure 6B). The observed effects of these MAPK inhibitors on activation of the c-fos promoter and c-Fos protein expression were highly correlative in these human keratinocytes.
Figure 6.
Effects of SB202190 and SP600125 on UVA-induced c-Fos protein expression. HCL14 cells were pretreated with a MAPK inhibitor in serum-free medium for 1 hour, mock-irradiated or irradiated with 250 kJ/m2 UVA, and then continued in serum-free medium supplemented with the respective inhibitor for 1 hour. Forty micrograms of total cell lysate was electrophoresed on 12.5% SDS polyacrylamide gels, transferred to Immobilon-P membranes, and immunodetected using optimal primary and secondary antibody concentrations for c-Fos. Western blot data are representative of two independent experiments. (A) SB202190; and (B) SP600125.
Discussion
A prominent role for UVA in skin tumor promotion has recently emerged, involving altered expression of mammalian genes through activation of transcription factors such as AP-1, AP-2, NFκB, and STATs [10,13–19]. UVA irradiation was previously shown to induce AP-1 DNA binding and AP-1 transactivation in the human keratinocyte cell line HaCaT. This induction correlated with UVA-induced c-fos promoter activation and c-Fos protein expression [15]. To further delineate the UVA signaling pathway(s) in these immortalized keratinocytes, we examined potential roles for p38, JNK, and ERK MAPKs in the mediation of UVA-induced AP-1 and c-fos promoter activations as well as UVA-induced c-Fos expression.
UVA irradiation (250 kJ/m2) rapidly and transiently induced p38 and JNK activation but inhibited ERK activation. Although peak activation or inhibition of UVA-induced MAPK activities differed slightly, all three kinases returned to basal levels by 2 hours postirradiation. These combined effects of UVA on MAPK activities may have temporarily halted proliferative signaling and allowed the keratinocytes to respond to the environmental stress through the p38 and JNK pathways before progressing through the cell cycle. Interestingly, basal levels of ERK activity were induced up to 15 minutes post-UVA irradiation. This induction may have been due to the environmental change experienced by the cells after the long irradiation period. Despite this induction, ERK activity was consistently lower in UVA-irradiated samples compared to controls. Basal levels of activated p38 and JNK were not affected by the transition. Total MAPK protein levels were not affected by UVA irradiation.
Studies that have addressed the potential roles of MAPKs in UVA signaling have been inconsistent. In the NCTC 2544 human keratinocyte cell line, UVA-induced ERK, JNK, and p38 activation through the generation of oxidative stress [14,41]. UVA-mediated AP-1 DNA binding and transcriptional activity through the Raf/ERK cascade, with dose-dependent ERK activation considerably declining up to 240 kJ/m2 UVA [14]. In mouse epidermal JB6 promotion-sensitive Cl 41 cells, UVA also activated ERK, JNK, and p38 MAPKs at doses between 40 and 160 kJ/m2 [42,43]. Klotz et al. examined MAPK activation patterns induced by singlet oxygen and UVA in human skin fibroblasts. With 300 kJ/m2 UVA, the authors reported a rapid and transient induction of p38 and JNK but no effect on ERK activation [12,55]. Although UVA irradiation did not appear to mediate ERK activity, unirradiated controls for each time point were not reported. Interestingly, intracellularly generated singlet oxygen activated p38 and JNK but appeared to inhibit ERK activity [12]. Similarly, Zhang et al. observed the UVA-induced activation of p38 and JNK but no activation of ERK in the normal human lymphoblast cell line JY. At 60 minutes post-UVA irradiation, however, these cells appeared to have decreased levels of ERK phosphorylation in comparison to controls [56].
The pyridinyl imidazole SB202190 and the anthrapyrazolone SP600125 were selected to implicate p38α/β and JNK, respectively, as components in the UVA-induced signaling pathways leading to transcriptional activation of AP-1-responsive genes. As potential modulators of AP-1 transactivation and c-Fos expression, these MAPK pharmacologic inhibitors could be clinically useful in skin cancer chemopreventive strategies. As reported previously, UVA irradiation of HaCaT cells significantly induced both AP-1 transactivation and c-fos transactivation [15]. Suppression of JNK with SP600125 and of p38α/β with SB202190 resulted in the abrogation of both AP-1 and c-fos activities in dose-dependent manners. Additionally, SB202190 and SP600125 dose-dependently decreased UVA-induced levels of c-Fos protein, the primary component in UVA-induced AP-1 DNA binding in these cells [15]. The doses of SB202190 and SP600125 that inhibited these downstream targets were shown to specifically reduce their respective UVA-induced MAPK activities.
