Abstract
(—)-Epigallocatechin-3-gallate (EGCG) has been shown to have potent antiphotocarcinogenic activity, but it was required to develop a cream-based formulation for topical application. For topical application, we tested hydrophilic creamas a vehicle forEGCG. Treatment with EGCG (≈ 1 mg/cm2 skin area) in hydrophilic cream resulted in exceptionally high protection against photocarcinogenesis when determined in terms of tumor incidence, tumor multiplicity, and tumor size in a SKH-1 hairless mouse model. EGCG also inhibited malignant transformation of ultraviolet B (UVB)-induced papillomas to carcinomas. In order to determine the mechanism of prevention of photocarcinogenesis, we determined the effect of EGCG on global DNA methylation pattern using monoclonal antibodies against 5-methyl cytosine and DNA methyltransferase in the long-term UV-irradiated skin because altered DNA methylation silencing is recognized as a molecular hallmark of human cancer. We found that treatment with EGCG resulted in significant inhibition of UVB-induced global DNA hypomethylation pattern. Longterm application of EGCG did not show any apparent sign of toxicity inmice when determined in terms of skin appearance, lean mass, total bone mineral content, and total bone mineral density but showed reduction in fat mass when analyzed using dual-energy X-ray absorptiometry. These data suggest that hydrophilic cream could be a suitable vehicle for topical application of EGCG, and that EGCG is a promising candidate for future cancer therapies based on its influence on the epigenetic pathway.
Keywords: DNA methylation, green tea, skin cancer prevention, (—)-epigallocatechin-3-gallate, ultraviolet radiation
Introduction
The incidence of skin cancer among high-risk human populations is continuing to grow at an alarming rate, and approximately 1.3 million new cases of skin cancer, including basal and squamous cell carcinomas, are diagnosed each year in the United States [1]. Epidemiological, clinical, and biochemical studies have implicated solar ultraviolet (UV) radiation, particularly UVB (290–320 nm) spectrum, as a complete carcinogen and repeated exposures can lead to the development of melanoma and nonmelanoma skin cancers [1–4]. The increased risk of skin cancer is expected to continue as the population ages and larger amounts of UV radiation reach the surface of the Earth because of depletion of the ozone layer [2–5]. Moreover, the increased tendency of individuals to obtain a rapid tan and the use of tanning booths are also implicated in the high risk of melanoma and nonmelanoma skin cancers [1–5], the occurrence of which has a tremendous impact on public health and healthcare expenditures. Therefore, it is desired to develop newer and effective chemopreventive agents and strategies, which can inhibit or slow down the UV-induced risk of melanoma and nonmelanoma skin cancers among high-risk human populations.
Cancer chemoprevention can prevent or delay the occurrence of cancer in high-risk human populations using dietary or chemical intervention approaches. The worldwide interest is considerably increasing in the use of naturally occurring botanical supplements, which can be used as cancer chemopreventive agents and/or as complementary and alternative medicine. In earlier studies, polyphenols from green tea, a widely consumed beverage worldwide, have been shown to have anticarcinogenic activity in several in vitro and in vivo models [6]. In earlier studies, it has been shown that topical treatment of (—)-epigallocatechin-3-gallate (EGCG), a polyphenolic constituent from green tea, in organic solvents such as acetone onto the mouse skin inhibited 7,12-dimethylbenz[a]-anthracene-initiated and 12-O-tetradecanoylphorbol-13-acetate-promoted skin tumorigenesis [7], and also inhibited UV-induced adverse biological effects associated with skin carcinogenesis [8–11]. Use of organic solvents in skin care products, or for topical treatment of pharmacologic agents for human use, does not seem to be clinically appropriate. Dvorakova et al. [12] have reported that topical treatment of EGCG with hydrophilic cream achieved a high concentration in the skin. Subsequently, very recently, we tested the efficacy of EGCG in hydrophilic cream on SKH-1 hairless mouse skin against UVB-induced oxidative stress and oxidative stress-mediated cell signaling pathways, such as phosphorylation of mitogen-activated protein kinases [13]. We found that treatment of EGCG in hydrophilic cream resulted in exceptionally high protection against UVB-induced oxidative stress and phosphorylation of mitogen-activated protein kinases [13]. The results from this in vivo study encourage us to determine whether topical treatment with EGCG in hydrophilic cream would provide exceptionally high protection against UVB-induced skin tumorigenesis in mouse models compared to previous observations, and the suitability of this formulation for the safer use of EGCG against UVB-induced adverse biological effects including skin cancer.
In carcinogenesis, epigenetic alterations play a critical role in the regulation of gene expression and are mediated through modulation of heritable transcription superimposed on the primary DNA sequence. Thus, without changing the sequence of the DNA, epigenetic mechanisms such as DNA methylation [e.g., 5-methylcytosine (5-mc) content of DNA] can change the expression of proteins at transcriptional levels [14]. Both hypermethylation and hypomethylation can contribute to carcinogenesis by silencing of tumor suppressor genes, upregulation of oncogenes, and/or decreased genomic stability [15,16]. Overall global DNA hypomethylation and hypermethylation at GC-rich regions are typical characteristics of tumors that could alter the expression of genes [17]. Changes in methylation pattern precede tumor formation, indicating that these alterations might contribute to tumorigenesis [18]. The enzyme DNA methyltransferase (DNMT) catalyzes the transfer of a methyl moiety from S-adenosyl-l-methionine to cytosine principally in the cytosine guanine dinucleotide [19,20]. Cancers with aberrant methylation patterns have been observed to have concurrent significant changes in their DNMT activities [21–23]. There has been limited investigation on DNA methylation in mouse skin, but a few reports indicate methylation differences between normal skin and tumor tissues [24,25]. As we believe, there have been no reported studies as yet on the effect of UV irradiation on DNA methylation patterns in murine skin, and chemopreventive effects of dietary constituents such as EGCG on UVB-induced alterations in DNA methylation pattern.
Therefore, the present study was designed to determine the chemopreventive effect of topical application of EGCG in hydrophilic cream (≈ 1 mg/cm2) on: 1) photocarcinogenesis in terms of tumor incidence, tumor multiplicity, and tumor growth; and 2) malignant transformation of benign papillomas to carcinomas in SKH-1 hairless mouse models. We also determined 3) whether prevention of photocarcinogenesis by EGCG is mediated by inhibition of UV-induced changes in global DNA methylation pattern. Additionally, for the first time, we also determined 4) the effect of longterm topical treatment of EGCG on body composition such as bone free soft lean tissue mass, fat mass, total bone mineral content (TBMC), and total bone mineral density (TBMD) using dual-energy X-ray absorptiometry (DXA) in experimental animals.
Materials and Methods
Animals
Six- to seven-week-old female SKH-1 hairless mice, which were obtained from Charles River Laboratories (Wilmington, MA), were used in this study. Mice were housed five per cage and were acclimatized for at least 1 week before use in animal facility and were maintained at standard conditions of 12-hour light/12-hour dark cycle, 24 ± 2°C temperature, and 50 ± 10% relative humidity. Animals were fed Teklad Chow diet and water ad libitum. The animal protocol for this study was approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham, in accordance with the current US Department of Agriculture, Department of Health and Human Service regulations and standards.
Chemicals and Antibodies
Diaminobenzidine substrate set and peroxidase-labeled streptavidin were purchased from Kirkgaard and Perry (Gaithersburg, MD). 5-mc Ab was a gift from Dr. Alain Niveleau at the University Joseph Fourier of Grenoble (Grenoble, France). All chemicals employed in this study were of analytical grade and purchased from Sigma Chemical Co. (St. Louis, MO).
Preparation of EGCG Formulation in Hydrophilic Cream
Purified EGCG (> 98% pure) was procured from Tokyo Food Techno Co. Ltd. (Shizuoka, Japan). EGCG was uniformly mixed in hydrophilic cream (Fougera and Co., Melville, NY) and was topically applied (≈ 1 mg/cm2 skin area) onto the mouse skin 20 to 25 minutes before each UVB exposure. This hydrophilic cream is prescribed for external use as an ointment or cosmetic base. Formulation was prepared on a weekly basis and stored at 4°C.
