Abstract
Posttranslational regulation of nitrogenase, or switch-off, in the methanogenic archaeon Methanococcus maripaludis requires both nifI1 and nifI2, which encode members of the PII family of nitrogen-regulatory proteins. Previous work demonstrated that nitrogenase activity in cell extracts was inhibited in the presence of NifI1 and NifI2, and that 2-oxoglutarate (2OG), a potential signal of nitrogen limitation, relieved this inhibition. To further explore the role of the NifI proteins in switch-off, we found proteins that interact with NifI1 and NifI2 and determined whether 2OG affected these interactions. Anaerobic purification of His-tagged NifI2 resulted in copurification of NifI1 and the dinitrogenase subunits NifD and NifK, and 2OG or a deletion mutation affecting the T-loop of NifI2 prevented copurification of dinitrogenase but did not affect copurification of NifI1. Similar results were obtained with His-tagged NifI1. Gel-filtration chromatography demonstrated an interaction between purified NifI1,2 and dinitrogenase that was inhibited by 2OG. The NifI proteins themselves formed a complex of ≈85 kDa, which appeared to further oligomerize in the presence of 2OG. NifI1,2 inhibited activity of purified nitrogenase when present in a 1:1 molar ratio to dinitrogenase, and 2OG fully relieved this inhibition. These results suggest a model for switch-off of nitrogenase activity, where direct interaction of a NifI1,2 complex with dinitrogenase causes inhibition, which is relieved by 2OG. The presence of nifI1 and nifI2 in the nif operons of all nitrogen-fixing Archaea and some anaerobic Bacteria suggests that this mode of nitrogenase regulation may operate in a wide variety of diazotrophs.
Keywords: ammonia switch-off, Archaea, Methanococcus maripaludis, NifI, posttranslational regulation
Biological nitrogen fixation is a key step in the global nitrogen cycle and is carried out by a variety of Bacteria and methanogenic Archaea (1). This process is catalyzed by the nitrogenase complex, which consists of dinitrogenase (an α2β2 heterotetramer of the NifD and NifK proteins), the site of substrate reduction, and its specific electron-donor dinitrogenase reductase (a homodimer of the NifH protein) (2). Nitrogen fixation is energetically demanding and highly regulated, often at multiple levels. Posttranslational regulation of nitrogenase activity in response to conditions such as addition of ammonium is termed switch-off and is present in some free-living diazotrophs (1). The best characterized mechanism of switch-off involves the reversible ADP-ribosylation of an arginine residue in NifH by the dinitrogenase reductase ADP ribosyltransferase/dinitrogenase reductase-activating glycohydrolase (DraT/DraG) system (3). This mode of regulation occurs in various Proteobacteria, however other diazotrophs apparently do not effect switch-off by this process (4–7).
PII proteins are widespread and ancient nitrogen sensors that function in nearly all aspects of nitrogen regulation (reviewed in refs. 8 and 9), including ADP-ribosylation of NifH (10–12). The best studied examples, GlnB and GlnK of Escherichia coli and other Proteobacteria, are homotrimers (13). Studies have shown that E. coli GlnB binds ATP and 2-oxoglutarate (2OG), and is covalently modified in response to low glutamine levels (14, 15). Depending on its state of ligand binding and modification, GlnB participates in transcriptional and posttranslational regulation of nitrogen metabolism through its interaction with a variety of other proteins (16). The T-loop domain, also the site of covalent modification, has been shown to be important in these interactions (17). The roles of PII proteins in regulation of nitrogen metabolism have also been studied in cyanobacteria (18) and Gram-positive bacteria (19–21), as well as in Archaea (22) and plastids (23). Although glutamine seems to be the primary nitrogen signal (reflecting excess) sensed by PII proteins in enteric bacteria (24), 2OG may be the main signal (reflecting nitrogen deficiency) in some cyanobacteria (25).
NifI1 and NifI2 form two distinct subfamilies of PII proteins, whereas all other known PII proteins, including GlnB and GlnK, form a third subfamily (26). The genes encoding these proteins are present in the nif operons of all nitrogen-fixing Archaea as well as some anaerobic nitrogen-fixing Bacteria, including Chlorobium tepidum, Dehalococcoides ethanogenes (27), Heliobacterium chlorum, and some members of the Clostridia and δ-Proteobacteria (26). In contrast to other members of the PII family, the functions of nifI1 and nifI2 have been studied only in the model methanogenic archaeon Methanococcus maripaludis, where both genes were essential for nitrogenase switch-off (28). Previous work with crude extracts of M. maripaludis demonstrated that addition of 2OG relieved a nifI1- and nifI2-dependent inhibition of nitrogenase activity (29). In this study, we extend these findings by identifying proteins that interact with NifI1 and NifI2 and determining the effect of 2OG on these interactions.
Results
Copurification of Proteins with His-Tagged NifI1 and NifI2.
Because other PII proteins regulate nitrogen metabolism by protein–protein interactions, often mediated by the T-loop domain, we attempted to find proteins that might specifically copurify with NifI1 and NifI2 and to determine whether any copurification was affected by 2OG or mutations in the T-loop. His-tagged NifI1 and His-tagged NifI2, with and without deletion mutations affecting the T-loops, were constructed and expressed on low-copy plasmids in M. maripaludis strains containing null mutations in nifI1 and nifI2, respectively (see Materials and Methods). Expression of C-terminal His-tagged NifI1 and N-terminal His-tagged NifI2 restored switch-off in ΔnifI1 and ΔnifI2 background strains, respectively. A Δ43–49 mutation in the T-loop of His-tagged NifI1 was deficient in switch-off and a Δ48–52 mutation in the T-loop of His-tagged NifI2 was only slightly capable of effecting switch-off (data not shown).