The JNK inhibitor SP600125 consistently blocked c-fos transactivation at a dose of 62 nM, whereas AP-1 transactivation consistently required doses of 125 nM. Although c-Jun was a component of the UVA-induced AP-1 DNA-binding complex in these cells, other Jun family members that could compete for dimerization and AP-1 site binding were also present [15]. We demonstrated that JNK inhibition by SP600125 reduced not only c-Jun activity and expression, but also c-Fos expression. Preexisting c-Fos protein, however, could still be activated by c-Fos-regulating kinase [30,57]. Therefore, higher concentrations of inhibitors may have been required to effectively inhibit AP-1 activation. In contrast, JNK activates ternary complex factor proteins such as Elk-1 and STAT proteins, which bind directly to transcriptional control elements in the c-fos promoter [18,28,30]. Therefore, lower doses of SP600125 may be sufficient to see an effect on this promoter construct.
SP600125 treatment of HaCaT cells with UVA irradiation not only inhibited the phosphorylation of c-Jun on serines 63 and 73 but also decreased total c-Jun protein levels. The c-Jun promoter contains two AP-1-binding sites that allow c-Jun protein to regulate its own transcription [54]. Therefore, inhibition of c-Jun phosphorylation may also affect total c-Jun protein levels.
The doses of SP600125 that inhibited UVA-induced c-Jun activity and expression were considerably lower than IC50 doses reported in primary human monocytes stimulated with lipopolysaccharide and Jurkat T cells stimulated with phorbol-12-myristate-13-acetate plus anti-CD3 and anti-CD28 (5–10 µM) [46]. Additionally, although doses of SB202190 as high as 15 µM have been used in HaCaT cells to determine the role of p38α/β in UV signaling, much lower doses were capable of inhibiting UVA-induced p38 activity. In addition to potentially diverse signaling pathways between cell types, different stimuli can also alter the effects of an inhibitor on cells. The combination of 250 kJ/m2 UVA with low doses of either SB202190 or SP600125 resulted in dramatic morphologic changes and increased cell death that were visibly detectable approximately 4 hours post-UVA irradiation (unpublished observations). We are currently exploring the biologic consequences and significance of these synergistic effects in HaCaT cells as well as in primary human keratinocytes.
Although ERK activation did not play a role in the UVA-induced AP-1 transactivation in these human keratinocytes, treatment with the MEK1/2 inhibitor PD98059 dose-dependently blocked both basal and UVA-induced c-fos activation and c-Fos protein levels (data not shown). The dose-dependent drop in c-fos activity observed in the UVA-treated groups may have reflected the modulation of basal c-fos activity. We suggest that ERK activity may contribute to basal levels of c-fos expression in HaCaT cells.
Our increased elucidation and understanding of cellular signaling pathways allow mechanistic approaches to chemoprevention and chemotherapy. The availability of potent, selective inhibitors of specific signaling proteins may allow researchers to further dissect cellular signaling pathways, to individually genetically tailor preventive strategies, and to reduce adverse toxicologic responses elicited by the screening of natural products with multiple mechanisms of action [58,59].
Our results demonstrated for the first time that activation of the stress-activated kinases p38 and JNK played important roles in UVA-mediated AP-1 transactivation and c-fos transactivation, and that these kinases are involved in UVA-induced c-Fos expression, a primary component of the UVA-induced AP-1 DNA-binding complex [15]. Because deregulated expression of AP-1 has been shown to play an important role in the neoplastic transformation of epidermal cells in both cell culture and animal models [23–26], targeted inhibition of the upstream kinases p38 and JNK with the pyridinyl imidazole SB202190 and the anthrapyrazolone SP600125, respectively, may be effective chemopreventive strategies for UVA-induced nonmelanoma skin cancer.