UVB Irradiation of Mice
UVB irradiation of mice was performed as described previously [26,27]. Briefly, dorsal skin was exposed to UV radiation from a band of four FS-20 fluorescent lamps from which short wavelengths of UV (< 290 nm) not normally present in natural solar light were filtered out using Kodacel cellulose film (Eastman Kodak Co., Rochester, NY). The majority of the resulting wavelengths after Kodacel filtration were in UVB (290–320 nm) and UVA range with peak emission at 314 nm, as monitored. The UVB emission was monitored before each UVB irradiation with an IL-1700 phototherapy radiometer equipped with an IL SED 240 detector fitted with a W side-angle quartz diffuser and a SC5 280 filter (both from International Light, Newburyport, MA). During UVB irradiation, mice were held in dividers separated by Plexiglas.
Photocarcinogenesis Experiment and Protocol
At least 1 week after their arrival in the animal facility, the mice were divided into different treatment groups with 20 mice in each group. Long-term photocarcinogenesis protocol (including both UVB-induced tumor initiation and promotion stages) was employed to determine the photoprotective effect of EGCG as described previously [26,27]. Briefly, mice were divided into two groups. Mice in group 1 were topically treated with vehicle (hydrophilic cream) only, whereas group 2 was topically treated with EGCG in hydrophilic cream prior to each UVB irradiation. Initially, mice in group 2 were topically treated with EGCG for 14 days and, thereafter from day 15, the mice belonging to both groups 1 and 2 were irradiated everyday with UVB (180 mJ/cm2) and continued for a total of 10 days. This period of UVB irradiation was termed as tumor initiation stage. One week after the last UVB exposure of tumor initiation, the mice were again UVB-irradiated with the same dose (180 mJ/cm2) three times a week to achieve tumor promotion stage.
During the experimental protocol, skin papillomas or suspected carcinomas were observed and recorded weekly. Tumors larger than 1 mm in diameter that persisted for 2 weeks or more were recorded. Tumor data were recorded until their yield and size were stabilized. At this time point, the dimensions of all the tumors on each mouse were recorded. Tumor volumes were calculated by the hemiellipsoid model formula: tumor volume = 1/2(4π/3)(1/2)(w/2)h, where l = length, w = width, and h = height, as followed earlier [26,27]. Carcinoma incidence and multiplicity were recorded until 30 weeks of the experimental protocol. The diagnosis of carcinoma was confirmed histologically either at the time when carcinoma-bearing mice died, or at the termination of the experiment at 30 weeks. Because of ulcerations and the larger size of carcinomas, the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham did not allow these experiments for a longer time; therefore, experiments were stopped at this stage. At this time, carcinoma incidence, multiplicity, and sizes were finally recorded.
Histology of the Skin
To evaluate the chemopreventive effect of topical treatment of EGCG on long-term UVB irradiation-induced adverse changes in skin morphology, mice were sacrificed at the termination of the experiment at 30 weeks and dorsal skin biopsies were collected, fixed in 10% buffered formalin, and processed for H&E staining for microscopic evaluation.
Immunohistochemical Detection of DNA Methylation Pattern
Paraffin-embedded skin sections (6 µm thick) were stained to detect DNA methylation patterns following antigen retrieval methods, as described earlier with specific modifications [28]. Briefly, the sections were placed in 0.01 mol/l citric acid (pH 6.0) in a microwave oven set at full potency for 10 minutes. After antigen retrieval, the slides were immersed in 3.5 N HCl for 15 minutes at room temperature to expose the CpG. The sections were then treated with 3% H2O2 for 5 minutes to quench endogenous peroxidase. Sections were incubated with preimmune goat serum (3%) for 30 minutes followed by incubation with 40 µg/ml AffiPure Fab (Jackson Immunoresearch, Marseille, France) fragment goat anti-mouse IgG (H + L) diluted in phosphate-buffered saline for 30 minutes to suppress nonspecific staining. The sections were subsequently incubated with a hybridoma supernatant containing anti-5-mc monoclonal antibody (7.5 µg/ml) for 1 hour. After washing the slides, sections were incubated with a biotin-streptavidin detection system (Signet, Dedham, MA). Companion matching slides processed identically and stained without primary antibody served as controls (deletes). The substrate diaminobenzidene tetrahydrochloride was used to detect the antigen-antibody complex, and sections were counterstained with hematoxylin.
Assay for DNMT
Quantitative assay was performed to determine levels of DNMT in skin tissues collected from different treatment groups at the termination of the photocarcinogenesis experiment. Age-matched normal (non-UV) skin was used as a control. Details of the assay are described below.
Oligonucleotide DNA Substrates
Oligonucleotides for the DNMT assay were synthesized as reported earlier [29]. Briefly, the sequence of 60-mer oligonucleotide template was 5′-CATGGCCTAAGCAGGACTGAATGAGCAAGCTTCCGGAGAATTCTGCAGGACTGCAGATGC-3′ containing one centrally located CpG dinucleotide (underlined) in a HpaII/MspI recognition sequence (CCGG). For synthesis of the nonmethylated template used for de novo methylation analysis, complementary sequences were mixed at 500 µg/ml each, heated to 75°C for 10 minutes in 20 mM Tris-HCl (pH 7.5) and 50 mM NaCl, and annealed by slow cooling to room temperature [29]. For synthesis of the hemimethylated template to assess maintenance methylation, 5-mc replaced the cytosine of the one centralized CpG dinucleotide, and this oligonucleotide was annealed with the nonmethylated complementary oligonucleotide to create a hemimethylated CpG in the central region of the oligonucleotide. All double-stranded oligonucleotides were analyzed for annealing efficiency on 3% (wt/vol) agarose gels and used only if the annealing process was complete.
Determination of DNMT Activity
Tissue extracts were prepared by pooling together the skin biopsies from at least four mice from each group, and suspending them in approximately five times (wt/vol) that of the lysis buffer (10 mM Tris, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM HEPES, 1% Triton X-100, 2 µg/ml leupeptin, 2 µg/µl aprotinin, 2 mM sodium orthovanadate, 0.2 mM PMSF). This was followed by homogenization for 10-second bursts, up to a maximum of five times on ice. The homogenate was centrifuged for 30 minutes at 12,000g at 4°C, and the supernatant was collected and stored at -80°C for estimation of methyltransferase enzymes, as described [30]. Briefly, the reaction mix of 30 µl—containing 5 µM H3-S-adenosyl-l-methionine (15 Ci/mmol; NEN, Boston, MA), 1 µg of DNA substrate, and 18 µl of supernatant—was prepared and incubated for 2 hours at 30°C. The reaction mixture was kept at 4°C to stop further reaction. Now 30 µl of the sample was applied onto Whatman (Whatman International, Maidstone, UK) GF/C filters. The filters were then washed with graded concentration of 50%, 20%, and 10% cold trichloroacetic acid. The final rinse with absolute alcohol was done and the filters were allowed to dry overnight at room temperature. The next day, 3 ml of scintillation fluid was added to the filters that had been taken in the scintillation vial in order to quantify the units of radioactivity present in each sample by using a liquid scintillation counter (LS 6500 multipurpose; Beckman, Fullerton, CA). Enzyme activity was expressed as counts per minute per microgram of protein. The 60-mer oligonucleotide templates, either containing a centrally located de novo methylation activity or a hemimethylated CpG to measure maintenance DNMT activity, were used as substrates in DNMT assays. Total protein content in the samples was estimated using the DC protein assay kit (BioRad, Hercules, CA). Units of activity were expressed as counts per minute per microgram of protein.
Analysis of Cells Positive for 5-mc Staining
To determine the chemopreventive effect of EGCG on UVB-induced global DNA methylation pattern, cells positive for 5-mc in the epidermis were counted at random at six to eight places in each section using an ocular micrometer grid with x 200 magnification under Zeiss Axiophot microscope and Zeiss (Göttingen, Germany) Plan-Neofluar objective. The ocular micrometer grid corresponds to 0.0625 mm2. After counting the number of 5-mc+ cells in three sections per individual specimen, the number of 5-mc+ cells in each treatment group was expressed as a percentage ± SD of the mean count from at least three different animals per group. The counting of 5-mc+ cells was performed blindly by two independent observers.
