Abstract
Hsp90 is required for the normal activity of steroid receptors, and in steroid receptor complexes it is typically bound to one of the immunophilin-related co-chaperones: the peptidylprolyl isomerases FKBP51, FKBP52 or CyP40, or the protein phosphatase PP5. The physiological roles of the immunophilins in regulating steroid receptor function have not been well defined, and so we examined in vivo the influences of immunophilins on hormone-dependent gene activation in the Saccharomyces cerevisiae model for glucocorticoid receptor (GR) function. FKBP52 selectively potentiates hormone-dependent reporter gene activation by as much as 20-fold at limiting hormone concentrations, and this potentiation is readily blocked by co-expression of the closely related FKBP51. The mechanism for potentiation is an increase in GR hormone-binding affinity that requires both the Hsp90-binding ability and the prolyl isomerase activity of FKBP52.
Keywords: FKBP52/glucocorticoid receptor/Hsp90/immunophilin/peptidylprolyl isomerase
Introduction
Steroid receptors require continuous interactions with molecular chaperones to establish and maintain competence as ligand-dependent transcription factors (Pratt and Toft, 1997; Cheung and Smith, 2000). Studies of the assembly of receptor–chaperone complexes in cell-free systems have revealed an ordered dynamic pathway involving the obligate participation of major chaperone components (Hsp40, Hsp70 and Hsp90) and various co-chaperones. Native Hsp90–receptor complexes typically contain one of the Hsp90-bound immunophilins: the FK506-binding proteins FKBP52 and FKBP51, the cyclosporin A-binding protein cyclophilin 40 (CyP40) or the protein phosphatase PP5. A common feature of these co-chaperones is a tetratricopeptide repeat (TPR) domain, which forms the Hsp90-binding site, linked to another functional domain, a peptidylprolyl isomerase/drug-binding domain in the FKBPs and CyP40 or a protein phosphatase domain in PP5.
In cell-free assembly reactions, the immunophilins are not required for receptors to gain hormone-binding ability (Dittmar and Pratt, 1997; Kosano et al., 1998), but immunophilins do influence steroid response pathways. Receptor-associated FKBP52 (Czar et al., 1995; Galigniana et al., 2001 and references therein) and PP5 (Dean et al., 2001) may play a role in nuclear targeting of glucocorticoid receptor (GR). Overexpression and structural alterations of FKBP51 may largely account for the cortisol insensitivity observed in many New World primates (Reynolds et al., 1999; Scammell, 2000). Additionally, human FKBP51 can lower GR hormone binding and transactivation (Denny et al., 2000) and is transcriptionally upregulated by glucocorticoids (Baughman et al., 1995). Still, detailed analysis of the function of individual immunophilins in vertebrate cells is confounded by the co-expression of several immuno philins at relatively high levels, and so we sought an alternative model system.
Genetic approaches to analyzing the roles of chaperones in steroid hormone signaling have favored the genetically tractable yeast Saccharomyces cerevisiae. Although yeasts do not express steroid receptors, co-transformation of S.cerevisiae with cDNAs for a steroid receptor and a suitable hormone-responsive reporter is sufficient to establish a biological response (Schena and Yamamoto, 1988). Selected gene disruptions in S.cerevisiae have provided some of the best evidence for the physiological roles of Hsc82/Hsp90 (Picard et al., 1990a; Bohen and Yamamoto, 1993; Nathan and Lindquist, 1995), Ydj1/Hsp40 (Caplan et al., 1995; Kimura et al., 1995) and the co-chaperones Sti1/Hop (Chang et al., 1997) and Sba1/p23 (Freeman et al., 2000) in steroid hormone signaling. In another case, disruption of the gene for Cpr7, one of two CyP40 homologs in yeast, reduces glucocorticoid responses (Duina et al., 1996). Saccharomyces cerevisiae also contains an ortholog for mammalian PP5 (PPT1/YGR123C), but there have been no reported characterizations of the role of this gene in steroid signaling. Finally, whereas S.cerevisiae harbors several small FKBP proteins, it does not contain any of the large TPR-containing FKBPs that bind to Hsp90. Thus S.cerevisiae provides a null background for testing in vivo the function of FKBP52 and FKBP51 in steroid signaling.
Results
Saccharomyces cerevisiae reporter model
To assess the in vivo function of receptor-associated immunophilins, we constructed S.cerevisiae reporter strains that harbor expression plasmids for a vertebrate steroid receptor, one of the receptor-associated human immunophilins and a lacZ reporter plasmid (Figure 1A). Hormone signaling in S.cerevisiae has previously been measured by assaying the accumulation of the reporter gene product β-galactosidase at a fixed time interval after hormone addition (ranging from 1 to 12 h). While the amount of β-galactosidase is strongly influenced by the rate of synthesis, other factors, such as cell density, growth rate and stability, also influence its levels. Taking advantage of a commercially available β-galactosidase assay that is rapid and sensitive, we modified the typical assay to measure responses soon after hormone addition while accounting for cell growth.
