Abstract
Phylogenetically diverse polar and subpolar marine teleost fishes have evolved antifreeze proteins (AFPs) or antifreeze glycoproteins (AFGPs) to avoid inoculative freezing by internalized ice. For over three decades since the first fish antifreeze (AF) protein was discovered, many studies of teleost freezing avoidance showed hepatic AF synthesis and distribution within the circulation as pivotal in preventing the blood, and therefore the fish, from freezing. We have uncovered an important twist to this long-held paradigm: the complete absence of liver synthesis of AFGPs in any life stage of the Antarctic notothenioids, indicating that the liver plays no role in the freezing avoidance in these fishes. Instead, we found the exocrine pancreas to be the major site of AFGP synthesis and secretion in all life stages, and that pancreatic AFGPs enter the intestinal lumen via the pancreatic duct to prevent ingested ice from nucleating the hyposmotic intestinal fluids. AFGPs appear to remain undegraded in the intestinal milieu, and the composition and relative abundance of intestinal AFGP isoforms are nearly identical to serum AFGPs. Thus, the reabsorption of intact pancreas-derived intestinal AFGPs, and not the liver, is the likely source of circulatory AFGPs in notothenioid fishes. We examined diverse northern fish taxa and Antarctic eelpouts with hepatic synthesis of bloodborne AF and found that they also express secreted pancreatic AF of their respective types. The evolutionary convergence of this functional physiology underscores the hitherto largely unrecognized importance of intestinal freezing prevention in polar teleost freezing avoidance, especially in the chronically icy Antarctic waters.
Keywords: antifreeze glycoprotein-null liver, antifreeze paradigm shift, evolutionary adaptation, intestinal freeze avoidance, functional convergence
The predominant endemic Antarctic marine teleost group, the notothenioid fishes (1), inhabit the world’s coldest and iciest marine waters (2, 3) and are endowed with the highest known levels (20–35 mg/ml) of protein antifreeze (AF) (a family of AF glycoproteins; AFGPs) in their blood and other body fluids (4, 5). The synthesis of blood AFGPs in Antarctic notothenioids has historically been attributed to the liver (6, 7), because the vertebrate liver is well known as the major source of secreted plasma proteins (8, 9), and thus there were no a priori reasons to invoke a different source for the abundant plasma AFGPs. Also contributing to the prevailing notion of universal hepatic AF synthesis is the readily demonstrable liver expression of AF mRNA by Northern blots and/or cDNA cloning in all other AF protein (AFP)-bearing fish taxa (AFP type I, II, III, or IV) (10–14) and in northern hemisphere AFGP-bearing polar cod (15). However, definitive verification of liver biosynthesis of AFGPs in Antarctic notothenioids has been lacking. Early radioactive-tracer investigations of notothenioid AFGP biosynthesis could not rule out a nonhepatic synthesis site, because the appearance of labeled AFGPs and non-AFGP plasma proteins in the blood was drastically asynchronous (AFGPs lagged by 8–18 h) (6). Northern blot analysis of liver RNA of the Antarctic nototheniid Notothenia coriiceps showed AFGP mRNA hybridization, but an unusually large amount (50 μg) of polyA+ RNA was required (16), contradictory to liver being a strong expression site. If there is little or no hepatic AFGP biosynthesis, the tissue origin of the abundant bloodborne AFGPs in Antarctic notothenioids returns as an unsolved fundamental question >30 years after the discovery of the protein (5). In our prior elucidations of the evolution of the notothenioid AFGP gene from a pancreatic trypsinogen-like protease (TLP) gene, AFGP and chimeric AFGP/TLP cDNAs were obtained from exocrine pancreas RNA, indicating that exocrine pancreas is an AFGP expression site (17, 18). In this comprehensive study, we confirm that the exocrine pancreas is the major AFGP synthesis site in Antarctic notothenioid fishes from hatching through adulthood, whereas the liver is AFGP-expression null in all life stages. We show that pancreatic AFGPs are secreted into the intestine, and, with additional AFGP contribution from the anterior portion of the stomach (the only other major expression site), they prevent the freezing of the hyposomotic intestinal fluid that is at high risk of ice inoculation in the chronically icy Antarctic waters. The apparently undegraded intestinal AFGPs raise the possibility that plasma AFGPs in the notothenioids are derived from reabsorption of these intact macromolecules, which would resolve the conundrum of high plasma AFGP concentrations despite the absence of liver AF synthesis and secretion. In addition, we examine diverse AF-bearing species from both north- and south-polar and subpolar regions and confirm that they have converged on pancreatic AF expression regardless of their AF type, which attests to intestinal freezing prevention as a vital and integral component of the repertoire of teleost freeze-avoidance strategies.
Results
AFGP mRNA Expression in Adult Notothenioid Tissues.