Evaluation of Nontoxicity of EGCG in Hydrophilic Cream: In Vivo Analyses of TBMD, TBMC, Lean Mass, and Fat Mass in Mice by DXA
We were interested to assess the long-term effect of the topical application of EGCG in hydrophilic cream on mice; therefore, we determined some physico-chemical parameters such as bone free soft lean tissue mass, TBMD, TBMC, and fats in mice of different treatment groups using the DXA analysis technique, as described earlier [31]. These parameters were analyzed at the end of the 30th week of photocarcinogenesis experiment. For this purpose, mice were sacrificed and kept at -80°C until they were analyzed. The head part of the mouse was not included in any of these measures, and DXA analysis was performed on thawed animals.
Statistical analysis
In tumorigenesis experiments, the statistical significance of differences between the UVB-alone and EGCG + UVB treatment groups in terms of tumor incidence and tumor multiplicity was evaluated by chi-square analysis and Wilcoxon rank sum test, respectively. An advantage of Wilcoxon rank sum test is that its validity does not depend on any assumption about the shape of the distribution of tumor multiplicities. Kinetics of tumor multiplicity was analyzed by using the Fisher-Irwin exact test. Student's t-test was used to determine the statistical significance of differences in tumor volume, fat content, percent cells positive for 5-mc (global DNA methylation+), and DNMT data analysis between UVB-alone and EGCG + UVB-treated groups.
Results
Topical Application of EGCG in Hydrophilic Cream Affords Exceptionally High Protection of Photocarcinogenesis
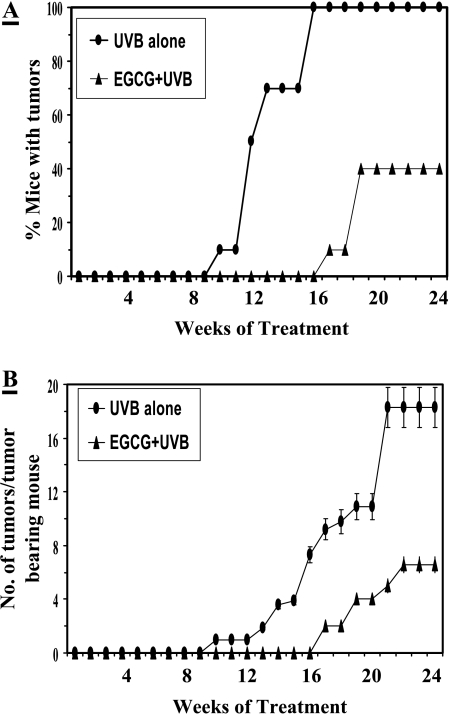
Our prior study indicated that topical application of EGCG in hydrophilic cream significantly inhibited UVB-induced oxidative stress and phosphorylation of MAPK proteins in in vivo mouse skin [13]. Prevention by EGCG treatment in this formulation was greater than that of previous reports (reviewed in Ref. [8]). Because UVB-induced oxidative stress and activation of MAPK pathway play an important role in UV carcinogenesis, we performed studies to determine whether the degree of prevention of photocarcinogenesis by EGCG in this formulation is greater than that of previous observations (reviewed in Refs. [6,8]). Following complete photocarcinogenesis protocol (UVB-induced both initiation and promotion) in SKH-1 hairless mouse models, topical application with EGCG resulted in a significant reduction in UV-induced tumorigenesis when expressed in terms of tumor incidence (Figure 1, Panel A) and tumor multiplicity (Figure 1, Panel B) compared to that of non-EGCG-treated but UVB-irradiated control animals. Treatment of EGCG resulted in 60% (P < .001) inhibition of tumor incidence (percent of mice with tumors) at the termination of the experiment at 24 weeks as compared to non-EGCGtreated mice (Figure 1, Panel A). Non-EGCG-treated animals achieved 100% tumor incidence on the 16th week of tumor promotion, whereas EGCG-treated mice could not achieve 100% tumor incidence up to the end of the 24th week when the tumor incidence was stabilized. Furthermore, treatment of EGCG increased the latency period of tumors by 7 weeks during the 24-week-long tumor protocol. In this photocarcinogenesis protocol, a total of 360 tumors (18 ± 2.5 tumors/tumor-bearing animal) was recorded in UVB-alone-irradiated (non-EGCG) group of mice, whereas only 48 tumors (6 ± 0.5 tumors/tumor-bearing mouse) were recorded with treatment of EGCG (Figure 1, Panel B). Each group contained 20 mice. Thus, topical treatment of EGCG in hydrophilic cream resulted in significant inhibition of UV-induced tumor multiplicity (86%, P < .001) compared to that of UVB-alone-irradiated control group (Figure 1, Panel B and Table 1). Furthermore, the rate of tumor appearance and their development in EGCG-treated animals were significantly decreased (P < .01, Fisher-Irwin exact test) throughout the experimental protocol compared to the non-EGCG-treated control group. As summarized in Table 1, the growth or size of tumors in the EGCG-treated group was also significantly inhibited when measured in terms of total tumor volume/group, tumor volume/tumor-bearing mouse, and average tumor volume/tumor, respectively by 95% (P < .001), 65% (P < .001), and 62% (P < .001) compared to that of the non-EGCG-treated group. The treatment of hydrophilic cream alone onto the mouse skin for 30 weeks did not induce tumor formation (data not shown).
Figure 1.
Chemopreventive effects of topical application of EGCG (1 mg/cm2) in hydrophilic cream on UVB-induced skin tumorigenesis in SKH-1 hairless mice. The details of the experimental protocols are described in Materials and Methods section. The percent of mice with tumors (Panel A) and the number of tumors per tumor-bearing mouse (Panel B) were plotted as a function of the number of weeks of treatment. Each treatment group contained 20 mice, and tumor data were recorded until the 24th week of UVB-induced tumor promotion when tumor yield was stabilized. The number of tumors per tumor-bearing mouse has been shown as mean ± SD.
Table 1.
Protective Effect of Topical Application of EGCG in Hydrophilic Cream on UVB-Induced Tumors in Mice.
| Physical Characteristics* | >Treatment Groups† | % Inhibition | |
| UVB alone | EGCG + UVB | ||
| Total number of tumors/group | 360 | 48 | 86‡ |
| Total tumor volume/group | 9230 | 490 | 95‡ |
| Tumor volume/ tumor-bearing mouse | 462 ± 44§ | 162 ± 10 | 65‡ |
| Average tumor volume/tumor (mm3) | 26 | 10 | 62‡ |
Total number of tumors and tumor volume in different treatment groups were recorded at the end of 24 weeks when tumor yield and size were stabilized.
Details of treatment groups are provided in Materials and Methods section. EGCG was administered topically (1 mg/cm2 skin area), 20 to 25 minutes prior to each UVB irradiation.
Highly significant versus UVB alone (P <.001).
Mean ± SD obtained from 20 animals in each group at the time of data recording.