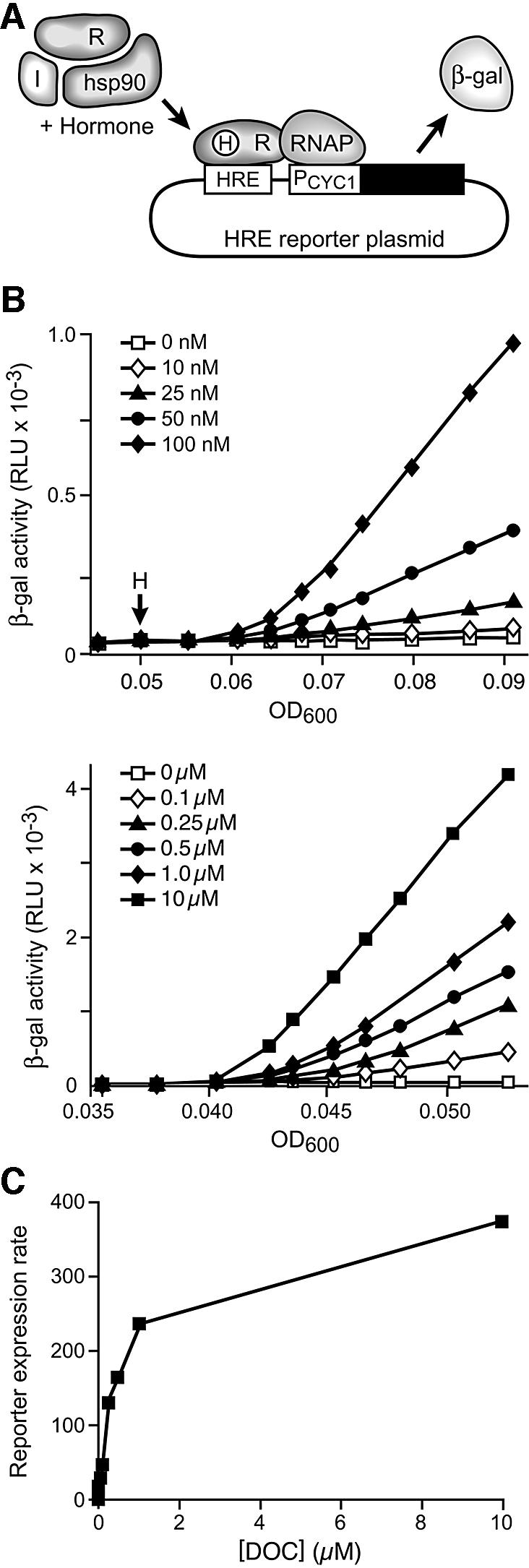
Fig. 1. Measurement of hormone-induced reporter activity. (A) Yeast strains were transformed with a hormone receptor expression plasmid, an immunophilin expression plasmid and a plasmid carrying a corresponding reporter gene. The receptor and immunophilin genes are transcribed from the strong constitutive glycerol phosphate dehydrogenase promoter. The reporter plasmid contains the lacZ gene transcribed from a truncated yeast cytochrome C1 promoter (PCYC1) downstream of receptor-specific hormone response elements (HRE). Receptor complexes, which contain yeast Hsp90 and other chaperones plus an immunophilin, are activated by hormone binding. The active receptor binds to HRE and recruits RNA polymerase complex (RNAP) to drive transcription from the reporter plasmid. (B) β-galactosidase was induced in the GR reporter strain by addition of deoxycorticosterone (H) at the concentrations indicated. β-galactosidase activities, measured in chemiluminescent relative light units (RLU), are plotted as a function of OD600. (C) To generate a DOC dose–response curve for the reporter gene expression rate, the slope (ΔRLU/ΔOD600) was calculated for the linear portion of each induction curve in (B) and plotted relative to hormone concentration.
Attention was focused on the GR reporter system since this has been the most thoroughly investigated steroid receptor system in yeast models. First, we established a dose–response curve for hormone-induced β-galactosidase activity (Figure 1B and C). Replicate cultures were treated with a range of concentrations of deoxycorticosterone (DOC), a favored GR agonist in yeast models (Garabedian and Yamamoto, 1992; Kralli et al., 1995). The optical density at 600 nm (OD600) of the culture, a measure of yeast number and thus total protein, was monitored and aliquots were assayed for β-galactosidase activity. By plotting the β-galactosidase activity of each sample against its OD600, plots of β-galactosidase synthesis relative to total protein synthesis are generated (Figure 1B). After an initial 20–30 min lag, the rate of β-galactosidase expression, as reflected by the linear slope of each plot, remains constant for several hours. To generate a dose–response curve (Figure 1C), the calculated slope for each data set was plotted relative to DOC concentration. Since immunophilin influences on hormone signaling are likely to be most physiologically relevant at a limiting concentration of hormone and 25 nM DOC is as near as practical to the physiological range of GR agonists while still being sufficient for reliable data generation, 25 nM was selected as the hormone concentration used in most single-dose assays, but similar results were obtained in experiments using 50 or 100 nM concentrations of hormone.
Immunophilin influence on hormone responses
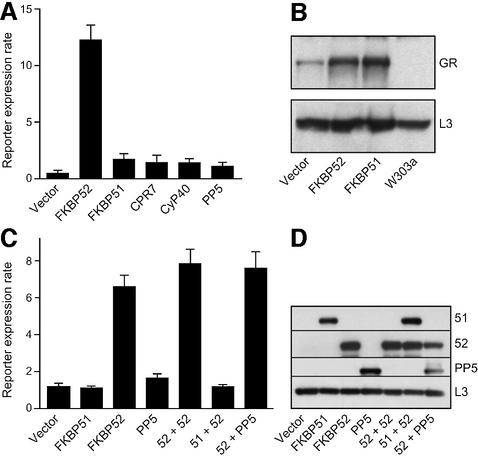
To test whether the receptor-associated immunophilins influence basal hormone signaling, we compared GR reporter strains transformed with an empty vector or with vectors individually expressing the human immunophilins FKBP51, FKBP52, PP5 or CyP40, or overexpressing CPR7, a yeast ortholog of CyP40 (Figure 2A). Reporter activity increased ∼2-fold over basal levels with any of the immunophilins, but only FKBP52 conferred a markedly higher response to hormone. Since the limited enhancement of DOC response seen with FKBP51, which is most closely related to FKBP52, is typical of the other immunophilins, FKBP51 served as a control for FKBP52-specific effects. To determine whether the immunophilins differentially influence the steady state level of GR protein, extracts from cells lacking GR or GR-plus cells expressing FKBP52, FKBP51 or no added immunophilin were compared by western immunostain (Figure 2B). GR protein level was increased 2- to 3-fold in the presence of FKBP52, but an equal increase was observed with FKBP51. Perhaps elevation of GR protein could account for the modest increase in hormonal responses observed with each immunophilin (Figure 2A), but this does not account for the greater potentiation observed with FKBP52.
Fig. 2. Potentiation of GR signaling by the immunophilins. (A) Reporter expression rates of the GR reporter cells transformed with an empty vector or with plasmids expressing FKBP52, FKBP51, CPR7, CyP40 or PP5. Each bar is the average expression rate (±SD) from quadruplicate cultures of a representative isolate. (B) Steady state levels of GR in the parental strain W303a and GR reporter strains in FKBP51, FKBP52 or control vector backgrounds. Extracts from each strain were analyzed by western blotting for GR (upper panel) and for the ribosomal protein L3 as a loading control (lower panel). (C) Similar to (A) except that GR reporter cells were transformed with a single vector or two vectors (52+52, 51+52 or 52+PP5) for co-expression of immunophilins. (D) For each strain shown in (C), the total cell extract was immunostained for the proteins indicated.