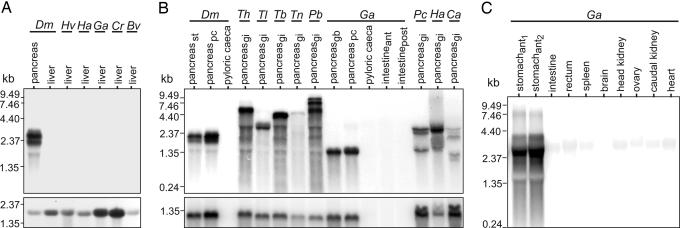
To determine the tissue site or sites of AFGP synthesis in adult notothenioids, Northern blots of total RNA from different tissues were hybridized to an AFGP gene (coding region only) probe (Fig. 1). Like the phyletically basal AFGP-null New Zealand notothenioid Bovichtus variegatus, liver RNA from representative AFGP-bearing species of each of the five endemic Antarctic notothenioid families (Nototheniidae, Artedidraconidae, Harpagiferidae, Bathydraconidae, and Channichthyidae; detailed species information in Table 1, which is published as supporting information on the PNAS web site) showed no hybridization, as opposed to the intense hybridization in pancreatic RNA sampled from the Antarctic nototheniid Dissostichus mawsoni included in the blot (Fig. 1A Upper). As a control, the liver RNA samples showed hybridization with a cDNA probe of a constitutively expressed nototheniid elongation factor 1-α subunit (Fig. 1A Lower).
Fig. 1.
Northern blot analysis of AFGP mRNA expression in adult notothenioid fish tissues. (A) Absence of liver AFGP expression in five species representing the five Antarctic notothenioid families (lanes 2–5) and the basal AFGP-null New Zealand species Bv (B. variegatus) (lane 6), versus strong pancreatic expression in the Antarctic species Dm (D. mawsoni) (lane 1) (Upper). Positive hybridization with an elongation factor 1-α subunit probe serves as control for AFGP-negative liver samples (Lower). (B) AFGP expression in pancreatic tissue isolates from selected species from all five Antarctic notothenioid families and no expression in nonpancreatic GI components (Upper). Location of pancreatic tissue isolates: st, stomach; pc, pyloric ceca; gb, gall bladder; gi, GI locations; ant, anterior; post, posterior. Hybridization of pancreatic RNA samples to a pancreatic trypsinogen probe verifies presence of exocrine pancreas RNA (Lower). (C) AFGP expression in anterior stomach (lanes 1 and 2) and absence of expression in all other tissues from Ga (G. acuticeps). (Detailed species information is in Table 1.)
In contrast to liver RNA, Northern blot of RNA from pancreatic tissues of species representing the five endemic Antarctic notothenioid families showed strong AFGP mRNA expression (Fig. 1B Upper). Because adult teleost exocrine pancreas is commonly diffuse and scattered beneath the surface or between layers of mesenteric (peritoneal or serosal) and fatty tissues associated with abdominal organs and the biliary and pancreatic ducts (19, 20), we confirmed the presence of pancreatic mRNA in our pancreatic tissue isolates based on the positive hybridization with a probe derived from a D. mawsoni pancreatic trypsinogen cDNA (Fig. 1B Lower). Lower gastrointestinal (GI) tract components (pyloric ceca and intestine) with surface mesentery removed showed no AFGP expression (Fig. 1B Upper). The sizes of pancreatic AFGP mRNAs ranged from ≈1.35 kb to ≈9.5 kb (Fig. 1B Upper), reflecting the large size of the AFGP polyprotein precursor (16, 17) and potential chimeric AFGP-TLP genes found in extant Antarctic notothenioid genomes (18). Northern blot analysis of RNA of ten tissues from the Antarctic bathydraconid Gymnodraco acuticeps identified the anterior portion of the stomach immediately next to the esophagus-stomach junction as the only other strong expression site of AFGP mRNA in adults (Fig. 1C).
AFGP Expression in Larval Notothenioid Fish.
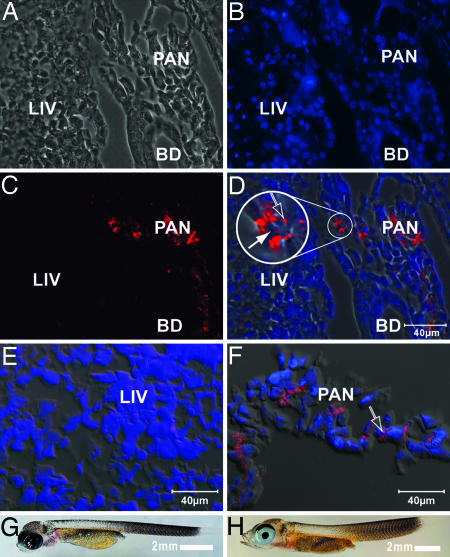
Immunodetection of mature AFGPs using polyclonal rabbit anti-AFGP antibodies and fluorescently labeled secondary goat anti-rabbit IgG in tissue sections of a 1-day-old G. acuticeps hatchling (Fig. 2 A–D and G) and a 4-month-old larva (Fig. 2 E, F, and H) showed no immunoreactive AFGPs in the liver cells of either age, but both ages revealed a high degree of AFGP expression in pancreatic acinar cells, particularly within apical zymogen granules. Pancreatic AFGP-positive and hepatic AFGP-null expression is therefore not an adult-only condition, but occurs at hatching and in the early life stages of notothenioid fishes.
Fig. 2.
Immunodetection of AFGP expression in Antarctic notothenioid G. acuticeps larvae. (A–D) One-day-old larva. (A) Phase-contrast image showing liver (LIV) and developing pancreas (PAN) spreading over the bile duct (BD). (B) DAPI-stained image illustrating the position of nuclei (blue). (C) Immunolabeled AFGP (red) in the pancreas. (D) Composite image of A–C (phase contrast, DAPI, and immunofluorescence). The inset (3× magnification) shows AFGP localization in some (open arrow) but not all zymogen granules (filled arrow) of pancreatic acinar cells. (E and F) Four-month-old larva. (E) Composite image of the liver. Immunostained AFGP is lacking. (F) Composite image of the exocrine pancreas. Immunostained AFGP (red) is prominent in apical zymogen granules of acinar cells (open arrow). (G) Image of a 1-day-old hatchling. (H) Image of a 4-month-old larva.