Prevention of Malignant Transformation of Papillomas to Carcinomas
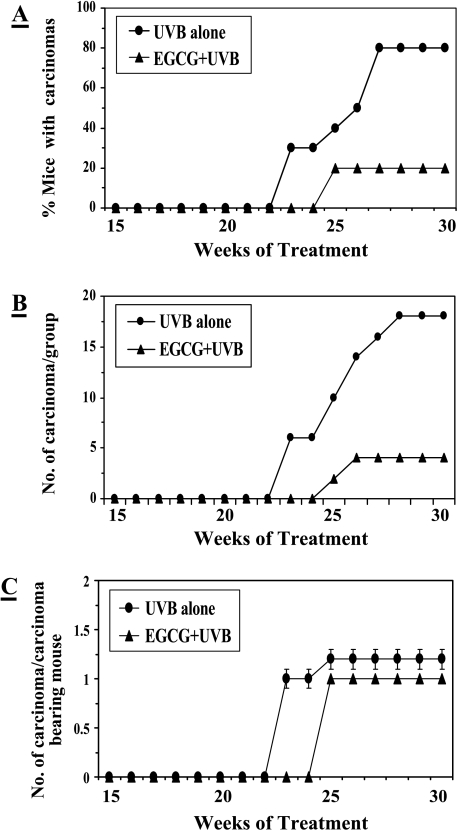
After observing that topical application of EGCG significantly inhibited UVB radiation-induced skin tumorigenesis, we continued to look at the chemopreventive efficacy of EGCG on malignant transformation of benign papillomas to carcinomas. Therefore, this experiment was extended up to 30 weeks. Histological observations indicated that papillomas have started transforming into carcinomas at the 23rd week of UVB-induced tumor promotion stage. As shown in Figure 2, 80% of mice developed carcinoma in the non-EGCG-treated group compared with that of only 20% mice with carcinomas in the EGCG-treated group. Thus, 60% (P < .001) prevention was observed in terms of carcinoma incidence by topical application of EGCG (Figure 2, Panel A). The weekly progression of carcinoma multiplicity in terms of total number of carcinomas per group and number of carcinomas per carcinoma-bearing mouse was recorded as shown in Panels B and C, respectively (Figure 2). Analysis of data indicated that 18 papillomas were transformed into carcinomas in the non-EGCG-treated but UVB-irradiated group of mice, whereas only four papillomas were transformed into carcinomas in the EGCG + UVB-treated group. These data indicated that topical application of EGCG resulted in 78% (P < .001) prevention of UVB-induced transformation of benign papillomas to carcinomas (Table 2). When data were analyzed in terms of number of carcinomas per carcinoma-bearing mouse, the protective effect of EGCG was not significant compared to that of the non-EGCG-treated (UVB-alone) group. Furthermore, topical application of EGCG inhibited the growth of carcinoma. As evidenced by the analysis of physical characteristics of carcinomas (Table 2), topical application with EGCG to UVB-irradiated mice resulted in significant inhibition in terms of total carcinoma volume/group and average carcinoma volume/carcinoma by 88% and 87% (P < .001), respectively, compared to that of the non-EGCG-treated group of mice.
Figure 2.
Chemopreventive effects of topical application of EGCG (1 mg/cm2) in hydrophilic cream on UVB-induced malignant transformation of papillomas to carcinomas during UVB-induced skin tumorigenesis protocol in SKH-1 hairless mice. The details of the experiment are described in Materials and Methods section. The percent of mice with carcinomas (Panel A), the number of carcinomas per group (Panel B), and the number of carcinomas per carcinoma-bearing mouse (Panel C) were plotted as a function of the number of weeks of treatment. Each treatment group contained 20 mice, and the number of carcinoma/carcinoma-bearing mouse has been shown as mean ± SD.
Table 2.
Protective Effect of Topical Application of EGCG in Hydrophilic Cream on UVB-Induced Carcinomas at the Termination of the Photocarcinogenesis Protocol in Mice.
| Physical Characteristics* | Treatment Groups | % Inhibition | |
| UVB alone | EGCG + UVB | ||
| Total number of carcinomas/group | 18 | 4 | 78† |
| Total carcinoma volume/group (mm3) | 8010 | 950 | 88† |
| Carcinoma volume/carcinoma-bearing mouse (mm3) | 501 ± 25‡ | 238 ± 12 | 53§ |
| Average carcinoma volume/carcinoma (mm3) | 445 ± 29 | 60 ± 1 | 87† |
Total number of carcinoma and carcinoma volume were recorded at the termination of the experiment at 30 weeks when carcinoma yield and size were stabilized.
Highly significant versus UVB alone (P < .001).
Mean ± SD were obtained from 20 animals in each group at the time of data recording.
Significant versus UVB alone (P < .01).
Topical Application of EGCG in Hydrophilic Cream Prevents UVB-Induced Adverse Morphological Changes in the Skin
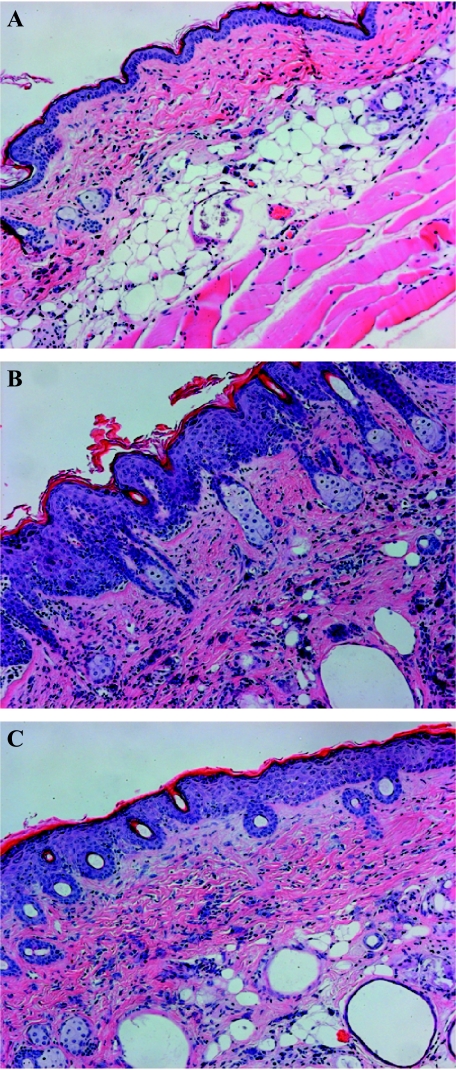
Chronic exposure of mouse skin to UV radiation induced morphological changes, which make skin susceptible to various skin disorders [5]. As shown in Figure 3, long-term exposure of the skin to UVB for 30 weeks induced markers of inflammatory responses, such as infiltration of inflammatory leukocytes, edema development, and hyperplastic response (increased number of epidermal cell layers) compared to that of non-UVB-exposed skin. UV-induced infiltrating leukocytes are the major source of oxidative stress [32], and thus a contributing factor in skin carcinogenesis. Treatment of EGCG markedly inhibited UVB-induced edema (bifold skin thickness), hyperplastic response, and leukocyte infiltration (Figure 3, Panel C) compared to that of non-EGCG-treated but UVB-irradiated skin, as can be seen in Figure 3 (Panel B). Thus, it can be suggested that inhibition of UVB-induced leukocyte infiltration could inhibit oxidative stress and thus could be responsible for the prevention of adverse biological effects of UVB radiation.
Figure 3.
Topical application of EGCG in hydrophilic cream inhibits UVB-induced adverse biochemical changes in the skin of SKH-1 hairless mice. H&E staining was performed on skin tissue sections to observe the changes as detailed in Materials and Methods section. Panel A: Normal skin (non-UVB-irradiated). Panel B: UVB-alone-irradiated skin showing hyperplastic response and spongiosis (edematous) in epidermis and dermis, and larger number of infiltrating leukocytes compared to normal skin. Leukocytes are shown by black nuclei. Panel C: UVB-irradiated skin pretreated with EGCG. Treatment of EGCG inhibits UVB-induced hyperplastic response and spongiosis in the epidermis and dermis, and lesser number of infiltrating leukocytes compared to UVB alone. Magnification, x 10.