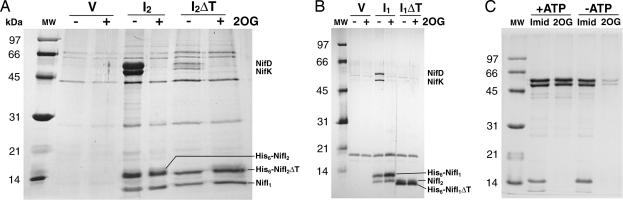
Immobilized metal-ion-affinity chromatography was used to purify the His-tagged constructs under conditions similar to those used for measuring nitrogenase activity in vitro (anaerobic, presence of dithionite, ATP and Mg+2; see Materials and Methods). When an ATP-regenerating system (creatine phosphate and creatine phosphokinase) and acetylene were included in the binding step, nitrogenase activity was observed and was enhanced ≈3-fold with 10 mM 2OG (data not shown), similar to previous results in crude protein extracts (29). After washing, proteins bound to the Ni-NTA agarose were eluted with imidazole, and copurifying proteins were identified by MS. Results for His-tagged NifI2 are shown in Fig. 1A. NifI1 copurified with both the full-length and Δ48–52 His-tagged NifI2, regardless of 2OG. The subunits of dinitrogenase, NifD, and NifK also copurified with His-tagged NifI2 but only in the absence of 10 mM 2OG. Copurification of dinitrogenase was significantly decreased with the Δ48–52 NifI2 but, similar to wild-type NifI2, could still be eliminated in the presence of 2OG. No other proteins were observed to consistently copurify with the His-tagged constructs. Similar and complementary results were obtained with the His-tagged NifI1 (Fig. 1B): NifI2 copurified with NifI1, and copurification of dinitrogenase was observed only in the absence of 2OG. The Δ43–48 mutation in NifI1 prevented any detectable copurification of dinitrogenase. These data suggest that NifI1 and NifI2 act as a heteromeric complex (NifI1,2) that binds to dinitrogenase, that 2OG inhibits this binding, and that the T-loops of both NifI1 and NifI2 are important for binding.
Fig. 1.
Copurification of proteins with His-tagged NifI1 and NifI2 and interaction of NifI1,2 with dinitrogenase. Extracts were bound to Ni-NTA agarose, and elution fractions were analyzed by gel electrophoresis. (A) NifI2 interactions: V, strain Mm1012 [ΔnifI2 (null) background containing pLW40neo (vector control)]; I2, strain Mm711 (ΔnifI2 background containing pLW40neo nifI2 expressing His-tagged NifI2); I2ΔT, strain Mm1017 (ΔnifI2 background containing pLW40neo nifI2ΔT expressing His-tagged NifI2 with a deletion in the T-loop). Extracts were bound to Ni-NTA agarose in the absence (−) or presence (+) of 10 mM 2OG and eluted with 100 mM imidazole. Proteins corresponding to the indicated bands were identified by 2D gel electrophoresis and MALDI-TOF MS. Masses of the molecular weight markers (MW) are shown on the left. (B) NifI1 interactions: V, strain Mm1050 [ΔnifI1 (null) background containing pLCW40neo (vector control)]; I1, strain Mm1051 (ΔnifI1 background containing pLCW40 nifI1 expressing his-tagged NifI1); I1ΔT, strain Mm1067 (ΔnifI1 background containing pLCW40neo nifI1ΔT expressing His-tagged NifI1 with a deletion in the T-loop). Binding and elution are as in A. The band corresponding to NifI2 is partially obscured by His-tagged NifI1ΔT but was separated and identified on 2D gels. (C) Effect of ATP on NifI2 interactions using extract of strain Mm711. Binding, washing, and elution were done in the presence (+) or absence (−) of 5 mM ATP. Elution was done with either 100 mM imidazole (Imid) or 10 mM 2OG. NifI1 is present at the dye front of the gel.
Binding of ATP to PII proteins has been shown to increase their affinity for 2OG (14). To determine whether ATP might affect the formation or stability of the NifI1,2–NifDK complex, purification of the His-tagged NifI2 was repeated with (as before) or without 5 mM ATP in the binding, wash, and elution buffers (Fig. 1C). By comparing the imidazole elutions, lack of ATP did not significantly affect the amount of dinitrogenase that copurified with the His-tagged NifI2, suggesting that the complex is stable in the absence of ATP. Elution with 10 mM 2OG instead of imidazole in the presence of ATP resulted in the elution of dinitrogenase because of its separation from NifI1,2, showing that the NifI1,2–NifDK complex was disrupted by 2OG. In the absence of ATP, however, much less dinitrogenase was eluted with 2OG, suggesting that ATP is required for 2OG to efficiently disrupt the NifI1,2–NifDK complex.
Other factors that affected the copurification were explored. A marked decrease in copurification of dinitrogenase with His-tagged NifI2 was observed when dithionite was not included in the wash and elution steps, and no copurification was detected when the sodium chloride concentration was increased from 0.1 to 1 M. Glycerol (10%) did not alter the copurification of dinitrogenase, and none of the above conditions had an effect on the copurification of NifI1 with His-tagged NifI2. Attempts at purification of His-tagged NifI2 under aerobic conditions resulted in copurification of NifI1, but no copurification of dinitrogenase or other proteins was detected with or without 2OG (data not shown). These results suggest that the interaction between the NifI1,2 and dinitrogenase may be ionic in character and sensitive to redox conditions. When expressed in a ΔnifI1ΔnifI2 background, His-tagged NifI2 was not eluted from the Ni-NTA and could not be detected in cell extracts by Western blotting with an antibody for the His-tag (data not shown), suggesting that it is not stable in the absence of NifI1.
Purification of NifI1,2 and Nitrogenase.