Abbreviations
- AP-1
activator protein-1
- AP-2
activator protein-2
- ATF-2
activating transcription factor-2
- DMEM
Dulbecco's modified Eagle's medium
- DTT
dithiothreitol
- EDTA
ethylenediamine-tetraacetic acid
- EGTA
ethylene glycol-bis(β-aminoethly ether)-N,N,N′,N′-tetraacetic acid
- ERK
extracellular signal-regulated kinase
- HRP
horseradish peroxidase
- ICAM-1
intercellular adhesion molecule-1
- JNK
c-Jun NH2-terminal kinase
- MAPK
mitogen-activated protein kinase
- MAPKAPK2
MAPK-activated protein kinase-2
- MEF
MADS box transcription enhancer factor
- MEK
MAPK/ERK kinase
- NFκB
nuclear factor kappa-B
- PBS
phosphate-buffered saline
- SAP
serum response factor accessory protein
- SDS
sodium dodecyl sulfate
- SRE
serum response element
- STAT
signal transducer and activator of transcription
- TBST
Tris-buffered saline with 0.05% Tween 20
- TPA
12-O-tetradecanoylphorbol-13-acetate
- TRE
TPA response element
- UVA
ultraviolet A
Footnotes
This work was supported, in part, by the National Cancer Institute-funded grant R25CA78447 through the Cancer Prevention and Control Program at the Arizona Cancer Center, Tucson, AZ. This work was also supported by the National Institutes of Health grants CA27502 and CA23074.
References
- 1.Hall EJ, Astor M, Bedford J, Borek C, Curtis SB, Fry M, Geard C, Hei T, Mitchell J, Oleinick N, Rubin J, Tu A, Ullrich R, Waldren C, Ward J. Basic radiobiology. Am J Clin Oncol. 1988;11:220–252. doi: 10.1097/00000421-198806000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Moan J. UV-A radiation, melanoma induction, sunscreens, solaria and ozone reduction. J Photochem Photobiol B Biol. 1994;24:201–203. doi: 10.1016/1011-1344(94)07022-9. [DOI] [PubMed] [Google Scholar]
- 3.Madronich S. The atmosphere and UV-B radiation at ground level. In: Young AR, Bjorn LO, Moan J, Nultsch W, editors. Environmental UV Photobiology. New York: Plenum; 1993. pp. 1–39. [Google Scholar]
- 4.Kelfkens G, de Gruijl FR, van der Leun JC. Ozone depletion and increase in annual carcinogenic ultraviolet dose. Photochem Photobiol. 1990;52:819–823. doi: 10.1111/j.1751-1097.1990.tb08687.x. [DOI] [PubMed] [Google Scholar]
- 5.de Laat A, van der Leun JC, de Gruijl FR. Carcinogenesis induced by UVA (365-nm) radiation: the dose-time dependence of tumor formation in hairless mice. Carcinogenesis. 1997;18:1013–1020. doi: 10.1093/carcin/18.5.1013. [DOI] [PubMed] [Google Scholar]
- 6.Kligman LH. The hairless mouse and photoaging. Photochem Photobiol. 1991;54:1109–1118. doi: 10.1111/j.1751-1097.1991.tb02134.x. [DOI] [PubMed] [Google Scholar]
- 7.Kelfkens G, de Gruijl FR, van der Leun JC. Tumorigenesis by short-wave ultraviolet A: papillomas versus squamous cell carcinomas. Carcinogenesis. 1991;12:1377–1382. doi: 10.1093/carcin/12.8.1377. [DOI] [PubMed] [Google Scholar]
- 8.Sterenborg HJ, van der Leun JC. Tumorigenesis by a long wavelength UV-A source. Photochem Photobiol. 1990;51:325–330. doi: 10.1111/j.1751-1097.1990.tb01718.x. [DOI] [PubMed] [Google Scholar]
- 9.Basu-Modak S, Tyrrell RM. Singlet oxygen: a primary effector in the ultraviolet A/near-visible light induction of the human heme oxygenase gene. Cancer Res. 1993;53:4505–4510. [PubMed] [Google Scholar]
- 10.Grether-Beck S, Olaizola-Horn S, Schmitt H, Grewe M, Jahnke A, Johnson JP, Briviba K, Sies H, Krutmann J. Activation of transcription factor AP-2 mediates UVA radiation- and singlet oxygen-induced expression of the human intercellular adhesion molecule 1 gene. Proc Natl Acad Sci USA. 1996;93:14586–14591. doi: 10.1073/pnas.93.25.14586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wlaschek M, Briviba K, Stricklin GP, Sies H, Scharffetter-Kochanek K. Singlet oxygen may mediate the ultraviolet A-induced synthesis of interstitial collagenase. J Invest Dermatol. 1995;104:194–198. doi: 10.1111/1523-1747.ep12612751. [DOI] [PubMed] [Google Scholar]