Topical Application of EGCG in Hydrophilic Cream Prevents UVB-Induced Global DNA Hypomethylation
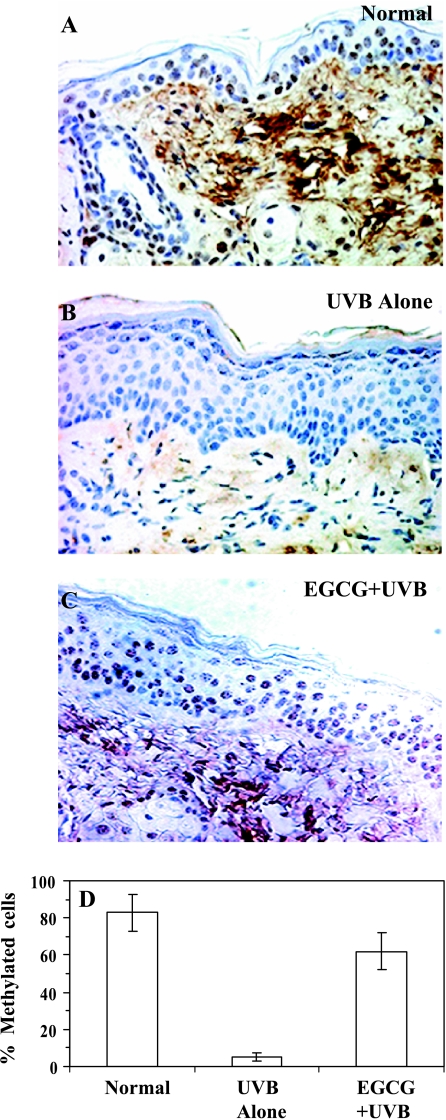
In order to determine the mechanism of protection of photocarcinogenesis by topical application of EGCG in the mouse skin, we performed experiments to detect whether topical application with EGCG affects UVB-induced alterations in global DNA methylation pattern. For the first time, we showed the effect of chronic UV exposure on DNA methylation pattern using immunohistochemical detection technique. We observed that chronic exposure of UV radiation at the end of the photocarcinogenesis protocol (at the 30th week) resulted in global DNA hypomethylation (Figure 4, Panel B) when compared with that of age-matched non-UVB-exposed skin (Figure 4, Panel A). UV-induced hypomethylation or hypermethylation patterns of DNA have been implicated in carcinogenesis [33]. This is the first observation or report which identified that chronic UVB irradiation resulted in the hypomethylation pattern of DNA (5-methyl cytosine-specific) in mouse skin (Figure 4). Non-UVB-exposed skin showed cells positive for 5-mc. The antibody specific to 5-mc is not specific to a particular cell type. Topical application of EGCG throughout the experimental protocol for 30 weeks prior to each UVB irradiation resulted in inhibition of UVB-induced global DNA hypomethylation (Figure 4, Panel C). Cells positive to 5-mc were counted in the epidermis at random at six to eight sites in each section of different treatment group under a microscope. We counted the percentage of cells positive to 5-mc (n = 3) in the epidermis, which were approximately 83 ± 10%, 5 ± 2% ,and 62% ± 10%, respectively, in normal (non-UVB), UVB-alone, and EGCG + UVB-irradiated skin (Figure 4, Panel D). Thus, topical treatment of EGCG inhibited UVB-induced global DNA hypomethylation pattern by 69% (P < .001) when determined in terms of 5-mc+ cells. These data indicated that topical application of EGCG abrogated UVB-induced global DNA hypomethylation, thus suggesting a possible mechanism of prevention of photocarcinogenesis in this mouse model.
Figure 4.
Topical treatment of EGCG in hydrophilic cream inhibits UVB-induced global DNA hypomethylation pattern in chronically UVB-exposed mouse skin for 30 weeks. Immunohistochemical detection of DNA methylation pattern was performed using anti-5-mc monoclonal antibody as detailed in Materials and Methods section. Panel A: Normal skin (non-UVBexposed). Panel B: UVB irradiation alone. Panel C: EGCG + UVB-irradiated skin. Skin biopsies were subjected to staining for DNA methylation using antibody specific to 5-mc. Cells positive for 5-mc staining appears brown in color. Normal skin showed the presence of numerous positive cells in the epidermis (Panel A) whereas chronic exposure of UVB radiation induced global DNA hypomethylation pattern in the skin (Panel B). Pretreatment with EGCG prevented chronic UVB irradiation-induced global DNA hypomethylation in the skin (Panel C). Magnification, x 40. Panel D: The percentage of cells positive for 5-mc in the epidermis was plotted against different treatment groups as mean ± SD from four animals in each group.
Topical Application of EGCG in Hydrophilic Cream Maintains DNMT in UVB-Exposed Skin
After observing the abrogating effect of EGCG on UVB-induced global DNA hypomethylation in the skin of mice, it was imperative to determine the levels of DNA methyltransferase enzymes, which are responsible for maintaining the methylation status of the DNA. At the termination of the photocarcinogenesis experiment at 30 weeks, skin homogenates were assayed for DNMT activities. The normal skin showed constitutive activity of 79 ± 7 cpm/µg protein DNMT1 enzyme (Table 3)—the enzyme responsible for maintaining the hemimethylation status of the DNA after replication. In the UVB-irradiated skin, the activity of DNMT1 dropped by 41% (P < .01) as compared to that of normal skin. Treatment with EGCG had significantly attenuated UVB-induced depletion of DNMT activity by 57% (P < .01) as compared to that of non-EGCG-treated but chronic UVB-irradiated skin. The assays of DNMT3a and DNMT3b activities, which are responsible for the de novo methylation in the cell resulting in aberrant methylation at unmethylated sites, revealed that on chronic UVB irradiation, the activity had been upregulated by 26% (78.8 ± 7, P < .05) as compared to the normal (non-UVB) skin (62.8 ± 6) (Table 3). But on EGCG treatment, UVB-induced changes in the de novo methyltransferase activity had been reduced nearly to 54.8 ± 5 cpm/µg protein. The ratio of the maintenance methyltransferase activity to the de novo methyltransferase activity plays a crucial role in the maintenance of DNA methylation pattern [33,34]. The ratio of these enzymes in the normal skin was found to be 1.26. It was observed that chronic UVB irradiation resulted in a significantly low ratio (0.59, P < .05) as compared to that of normal (non-UV) skin. Continuous treatment with EGCG on UVB-irradiated mice resulted in an approximately normalized ratio of maintenance and de novo methyltransferase activities (1.2) as compared to that of the control group of mice.
Table 3.
Protective Effect of Topical Application of EGCG in Hydrophilic Cream on the DNA Methyltransferase Activity at the Termination of the Photocarcinogenesis Protocol*.
| Treatment Groups† | Maintenance Methylation‡ (cpm/g protein) | De Novo Methylation§ (cpm/g protein) |
| Normal | 79.1 ± 7 | 62.8 ± 6 |
| UVB alone | 46.5 ± 4¶ | 78.8 ± 7¶ |
| EGCG + UVB | 65.2 ± 6¶,# | 54.8 ± 5¶ |
The skin lysates were pooled from four mice from each treatment group at the termination of the experiment, and methyltransferase activity was analyzed in triplicate.
The details of the experiment are described in Materials and Methods section. EGCG was administered topically (1 mg/cm2), 20 to 25 minutes prior to each UVB irradiation.
Maintenance methylation (DNMT1) was assessed in triplicate using a 60-mer double-stranded oligonucleotide containing one centralized hemimethylated CpG. Counts-per-minute values represent the amount of radioactivity transferred by the enzyme from titrated S-adenosyl-l-methionine to the double-stranded template in 2 hours. Values are presented as mean ± SEM.
De novo methylation (DNMT3) was assessed in triplicate as for maintenance methylation except using a 60-mer oligonucleotide containing one centralized CpG with no preexisting methylation in the template. Values are presented as mean ± SEM.
Significant difference versus UVB alone (P < .05).
Significant difference versus UVB-induced depletion of maintenance methylation, (P < .01).
Long-Term Application of EGCG in Hydrophilic Cream Has No Apparent Toxicity to Skin, TBMC, and TBMD But Decreases Fat Content
To determine whether long-term use of EGCG in hydrophilic cream has any apparent toxic effect in animals, animals were subjected to assessment of skin appearance by blind observers, and they assessed the effects on lean mass (bone-free tissue mass), fat mass, TBMC, and TBMD, and compared them with non-EGCG-treated animals as well as UVB-alone-irradiated animals. As observed by three independent observers, the visual skin appearance of animals treated with EGCG was better than that of non-EGCG-treated animals. DXA analysis revealed that there was no significant difference in TBMD, TBMC, and lean mass in EGCG-treated (EGCG + UVB) animals compared with that of non-EGCG-treated (UVB-alone and control) mice (Table 4). Intriguingly, long-term topical application with EGCG resulted in a significant reduction in total tissue fat content (21–25%, P < .05) compared to that of non-EGCG-treated animals. Analysis was performed at the end of the experiment. Head was not included in this body composition analysis by DXA [31]. Body composition data suggested that reduction in fat mass could also have a role, at least in part, in the prevention of UV carcinogenesis by EGCG in this animal model.