Since FKBP51 has been shown to inhibit GR activity in mammalian systems, we anticipated that an attenuation of GR signaling might be observed in the yeast model, but this was not the case (Figure 2A). This unexpected finding suggested that the effect of FKBP51 observed in mammalian systems might be an indirect consequence of blocking FKBP52 potentiation. To test whether FKBP51 can block this potentiation, immunophilins were co-expressed in the GR reporter strain (Figure 2C). Co-expression of two FKBP52 plasmids did not significantly increase potentiation observed with a single plasmid. However, co-expression of FKBP51 with FKBP52 efficiently blocked potentiation. Since FKBP51 competes with FKBP52 for Hsp90 binding and incorporation into GR complexes, simple displacement of FKBP52 from GR complexes might account for the loss of potentiation. This possibility is discounted by the observation that PP5, which also competes for Hsp90 binding and assembly with receptor complexes, fails to block FKBP52-mediated potentiation. Western immunoassays were performed to show that the FKBP52 protein level in dual transformations is similar to the level obtained in yeast expressing FKBP52 alone (Figure 2D). Therefore it appears that FKBP51 functions in a specific and efficient manner to counteract the effects of FKBP52 on GR signaling.
FKBP52 and GR hormone binding
To gain a better understanding of how FKBP52 enhances GR signaling, reporter gene expression was measured over a broad range of DOC concentrations to generate full hormone response curves for the FKBP strains and the vector control strain (Figure 3A). In the presence of FKBP52, there is a distinct shift in the hormone response curve with a DOC EC50 of ∼70 nM, 3-fold less than the FKBP51 strain. In this respect, FKBP52 appears to mimic the effect of a GR mutation GR-F620S, a serine substitution for phenylalanine at position 620, which was identified in yeast genetic screens for elevated hormone responsiveness (Garabedian and Yamamoto, 1992). To determine whether FKBP52 also potentiates GR-F620S, similar hormone response curves were generated for cells expressing this receptor (Figure 3B). As with wild-type GR, the GR-F620S response was further shifted in the presence of FKBP52 (EC50 ≈ 15 nM), suggesting that the mechanism for enhanced basal hormone binding in GR-F620S is distinct from the mechanism for FKBP52-dependent potentiation.
Fig. 3. Hormone dose–response curves. (A) GR reporter cells expressing FKBP51 (GR/51) or FKBP52 (GR/52), or transformed with empty vector (GR/vector), were treated over a range of DOC concentrations. Reporter expression rates are normalized to the rate at the highest hormone concentration. Each data point is the average expression rate (n = 4 ± SD) that is representative of two independent isolates. (B) Reporter cells expressing wild-type GR or the F620S GR mutant were transformed with an expression plasmid for FKBP51 or FKBP52. Typical hormone dose–response curves are shown.
The left shift in the DOC dose–response curve stimulated by FKBP52 is consistent with an increase in GR hormone-binding affinity. To test this more directly, radiolabeled hormone-binding assays were performed on intact cells. To minimize the technical challenges others have encountered with GR-binding measurements in yeast, mutant GR-F620S was used since it has a higher affinity for hormone and also responds to FKBP52 in a similar manner to wild-type GR. Generating full-saturation binding curves was still not practical, and so we compared the levels of bound hormone at 25 nM, 250 nM and 2.5 µM [3H]corticosterone. At the lowest concentration, corticosterone binding was 5-fold greater in the presence of FKBP52 (21 ± 2 fmol/OD unit cells) compared with FKBP51 (4 ± 1 fmol/OD unit). At the highest concentration, the difference was reduced to <1.5-fold (549 ± 110 versus 388 ± 43 fmol/OD unit), indicating that binding in both strains approached a similar saturation level. This hormone-binding pattern is fully consistent with an FKBP52-dependent increase in GR hormone-binding affinity, as opposed to an increase in receptor number. As seen with wild-type GR (Figure 2B), western analysis confirmed that the steady state levels of GR-F620S protein were identical in the two FKBP strains (data not shown). We conclude that FKBP52 stimulates an increase in GR hormone-binding affinity to account, at least in part, for the potentiation of reporter activity.
Selectivity of FKBP52 for GR
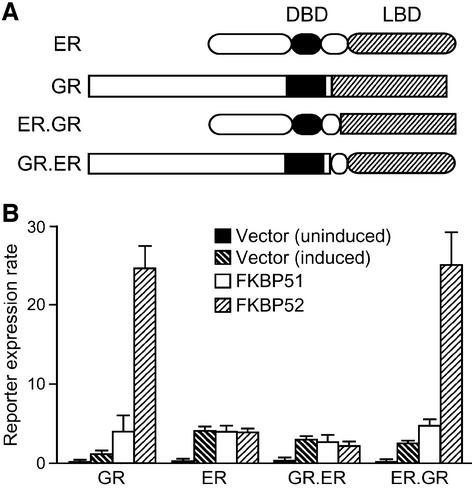
Each of the receptor-associated immunophilins can assemble with any of the five steroid receptors, although there are clear preferences for particular immunophilins in different receptor complexes (Barent et al., 1998). To determine whether FKBP52 enhances all steroid receptors, FKBP52 was tested in yeast reporter strains expressing GR, estrogen receptor (ER) or chimeric receptors (Figure 4). In contrast with GR reporter cells, no potentiation of ER activity was observed in yeast expressing FKBP52. Similarly, FKBP52 did not enhance reporter gene activity stimulated by androgen or progesterone receptor (data not shown). Based on the different results with GR and ER, we took advantage of chimeric receptors in which ligand-binding domains (LBDs) were exchanged between these receptors (Bunone et al., 1996) to examine whether FKBP52 potentiation maps to the GR LBD or to the GR DNA-binding domain (DBD) and upstream sequences (Figure 4). Clearly, potentiation is specific for the GR LBD, as is consistent with our observation that FKBP52 elevates GR hormone-binding affinity.
Fig. 4. Selectivity of FKBP52-dependent potentiation. (A) Yeast report er strains were prepared that express human ER (oval features), rat GR (rectangular features) or the ER.GR or GR.ER chimera. The approximate positions for the DBD (solid fill) and the LBD (hatched fill) in each receptor construct are illustrated. (B) Reporter strains expressing FKBP51 or FKBP52 or transformed with empty vector were compared for hormone-dependent β-galactosidase activity. The background level of reporter activity is shown for uninduced vector-only cells. Other bars represent reporter activity in vector, FKBP51 and FKBP52 cells induced with the following hormone concentrations: GR strains, 25 nM DOC; ER strains, 10 pM 17-β-estradiol; GR.ER, 450 pM 17-β-estradiol; ER.GR, 400 nM DOC. These data are the average (n = 4 ± SD) of duplicate assays from two independent isolates.