- 12.Klotz LO, Pellieux C, Briviba K, Pierlot C, Aubry JM, Sies H. Mitogen-activated protein kinase (p38-, JNK-, ERK-) activation pattern induced by extracellular and intracellular singlet oxygen and UVA. Eur J Biochem. 1999;260:917–922. doi: 10.1046/j.1432-1327.1999.00255.x. [DOI] [PubMed] [Google Scholar]
- 13.Djavaheri-Mergny M, Mergny JL, Bertrand F, Santus R, Maziere C, Dubertret L, Maziere JC. Ultraviolet-A induces activation of AP-1 in cultured human keratinocytes. FEBS Lett. 1996;384:92–96. doi: 10.1016/0014-5793(96)00294-3. [DOI] [PubMed] [Google Scholar]
- 14.Djavaheri-Mergny M, Dubertret L. UV-A-induced AP-1 activation requires the Raf/ERK pathway in human NCTC 2544 keratinocytes. Exp Dermatol. 2001;10:204–210. doi: 10.1034/j.1600-0625.2001.010003204.x. [DOI] [PubMed] [Google Scholar]
- 15.Silvers AL, Bowden GT. UVA irradiation-induced activation of activator protein-1 is correlated with induced expression of AP-1 family members in the human keratinocyte cell line HaCaT. Photochem Photobiol. 2002;75:302–310. doi: 10.1562/0031-8655(2002)075<0302:uiiaoa>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 16.Djavaheri-Mergny M, Gras MP, Mergny JL, Dubertret L. UVA-induced decrease in nuclear factor-kappaB activity in human keratinocytes. Biochem J. 1999;338:607–613. [PMC free article] [PubMed] [Google Scholar]
- 17.Vile GF, Tanew-Ilitschew A, Tyrrell RM. Activation of NF-kappa B in human skin fibroblasts by the oxidative stress generated by UVA radiation. Photochem Photobiol. 1995;62:463–468. doi: 10.1111/j.1751-1097.1995.tb02369.x. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Liu G, Dong Z. MSK1 and JNKs mediate phosphorylation of STAT3 in UVA-irradiated mouse epidermal JB6 cells. J Biol Chem. 2001;276:42534–42542. doi: 10.1074/jbc.M106044200. [DOI] [PubMed] [Google Scholar]
- 19.Maziere C, Dantin F, Dubois F, Santus R, Maziere J. Biphasic effect of UVA radiation on STAT1 activity and tyrosine phosphorylation in cultured human keratinocytes. Free Radic Biol Med. 2000;28:1430–1437. doi: 10.1016/s0891-5849(00)00264-1. [DOI] [PubMed] [Google Scholar]
- 20.Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991;1072:129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- 21.Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001;20:2390–2400. doi: 10.1038/sj.onc.1204383. [DOI] [PubMed] [Google Scholar]
- 22.Briata P, D'Anna F, Franzi AT, Gherzi R. AP-1 activity during normal human keratinocyte differentiation: evidence for a cytosolic modulator of AP-1/DNA binding. Exp Cell Res. 1993;204:136–146. doi: 10.1006/excr.1993.1018. [DOI] [PubMed] [Google Scholar]
- 23.Dong Z, Birrer MJ, Watts RG, Matrisian LM, Colburn NH. Blocking of tumor promoter-induced AP-1 activity inhibits induced transformation in JB6 mouse epidermal cells. Proc Natl Acad Sci USA. 1994;91:609–613. doi: 10.1073/pnas.91.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenhalgh DA, Yuspa SH. Malignant conversion of murine squamous papilloma cell lines by transfection with the fos oncogene. Mol Carcinog. 1988;1:134–143. doi: 10.1002/mc.2940010209. [DOI] [PubMed] [Google Scholar]
- 25.Saez E, Rutberg SE, Mueller E, Oppenheim H, Smoluk J, Yuspa SH, Spiegelman BM. c-fos is required for malignant progression of skin tumors. Cell. 1995;82:721–732. doi: 10.1016/0092-8674(95)90469-7. [DOI] [PubMed] [Google Scholar]