Osmolarity and AF Activity of GI Fluids.
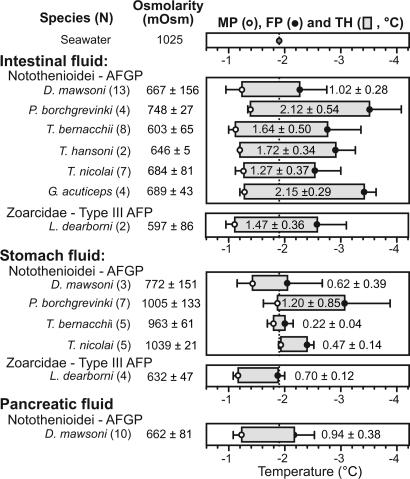
The strong AFGP mRNA expression in the adult exocrine pancreas and anterior stomach (Fig. 1) is associated with the presence of significant AF activity (measured as thermal hysteresis, the difference between melting and freezing points) in the pancreatic, stomach, and intestinal fluids of Antarctic notothenioid fishes (Fig. 3, and numerical data in Table 2, which is published as supporting information on the PNAS web site), indicating that transcripts are translated into secreted proteins. The stomach fluid is isosmotic or moderately hyposmotic (1,039±21 to 772±151 mOsm) to seawater (1,025 mOsm), reflecting undiluted salty ingesta (seawater and/or marine food) or limited dilution by the incipient digestion in the stomach. Intestinal fluid (IF) is significantly hyposmotic (603±65 to 748±27 mOsm) to seawater, reflecting dilution by GI secretions, late-stage digestion, and intestinal salt absorption (21). The presence of AFGPs in the stomach fluid and IF depresses their freezing points to below that of seawater (−1.91°C) to −2.01±0.14°C to −3.45±0.21°C, and thus neither fluid risks inoculative freezing by ingested ice. Thermal hysteresis of the IF is especially large (1.02±0.28°C to 2.15±0.29°C), indicating the presence of abundant AF. Pancreatic fluid (PF) was only obtained from the large nototheniid D. mawsoni in which the pancreatic duct (distinct from the bile duct) visibly distends into a small reservoir at its base above its junction with the intestine. D. mawsoni PF displays a large thermal hystersis (0.94±0.38°C), indicative of the presence of substantial AFGPs before secretion into the intestinal lumen.
Fig. 3.
Osmolarity, melting point (MP), freezing point (FP), and thermal hysteresis (TH, or AF activity) of intestinal, stomach, and pancreatic fluids of Antarctic notothenioids and Antarctic eelpout. Figure is graphical representation of data in Table 2. Values of ambient McMurdo Sound seawater are given at the top for reference. TH represented by gray box, and indicated value is the difference between MP and FP in degrees Celsius.
AFGPs from PF, IF, and Serum.
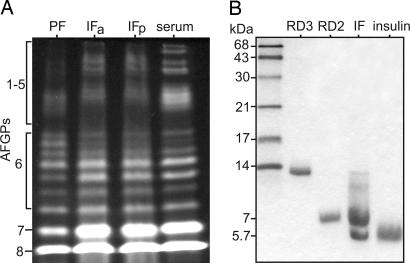
Notothenioid AFGPs occur as a family of size isoforms (15, 22), and the profiles of electrophoretically resolved AFGPs purified from the PF and IF of one D. mawsoni specimen show they comprise the full complement of isoforms similar to serum AFGPs of the same specimen in terms of size heterogeneity and relative abundance (Fig. 4A). The similar PF and IF AFGP profiles support the pancreas as a major source of intestinal AFGPs, with the input of stomach AFGPs to the IF contributing to the observed IF profile (Fig. 4A). Intestinal AFGP molecules appear to remain intact as they transit the intestinal tract, as seen in the identical profiles of AFGPs purified from IF obtained from the anterior and posterior half of the intestine respectively (Fig. 4A). Profiles of IF and serum AFGPs are nearly identical, suggesting the possibility that the latter may be derived from the former via reabsorption. For species of the other four Antarctic notothenioid families, which are too small for PF sampling, we cloned and sequenced partial pancreatic AFGP cDNAs from a representative member of each family. All pancreatic cDNAs encode AFGPs that share a conserved signal peptide sequence (Fig. 6, which is published as supporting information on the PNAS web site), indicating their translation into secreted proteins. Pancreatic AFGP expression and secretion therefore occurs throughout members of all the Antarctic notothenioid families.
Fig. 4.
Electrophoretically resolved AF proteins purified from fish serum and GI fluids. (A) Fluorescently labeled AFGPs purified from pancreatic fluid (PF), intestinal fluid (IF) in anterior half (IFa) and posterior half (IFp) of intestine, and serum of the same D. mawsoni specimen. (B) Coomassie blue-stained type III AFPs from Antarctic eelpout L. dearborni IF showing prominent 7-kDa isoform and a small amount of the ≈14-kDa isoform. Purified serum AFP isoform counterparts (7-kDa RD2 and 14-kDa RD3) (24) were coelectrophoresed as reference. Insulin (5.7 kDa) was used as a low molecular weight standard. The ≈5.7-kDa peptide band in IF may be partly digested fragment of the 7-kDa or 14-kDa AFP isoform.