In addition, we tested for FKBP effects on hormone-independent reporter activity by co-expressing FKBP52 or FKBP51 with GR-N525, a GR truncation mutant lacking an LBD that constitutively transactivates the GRE-regulated reporter (Picard et al., 1990a). Neither FKBP affected the constitutive level of β-galactosidase activity mediated by GR-N525 (results not shown). These observations highlight the role of the LBD in FKBP52-mediated potentiation and minimize the likelihood that FKBP52 acts additionally on the DBD or at the level of general transcriptional enhancement.
Mechanism of FKBP52 action
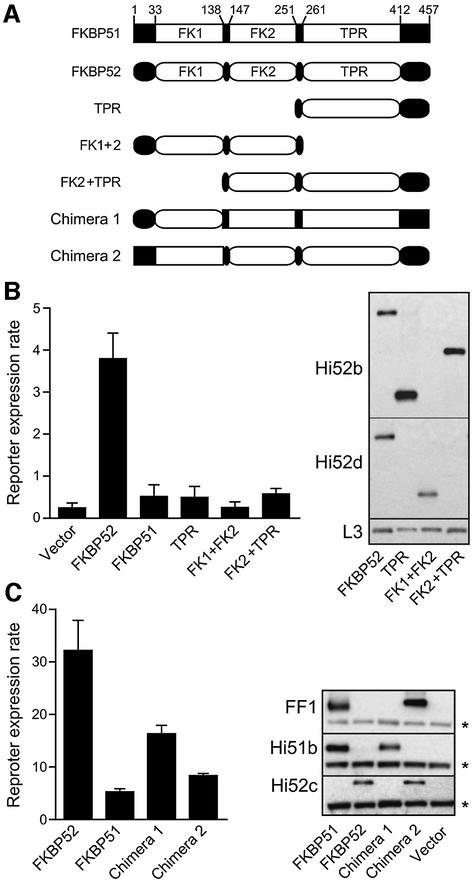
To assist in the design of FKBP52 mutants for functional mapping, the crystal structure of FKBP51 (Sinars et al., 2002) was used as a guide (Figure 5A). FKBP51 contains three major structural domains: two FKBP12-like domains, termed FK1 and FK2, plus a typical Hsp90-binding TPR domain. Peptidylprolyl isomerase (PPIase) activity and FK506 binding are restricted to FK1 (Sinars et al., 2002). The domain organization of FKBP52 is likely to resemble FKBP51 since they share ∼75% sequence similarity.
Fig. 5. The role of Hsp90 binding in FKBP52-mediated potentiation. (A) Major structural domains of human FKBP51. FKBP51 contains two FKBP12-like domains, FK1 and FK2, that have predominant β-sheet structure. FK1 forms the FK506-binding site and has PPIase activity; the 80S and 40S loop regions characteristic of FKBP12 are indicated. Six adjacent amino acids in the FK1 domain (broken lines) that correspond to the 40S loop of FKBP12 are unresolved. The N- and C-terminal ends of the molecule, as indicated, are also unresolved. FK2, which is structurally similar to FK1, lacks PPIase and FK506-binding activities. The C-terminal TPR domain is predominantly α-helical and forms the Hsp90-binding site; mutation of Lys-352 within the TPR domain will abrogate Hsp90 binding. FKBP52 is likely to have an overall structure very similar to FKBP51. (B) The FKBP52 point mutant K354A (corresponding to Lys-352 in FKBP51) was compared with wild-type FKBP52 for binding to Hsp90. Radiolabeled FKBP52 and FKBP52-K354A were prepared in vitro (first two lanes); aliquots of each synthesis mixture were tested for co-immunoprecipitation with Hsp90 complexes (final two lanes). Samples were separated by SDS–PAGE and visualized by Coomassie Blue staining (upper panel) or autoradio graphy (lower panel). (C) Hormone-induced β-galactosidase activity in GR reporter cells containing vector or plasmids expressing FKBP52 or FKBP52-K354A. All data are the average (n = 4 ± SD) of duplicate assays from two independent isolates.
Does the increase in GR hormone-binding affinity require FKBP52 binding to Hsp90? As discovered by Chinkers and colleagues (Russell et al., 1999), a K354A point mutation in the TPR domain of FKBP52 (see Figure 5A for the equivalent position in FKBP51) abrogates binding to Hsp90 (Figure 5B). When FKBP52-K354A is substituted for wild-type FKBP52 in GR reporter cells, potentiation is virtually abolished (Figure 5C). This observation is consistent with previous evidence that the receptor-associated immunophilins primarily enter receptor complexes indirectly through interactions with Hsp90 (Renoir et al., 1990; Pratt and Toft, 1997).
Is the TPR domain sufficient for potentiation of GR activity? To address this question and extend the functional mapping of FKBP52, a series of FKBP52 domain truncation mutants (Figure 6A) were tested. All the domain truncations completely lacked the potentiation activity observed with full-length FKBP52 (Figure 6B). This indicates that the TPR alone is not sufficient and suggests an additional requirement for other domains. To test this possibility further, we took advantage of the structural similarity between FKBP52 and FKBP51, which lacks potentiation activity, to generate chimeric constructs in which the FK1 domain was exchanged (Figure 6A). Substituting FKBP51-FK1 for FKBP52-FK1 largely abrogated potentiation (Figure 6C, chimera 2), whereas the converse substitution on FKBP51 (chimera 1) caused a gain in potentiation that approximates half the activity seen with FKBP52. Western immunostains for each FKBP were performed to verify that wild-type and chimeric proteins are expressed at similar levels. The steady-state level of chimera 1, which is more active in potentiation of GR signaling than FKBP51 or chimera 2, is ∼30% lower than FKBP51; if anything, this suggests that the ability of chimera 1 to enhance GR responses may be underestimated. Chimera 2 levels are similar to FKBP52 and so loss of potentiation does not correspond to reduced protein. In another experiment, the N-terminal 30 amino acids preceding FK1 were exchanged with no effect on potentiation (results not shown). These observations demonstrate that the FK1 domain of FKBP52 is required for potentiation of GR signaling.