To investigate the interactions of the NifI proteins and nitrogenase by using purified components as well as to reconstitute switch-off in vitro, the NifI1,2 complex and the nitrogenase components dinitrogenase and dinitrogenase reductase were purified. The NifI1,2 complex and the dinitrogenase were purified separately by immobilized metal-ion-affinity chromatography (IMAC) from extracts of the strain expressing the His-tagged NifI2. NifI1,2 was purified by including 2OG in all purification steps, thereby preventing copurification of dinitrogenase. Dinitrogenase was purified by eluting with 2OG instead of imidazole. His-tagged dinitrogenase reductase was also purified by IMAC from a strain expressing His-tagged NifH from a plasmid in a ΔnifH (Nif−) background. Expression of this His-tagged NifH conferred a Nif+ phenotype to the background strain, indicating that it could form a functional nitrogenase. Components were purified to at least 95% purity, as assessed by Coomassie-stained gels. Sometimes, a second round of IMAC purification was used for the NifI1,2 complex to obtain the desired level of purity. Typical activity of purified nitrogenase was 100–300 nmol of C2H2 reduced per min−1 per mg protein−1, an approximate 400-fold increase in specific activity from crude extracts (29). When assayed individually, purified components had no activity. As observed for purified nitrogenase from Methanosarcina barkeri (5), activity of purified nitrogenase components decreased after prolonged storage, together or separately, on ice; however, about a third of the activity was present after storage in a 1:1 mass ratio at −20°C in 50% glycerol or frozen at −80°C for 2 weeks. All of the experiments shown here were performed by using freshly purified dinitrogenase, dinitrogenase reductase, and NifI proteins.
Interaction of Purified NifI1,2 and Dinitrogenase.
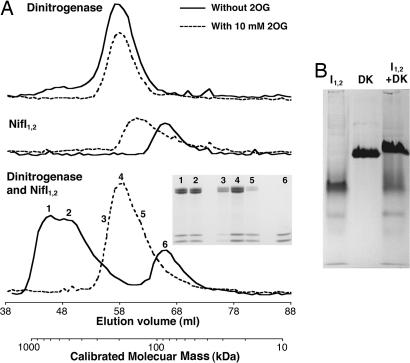
Gel filtration chromatography was used to confirm the interaction of NifI1,2 with dinitrogenase and to determine the effect of 2OG on this complex as well as to estimate the molecular masses of NifI1,2 and the complex (Fig. 2A). Because of the effects of ATP and anaerobiosis observed in the copurification experiments, gel filtration was performed under anaerobic conditions in the presence of ATP. In the absence of 2OG, dinitrogenase eluted mainly in a single peak corresponding to a molecular mass of ≈200 kDa, close to the predicted 208 kDa for an α2β2 heterotetramer of NifD (53.6 kDa) and NifK (50.5 kDa). A small amount of protein eluted in higher molecular mass fractions, possibly because of complex formation with small amounts of NifI1,2 present as impurities in the dinitrogenase preparation (see below). 2OG did not affect the elution profile of the dinitrogenase peak; however, no protein was observed in the higher-molecular-mass fractions. In the absence of 2OG, purified NifI1,2 eluted in a single peak corresponding to a molecular mass of ≈85 kDa. This peak eluted in earlier fractions when 2OG was present, at a molecular mass of ≈145 kDa, although the peak had a broad tail that overlapped with the NifI1,2 run without 2OG. These results are not consistent with NifI1,2 being a trimer (NifI1, 11.5 kDa; His-tagged NifI2, 14.8 kDa) as with other PII proteins but, instead, suggest that NifI1,2 is a larger complex, possibly a hexamer, that further oligomerizes in the presence of 2OG.
Fig. 2.
Interaction of purified NifI1,2 and dinitrogenase. (A) Gel filtration of dinitrogenase alone, NifI1,2 alone, or a mix of the two was performed anaerobically with ATP and MgCl2, with or without 10 mM 2OG. Protein concentration in fractions, measured as A600 nm, is shown versus the elution volume. The secondary x axis shows the molecular mass corresponding to the fraction volume calibrated by known protein standards. Protein in the fractions indicated by numbers 1–6 were concentrated and run on SDS/PAGE, and the resulting Coomassie-stained gel is shown (Inset). Amounts of protein loaded on the column were dinitrogenase, 1 mg and 2 mg with and without 2OG, respectively; NifI1,2, 0.75 mg and 0.5 mg with and without 2OG, respectively; and dinitrogenase:NifI1,2 mix, 2:1 mg and 3:1.5 mg with and without 2OG, respectively. (B) Purified NifI1,2 (I1,2), dinitrogenase (DK), or a 1:2 mix (NifI1,2/NifDK, by mass) run on an 8% native PAGE gel and stained with Coomassie.
When a 2:1 (by mass) mixture of dinitrogenase and NifI1,2 was loaded on the column in the absence of 2OG, a peak corresponding to dinitrogenase alone was not observed; however, most of the protein eluted as a broad peak corresponding to a higher molecular mass (500–700 kDa). SDS/PAGE revealed that both dinitrogenase and NifI1,2 were present in this higher-molecular-mass peak (Fig. 2A Inset, lanes 1 and 2). An amount of NifI1,2 corresponding to approximately half of the NifI1,2 loaded eluted in the same fractions as NifI1,2 run alone (Fig. 2A Inset, lane 6). When the mixture was run in the presence of 2OG, the higher-molecular-mass peak was not observed, and protein eluted in the same fractions as dinitrogenase and NifI1,2 run alone with 2OG. This peak was similar in shape to that obtained by summing the peaks of the dinitrogenase and NifI1,2 run alone in the presence of 2OG, and the dinitrogenase and NifI1,2 were unevenly distributed within this peak, with more dinitrogenase in the earlier fractions and more NifI1,2 in the later fractions (Fig. 2A Inset, lanes 3–5). This peak, therefore, likely represents the NifI1,2 and dinitrogenase running through the column independently rather than as a complex. These results are consistent with NifI1,2 and dinitrogenase forming a complex that is disrupted by 2OG.