Pancreatic AF Expression in Nonnotothenioid AF-Bearing Fishes.
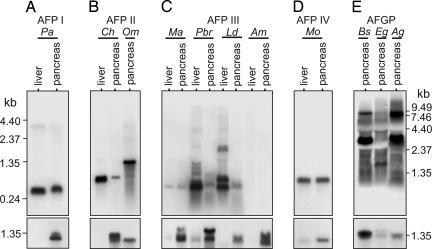
The need for protecting hyposmotic IF from freezing also applies to other polar fishes; thus, we examined diverse species (eleven) from both north- and south-polar (and subpolar) regions bearing all other known types of AFs (species and AF information in Table 1). Northern blots of RNA from fish bearing each type of AF hybridized with the corresponding AF cDNA (or gene) probe showed pancreatic AF expression (Fig. 5 Upper) in addition to liver expression (Fig. 5 Upper and refs. 10–15). The pancreatic AFP mRNA of type I, II, III, and IV AFP-bearing species corresponds in size to a liver AFP mRNA (Fig. 5 A–D Upper), indicating expression of a similar isoform in both tissues. Hybridization intensity is variable for type III AFP mRNA in zoarcoid fishes (pouts and wolffish) (Fig. 5C), likely due to the tissue heterogeneity of the pancreatic isolates and/or seasonal variation of synthesis in the northern hemisphere species (23) (Atlantic ocean pout Macrozoarces americanus and spotted wolffish Anarhichas minor sampled in late spring). Strong pancreatic AFGP mRNA expression is seen in the three northern gadids examined, and their AFGP mRNAs range from ≈1 kb to ≈9 kb, with predominant transcripts at ≈2 kb for the saffron cod Eleginus gracilis and ≈3 kb and 9 kb for the high-latitude polar cod Boreogadus saida and the icecod Arctogadus glacialis (Fig. 5E Upper), reflecting AFGP multigene families and the large polyprotein structure of the transcribed genes (15). Regardless of hybridization intensity, all RNA samples yielded AF cDNAs upon RT-PCR amplification, which we cloned and sequenced. Alignments of the translated sequences of pancreatic and liver AFP cDNAs from each species show high amino acid identities inclusive of the conserved signal peptide (Fig. 7 A–E, which is published as supporting information on the PNAS web site), indicating pancreatic AFPs are secreted molecules. Likewise, a signal peptide sequence precedes the AFGP encoded in the pancreatic AFGP cDNA from the saffron cod (Fig. 7F); thus, gadid pancreatic AFGPs are also secreted proteins.
Fig. 5.
Northern blot analysis of AF mRNA expression in pancreas and liver of nonnotothenioid AF-bearing fishes. (Upper) (A) Type I AFP, winter flounder. (B) Type II AFP, herring and smelt. (C) Type III AFP, zoarcoid fishes. Ma and Am are late spring specimens that have greatly reduced AFP synthesis. (D) Type IV AFP, longhorn sculpin. (E) AFGP, northern hemisphere gadids. (Lower) Same blots hybridized with pancreatic trypsinogen cDNA to verify presence of exocrine pancreas in pancreatic isolates. Faint trypsinogen hybridization in liver RNA (Pbr, Mo) is likely a result of pancreatic infiltration in liver (32). (Detailed species information is in Table 1.)
In addition to verifying pancreatic expression at the transcriptional level in nonnotothenioid AF-bearing taxa, we sampled the GI fluids of the type III AFP-bearing Antarctic eelpout Lycodichthys dearborni and determined the presence of stomach and intestinal AFP on the basis of significant thermal hysteresis in the respective fluid (Fig. 3). Furthermore, we isolated apparently intact 7-kDa type III AFP from the IF similar to the serum isoform RD2 (24) (Fig. 4B), supporting the entry of the secreted stomach and pancreatic AFPs into the intestinal lumen. A ≈5.7-kDa peptide is also present in the IF, which may represent a partially digested fragment of the 14-kDa (RD3) (24) or 7-kDa isoform.
Discussion
The conventional belief that the vertebrate liver is the major source of plasma proteins (8, 9), and that hepatic synthesis and secretion is responsible for the high circulatory levels of AF protein in AF-bearing polar and subpolar fishes (10–15), is based on documented evidence. It is therefore natural to assume that the same applies to the bloodborne AFGPs of the Antarctic notothenioid fishes. Our results, however, provide clear evidence to the contrary; hepatic AFGP synthesis does not occur in the Antarctic notothenioid fishes, the predominant teleost group endemic to the Southern Ocean (1). The absence of liver AFGP synthesis in the day-old hatchling and 4-month-old G. acuticeps larva (Fig. 2) indicates that no hepatic contribution to blood AFGPs occurs during the early posthatch life stages, although blood AF in these AF-deficient larvae steadily accumulates to adult levels during this time (from hatching to 5 months) (25). The absence of liver AF synthesis during this period of accrual to adult plasma AF concentrations, together with the absence of hepatic AFGP expression in adults (Fig. 1A), confirms that liver plays no role in AFGP biosynthesis throughout the life of notothenioid fishes. Our Northern blot results indicating the absence of hepatic AFGP synthesis in adults is corroborated by expressed sequence tags (EST) sequencing projects of two adult Antarctic notothenioid species included in our analysis (Fig. 1A), D. mawsoni (L. Chen and C.-H.C.C., unpublished results) and Harpagifer antarcticus (Melody Clark, British Antarctic Survey, personal communication), which revealed no AFGP cDNA from the liver. For D. mawsoni, none of the ≈4,700 unique cDNAs obtained from ≈12,000 sequenced clones code for AFGP (L. Chen and C.-H.C.C., unpublished results). Together with this study, we have established a body of compelling evidence demonstrating the AFGP-synthesis null condition of the Antarctic notothenioid liver.