Fig. 6. Mapping of FKBP52 domains required for potentiation. (A) Domain structures are illustrated for full-length FKBPs, FKBP52 truncation mutants and FKBP chimeric proteins. (B) Reporter expression rates were measured in separate GR reporter strains containing empty vector or expressing the indicated immunophilin product. Expression data are the average (n = 4 ± SD) of duplicate assays from two independent isolates. Expression levels for FKBP52 and truncation mutants were compared by western immunostaining. Owing to the loss of individual epitopes in truncation mutants, two anti-FKBP52 antibodies (Hi52b and Hi52d) were used along with an L3 probe as loading control. (C) Reporter expression rates were similarly determined in GR reporter strains expressing wild-type proteins or full-length chimeras containing the FK1 domain from FKBP52 (chimera 1) or FKBP51 (chimera 2). Expression of each form was verified by western immuno staining with anti-FKBP51 antibodies FF1 or Hi51b and anti-FKBP52 Hi52c. Note that FF1, which has an epitope in FK1 of FKBP51, detects wild-type FKBP51 and chimera 2, Hi51b, whose epitope is in the C-terminal region of FKBP51, detects FKBP51 and chimera 1, and Hi52c, with an epitope in FK1 of FKBP52 detects wild-type FKBP52 and chimera 2. Immunostaining of L3 (asterisk) provided an internal loading reference for each sample.
Pratt and colleagues have reported that the FK1 region of FKBP52 binds to dynein and have proposed that, through the mutual interaction of FKBP52 with dynein and receptor-bound Hsp90, FKBP52 assists in transporting GR from the cytoplasm to the nucleus (Pratt et al., 1999). Saccharomyces cerevisiae contains a single gene for dynein (DYN1), and cells containing a dyn1 null allele are viable (Eshel et al., 1993; Saunders et al., 1995). To test whether DYN1 contributes to FKBP52-dependent potentiation, GR reporter strains were constructed in the dyn1 null background and compared with reporter strains in the wild-type background (Figure 7A). FKBP52-dependent potentiation was unaffected by the absence of dynein.
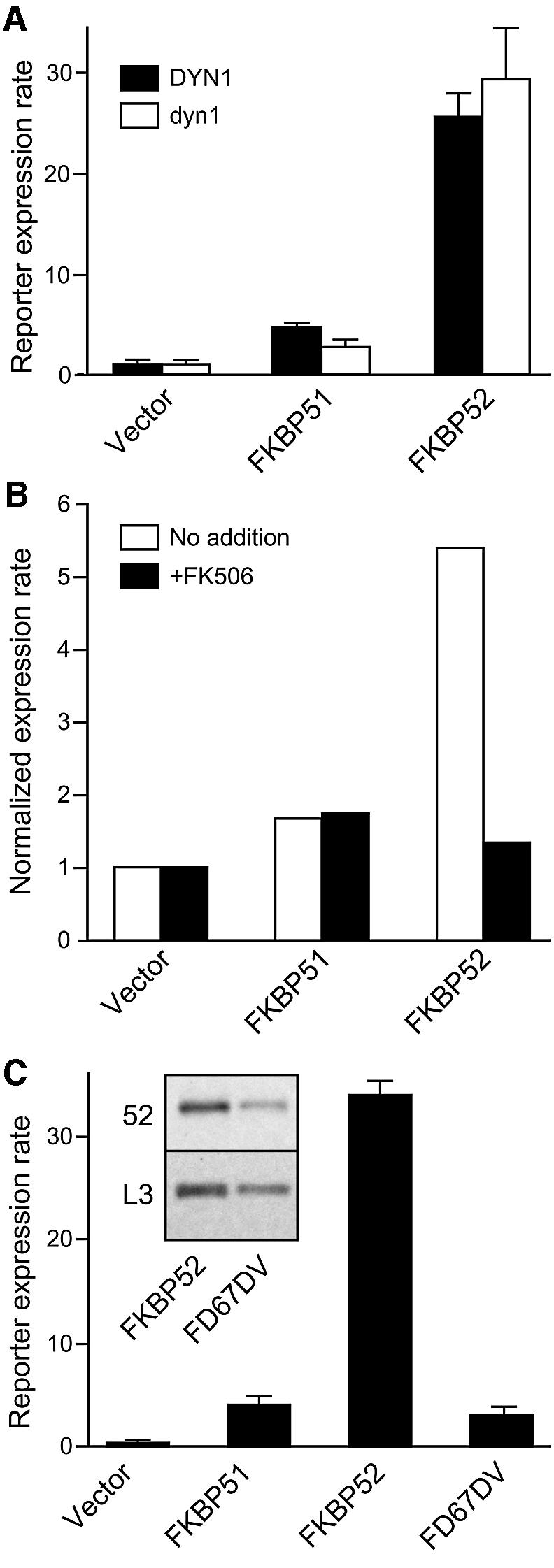
Fig. 7. Roles of dynein binding and PPIase activity in potentiation. (A) Reporter expression in cells containing empty vector, FKBP51 or FKBP52 in a wild-type DYN1 or dyn1-null background in response to 50 nM DOC. All data are the average (n = 4 ± SD) of duplicate assays from two independent isolates. (B) Cultures of GR reporter cells expressing the indicated immunophilin were treated with FK506 (10 µg/ml) for 30 min before addition of DOC (50 nM) and measurement of reporter activity (A and B normalized to the level of expression in vector-only cells). (C) Expression rates in GR reporter cells containing empty vector, FKBP51, FKBP52 or the PPIase-deficient mutant FKBP52-FD67DV. All data are the average (n = 4 ± SD) of duplicate assays from two independent isolates. The inset shows a western immunostain for FKBP52 or L3 which compares FKBP52 expression levels in the wild-type and mutant strains.
Since PPIase activity resides in FK1, its potential role was examined both pharmacologically and genetically. The immunosuppressive drug FK506 binds FK1 and efficiently blocks the PPIase activity of FKBP52 (Peattie et al., 1992). Addition of FK506 to cells prior to hormone addition completely blocked FKBP52-mediated enhancement of reporter expression (Figure 7B). However, apart from inhibition of PPIase activity, FK506 could, conceivably, sterically interfere with FK1 interactions or alter the conformation of FK1 and disrupt interactions that occur away from the PPIase active site. As an alternative approach, we tested the FKBP52 mutant FD67DV, which has substitutions in two residues that are highly conserved in the FKBP family and are critical for PPIase activity in FKBP12 (DeCenzo et al., 1996) and FKBP51 (Barent et al., 1998). Similar to FK506, the FD67DV double-point mutant was found to abrogate potentiation (Figure 7C). Since some mutations to FKBP12 have been shown to make it more susceptible to proteolysis in vitro (Dolinski et al., 1997), we were concerned that FKBP52-FD67DV may not accumulate to appreciable levels in yeast. However, western immunostains (Figure 7C, inset) revealed that mutant and wild-type proteins, when normalized to the L3 level in each cellular extract, were similar. We conclude that the PPIase activity of FKBP52 is required for the elevation of GR hormone-binding affinity and potentiation of hormonal signaling.