Anaerobic native-gel electrophoresis was also used to confirm the interaction between NifI1,2 and dinitrogenase (Fig. 2B). When run alone, dinitrogenase and NifI1,2 each formed a single major band. Combined with the results from the gel filtration above, this result suggests that NifI1,2 exists primarily as a single, defined complex. Interaction of NifI1,2 with dinitrogenase was observed as a retardation in the mobility of the band corresponding to dinitrogenase when NifI1,2 was present. The 2OG did not prevent the gel shift when included in the loaded samples (data not shown); however, neither 2OG nor ATP were included in the gel or running buffer.
Effects of NifI1,2 and 2OG on Nitrogenase Activity.
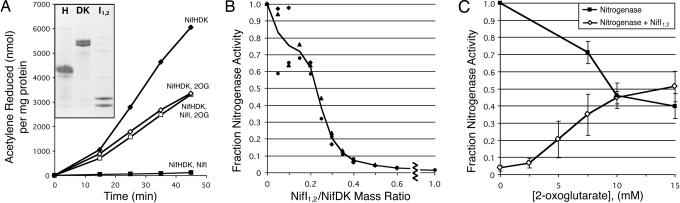
To try to reconstitute switch-off in vitro, the effects of 2OG and purified NifI1,2 on the activity of purified nitrogenase were determined. In all of these assays, high levels of ATP were maintained (see Materials and Methods). Fig. 3A shows a sample of data from one of these experiments. Nitrogenase activity was linear from 15 min to at least 45 min after the start of the reaction, and activity was calculated from this portion. The addition of NifI1,2 resulted in a decrease in activity of >50-fold. The presence of 2OG (10 mM) in reactions containing NifI1,2 resulted in the restoration of activity to the level of nitrogenase alone. For unknown reasons, 2OG had a negative effect on activity of nitrogenase alone, reducing it by about half. These results show that switch-off can be reconstituted in vitro, with a NifI1,2-dependent inhibition of nitrogenase activity that is fully relieved by 2OG.
Fig. 3.
Effects of NifI1,2 and 2OG on nitrogenase activity. (A) Acetylene (C2H2) reduced per milligram of nitrogenase (75 μg each of NifH and NifDK) as a function of time, with or without 10 mM 2OG or 60 μg of NifI1,2. Data are from a representative experiment. (Inset) A Coomassie-stained SDS/PAGE gel of the purified NifH, NifDK, and NifI1,2. (B) The effect on nitrogenase activity of increasing amount of NifI1,2 as a fraction of the nitrogenase activity in the absence of NifI1,2. The concentration of NifI1,2 is expressed as a ratio to the total amount of dinitrogenase (NifDK, 75 μg) in the reaction. Results from three independent experiments (circles, diamonds, and triangles) and the average (solid line) are shown. (C) Nitrogenase activity without (filled squares) or with (open diamonds) 60–75 μg of NifI1,2 as a function of 2OG concentration, expressed as the fraction of activity without NifI1,2 or 2OG. Error bars show the SEM of at least three independent experiments.
To determine the amount of NifI1,2 needed to fully inhibit nitrogenase and to infer the stoichiometry of the complex, the effect of increasing NifI1,2 concentrations on nitrogenase activity was determined (Fig. 3B). Because the exact oligomeric structure of NifI1,2 is not known, the amount of NifI1,2 added is expressed as a fraction of the mass of dinitrogenase present in the reaction. Although the effect of small amounts of NifI1,2 was variable, nitrogenase activity decreased consistently with addition of NifI1,2 above a 0.1 mass ratio. Activity was decreased by half between 0.2 and 0.25 mass ratio, and loss of >90% of the activity was achieved at a mass ratio of 0.4. Combined with the molecular masses calculated from the gel filtration (85 kDa for NifI1,2 and 200 kDa for dinitrogenase), these results suggest that ≈1 mol of NifI1,2 is sufficient to almost completely inhibit the activity of 1 mol of dinitrogenase.
Once an inhibitory level of NifI1,2 was established, the effect of increasing concentrations of 2OG on nitrogenase activity was determined in the presence and absence of NifI1,2 (Fig. 3C). Addition of 2OG from 2.5 to 10 mM resulted in a steady increase in nitrogenase activity in the presence of NifI1,2, reaching 100% of the activity of nitrogenase alone at 10 mM. The 2OG had a negative effect on nitrogenase activity alone, decreasing the activity by ≈50% at 10 mM. In all but one of the experiments, 2OG concentrations >10 mM did not increase the activity of nitrogenase with NifI1,2 above the activity of nitrogenase alone, suggesting that the positive effect of 2OG on activity is solely the relief of inhibition by NifI1,2. Addition of other potential signaling molecules, glutamine, glutamate, or pyruvate at 7.5 mM, did not increase activity in the presence of NifI1,2. When equal amounts (7.5 mM) of glutamine and 2OG were present, the activity was the same as with 2OG alone. Thus, 2OG appears to be the main nitrogen signal sensed by NifI1,2.
Discussion
Results presented here support a model for regulation of nitrogenase activity in M. maripaludis: NifI1,2, a heteromeric nitrogen sensor composed of subunits related to PII proteins, inhibits nitrogenase activity by direct binding to dinitrogenase. This binding is prevented, and inhibition thus relieved, by high levels of 2OG, which act as a signal of nitrogen limitation. The model is supported by copurification experiments and gel filtration with purified components that demonstrate the protein–protein interactions and in vitro nitrogenase assays with purified components. The mechanism is relatively simple and contrasts with the more complex regulation found in some Proteobacteria involving ADP-ribosylation of nitrogenase reductase by DraT and DraG (3), binding and membrane sequestration of DraG to the ammonia transporter AmtB by PII proteins, and covalent modification of the PII proteins by the glutamine sensor GlnD (12). The wide phylogenetic distribution of nifI1 and nifI2 genes, and their almost exclusive linkage to nifHDK genes, suggests that this mode of regulation may be conserved in a variety of diazotrophic Archaea and Bacteria (30).