Without hepatic AFGP synthesis, we come full circle to the fundamental question of the source of the abundant AFGPs in the blood, decades after the protein was discovered in the Antarctic notothenioids (5). From the evidence in this study, we propose that reabsorption of pancreatic- and stomach-derived intestinal AFGPs is the source of circulatory AFGPs in Antarctic notothenioids, through the following deduction. We have shown the exocrine pancreas to be the major AFGP expression site from hatching to adulthood by Northern blot and immunodetection (Figs. 1 and 2), and Northern blot analysis reveals the anterior stomach as the only additional site (Fig. 1). No other tissues we examined by Northern blot analysis showed AFGP mRNA expression, including hemopoietic tissues (head kidney and spleen) and circulating blood cells (data not shown) that could potentially contribute to bloodborne AFGPs (Fig. 1C). We have also shown that the AFGPs are secreted into the GI tract by the presence of AF activity in the stomach fluid, PF, and IF (Fig. 3), and the isolation of similar complements of AFGP isoforms from the PF and IF (Fig. 4A); both pancreatic and gastric AFGPs expectedly reach the intestine through obvious anatomical connections. The intestinal AFGPs are derived from the pancreas and anterior stomach only, and not from the components of the lower GI tract, because pyloric ceca and intestine cleaned of surface mesentery (which often contains pancreatic tissue) show no AFGP mRNA expression (Fig. 1 B and C). We observed that the intestinal AFGPs remain largely undegraded as they transit the intestinal tract (Fig. 4A), likely because of their known resistance to acid and alkali by virtue of high sugar content (≈60% by mass) and the lack of cleavage sites for known digestive proteases in their repetitive peptide backbone (Thr-Ala/Pro-Ala-)n (4, 22). The nearly identical intestinal and serum AFGP profiles suggest that serum AFGPs could be derived from intestinal AFGPs. Reuptake mechanisms for intestinal AFGPs may exist because rectal uptake of the protein macromolecule ferritin introduced into the GI tract has been demonstrated for the Antarctic nototheniid Notothenia neglecta (26), and a body of experimental evidence exists showing intestinal absorption of macromolecular proteins for intracellular processing in teleost fishes (27). Collectively, the above reasoning leads us to propose that pancreatic- and gastric-derived intestinal AFGPs may be returned to the blood by intestinal and/or rectal absorption. The secretion of pancreatic and gastric AFGPs, their transit into the intestine, and subsequent intestinal/rectal absorption and transport of intestinal AFGPs to blood is a circuitous route that would take considerable time, and some excretory loss of intestinal AFGPs likely occurs. These factors conceivably contribute to the slow accumulation of plasma AF to adult levels (5 months posthatching) observed in the nascent AF-deficient G. acuticeps larvae (25). Once in the blood, the aglomerular kidneys, a derived character in the Antarctic notothenioids that correlates with AFGP production (28, 29), serve to conserve AFGPs in the circulation. Renal AFGP conservation and high chemical stability of the AFGPs imply that, once sufficient plasma levels are achieved, only maintenance levels of input of GI AFGPs to blood would be necessary, which could be readily provided by the GI-to-blood transport even if the process lacks efficiency. Inefficient GI-to-blood transport would mean significant loss of intestinal AFGPs, which seems energetically wasteful. Some fraction of intestinal AFGPs may indeed be “recycled” via a blood-to-bile-to-intestine pathway, because AF activity is found in the gall bladder bile of notothenioids (results not shown; ref. 35). Given that the liver does not synthesize AFGPs, bile AFGPs are likely derived from plasma AFGP via paracellular and/or transhepatocyte blood-to-bile transport in the liver, known to occur for plasma proteins in mammalian liver (30, 31). However, strong pancreatic AFGP mRNA expression appears to be a constitutive condition in notothenioids (regardless of age and time of pancreatic tissue sampling), suggesting that continuous resynthesis to replenish intestinal loss may be an obligatory energetic cost for ensuring intestinal freezing avoidance.
Our discovery of the AFGP-synthesis active exocrine pancreas and AFGP-null liver in Antarctic notothenioids establishes that the historical attribution to liver as the source of plasma AFGPs in these fishes (6, 7, 16) is erroneous, and that the reported observations of hepatic AFGP expression might be of pancreatic origin. The exocrine pancreas in almost all adult teleost fishes, including Antarctic notothenioids, is not a discrete organ but highly scattered, associated with surfaces of the abdominal organs, adipose tissue, mesentery, blood vessels, and ducts, on and in the gall bladder wall, and, in some fishes, it also infiltrates the liver along hepatic portal blood vessels forming hepatopancreas islets (19, 20, 32). Thus, the reported AFGP synthesis in primary cultures of isolated hepatocytes by radioactive labeling (7) and detection of liver AFGP mRNA in Northern blot with an unusually large amount (50 μg) of polyA+ RNA applied (16) (equivalent to 5 mg total RNA assuming 1% mRNA abundance, as opposed to 5–15 μg total RNA per sample used in Northern blots in this study) could have resulted from contaminant exocrine pancreatic tissue commonly found near the converging biliary ductules exiting the notothenioid liver (19). The protracted delay (8–18 h) of the appearance of labeled AFGPs in the blood behind non-AFGP plasma proteins in radioactive tracer studies (6) could be reconciled by our proposal that the source of plasma AFGPs is the secretion and reabsorption of intestinal AFGPs, expectedly a slow process, and therefore not synchronized with liver synthesis and secretion of other plasma proteins.