Discussion
Previous studies have established that the cellular chaperone and transcriptional machineries of S.cerevisiae are competent to support hormone-dependent activation of a vertebrate GR reporter system. GR requires Hsp90, Hsp70 and other chaperone components to establish basal hormone-binding ability (reviewed in Pratt and Toft, 1997), and yeast chaperone orthologs can fulfill this basic requirement (Picard et al., 1990a; Kimura et al., 1995; Chang et al., 1997). However, we show here that human FKBP52, which is a native component of GR complexes in vertebrates and has no ortholog in S.cerevisiae, can significantly boost GR activity in yeast. At limiting hormone concentrations and soon after hormone exposure, conditions that are physiologically germane, reporter read-out is elevated 10-fold or greater in the presence of FKBP52 as compared with yeast lacking FKBP52 (Figure 2A). This effect is specific for FKBP52, since FKBP51, a closely related protein, and other introduced immunophilins boost signaling to a minimal extent. However, FKBP51, but not PP5, can efficiently block FKBP52-mediated potentiation (Figure 2C). Hormone response curves (Figure 3) and radiolabeled hormone-binding assays indicate that FKBP52 raises GR hormone-binding affinity; consistent with this mechanism of action, the FKBP52 effect localizes to the GR LBD (Figure 4). FKBP52 is selective for GR, as we do not observe a similar enhancement of signaling with ER (Figure 4) or other steroid receptors. Finally, potentiation of GR signaling requires Hsp90 binding (Figure 5) plus the active PPIase domain of FKBP52 (Figures 6 and 7). As discussed below, the novel function of FKBP52 in GR activity shines new light on the intersection of steroid signaling and molecular chaperone pathways.
Modulation of steroid receptor activity
Steroid signaling is physiologically regulated at many levels, from hormone metabolism to expression and modification of transcriptional co-regulatory proteins that bind receptors. Few instances of regulation at the level of receptor hormone-binding affinity have been described, yet small differences in hormone-binding affinity are physiologically meaningful. For example, mutations in steroid receptors that shift hormone-binding affinity over a narrow range can have significant clinical ramifications, such as cortisol resistance and resulting sequelae in patients with mutant GR (Lamberts, 2001). Interestingly, the apparent cortisol resistance that is typical of New World primates (Brandon et al., 1989) can be largely attributed to constitutive overexpression of FKBP51 rather than a receptor mutation (Reynolds et al., 1999; Scammell et al., 2001). Human GR has been shown to have a lowered affinity for hormone when associated with FKBP51 (Reynolds et al., 1999; Denny et al., 2000), and the human FKBP51 gene is highly inducible by glucocorticoids (Baughman et al., 1991; Yoshida et al., 2002). These observations have suggested a feedback model in which cellular responsiveness to glucocorticoids can be down-modulated by hormone-induced expression of FKBP51 (Cheung and Smith, 2000; Scammell, 2000). Our results here show that FKBP51 does not inhibit GR function in yeast that lack FKBP52, although FKBP51 can efficiently block the potentiation attributable to FKBP52. Since FKBP52 acts through intrinsic PPIase activity and Hsp90 to elevate GR hormone-binding affinity, it appears that FKBP51 is somehow able to counter this increase and return GR to a basal hormone-binding affinity.
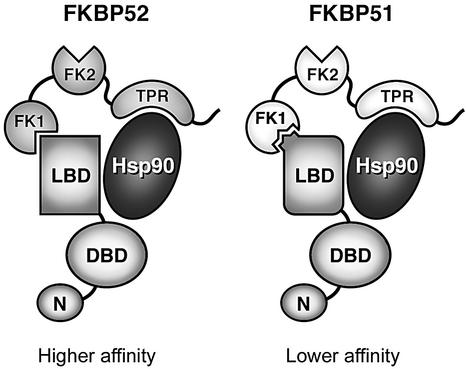
How FKBP51 lowers GR hormone-binding affinity while FKBP52 raises affinity is unresolved. FKBP52 and FKBP51 share >70% amino acid sequence similarity and comparable PPIase activity toward a model peptide substrate (Pirkl and Buchner, 2001). However, the respective FK1 domains differentially affect GR activity (Figure 6C). A mutagenic study with two small FKBP family members demonstrated that amino acids surrounding the PPIase pocket can influence specificity for protein substrates (Xin et al., 1999), and by analogy FKBP52 and FKBP51 differ at several amino acid sites that are positioned around the PPIase pocket. In a working model (Figure 8), we propose that FKBP52 and FKBP51 interact with one or more sites in the GR LBD, alternately favoring distinct conformations with higher or lower affinity, respectively, for hormone. By understanding these mechanisms and taking advantage of the FKBPs as targets for FK506 and related drugs, there is the prospect of novel pharmacological approaches to therapeutic intervention in glucocorticoid-mediated processes.
Fig. 8. Model for FKBP-mediated changes in GR hormone-binding affinity. FKBP52 or FKBP51 assemble with GR complexes through an interaction with Hsp90. The three major FKBP domains (FK1, FK2 and TPR) are indicated, as are the major domains of GR (N-terminal domain, DBD and LBD). Hsp90 is a multidomain protein that normally functions as a dimer but, for simplicity, is illustrated as a single entity. When FKBP52 is present in the GR complex, the active PPIase domain (FK1) of FKBP52 interacts with the LBD of GR, stimulating a conformational change and increase in hormone-binding affinity. Conversely, FKBP51 in the GR complex favors an alternative lower-affinity LBD conformation. The distinction between FKBP52 and FKBP51 is perhaps not in PPIase activity per se, but in the specificity of each for sequences within the LBD.