Table 4.
Effect of Topical Application of EGCG in Hydrophilic Cream on Bone Mineral Density, Bone Mineral Content, Lean Mass and Fat in Mice*.
| Treatment Group | TBMD (mg/cm2) | TBMC (mg) | Lean (g) | Fat (g) |
| Control (non-EGCG) | 65 ± 4† | 582 ± 13 | 19.9 ± 1.9 | 5.3 ± 1.3 |
| EGCG alone | 61 ± 2 | 561 ± 33 | 20.3 ± 1.7 | 4.2 ± 0.8(21)‡ |
| UVB alone | 57 ± 4 | 539 ± 57 | 20.4 ± 2.4 | 5.3 ± 1.2 |
| EGCG + UVB | 60 ± 3 | 542 ± 47 | 20.9 ± 1.4 | 4.0 ± 0.9(25)§ |
The values in parentheses indicate percent reduction in fat content by EGCG treatment.
Photocarcinogenesis experiment was continued for 30 weeks. At the end of the experiment, mice were sacrificed and subjected to DXA analysis. A brief description of analysis is described in Materials and Methods section. Each treatment group contained 20 mice. TBMD = total bone mineral density (bone mineral content/bone area); TBMC = total bone mineral content.
Data are expressed as mean ± SD from 20 animals in each group.
Significant reduction in fat content, EGCG alone versus non-EGCG treatment (P < .05).
Significant reduction in fat content, EGCG + UVB versus UVB alone (P < .05).
Discussion
The present study is an extension of our recent published report wherein we have shown that topical application of EGCG in hydrophilic cream afforded exceptionally high protection against UVB-induced oxidative stress and phosphorylation of MAPK proteins, which play an important role in cellular differentiation, proliferation, and leads to carcinogenesis [13]. In the present study, topical application of EGCG in hydrophilic cream resulted in exceptionally high protection against photocarcinogenesis in SKH-1 hairless mouse models. Photocarcinogenesis was assessed in terms of tumor incidence and tumor multiplicity (on a weekly basis) and tumor size, which was measured at the termination of the experiment (Figure 1 and Table 1). We tried to develop a cream-based formulation for the topical treatment of EGCG for future human use, and the data obtained in this experiment revealed that the chemopreventive effect of EGCG was superior to all earlier studies where mostly organic solvent acetone was used as a vehicle for the administration of EGCG [6,8,32,35]. It may be because of the fact that the use of hydrophilic cream enhanced the penetrating ability of EGCG in the skin [12]. With respect to the nature of UV radiation, it is worth mentioning that UV radiation has both tumor-initiating and tumor-promoting activities as it causes “signature” mutations at the genomic level as well as epigenetic changes. Therefore, it is not possible to design a clean UVB-induced initiating and/or promoting protocol; however, to study the causes, mechanisms, and preventive approaches of UV carcinogenesis, multistage photocarcinogenesis protocols are generally employed [5,26,27]. Earlier, we have reported that treatment of EGCG inhibited UV irradiation-induced oxidative stress in both animal and human skin [32,36], and that oxidative stress plays a crucial role in tumor promotion as well as in tumor initiation. Thus, the prevention of oxidative stress by EGCG could be a possible mechanism of prevention of photocarcinogenesis. Moreover, chronic inflammation is also considered a biomarker of tumor promotion [5], chronic UV irradiation-induced hyperplastic response, and infiltration of inflammatory leukocytes in the skin (Figure 3, Panel B). Prevention of these events by EGCG would decrease the risk of UV carcinogenesis (Figure 3, Panel C).
The present study provides additional new information that topical application of EGCG inhibits malignant transformation of benign papillomas to carcinomas (Figure 2 and Table 2). Transformation of benign papilloma to carcinoma requires further genetic and epigenetic changes in the tumor cells and this can be achieved by using free radical-generating agents [37,38] or genotoxic substances [39]. It appeared that chronic exposure of UVB radiation performed these alterations/functions. The increase in the rate of malignant transformation by free radical-generating agents may be related to free radical-mediated enhancement of genetic instability [40]. Therefore, the results of this study suggest that EGCG might have inhibited carcinoma incidence and multiplicity by inhibiting UVB-induced free radical generation and therefore free radical-mediated enhancement of genetic instability. It has been documented that transformation of benign papillomas to carcinomas requires further genetic changes in papillomas, which can be achieved by tumor-initiating agents [39,41]. In the present photocarcinogenesis protocol, repeated exposure of UVB to papillomas would result in further genetic changes in tumor cells [5], which would enhance the malignant conversion of benign papillomas to carcinomas. Therefore, it can be suggested that EGCG affords protection against genetic and epigenetic alterations caused by chronic UVB irradiation. It is important to mention that EGCG shows a UV absorption peak at or near 270 to 273 nm, and therefore, it is likely that EGCG could block some short wavelengths (< 290 nm) of UVB spectra. Similar chemopreventive observations were also reported when topical treatment of green tea polyphenols (mixture of polyphenols from green tea) inhibited benzoyl peroxide-induced (free radical-generating agent) and 4-nitroquinoline-N-oxide-induced (initiating agent) malignant transformations of chemically induced papillomas into carcinomas [42].
Cancer is a manifestation of both abnormal genetic and epigenetic events. The importance of epigenetic events is that it represents a mechanism by which gene function is selectively activated or inactivated. Because epigenetic events are susceptible to change, they represent excellent targets to explain how environmental factors, including dietary constituents/supplements/chemopreventive agents, may modify cancer risk and tumor behavior. DNA methylation is such a marker of epigenetic events, and is a fundamental process that not only modulates gene expression, but is also key to regulating chromosomal stability [33,34]. Abnormal DNA methylation, both hypermethylation and hypomethylation patterns, is a hallmark of most cancers, including colon, lung, prostate, and breast cancers, and can contribute to carcinogenesis by silencing of tumor suppressor genes, upregulation of oncogenes, and/or decreased genomic stability [15,16,33,34]. Changes in methylation pattern precede tumor formation, indicating that these alterations might contribute to tumorigenesis [18,33,34]. In view of these facts, we studied the pattern of global DNA methylation in chronically UV exposed skin, which may have a role in skin cancer, and also determined the effect of nutrients such as EGCG on this epigenetic marker. We observed that chronically UV-exposed skin for 30 weeks shows global DNA hypomethylation pattern and lowering of maintenance methylation in the mouse skin (Figure 4 and Table 3). Treatment of EGCG prior to each UV exposure resulted in a significant inhibition of UV-induced global DNA hypomethylation and reversed the effect on DNMT activities in chronically UVexposed skin (Figure 4 and Table 3). Because methylation changes are reversible, they may also lead to the development of novel therapeutic strategies to reverse or inhibit the transformed phenotype through the use of known or novel chemopreventive agents, such as EGCG, to correct aberrant methylation patterns and restore growth control in tumor cells and/or against the adverse effects of solar UV radiation. The exact mechanism of altered DNA methylation pattern by EGCG is not known at this stage; however, it can be assumed that EGCG may influence the supply of methyl groups for the formation of S-adenosylmethionine, and/or modify utilization of methyl groups by processes including shifts in DNMT activity. However, more studies on this aspect are required to give appropriate answers on these questions.