The historical focus of polar teleost freeze avoidance studies has been the primary importance of preventing the blood from freezing, ostensibly shaped by the conspicuously high plasma levels of AFs in the adult fishes. Less obvious and far under-recognized is that freezing prevention of the hyposmotic fluids of the alimentary canal is equally crucial to survival. The endowment of AFs in the stomach and intestinal fluids (particularly ample in the highly hyposmotic IF) in unrelated Antarctic notothenioid and zoarcid (eelpout) fishes (Figs. 3 and 4) and the functional convergence of pancreatic AF expression in all AF-bearing fishes regardless of AF type (Figs. 1 and 5) attest to the universal need for preventing the hyposmotic GI fluids from freezing. This need inevitably arises from the obligatory active seawater drinking of marine teleost fishes for osmoregulation (21, 33), which generates an avenue for the steady entry of environmental ice into the GI tract for the Antarctic notothenioids confined to chronically ice-laden freezing waters (3, 22) and for north-polar fishes when their habitats reach freezing temperatures and become icy. Intake of ice-associated food in icy habitats adds further risks of inoculative freezing of the hyposmotic GI fluids, which must be prevented to ensure organismal survival.
The presence of high levels of AFGPs in the IF of Antarctic notothenioids has been recognized in earlier studies (34, 35), but the source was unknown until the present study. For fishes that possess other types of AF protein, presence of intestinal AF is demonstrated for the type III AFP-bearing Antarctic eelpout L. dearborni (Figs. 3 and 4B) in this study, and recently for the AFGP-bearing Arctic ice cod A. glacialis (36), both of which show pancreatic mRNA expression of their respective AF (see L. dearborn and A. glacialis in Fig. 5). It follows that the intestinal AF originates from pancreatic AF secretions through the direct pancreatic duct connection between the exocrine pancreas and intestine in these species, as well as in fishes bearing type I, II, and IV AFP (Fig. 5), which show pancreatic expression of AF mRNA carrying a secretory signal sequence (Fig. 7). Furthermore, besides Antarctic notothenioids, we found type III AFP mRNA expression in the anterior stomach of L. dearborni (data not shown) and AF activity in the stomach fluid (Fig. 3), and stomach expression of the mRNA of the secreted type of AFP I by winter flounder has also been observed (12), suggesting that this additional GI source of AF may also be common among AF-bearing fishes.
The evolutionary confinement of Antarctic notothenioids to persistently icy conditions in the Southern Ocean, where ice ingestion is recurrent and arresting ice growth in their hyposmotic GI fluids a constant necessity (22), combined with the absence of liver AFGP expression, suggests that natural selection acted first and foremost on preventing GI freezing in these fishes. Consistent with the selection for GI freeze avoidance is the evolution of the AFGP gene from a pancreatic trypsinogen-like protease (TLP) gene (17, 18) and the exclusive role of GI components in AFGP expression (this study). Experimentally, wild-caught notothenioid fishes rendered free of associated ice by warming at 1°C acquire ice in their IF much faster than in their blood after being returned to the ambient icy waters of McMurdo Sound, Antarctica (22), underscoring the more imminent danger of inoculative freezing of the GI fluids over other body fluids. The eventual acquisition of ice in the blood means continual protection of the hyposmotic vascular fluid is also necessary. Why the expression of AFGP genes persists in the pancreas and anterior stomach and has not spread to the liver over evolutionary time for more expeditious delivery of AFGPs into the blood seems to defy reasonings of evolutionary parsimony. A possibility resides in the expression of the evolutionarily related AFGP and TLP genes being potentially still under common transcriptional control, as indicated by the chimeric AFGP/TLP genes that are transcriptionally active in the pancreas (18). Without separate transcriptional control, concomitant expression of high levels of a serine protease by the liver could lead to unfavorable physiological consequences. Another aspect of Antarctic notothenioid physiology regarding freeze avoidance, alluded to earlier, is the AF-deficient (up to 3 months posthatching) but paradoxically freeze-resistant condition of young larvae that apparently rely on intact skin and under-developed gill structures as effective physical barriers to ice entry during that time, instead of fully AFGP-fortified blood (25). Collectively, these physiologies highlight a general need for a greater awareness of evolutionary creativity in organismal adaptations.
This study establishes active pancreatic AFGP synthesis and the AFGP-expression null state of the liver in Antarctic notothenioid fishes, bringing a significant perspective to teleost freeze-avoidance physiology. It also reveals that the long-held paradigm of hepatic-based AF synthesis and secretion is no longer universally applicable. Our proposal of reabsorption of intestinal AFGP molecules as the source of blood AF in Antarctic notothenioids is an experimentally testable hypothesis that may further our knowledge of transport physiology of macromolecules. Although freeze avoidance of the blood remains crucial, our finding that divergent AF-bearing fish taxa from both polar regions display exocrine pancreatic expression of secreted AF regardless of AF type attests to freezing prevention of the hyposmotic GI fluids as a vital component of teleost freeze avoidance strategies. The universal pancreatic AF mRNA expression in all nonnotothenioid AF-bearing fishes we examined, and the presence of stomach and intestinal AF in some of these species, will expectedly stimulate further investigations into the details of GI freeze avoidance across other fish taxa and life stages.