Co-operative chaperone activities
Some of the Hsp90 co-chaperones have an Hsp90-independent ability to prevent aggregation of misfolded model protein substrates (Bose et al., 1996; Freeman et al., 1996; Pirkl and Buchner, 2001), but many actions occur through Hsp90. The Hsp90 co-chaperones have been shown to play roles in regulating Hsp90 binding to client proteins (Johnson and Toft, 1995; Dittmar et al., 1997), regulating Hsp90 ATPase activity (Prodromou et al., 1999; McLaughlin et al., 2002), recruiting Hsp90 to Hsp70 client complexes (Chen and Smith, 1998) or docking Hsp90 client protein complexes to the cytoskeleton (Czar et al., 1995; Pratt et al., 1999). The Hsp90-binding immunophilins each have an enzymatic domain, either a PPIase or protein phosphatase domain, so one would expect that these enzymatic activities could influence Hsp90 or client protein function, but there have been surprisingly few demonstrations of this. Sharing some similarities with our finding that FKBP52 PPIase can alter GR function, FKBP52 has also been found (Mamane et al., 2000) to alter transcriptional transactivation activity of interferon regulatory factor-4 (IRF-4). Change in IRF-4 function required the PPIase activity of FKBP52, but the IRF-4 example differs from GR in that FKBP52 binds directly to IRF-4 in a manner independent of Hsp90 and other chaperones. With GR complexes, FKBP52 is one of several components in the chaperone machinery whose interactions must be closely co-ordinated to permit FKBP52 to elicit its effect. Despite the similar assembly of FKBP52 into all steroid receptor complexes, the activity we observe is specific for GR. Another Hsp90-binding FKBP family member, XAP2/AIP, specifically influences aryl hydrocarbon receptor function (Ma and Whitlock, 1997; Meyer et al., 1998), but XAP2 activity is PPIase independent and this immunophilin has not been observed in steroid receptor complexes. The selective action of FKBP52 toward GR supports a dynamic sampling model (Nair et al., 1997) in which Hsp90 presents various co-chaperone activities to a client protein, effectively allowing the client to pick and choose from these activities in a client-specific manner. It follows from the sampling model that the mere presence of a co-chaperone in a particular client complex does not necessarily imply function. Conversely, sampling provides an efficient mechanism by which a broad range of Hsp90 client proteins can be accommodated, insuring that each client will have the opportunity for productive interactions from a spectrum of co-chaperone activities.
Materials and methods
Yeast methods and strains
The parent of all strains, except for dynein studies, was W303a (MATa leu2-112 ura3-1 trp1-1 his3-11,15 ade2-1 can1-100 GAL SUC2). For dynein studies, the strain BY4742-YKR054C (dyn1Δ::kanMX4) and its DYN1 parent BY4742 were obtained from the American Type Culture Collection (Manassas, VA). Plasmids were introduced into yeast using the lithium acetate–polyethylene glycol protocol. At least two to four independent transformants of each strain construction were analyzed. Strains were propagated in minimal media consisting of 0.67% (w/v) yeast nitrogen base without amino acids, 2% (w/v) glucose, the appropriate SC supplement mixture (Q-biogene, Carlsbad, CA) and 1.6% (w/v) agar for plates. GR was detected with the mouse monoclonal antibody MA1-510 (Affinity Bioreagents, Golden, CO), and yeast ribosomal protein L3 (as a loading control) was detected with a mouse monoclonal antibody (Vilardell and Warner, 1997). The strains used for the in vivo hormone-binding measurements contained the GR-F620S receptor and either FKBP51 or FKBP52. Cultures were grown at room temperature in selective medium to an OD600 of ∼1, and then harvested and resuspended in selective medium supplemented with 25 mM β-mercaptoethanol. Eight aliquots were tested for each hormone concentration: four contained labeled hormone to determine total disintegrations per minute (dpm) bound, and four contained labeled hormone and a 400-fold excess of unlabeled hormone to determine the dpm of non-specifically bound hormone. Each aliquot contained [3H]corticosterone (79.0 Ci/mmol; Amersham, Piscataway, NJ), unlabeled hormone and 6 OD600 units of cells (1 OD unit is equivalent to 1 ml of culture having OD600 = 1) in 0.7 ml final volume. The cells were incubated at room temperature for 3 h, harvested and washed three times in room temperature phosphate-buffered saline (PBS) containing 25 mM β-mercaptoethanol. The washed cell pellet was resuspended in 1 ml PBS and the bound hormone determined by liquid scintillation counting. The OD600 of the labeled cell suspension was determined in order to normalize the bound hormone measurement to dpm/OD unit.
Plasmids
GR was expressed from pG/N795 (Schena and Yamamoto, 1988) and ER from pG/ER(G) (Liu and Picard, 1998). For immunophilin competition experiments, GR was cloned into p415GPD [this and other GPD series vectors (Mumberg et al., 1995) were obtained from the American Type Culture Collection]. The ER/GR chimeric receptors (Bunone et al., 1996) and the GR-F620S receptor (Garabedian and Yamamoto, 1992) were subcloned into the vector p424GPD. The reporter plasmids for GR and ER were pUCΔSS-26X (Louvion et al., 1996) and pUCΔSS-ERE (Picard et al., 1990b), respectively. Plasmids expressing the immunophilins or PP5 were generated in p423GPD by subcloning PCR-generated products containing the following cDNA with the indicated restriction sites: FKBP51, FKBP52, FKBP52–K354A and CyP40, EcoRI–SalI; CPR7, EcoRI–ClaI; and PP5, EcoRI–XhoI.
The FKBP52 truncation mutant TPR52 was created by making a F252M mutation and removing all upstream coding sequences. Likewise, a K138M substitution was used to generate the (FK2+TPR)52 construct. (FK1+FK2)52 was made by introducing a stop codon at position 257. The FKBP51–52 chimeras were constructed by PCR amplification of the two complementary FKBP51 and FKBP52 gene fragments from the yeast expression plasmids. Each product had ∼40 nucleotides of vector sequence at one end and a primer-encoded SapI site at the other end. The two PCR products were cut with SapI and ligated, and the chimeric gene was gel purified. The resulting chimeras, which contain vector sequences at each end, were co-transformed with a linearized expression vector into the GR signaling strain. Yeast colony PCR, western blotting and DNA sequencing verified gap-repair of the vector. Western analysis confirmed that these mutated FKBPs were as abundant as the wild-type FKBPs in the GR tester strains.