Two features of our results are anomalous but do not, in our opinion, diminish the evidence for our model of regulation. First, 2OG, in addition to reversing binding of NifI1,2 to dinitrogenase and preventing inhibition of nitrogenase activity, lowered nitrogenase activity when NifI1,2 was not present. This effect could be due to the action of 2OG as a weak chelator of either magnesium or iron, lowering the MgATP concentration or damaging the nitrogenase metal cofactors by removal of iron. Second, the amount of 2OG required for relief of NifI1,2 inhibition of nitrogenase, 2.5 mM to 10 mM, was ≈10-fold higher than the concentration range we estimated to be present in cells (29). A similar concentration was required to restore activity in crude extracts from wild-type M. maripaludis, suggesting that the high levels of 2OG needed were not simply an alteration of its binding to NifI1,2 because of the His-tag fusions. It is possible that some factor might increase the affinity of NifI1,2 for 2OG in vivo; however, this factor is apparently either not present or not active in the crude cell extracts.
The mechanism for regulation of nitrogenase activity proposed here has significant similarities with the proteobacterial mechanism. PII-related proteins are involved, and the T-loops are important for the interaction with the target protein. 2OG appears to act as an indicator of nitrogen deficiency. Also, ATP is required for the effect of 2OG (14). However, the two mechanisms have many contrasting features. The two PII-related proteins, NifI1 and NifI2, represent two subfamilies of PII that are distinct from all those that have been previously studied (26). Although heterotrimers of GlnB and GlnK have been observed (31), these proteins function primarily as homotrimers; in contrast, NifI1,2 appears to function as a heteromer. It is interesting that the T-loops of both NifI1 and NifI2 seem to be required for the interaction with dinitrogenase but are not required for the interaction of NifI1 with NifI2. The NifI1,2 heteromer has a molecular mass consistent with a hexamer and, in the presence of 2OG, appears to oligomerize to a higher molecular mass. The 2OG seems to indicate only the nitrogen state of the cell, not glutamine as well (32). We have found no evidence of covalent modification of NifI1 or NifI2 that would mediate an additional signal, as is the case with other PII-like proteins. The reconstitution of switch-off in vitro suggests either that modification is not necessary or that the purified NifI1 and/or NifI2 are already in their appropriately modified form. Finally, the immediate target of regulation is dinitrogenase rather than dinitrogenase reductase, and inhibition occurs by direct binding of NifI1,2 rather than a cascade of events leading to covalent modification.
It is interesting to speculate on how binding of NifI1,2 to dinitrogenase inhibits nitrogenase activity. NifI1,2 could occlude access of dinitrogenase reductase to dinitrogenase. Alternatively, binding of NifI1,2 could interfere with substrate binding or result in a conformational change that somehow prevents electron transfer within dinitrogenase. In any case, the mechanism of inhibition may shed more light on the function of nitrogenase in general. Further study of the interaction between NifI1,2 and dinitrogenase, as well as between NifI1 and NifI2 themselves, and how this interaction inhibits nitrogenase activity should yield intriguing results.
Materials and Methods
Cell Growth, Harvesting, and Extract Preparation.
Standard procedures for culturing methanogens were used (33). Strains were grown in N-free medium in either 150-ml volumes using 1-liter bottles (for copurification experiments), as described previously, (29) or 10-liter volumes (for protein purification) by using a Microferm fermenter (New Brunswick Scientific). Puromycin (2.5 μg/ml) and neomycin (1 mg/ml) were used for selection when necessary. Medium composition for fermenter growth was altered, by using 2 g/liter sodium bicarbonate and 0.5 g/liter cysteine. The cysteine allows for better growth in the fermenter but cannot be used as a sole nitrogen source when added after autoclaving (A. K. Haydock and J.A.L., unpublished results). Fermenter growth was at 37°C with an agitation rate of 200 rpm, and gassing rates were 500 ml/min H2, 200 ml/min CO2, 200 ml/min N2, and 20 ml/min 1% H2S (balance N2). Culture was harvested anaerobically from the fermenter at an OD660 of ≈1.0 via an 18-gauge syringe needle inserted into a 4-liter vacuum flask that was previously incubated in an anaerobic chamber and sealed with butyl rubber stoppers. Cells were pelleted anaerobically as described (29). Fermenter-grown cells were resuspended in a buffer containing 50 mM Hepes, pH 7.5, 50 mM NaCl, and 4 mM sodium dithionite and stored up to 8 months at −80°C in 5-ml serum vials (Wheaton Science Products, Millville, NJ) sealed with butyl rubber stoppers. Crude protein extracts were prepared by sonication as described (29). Protein quantitation was performed with Coomassie Plus Protein Assay reagent (Pierce) using BSA as a standard.
Strain Construction.