Materials and Methods
Animals and Sampling.
Fish were collected by traps or line through sea ice or in open water, or by trawls from research vessels. Specimens were anesthetized with MS222 (Sigma) before sampling. Blood was obtained from the caudal vein by using a needle and syringe, and tissues were dissected, frozen in liquid nitrogen, and stored at −80°C. Intestinal fluid from unfed fish was drained and collected from ligated intestines after they were blotted dry of AF-bearing peritoneal fluid and removed from the abdomen. Pancreatic fluid (up to ≈400 μl) from unfed D. mawsoni was sampled with an insulin syringe from the pancreatic duct, which distends into a visible small reservoir when filled with pancreatic secretion. All animal handling was carried out in accordance with institutionally approved protocols.
Northern Blot and RT-PCR Amplification.
Total RNA was isolated from tissues and 5–15 μg per sample were used in Northern blot analyses as described (15). Blots were stripped of hybridized probe by using 0.1% SDS at 100°C before hybridization with a second probe. Sequences of the primers used to RT-PCR amplify the cDNA of each type of AF protein, elongation factor 1-α subunit, and pancreatic trypsinogen are given in Table 3, which is published as supporting information on the PNAS web site. Amplified cDNA products were cloned into pGemTeasy (Promega) and sequenced with BigDye Terminator v.3 cycle sequencing (Applied Biosystems).
Osmolarity, Melting Point, and Freezing Point Determinations of Fish Fluids.
Osmolarity was measured with a Wescor (Logan, UT) vapor pressure osmometer, and melting and freezing points were determined by using single crystal seed ice in a Clifton nanoliter osmometer as described (25).
AF Protein Purification and Electrophoresis.
Fluid samples containing AFGPs were treated with trichloroacetic acid (5% final concentration), and the acid-resistant AFGPs were purified from the soluble fraction by dialysis and lyophilization. About 400 μg of purified AFGPs were fluorescently labeled with fluorescamine (Roche) and electrophoresed on a nondenaturing 10–15% gradient polyacrylamide gel as described (15). Type III AFP from fluid samples of Antarctic eelpouts were purified by G75 Sephadex (Amersham Pharmacia) gel filtration column chromatography. The AFP-containing column fractions were lyophilized and ≈30 μg were electrophoresed on 15% SDS/polyacrylamide gel as described (24).
Immunodetection of AFGPs.
Whole larvae or dissected tissues were fixed immediately in cold 4% paraformaldehyde prepared in notothenioid PBS (0.1 M sodium phosphate, pH 7.6, and adjusted to 450 mOsm with NaCl). Frozen sections (5–15 μm) were pretreated with PDB (notothenioid PBS containing 1% vol/vol DMSO and 1% wt/vol Ig-free BSA) for 20 min at 20°C, washed, and then incubated (2 h at 20°C) with a primary polyclonal rabbit anti-AFGP antibody (diluted 1:1000 with PDB). The sections were then washed four times for 5 min with PDB and incubated for 1 h at room temperature with an Alexa Fluor 546-labeled secondary goat anti-rabbit IgG (Molecular Probes) diluted 1:1000 with PDB. After a further four washes with PDB, and with or without treatment with 0.2 μg ml−1 DAPI for 5 min at 20°C between the first and second washes, the sections were mounted under a coverslip by using FluoroGuard (Bio-Rad). Control sections were treated with preimmune serum instead of the primary antibody and were consistently negative (results not shown).
Supplementary Material
Acknowledgments
We thank Clarabelle DeVries for assistance with the AFGP characterization and Vivian Ward with the immunodetection images. This work was supported by U.S. National Science Foundation Office of Polar Programs Grants OPP0002654 and OPP0231006 (to C.-H.C.C.). C.W.E. acknowledges additional support from the University of Auckland Research Committee.
Abbreviations
- AF
antifreeze
- AFGP
antifreeze glycoprotein
- AFP
antifreeze protein
- GI
gastrointestinal
- IF
intestinal fluid
- PF
pancreatic fluid
- TLP
trypsinogen-like protease.
Footnotes
Conflict of interest statement: No conflicts declared.
Data deposition: The 26 sequences reported in this paper have been deposited in the GenBank database (accession nos. DQ062435DQ062436DQ062437DQ062438DQ062439DQ062440DQ062441DQ062442DQ062443DQ062444DQ062445DQ062446DQ062447DQ062448DQ062449DQ062450DQ062451DQ062452DQ062453DQ062454DQ062455DQ062456DQ062457DQ062458–DQ062459 and DQ394083). Details listed in Table 4, which is published as supporting information on the PNAS web site.