Lastly, we were interested to determine the adverse effect of long-term topical treatment of EGCG with this formulation on the skin, if any. Mice were administered topically applied EGCG in hydrophilic cream for 30 weeks, and at the termination of the experiment, it was found that EGCG has no adverse apparent visual toxic effect on the skin. The suitability of EGCG in cream was also determined by assessing various physico-chemical parameters in EGCG-treated animals and compared with that of non-EGCG-treated animals. It was observed that during the period of 30 weeks, animals neither lost nor gained body weight (data not shown), which may have distorted their physical or metabolic activity. Most importantly, long-term treatment with EGCG did not affect lean mass, TBMD, and TBMC when analyzed in vivo by DXA (Table 4). These observations provide ample evidence for the first time that topical treatment of EGCG has no apparent toxic effects in animals. The most significant observation is that treatment of EGCG decreased total fat mass in animals, which may have a relationship with the inhibition of UV-induced tumor incidence, multiplicity, and transformation of papillomas to carcinomas. Although several dietary modifications are known to inhibit carcinogenesis, and some may decrease body fat levels in rodents (e.g., caloric restriction) [43–45], this study provides evidence that inhibition of photocarcinogenesis by EGCG has a relationship with the reduction of tissue fat levels. This may be attributed to the fact that EGCG increased lipolysis or decreased the synthesis of fat without changing the body mass. It may be of interest to determine the effects of EGCG on the profile of fatty acids in epidermal phospholipids and in the neutral fats of parametrial fat pads. EGCG-induced decrease in arachidonic acid level in fat could result in decreased levels of prostaglandin metabolites that are believed to play a role in skin carcinogenesis [5].
Acknowledgements
Thanks are due to Tim Nagy for providing assistance in analyzing the body composition of mice in vivo by DXA (P30AR46301), and Alain Niveleau for providing the antibody specific to 5-mc.
Abbreviations
- DXA
dual-energy X-ray absorptiometry
- EGCG
(—)-epigallocatechin-3-gallate
- TBMD
total bone mineral density
- TBMC
total bone mineral content
- UV
ultraviolet
Footnotes
This work was supported, in part, by the National Cancer Institute (CA94593, CA89738), the National Institute of Environmental Health Sciences (ES11421), and the Cancer Research and Prevention Foundation (S.K.K.).
References
- 1.O'Shaughnessy JA, Kelloff GJ, Gordon GB, Dannenberg AJ, Hong WK, Fabian CJ, Sigman CC, Bertagnolli MM, Stratton SP, Lam S, Nelson WG, Meyskens FL, Alberts DS, Follen M, Rustgi AK, Papadimitrakopoulou V, Scardino PT, Gazdar AF, Wattenberg LW, Sporn MB, Sakr WA, Lippman DD, Von Hoff DD. Treatment and prevention of intraepithelial neoplasia: an important target for accelerated new agent development. Recommendations of the American Association for Cancer Research Task Force on the Treatment and Prevention of Intraepithelial Neoplasia. Clin Cancer Res. 2002;8:314–346. [PubMed] [Google Scholar]
- 2.Setlow RB. Spectral regions contributing to melanoma: a personal view. J Invest Dermatol Symp Proc. 1999;4:46–49. doi: 10.1038/sj.jidsp.5640180. [DOI] [PubMed] [Google Scholar]
- 3.Johnson TM, Dolan OM, Hamilton TA, Lu MC, Swanson NA, Lowe L. Clinical and histologic trends of melanoma. J Am Acad Dermatol. 1998;38:681–686. doi: 10.1016/s0190-9622(98)70196-3. [DOI] [PubMed] [Google Scholar]
- 4.Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol. 1994;30:774–778. doi: 10.1016/s0190-9622(08)81509-5. [DOI] [PubMed] [Google Scholar]
- 5.Mukhtar H, Elmets CA. Photocarcinogenesis: mechanisms, models and human health implications. Photochem Photobiol. 1996;63:355–347. doi: 10.1111/j.1751-1097.1996.tb03040.x. [DOI] [PubMed] [Google Scholar]
- 6.Katiyar SK, Mukhtar H. Tea in chemoprevention of cancer: epidemiologic and experimental studies. Int J Oncol. 1996;8:221–238. doi: 10.3892/ijo.8.2.221. [DOI] [PubMed] [Google Scholar]
- 7.Katiyar SK, Agarwal R, Wood GS, Mukhtar H. Inhibition of 12-O-tetradecanoylphorbol-13-acetate-caused tumor promotion in 7,12-dimethylbenz[a]anthracene-initiated SENCAR mouse skin by a polyphenolic fraction isolated from green tea. Cancer Res. 1992;52:6890–6897. [PubMed] [Google Scholar]
- 8.Katiyar SK, Elmets CA. Green tea polyphenolic antioxidants and skin photoprotection (review) Int J Oncol. 2001;18:1307–1313. doi: 10.3892/ijo.18.6.1307. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal R, Katiyar SK, Khan SG, Mukhtar H. Protection against ultraviolet B radiation-induced effects in the skin of SKH-1 hairless mice by a polyphenolic fraction isolated from green tea. Photochem Photobiol. 1993;58:695–700. doi: 10.1111/j.1751-1097.1993.tb04954.x. [DOI] [PubMed] [Google Scholar]
- 10.Katiyar SK, Agarwal R, Mukhtar H. Inhibition of spontaneous and photo-enhanced lipid peroxidation in mouse epidermal microsomes by epicatechin derivatives from green tea. Cancer Lett. 1994;79:61–66. doi: 10.1016/0304-3835(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 11.Lu YP, Lou YR, Xie JG, Peng QY, Liao J, Yang CS, Huang MT, Conney AH. Topical application of caffeine or, (—)-epigallocatechin gallate, EGCG inhibit carcinogenesis and selectively increase apoptosis in UVB-induced skin tumors in mice. Proc Natl Acad Sci USA. 2002;99:12455–12460. doi: 10.1073/pnas.182429899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dvorakova K, Dorr RT, Valcic S, Timmermann B, Alberts DS. Pharmacokinetics of the green tea derivative, EGCG, by the topical route of administration in mouse and human skin. Cancer Chemother Pharmacol. 1999;43:331–335. doi: 10.1007/s002800050903. [DOI] [PubMed] [Google Scholar]
- 13.Vayalil PK, Elmets CA, Katiyar SK. Treatment of green tea polyphenols in hydrophilic cream prevents UVB-induced oxidation of lipids and proteins, depletion of antioxidant enzymes and phosphorylation of MAPK proteins in SKH-1 hairless mouse skin. Carcinogenesis. 2003;24:927–936. doi: 10.1093/carcin/bgg025. [DOI] [PubMed] [Google Scholar]
- 14.Holliday R. Epigenetics: an overview. Dev Genet. 1994;15:453–457. doi: 10.1002/dvg.1020150602. [DOI] [PubMed] [Google Scholar]
- 15.Goodman JI, Watson RE. Altered DNA methylation: a secondary mechanism involved in carcinogenesis. Annu Rev Pharmacol Toxicol. 2002;42:501–525. doi: 10.1146/annurev.pharmtox.42.092001.141143. [DOI] [PubMed] [Google Scholar]
- 16.Counts JL, Goodman JI. Alterations in DNA methylation may play a variety of roles in carcinogenesis. Cell. 1995;83:13–15. doi: 10.1016/0092-8674(95)90228-7. [DOI] [PubMed] [Google Scholar]
- 17.Gama-Sosa MW, Slagel VA, Trewyn RW, Oxenhandler R, Kuo KC, Gehrke CW, Ehrlich M. The 5-methylcytosine content of DNA from human tumors. Nucleic Acids Res. 1983;11:6883–6894. doi: 10.1093/nar/11.19.6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robertson KD, Jones PA. DNA methylation: past, present and future directions. Carcinogenesis. 2000;21:461–467. doi: 10.1093/carcin/21.3.461. [DOI] [PubMed] [Google Scholar]