Strains, plasmids, and oligonucleotide sequences used in this study appear in Table 1, which is published as supporting information on the PNAS web site. All strains are derivatives of M. maripaludis strain S2 (34). Two plasmids were constructed for expression of proteins with His6 tags at their N or C termini in M. maripaludis by insertion of a polylinker directly downstream of the Methanococcus voltae histone promoter present in the vector pWLG40NZ-R (35). Polylinkers were constructed by annealing the oligonucleotides WHN coding and WHN noncoding (for N terminus His-tagging) or WHC coding and WHC noncoding (for C terminus His-tagging) and ligated into pWLG40NZ-R digested with NsiI and BglII, creating pLW40neo and pLCW40neo, respectively. His-tagged NifI2 was constructed by PCR amplifying nifI2 with primers containing ApaI (at the 5′ end of nifI2) and AscI (3′ end) restriction sites and ligating into ApaI–AscI-digested pLW40neo. A similar process was used for nifI1 and nifH using pLCW40neo and digestion with NsiI instead of ApaI. The resulting constructs coded for the amino acid sequence MHHHHHHIEGRGP preceding the N-terminal methionine for NifI2 or GRAIEGRHHHHHH(stop) at the C terminus for NifI1 and NifH. Deletions in the T-loop domains were constructed by amplifying the 5′ and 3′ regions of the gene flanking the desired deletion with primers that contained a BamHI site at their 5′ ends (resulting in the insertion of codons for glycine and serine). PCR products were digested with BamHI, AscI, and either ApaI (for NifI2) or NsiI (for NifI1) and ligated with pLW40neo digested with ApaI and AscI (for NifI2) or pLCW40neo digested with AscI and NsiI (for NifI1). The resulting mutations were the replacement of amino acids 48–52 with a serine (the glycine codon in the BamHI site replaced a glycine codon in the wild-type nifI2) in NifI2 and the replacement of amino acids 43–49 with a glycine and serine in NifI1. A M. maripaludis strain (Mm1036) with an in-frame deletion in nifH (Δ4–268) was constructed from strain Mm900 for expression of the His-tagged NifH. Regions flanking nifH were PCR amplified and cloned into ApaI-XbaI digested pCRPrtNeo, and marker replacement was performed as described (36). All constructs were confirmed by sequencing. M. maripaludis strains Mm55 (ΔnifI2), Mm56 (ΔnifI1), and Mm1036 were transformed (37) with the following plasmids: Mm55 with pLW40neo, pLW40neo nifI2, and pLW40neo nifI2ΔT (creating strains Mm1012, Mm711, and Mm1017, respectively); Mm56 with pLCW40neo, pLCW40neo nifI1, and pLCW40neo nifI1ΔT (creating strains Mm1050, Mm1051, and Mm1067, respectively); and Mm1036 with pLCW40neo nifH (creating strain Mm1046).
Copurification Experiments.
Nickel-ion-affinity chromatography was used to identify proteins that copurify with the His-tagged NifI1 and NifI2 proteins. Binding reactions were prepared in 18-ml modified Balch tubes (shortened to 10-cm length at the Physics Department glass shop, University of Washington) in an anaerobic chamber (Coy Laboratory Products, Grass Lake, MI) with an atmosphere of H2/N2 (5:95). Protein extracts were bound to 0.25 ml of Ni-NTA agarose (Novagen) in binding buffer (25 mM Hepes, pH 7.5, 10 mM sodium dithionite, 100 mM NaCl, 10 mM imidazole, 5 mM ATP, 12.5 mM MgCl2, and 3.4 mg/ml phosphocreatine) with or without 10 mM 2OG. The total volume was 3 ml, with a protein concentration of 2–3 mg/ml. Tubes were stoppered, removed from the anaerobic chamber, and incubated with shaking at 30°C for 30–45 min. If nitrogenase activity was to be measured during the binding, 0.1 mg/ml creatine phosphokinase and 0.5 mg/ml BSA were included in the mixture, the headspace was flushed for 2 min with 100% argon gas, and 0.2 ml of 100% acetylene gas was added before incubation. Tubes were returned to the anaerobic chamber, and the binding mixture was applied to 10-ml PolyPrep Columns (Bio-Rad) drained by gravity flow. The Ni-NTA agarose was washed twice with 5 ml of binding buffer, and bound proteins were eluted with 1.5 ml of binding buffer containing 100 mM imidazole (for His-tagged NifI2) or 500 mM imidazole (for His-tagged NifI1). Elution fractions were run on 12% SDS/PAGE (Fig. 1 A and C) or 10% Tris-tricine gels (Fig. 1B), and proteins were visualized with Silver Stain Plus kit (Bio-Rad).
Protein Identification by 2D Gel Electrophoresis and MS.
Elution fractions (1 ml) were precipitated with 0.3 volumes of acetone, and 2D gel electrophoresis was performed as described (38), with the following modifications: pH 3–10 NL (nonlinear) immobilized pH gradient (IPG) strips (Amersham Pharmacia Biosciences) were used, 1% pH 3–10 NL buffer was included in the rehydration buffer, and 12% acrylamide gels were used for the SDS/PAGE. Gels were silver stained (39), and spots to be identified were excised by using a clean scalpel. As a negative control, a portion of the gel with no visible protein was also taken. In-gel digestion with sequencing-grade modified trypsin (Promega) was performed essentially as described (39), but without the treatment with iodoacetamide. Resulting peptide fragments were desalted by using C18 Zip-Tips (Millipore) according to the manufacturer’s instructions. MALDI-TOF MS was performed at the Medicinal Chemistry Mass Spectrometry Center, University of Washington, using α-cyano-4-hydroxy-cinnamic acid as a matrix. Peaks that were not in the mass spectrum of the negative control were used to identify the corresponding protein with peptidesearch version 3.0.5b0 (40) using the M. maripaludis strain S2 genome sequence (41) as an index file.
Purification of NifI and Nitrogenase Components.
Proteins were purified by nickel affinity essentially as described above for the copurification experiments, with some modifications. The volumes (binding, 12 ml; washes, 25 ml; elution, 3 ml) and the amount of Ni-NTA (1 ml) were increased, 20-cm length Econo-columns (Bio-Rad) were used, and protein concentrations were 10–15 mg/ml. Dinitrogenase (NifDK) and the NifI proteins (His-tagged NifI2 and NifI1) were purified from strain Mm711. For purification of NifI proteins, 20 mM 2OG was included in all of the steps to prevent dinitrogenase copurification, and elution was performed with imidazole as above. For dinitrogenase purification, elution was performed with 10 mM 2OG. His-tagged dinitrogenase reductase (NifH) was purified by using extracts from strain Mm1046. ATP, phosphocreatine, and MgCl2 were omitted, and elution was done with 100 mM imidazole. Elution fractions were concentrated to ≈100 μl by using Vivaspin 0.5-ml centrifugal concentrators with 10-kDa (for NifI proteins) or 30-kDa (for NifH and NifDK) molecular mass cut-off PES membranes, by using a Spectrafuge (Labnet International, Edison, NJ) microcentrifuge in the anaerobic chamber. Concentrated proteins were then desalted three times with 10 volumes of resuspension buffer (25 mM Hepes, pH 7.5, 25 mM NaCl, and 2–5 mM sodium dithionite) by using the centrifugal concentrators and stored on ice until use.