References
- 1.Eastman J. T. Polar Biol. 2005;28:94–107. [Google Scholar]
- 2.Dayton P. K., Robilliard G. A., DeVries A. L. Science. 1969;163:273–274. doi: 10.1126/science.163.3864.273. [DOI] [PubMed] [Google Scholar]
- 3.Hunt B. M., Hoefling K., Cheng C.-H. C. Antarct. Sci. 2003;15:333–338. [Google Scholar]
- 4.DeVries A. L. Annu. Rev. Physiol. 1983;45:245–260. doi: 10.1146/annurev.ph.45.030183.001333. [DOI] [PubMed] [Google Scholar]
- 5.DeVries A. L. Science. 1971;172:1152–1155. doi: 10.1126/science.172.3988.1152. [DOI] [PubMed] [Google Scholar]
- 6.Hudson A. P., DeVries A. L., Haschemeyer A. E. V. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1979;62:179–183. [Google Scholar]
- 7.O’Grady S. M., Clarke A., DeVries A. L. J. Exp. Zool. 1982;220:179–189. doi: 10.1002/jez.1402200207. [DOI] [PubMed] [Google Scholar]
- 8.Miller L. L., Bly C. G., Watson M. L., Bale W. F. J. Exp. Med. 1951;94:431–453. doi: 10.1084/jem.94.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haschemeyer A. E. V., Smith M. A. Biol. Bull. (Woods Hole, Mass.) 1979;156:93–102. doi: 10.2307/1541005. [DOI] [PubMed] [Google Scholar]
- 10.Ewart K. V., Rubinsky B., Fletcher G. L. Biochim. Biophys. Res. Commun. 1992;185:335–340. doi: 10.1016/s0006-291x(05)90005-3. [DOI] [PubMed] [Google Scholar]
- 11.Ewart K. V., Fletcher G. L. Mol. Mar. Biol. Biotechnol. 1993;2:20–27. [PubMed] [Google Scholar]
- 12.Gong Z., Ewart K. V., Hu Z., Fletcher G. L., Hew C. L. J. Biol. Chem. 1996;271:4106–4112. doi: 10.1074/jbc.271.8.4106. [DOI] [PubMed] [Google Scholar]
- 13.Wang X., DeVries A. L., Cheng C.-H. C. Mol. Mar. Biol. Biotechnol. 1995;4:135–147. [PubMed] [Google Scholar]
- 14.Deng G., Andrews D. W., Laursen R. A. FEBS Lett. 1997;402:17–20. doi: 10.1016/s0014-5793(96)01466-4. [DOI] [PubMed] [Google Scholar]
- 15.Chen L., DeVries A. L., Cheng C.-H. C. Proc. Natl. Acad. Sci. USA. 1997;94:3817–3822. doi: 10.1073/pnas.94.8.3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsiao K. C., Cheng C.-H. C., Fernandes I. E., Detrich H. W., DeVries A. L. Proc. Natl. Acad. Sci. USA. 1990;87:9265–9269. doi: 10.1073/pnas.87.23.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen L., DeVries A. L., Cheng C.-H. C. Proc. Natl. Acad. Sci. USA. 1997;94:3811–3816. doi: 10.1073/pnas.94.8.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng C.-H. C., Chen L. Nature. 1999;40:443–444. doi: 10.1038/46721. [DOI] [PubMed] [Google Scholar]
- 19.Eastman J. T., DeVries A. L. Polar Biol. 1997;17:1–13. [Google Scholar]
- 20.Smith L. S. In: Fish Nutrition. Halver J. E., editor. San Diego: Academic; 1989. pp. 331–421. [Google Scholar]
- 21.Kirsch R., Meister M. F. J. Exp. Biol. 1982;98:67–81. doi: 10.1242/jeb.98.1.67. [DOI] [PubMed] [Google Scholar]
- 22.DeVries A. L., Cheng C.-H. C. In: The Physiology of Polar Fishes. Farrell A. P., Steffensen J. F., editors. Vol. 22. San Diego: Elsevier; 2005. pp. 155–201. [Google Scholar]
- 23.Fletcher G. L., Hew C. L., Li X., Haya K., Kao M. H. Can. J. Zool. 1985;63:488–493. [Google Scholar]
- 24.Wang X., DeVries A. L., Cheng C.-H. C. Biochim. Biophys. Acta. 1995;1247:163–172. doi: 10.1016/0167-4838(94)00205-u. [DOI] [PubMed] [Google Scholar]
- 25.Cziko P. A., Evans C. W., Cheng C.-H. C., DeVries A. L. J. Exp. Biol. 2006;209:407–420. doi: 10.1242/jeb.02008. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez-Blazquez F. J., Cunha da Silva J. R. M. Can J. Zool. 1998;76:1247–1253. [Google Scholar]
- 27.Sire M. F., Vernier J.-M. Comp. Biochem. Physiol. A Physiol. 1992;103:771–781. [Google Scholar]
- 28.Eastman J. T., DeVries A. L. J. Fish Biol. 1986;29:649–662. [Google Scholar]
- 29.Eastman J. T. Antarctic Fish Biology: Evolution in a Unique Environment. San Diego: Academic; 1993. [Google Scholar]
- 30.Coleman R. Biochem. J. 1987;244:249–261. doi: 10.1042/bj2440249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LaRusso N. F. Am. J. Physiol. 1984;247:G199–G205. doi: 10.1152/ajpgi.1984.247.3.G199. [DOI] [PubMed] [Google Scholar]
- 32.Eurell J. A., Haensly W. E. J. Fish Biol. 1982;21:113–125. [Google Scholar]
- 33.Smith H. W. Am. J. Physiol. 1930;93:480–505. [Google Scholar]
- 34.O’Grady S. M., Ellory J. C., DeVries A. L. J. Exp. Biol. 1982;98:429–438. doi: 10.1242/jeb.98.1.429. [DOI] [PubMed] [Google Scholar]
- 35.O’Grady S. M., Ellory J. C., DeVries A. L. J. Exp. Biol. 1983;104:149–162. [Google Scholar]
- 36.Præbel K., Ramløv H. J. Exp. Biol. 2005;208:2609–2613. doi: 10.1242/jeb.01666. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.