- 26.Young MR, Li JJ, Rincon M, Flavell RA, Sathyanarayana BK, Hunziker R, Colburn N. Transgenic mice demonstrate AP-1 (activator protein-1) transactivation is required for tumor promotion. Proc Natl Acad Sci USA. 1999;96:9827–9832. doi: 10.1073/pnas.96.17.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson EJ, MacGowan J, Young MR, Colburn N, Bowden GT. A dominant negative c-jun specifically blocks okadaic acid-induced skin tumor promotion. Cancer Res. 2002;62:3044–3047. [PubMed] [Google Scholar]
- 28.Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 29.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- 30.Whitmarsh AJ, Davis RJ. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J Mol Med. 1996;74:589–607. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]
- 31.Hibi M, Lin A, Smeal T, Minden A, Karin M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993;7:2135–2148. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- 32.Derijard B, Hibi M, Wu IH, Barrett T, Su B, Deng T, Karin M, Davis RJ. JNK1: a protein kinase stimulated by UV light and Ha-ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 33.Minden A, Lin A, Smeal T, Derijard B, Cobb M, Davis R, Karin M. c-Jun N-terminal phosphorylation correlates with activation of the JNK subgroup but not the ERK subgroup of mitogen-activated protein kinases. Mol Cell Biol. 1994;14:6683–6688. doi: 10.1128/mcb.14.10.6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chou SY, Baichwal V, Ferrell JE., Jr Inhibition of c-Jun DNA binding by mitogen-activated protein kinase. Mol Biol Cell. 1992;3:1117–1130. doi: 10.1091/mbc.3.10.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen RH, Abate C, Blenis J. Phosphorylation of the c-Fos transrepression domain by mitogen-activated protein kinase and 90-kDa ribosomal S6 kinase. Proc Natl Acad Sci USA. 1993;90:10952–10956. doi: 10.1073/pnas.90.23.10952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ihle JN. STATs and MAPKs: obligate or opportunistic partners in signaling. Bioessays. 1996;18:95–98. doi: 10.1002/bies.950180204. [DOI] [PubMed] [Google Scholar]
- 37.Gupta S, Campbell D, Derijard B, Davis RJ. Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science. 1995;267:389–393. doi: 10.1126/science.7824938. [DOI] [PubMed] [Google Scholar]
- 38.Wilhelm D, van Dam H, Herr I, Baumann B, Herrlich P, Angel P. Both ATF-2 and c-Jun are phosphorylated by stress-activated protein kinases in response to UV irradiation. Immunobiology. 1995;193:143–148. doi: 10.1016/S0171-2985(11)80537-1. [DOI] [PubMed] [Google Scholar]
- 39.Han J, Jiang Y, Li Z, Kravchenko VV, Ulevitch RJ. Activation of the transcription factor MEF2C by the MAP kinase p38 in inflammation. Nature. 1997;386:296–299. doi: 10.1038/386296a0. [DOI] [PubMed] [Google Scholar]
- 40.Han TH, Prywes R. Regulatory role of MEF2D in serum induction of the c-jun promoter. Mol Cell Biol. 1995;15:2907–2915. doi: 10.1128/mcb.15.6.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maziere C, Conte MA, Leborgne L, Levade T, Hornebeck W, Santus R, Maziere JC. UVA radiation stimulates ceramide production: relationship to oxidative stress and potential role in ERK, JNK, and p38 activation. Biochem Biophys Res Commun. 2001;281:289–294. doi: 10.1006/bbrc.2001.4348. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y, Dong Z, Nomura M, Zhong S, Chen N, Bode AM. Signal transduction pathways involved in phosphorylation and activation of p70S6K following exposure to UVA irradiation. J Biol Chem. 2001;276:20913–20923. doi: 10.1074/jbc.M009047200. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, Zhong S, Dong Z, Chen N, Bode AM, Ma W. UVA induces Ser381 phosphorylation of p90RSK/MAPKAP-K1 via ERK and JNK pathways. J Biol Chem. 2001;276:14572–14580. doi: 10.1074/jbc.M004615200. [DOI] [PubMed] [Google Scholar]