- 19.Adams RL, McKay EL, Craig LM, Burdon RH. Mouse DNA methylase: methylation of native DNA. Biochim Biophys Acta. 1979;561:345–357. doi: 10.1016/0005-2787(79)90143-6. [DOI] [PubMed] [Google Scholar]
- 20.Ramsahoye BH, Biniszkiewicz D, Lyko F, Clark V, Bird AP, Jaenisch R. Non-CpG methylation is prevalent in embryonic stem cells and may be mediated by DNA methyltransferase 3a. Proc Natl Acad Sci USA. 2000;97:5237–5242. doi: 10.1073/pnas.97.10.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toyota M, Ahuja N, Ohe-Toyota M, Herman JG, Baylin SB, Issa JP. CpG island methylator phenotype in colorectal cancer. Proc Natl Acad Sci USA. 1999;96:8681–8686. doi: 10.1073/pnas.96.15.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toyota M, Ahuja N, Suzuki H, Itoh F, Ohe-Toyota M, Imai K, Baylin SB, Issa JP. Aberrant methylation in gastric cancer associated with the CpG island methylator phenotype. Cancer Res. 1999;59:5438–5442. [PubMed] [Google Scholar]
- 23.Strathdee G, Appleton K, Illand M, Millan DWM, Sargent J, Paul J, Brown R. Primary ovarian carcinomas display multiple methylator phenotypes involving known tumor suppressor genes. Am J Pathol. 2001;158:1121–1127. doi: 10.1016/S0002-9440(10)64059-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winter H, Rentrop M, Nischt R, Schweizer J. Tissue-specific expression of murine keratin K13 in internal stratified squamous epithelia and its aberrant expression during 2-stage mouse skin carcinogenesis is associated with the methylation state of a distinct CpG site in the remote 5′-flanking region of the gene. Differentiation. 1990;43:105–114. doi: 10.1111/j.1432-0436.1990.tb00436.x. [DOI] [PubMed] [Google Scholar]
- 25.Ramsden M, Cole G, Smith J, Balmain A. Differential methylation of the c-H-ras gene in normal mouse cells and during skin tumor progression. EMBO J. 1985;4:1449–1454. doi: 10.1002/j.1460-2075.1985.tb03801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mittal A, Elmets CA, Katiyar SK. Dietary feeding of proanthocyanidins from grape seeds prevents photocarcinogenesis in SKH-1 hairless mice: relationship to decreased fat and lipid peroxidation. Carcinogenesis. 2003;24:1379–1388. doi: 10.1093/carcin/bgg095. [DOI] [PubMed] [Google Scholar]
- 27.Katiyar SK, Korman NJ, Mukhtar H, Agarwal R. Protective effects of Silymarin against photocarcinogenesis in a mouse skin model. J Natl Cancer Inst. 1997;89:556–566. doi: 10.1093/jnci/89.8.556. [DOI] [PubMed] [Google Scholar]
- 28.Piyathilake CJ, Frost AR, Bell WC, Oelschlager D, Weiss H, Johannig A, Niveleau A, Heimburger DC, Grizzle WE. Altered global methylation of DNA: an epigenetic difference in susceptibility for lung cancer is associated with its progression. Hum Pathol. 2001;32:856–862. doi: 10.1053/hupa.2001.26471. [DOI] [PubMed] [Google Scholar]
- 29.Tollefsbol TO, Hutchison CA., III Control of methylation spreading in synthetic DNA sequences by the murine DNA methyltransferase. J Mol Biol. 1997;269:494–504. doi: 10.1006/jmbi.1997.1064. [DOI] [PubMed] [Google Scholar]
- 30.Tollefsbol TO, Hutchison CA., III Analysis in Escherichia coli of the effects of in vivo CpG methylation catalysed by the cloned murine maintenance methyltransferase. Biochem Biophys Res Commun. 1998;245:670–678. doi: 10.1006/bbrc.1998.8422. [DOI] [PubMed] [Google Scholar]
- 31.Nagy TR, Clair A-L. Precision and accuracy of dual-energy X-ray absorptiometry for determining in vivo body composition of mice. Obes Res. 2000;8:392–398. doi: 10.1038/oby.2000.47. [DOI] [PubMed] [Google Scholar]
- 32.Katiyar SK, Mukhtar H. Green tea polyphenol (—)-epigallocatechin-3-gallate treatment to mouse skin prevents UVB-induced infiltration of leukocytes, depletion of antigen presenting cells and oxidative stress. J Leukoc Biol. 2001;69:719–726. [PubMed] [Google Scholar]
- 33.Robertson KD. DNA methylation, methyltransferases, and cancer. Oncogene. 2001;20:3139–3155. doi: 10.1038/sj.onc.1204341. [DOI] [PubMed] [Google Scholar]
- 34.Baylin SB, Esteller M, Rountree MR, Bachman KE, Schuebel K, Herman JG. Aberrant patterns of DNA methylation, chromatin formation and gene expression in cancer. Hum Mol Genet. 2001;10:687–692. doi: 10.1093/hmg/10.7.687. [DOI] [PubMed] [Google Scholar]
- 35.Gensler HL, Timmermann BN, Valcic S, Wachter GA, Dorr R, Dvorakova DS, Alberts DS. Prevention of photocarcinogenesis by topical administration of pure epigallocatechin gallate isolated from green tea. Nutr Cancer. 1996;26:325–335. doi: 10.1080/01635589609514488. [DOI] [PubMed] [Google Scholar]
- 36.Katiyar SK, Afaq F, Perez A, Mukhtar H. Green tea polyphenol (—)-epigallocatechin-3-gallate treatment of human skin inhibits ultraviolet radiation-induced oxidative stress. Carcinogenesis. 2001;22:287–294. doi: 10.1093/carcin/22.2.287. [DOI] [PubMed] [Google Scholar]
- 37.Slaga TJ, Klein-Szanto AJP, Triplett LL, Yotti LP, Trosko JE. Skin tumor-promoting activity of benzoyl peroxide, a widely used free radical-generating compound. Science (Washington, DC) 1981;213:1023–1025. doi: 10.1126/science.6791284. [DOI] [PubMed] [Google Scholar]
- 38.O'Connell JF, Klein-Szanto AJP, DoGiovanni DM, Fries JW, Slaga TJ. Enhanced malignant progression of mouse skin tumors by the free-radical generator benzoyl peroxide. Cancer Res. 1986;46:2863–2865. [PubMed] [Google Scholar]
- 39.Hennings H, Shores R, Wenk ML, Spangler EF, Tarone R, Yuspa SH. Malignant conversion of mouse skin tumors is increased by tumor initiators and unaffected by tumor promoters. Nature (London) 1983;304:67–69. doi: 10.1038/304067a0. [DOI] [PubMed] [Google Scholar]
- 40.Athar M, Lloyd JR, Bickers DR, Mukhtar H. Malignant conversion of UV radiation- and chemically induced mouse skin benign tumors by free radical-generating compounds. Carcinogenesis (London) 1989;10:1841–1845. doi: 10.1093/carcin/10.10.1841. [DOI] [PubMed] [Google Scholar]
- 41.Hennings H, Shores R, Mitchell P, Spangler EF, Yuspa SH. Induction of papillomas with a high probability of conversion to malignancy. Carcinogenesis (London) 1985;6:1607–1610. doi: 10.1093/carcin/6.11.1607. [DOI] [PubMed] [Google Scholar]
- 42.Katiyar SK, Agarwal R, Mukhtar H. Protection against malignant conversion of chemically induced benign skin papillomas to squamous cell carcinomas in SENCAR mice by a polyphenolic fraction isolated from green tea. Cancer Res. 1993;53:5409–5412. [PubMed] [Google Scholar]
- 43.Tannenbaum A. The dependence of tumor formation on the degree of caloric restriction. Cancer Res. 1945;5:609–615. [Google Scholar]
- 44.Birt DF, Pelling JC, White LT, Dimitroff K, Barnett T. Influence of diet and calorie restriction on the initiation and promotion of skin carcinogenesis in the SENCAR mouse model. Cancer Res. 1991;51:1851–1854. [PubMed] [Google Scholar]
- 45.Birt DF, Pinch HJ, Barnett T, Phan A, Dimitroff K. Inhibition of skin tumor promotion by restriction of fat and carbohydrate calories in SENCAR mice. Cancer Res. 1993;53:27–31. [PubMed] [Google Scholar]