Gel Filtration.
Gel filtration was performed at room temperature in an anaerobic chamber using a Hi-Prep 16/60 Sephacryl S-300 column (Amersham Pharmacia Biosciences) with a flow rate of 0.5 ml/min. The buffer contained 50 mM Hepes, pH 7.5, 100 mM sodium chloride, 2.5 mM ATP, 6.25 mM MgCl2, and 2.5 mM sodium dithionite. For some runs, 10 mM 2OG was included. Before loading, proteins were incubated at room temperature for 10 min in resuspension buffer containing 5 mM ATP and 12.5 mM MgCl2, with or without 20 mM 2OG. Molecular mass calibration was done by using Low and High Molecular Weight Calibration kits (Amersham Pharmacia Biosciences). Protein in fractions (0.75 ml) eluted from the column was quantitated with Coomassie Plus Protein Assay reagent (Pierce). To identify the proteins present in certain peaks, corresponding fractions were concentrated by using 0.5-ml centrifugal concentrators with 3-kDa molecular mass cut-off membranes (Vivaspin), and SDS/PAGE was performed by using Precise 4–20% gradient gels (Pierce).
Native Gel Electrophoresis.
Native gel electrophoresis was performed anaerobically by using a modified version of the Laemmli method without SDS (42). Resolving gels (8% acrylamide) were cast aerobically and brought into an anaerobic chamber, where the stacking gels were cast and the gels were run. Sodium dithionite was used in the cast gels (0.5 mM) and the running buffer (2 mM), which was bubbled with 100% N2 gas to remove oxygen.
Nitrogenase Activity Assays.
In vitro nitrogenase activity assays, using an ATP regenerating system (phosphocreatine and creatine phosphokinase) and sodium dithionite as a reductant, were performed as described (29), with modifications. Reactions (0.5 ml) were prepared in 5-ml serum vials with 20 mM dithionite, 1 ml of 100% acetylene was added to the headspace, and 0.3-ml gas samples were taken by syringe at various times after the beginning of the 30°C incubation. Reactions typically contained 75 μg each of NifH and NifDK, and activities were calculated based on 150 μg of total nitrogenase.
Supplementary Material
Acknowledgments
We thank Drs. Murray Hackett, Tony Wang, and Yi Zhang for assistance with 2D gels and MS techniques. This work was supported by National Science Foundation (NSF) Grant MCB 0316251 and U.S. Department of Agriculture Grant ICGP:35319-09927. J.A.D. was generously supported by the Helen Riaboff Whiteley Fellowship Fund (University of Washington) and NSF Integrative Graduate Education and Research Traineeship Grant DGE-9870713.
Abbreviations
- 2OG
2-oxoglutarate.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Halbleib C. M., Ludden P. W. J. Nutr. 2000;130:1081–1084. doi: 10.1093/jn/130.5.1081. [DOI] [PubMed] [Google Scholar]
- 2.Rees D. C., Tezcan F. A., Haynes C. A., Walton M. Y., Andrade S., Einsle O., Howard J. B. Philos. Trans. R. Soc. London A. 2005;363:971–984. doi: 10.1098/rsta.2004.1539. [DOI] [PubMed] [Google Scholar]
- 3.Ludden P. W., Roberts G. P. Curr. Top. Cell. Regul. 1989;30:23–56. doi: 10.1016/b978-0-12-152830-0.50004-9. [DOI] [PubMed] [Google Scholar]
- 4.Yoch D. C., Li J. D., Hu C. Z., Scholin C. Arch. Microbiol. 1988;150:1–5. doi: 10.1007/BF00409708. [DOI] [PubMed] [Google Scholar]
- 5.Lobo A. L., Zinder S. H. J. Bacteriol. 1990;172:6789–6796. doi: 10.1128/jb.172.12.6789-6796.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Durner J., Böhm I., Hilz H., Böger P. Eur. J. Biochem. 1994;220:125–130. doi: 10.1111/j.1432-1033.1994.tb18606.x. [DOI] [PubMed] [Google Scholar]
- 7.Egener T., Martin D. E., Sarkar A., Reinhold-Hurek B. J. Bacteriol. 2001;183:3752–3760. doi: 10.1128/JB.183.12.3752-3760.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arcondéguy T., Jack R., Merrick M. Microbiol. Mol. Biol. Rev. 2001;65:80–105. doi: 10.1128/MMBR.65.1.80-105.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ninfa A. J., Jiang P. Curr. Opin. Microbiol. 2005;8:168–173. doi: 10.1016/j.mib.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y., Pohlmann E. L., Halbleib C. M., Ludden P. W., Roberts G. P. J. Bacteriol. 2001;183:1610–1620. doi: 10.1128/JB.183.5.1610-1620.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drepper T., Groß S., Yakunin A. F., Hallenbeck P. C., Masepohl B., Klipp W. Microbiology. 2003;149:2203–2212. doi: 10.1099/mic.0.26235-0. [DOI] [PubMed] [Google Scholar]
- 12.Huergo L. F., Souza E. M., Araujo M. S., Pedrosa F. O., Chubatsu L. S., Steffens M. B. R., Merrick M. Mol. Microbiol. 2006;59:326–337. doi: 10.1111/j.1365-2958.2005.04944.x. [DOI] [PubMed] [Google Scholar]
- 13.Xu Y., Carr P. D., Huber T., Vasudevan S. G., Ollis D. L. Eur. J. Biochem. 2001;268:2028–2037. doi: 10.1046/j.1432-1327.2001.02074.x. [DOI] [PubMed] [Google Scholar]