- 44.Chen W, Borchers AH, Dong Z, Powell MB, Bowden GT. UVB irradiation-induced activator protein-1 activation correlates with increased c-fos gene expression in a human keratinocyte cell line. J Biol Chem. 1998;273:32176–32181. doi: 10.1074/jbc.273.48.32176. [DOI] [PubMed] [Google Scholar]
- 45.English JM, Cobb MH. Pharmacological inhibitors of MAPK pathways. Trends Pharmacol Sci. 2002;23:40–45. doi: 10.1016/s0165-6147(00)01865-4. [DOI] [PubMed] [Google Scholar]
- 46.Bennett BL, Sasaki DT, Murray BW, O'Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y, Bhagwat SS, Manning AM, Anderson DW. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci USA. 2001;98:13681–13686. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mody N, Leitch J, Armstrong C, Dixon J, Cohen P. Effects of MAP kinase cascade inhibitors on the MKK5/ERK5 pathway. FEBS Lett. 2001;502:21–24. doi: 10.1016/s0014-5793(01)02651-5. [DOI] [PubMed] [Google Scholar]
- 49.Borsch-Haubold AG, Pasquet S, Watson SP. Direct inhibition of cyclooxygenase-1 and -2 by the kinase inhibitors SB 203580 and PD 98059. SB 203580 also inhibits thromboxane synthase. J Biol Chem. 1998;273:28766–28772. doi: 10.1074/jbc.273.44.28766. [DOI] [PubMed] [Google Scholar]
- 50.Chen W, Bowden GT. Activation of p38 MAP kinase and ERK are required for ultraviolet-B induced c-fos gene expression in human keratinocytes. Oncogene. 1999;18:7469–7476. doi: 10.1038/sj.onc.1203210. [DOI] [PubMed] [Google Scholar]
- 51.Chen W, Bowden GT. Role of p38 mitogen-activated protein kinases in ultraviolet-B irradiation-induced activator protein 1 activation in human keratinocytes. Mol Carcinog. 2000;28:196–202. doi: 10.1002/1098-2744(200008)28:4<196::aid-mc2>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 52.Chen W, Tang Q, Gonzales MS, Bowden GT. Role of p38 MAP kinases and ERK in mediating ultraviolet-B induced cyclooxygenase-2 gene expression in human keratinocytes. Oncogene. 2001;20:3921–3926. doi: 10.1038/sj.onc.1204530. [DOI] [PubMed] [Google Scholar]
- 53.Bachelor MA, Silvers AL, Bowden GT. The role of p38 in UVA-induced cyclooxygenase-2 expression in the human keratinocyte cell line, HaCaT. Oncogene. 2002;21:7092–7099. doi: 10.1038/sj.onc.1205855. [DOI] [PubMed] [Google Scholar]
- 54.Angel P, Hattori K, Smeal T, Karin M. The jun protooncogene is positively autoregulated by its product, Jun/AP-1. Cell. 1988;55:875–885. doi: 10.1016/0092-8674(88)90143-2. [DOI] [PubMed] [Google Scholar]
- 55.Klotz LO, Briviba K, Sies H. Singlet oxygen mediates the activation of JNK by UVA radiation in human skin fibroblasts. FEBS Lett. 1997;408:289–291. doi: 10.1016/s0014-5793(97)00440-7. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Y, Mattjus P, Schmid PC, Dong Z, Zhong S, Ma WY, Brown RE, Bode AM, Schmid HH. Involvement of the acid sphingomyelinase pathway in UVA-induced apoptosis. J Biol Chem. 2001;276:11775–11782. doi: 10.1074/jbc.M006000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deng T, Karin M. c-Fos transcriptional activity stimulated by H-Ras-activated protein kinase distinct from JNK and ERK. Nature. 1994;371:171–175. doi: 10.1038/371171a0. [DOI] [PubMed] [Google Scholar]
- 58.Shureiqi I, Reddy P, Brenner DE. Chemoprevention: general perspective. Crit Rev Oncol Hematol. 2000;33:157–167. doi: 10.1016/s1040-8428(99)00072-4. [DOI] [PubMed] [Google Scholar]
- 59.Stratton SP. Prevention of non-melanoma skin cancer. Curr Oncol Rep. 2001;3:295–300. doi: 10.1007/s11912-001-0080-x. [DOI] [PubMed] [Google Scholar]