- 14.Kamberov E. S., Atkinson M. R., Ninfa A. J. J. Biol. Chem. 1995;270:17797–17807. doi: 10.1074/jbc.270.30.17797. [DOI] [PubMed] [Google Scholar]
- 15.Jiang P., Peliska J. A., Ninfa A. J. Biochemistry. 1998;37:12795–12801. doi: 10.1021/bi9802420. [DOI] [PubMed] [Google Scholar]
- 16.Ninfa A. J., Atkinson M. R. Trends Microbiol. 2000;8:172–179. doi: 10.1016/s0966-842x(00)01709-1. [DOI] [PubMed] [Google Scholar]
- 17.Jiang P., Zucker P., Atkinson M. R., Kamberov E. S., Tirasophon W., Chandran P., Schefke B. R., Ninfa A. J. J. Bacteriol. 1997;179:4342–4353. doi: 10.1128/jb.179.13.4342-4353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forchhammer K. FEMS Microbiol. Rev. 2004;28:319–333. doi: 10.1016/j.femsre.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Hesketh A., Fink D., Gust B., Rexer H.-U., Scheel B., Chater K., Wohlleben W., Engels A. Mol. Microbiol. 2002;46:319–330. doi: 10.1046/j.1365-2958.2002.03149.x. [DOI] [PubMed] [Google Scholar]
- 20.Satomura T., Daisuke S., Asai K., Sadaie Y., Hirooka K., Fujita Y. J. Bacteriol. 2005;187:4813–4821. doi: 10.1128/JB.187.14.4813-4821.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nolden L., Ngouoto-Nkili C.-E., Bendt A. K., Krämer R., Burkovski A. Mol. Microbiol. 2001;42:1281–1295. doi: 10.1046/j.1365-2958.2001.02694.x. [DOI] [PubMed] [Google Scholar]
- 22.Ehlers C., Weidenbach K., Veit K., Forchhammer K., Schmitz R. A. Mol. Microbiol. 2005;55:1841–1854. doi: 10.1111/j.1365-2958.2005.04511.x. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y. M., Ferrar T. S., Lohmeir-Vogel E., Morrice N., Mizuno Y., Berenger B., Ng K. K. S., Muench D. G., Moorhead G. B. B. J. Biol. Chem. 2006;281:5726–5733. doi: 10.1074/jbc.M510945200. [DOI] [PubMed] [Google Scholar]
- 24.Ikeda T. P., Shauger A. E., Kustu S. J. Mol. Biol. 1996;259:589–607. doi: 10.1006/jmbi.1996.0342. [DOI] [PubMed] [Google Scholar]
- 25.Muro-Pastor M. I., Reyes J. C., Florencio F. J. J. Biol. Chem. 2001;276:38320–38328. doi: 10.1074/jbc.M105297200. [DOI] [PubMed] [Google Scholar]
- 26.Enkh-Amgalan J., Kawasaki H., Seki T. Int. J. System. Evol. Microbiol. 2006;56:65–74. doi: 10.1099/ijs.0.63815-0. [DOI] [PubMed] [Google Scholar]
- 27.Seshadri R., Adrian L., Fouts D. E., Eisen J. A., Phillippy A. M., Methe B. A., Ward N. L., Nelson W. C., Deboy R. T., Khouri H. M., et al. Science. 2005;307:105–108. doi: 10.1126/science.1102226. [DOI] [PubMed] [Google Scholar]
- 28.Kessler P. S., Daniel C., Leigh J. A. J. Bacteriol. 2001;183:882–889. doi: 10.1128/JB.183.3.882-889.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dodsworth J. A., Cady N. C., Leigh J. A. Mol. Microbiol. 2005;56:1527–1538. doi: 10.1111/j.1365-2958.2005.04621.x. [DOI] [PubMed] [Google Scholar]
- 30.Dandekar T., Snel B., Huynen M., Bork P. Trends Biochem. Sci. 1998;23:324–328. doi: 10.1016/s0968-0004(98)01274-2. [DOI] [PubMed] [Google Scholar]
- 31.Forchhammer K., Hedler A., Strobel H., Weiss V. Mol. Microbiol. 1999;33:338–349. doi: 10.1046/j.1365-2958.1999.01477.x. [DOI] [PubMed] [Google Scholar]
- 32.Jiang P., Peliska J. A., Ninfa A. J. Biochemistry. 1998;37:12802–12810. doi: 10.1021/bi980666u. [DOI] [PubMed] [Google Scholar]
- 33.Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Microbiol. Rev. 1979;43:260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitman W. B., Shieh J. S., Sohn S., Caras D. S., Premachandran U. System. Appl. Microbiol. 1986;7:235–240. [Google Scholar]
- 35.Lie T. J., Leigh J. A. Mol. Microbiol. 2003;47:235–246. doi: 10.1046/j.1365-2958.2003.03293.x. [DOI] [PubMed] [Google Scholar]
- 36.Moore B. C., Leigh J. A. J. Bacteriol. 2005;187:972–979. doi: 10.1128/JB.187.3.972-979.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tumbula D. L., Makula R. A., Whitman W. B. FEMS Microbiol. Lett. 1994;121:309–314. [Google Scholar]
- 38.Wang T., Zhang Y., Chen W., Park Y., Lamont R. J., Hackett M. Analyst. 2002;127:1450–1456. doi: 10.1039/b206157k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shevchenko A., Wilm M., Vorm O., Mann M. Anal. Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 40.Mann M., Wilm M. Anal. Chem. 1994;66:4390–4399. doi: 10.1021/ac00096a002. [DOI] [PubMed] [Google Scholar]
- 41.Hendrickson E. L., Kaul R., Zhou Y., Bovee D., Chapman P., Chung J., Conway de Macario E., Dodsworth J. A., Gillett W., Graham D. E., et al. J. Bacteriol. 2004;186:6956–6969. doi: 10.1128/JB.186.20.6956-6969.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gallagher S. R. Current Protocols in Molecular Biology. In: Ausubel F. M., Brent R., Kingston R. E., Moore D. D., Seidman J. G., Smith J. A., Struhl K., editors. New York: Wiley; 1999. p. 10.2B. 1–10.2B, and 11. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.