The binding of FKBP52 or FKBP52-K354A to Hsp90 was analyzed as follows. Anti-Hsp90 monoclonal antibody H90-10 (10 µg) was incubated with protein G–Sepharose (10 µl pellet) for 30 min at room temperature with rocking in 1 ml of binding buffer (20 mM Tris–HCl pH 7.4, 50 mM NaCl, 0.5% (v/v) Tween-20). The unbound antibody was removed from the resin with three washes in binding buffer. Radiolabeled FKBP52 and FKBP52-K354A were prepared using pSPUTK derivatives containing FKBP52 or FKBP52-K354A as templates in a reticulocyte lysate-based in vitro transcription/translation system (SP6 TnT system, Promega). Equal amounts of each labeled protein, as quantified by autoradiography of SDS–PAGE gels (∼10 µl of lysate, which contains substantial amounts of endogenous Hsp90), were added to antibody–resin pellets and the total volume was adjusted to 100 µl with binding buffer. These reactions were incubated for 30 min at 30°C with vortexing at 5 min intervals. After washing three times in binding buffer supplemented with 10 mM monothioglycerol, the proteins were separated by SDS–PAGE. The gel was stained with Coomassie blue and exposed to X-ray film.
Hormone induction assays
Yeast strains were grown in selective media at 25°C to an OD600 of 0.05–0.12 units. Growth was monitored spectrophotometrically for at least 30 min before hormone addition to ensure that the culture was in exponential phase. Hormone (DOC, 25 nM final concentration unless otherwise noted, or 17-β-estradiol) was added, and samples were withdrawn at 10 min intervals (typically 70–120 min after hormone addition) for β-galactosidase assays. Samples of 100 µl were immediately added to 100 µl of the chemiluminescent β-galactosidase assay reagent Gal-Screen™ (Tropix, Bedford, MA) in 96-well microtiter plates at room temperature. The entire plate was read in a luminometer 2 h after the last sample was collected.
To determine the rate of reporter expression, β-galactosidase induction curves were first generated by plotting RLU against the OD600 of the culture sample (such as in Figure 1B). Regression analysis of this linear portion of each dataset yielded a best-fit line (usually R2 > 0.98) whose slope is the growth-rate-normalized rate of β-galactosidase expression. For convenience, the reporter expression rate is defined as the slope of the induction curve divided by 1000.
Western immunostains
To prepare whole cell extracts, washed yeast cell pellets were resuspended in cracking buffer (8 M urea, 5% (w/v) sodium dodecyl sulfate, 40 mM Tris–HCl pH 7.5, 0.1 mM EDTA, 0.04% (w/v) bromophenol blue) at a rate of 4 ml/g of cells. The cells were homogenized with glass beads in a Mini Bead Beater (Biospec Products, Bartlesville, OK). After heating, samples were centrifuged to remove insoluble material. Typically, a 5 µl volume of supernatant was loaded per gel lane. Mouse monoclonal antibodies used were anti-GR BuGR2 (Affinity Bioreagents); anti-FKBP51 Hi51a, Hi51b and FF1; anti-FKBP52 Hi52c and Hi52d; anti-Hsp90 H90-10; and anti-L3 ribosomal subunit. Anti-PP5 is a rabbit polyclonal antibody.
Acknowledgments
Acknowledgements
We are grateful to Marija Tesic in the Gaber Laboratory for expert assistance with the development of yeast strains, Marv Ruona in the Mayo Visual Communications Laboratory for his assistance with figures, Jonathan Warner for the L3 antibody and Michael Chinkers for the anti-PP5 antibody. We would also like to thank Dr Susan Lindquist for her encouragement and critical insight. This work was supported by NIH R01 DK48218 and DK44923 (D.F.S.), and NSF MCB-0079249 (R.F.G.).
References
- Barent R.L., Nair,S.C., Carr,D.C., Ruan,Y., Rimerman,R.A., Fulton,J., Zhang,Y. and Smith,D.F. (1998) Analysis of FKBP51/FKBP52 chimeras and mutants for Hsp90 binding and association with progesterone receptor complexes. Mol. Endocrinol., 12, 342–354. [DOI] [PubMed] [Google Scholar]
- Baughman G., Harrigan,M.T., Campbell,N.F., Nurrish,S.J. and Bourgeois,S. (1991) Genes newly identified as regulated by glucocorticoids in murine thymocytes. Mol. Endocrinol., 5, 637–644. [DOI] [PubMed] [Google Scholar]
- Baughman G., Wiederrecht,G.J., Campbell,N.F., Martin,M.M. and Bourgeois,S. (1995) FKBP51, a novel T-cell-specific immunophilin capable of calcineurin inhibition. Mol. Cell. Biol., 15, 4395–4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohen S.P. and Yamamoto,K.R. (1993) Isolation of Hsp90 mutants by screening for decreased steroid receptor function. Proc. Natl Acad. Sci. USA, 90, 11424–11428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose S., Weikl,T., Bugl,H. and Buchner,J. (1996) Chaperone function of Hsp90-associated proteins. Science, 274, 1715–1717. [DOI] [PubMed] [Google Scholar]
- Brandon D.D., Markwick,A.J., Chrousos,G.P. and Loriaux,D.L. (1989) Glucocorticoid resistance in humans and nonhuman primates. Cancer Res., 49, 2203s–2213s. [PubMed] [Google Scholar]
- Bunone G., Briand,P.A., Miksicek,R.J. and Picard,D. (1996) Activation of the unliganded estrogen receptor by EGF involves the MAP kinase pathway and direct phosphorylation. EMBO J., 15, 2174–2183. [PMC free article] [PubMed] [Google Scholar]
- Caplan A.J., Langley,E., Wilson,E.M. and Vidal,J. (1995) Hormone-dependent transactivation by the human androgen receptor is regulated by a dnaJ protein. J. Biol. Chem., 270, 5251–5257. [DOI] [PubMed] [Google Scholar]
- Chang H.C., Nathan,D.F. and Lindquist,S. (1997) In vivo analysis of the Hsp90 cochaperone Sti1 (p60). Mol. Cell. Biol., 17, 318–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. and Smith,D.F. (1998) Hop as an adaptor in the heat shock protein 70 (Hsp70) and hsp90 chaperone machinery. J. Biol. Chem., 273, 35194–35200. [DOI] [PubMed] [Google Scholar]
- Cheung J. and Smith,D.F. (2000) Molecular chaperone interactions with steroid receptors: an update. Mol. Endocrinol., 14, 939–946. [DOI] [PubMed] [Google Scholar]
- Czar M.J., Lyons,R.H., Welsh,M.J., Renoir,J.M. and Pratt,W.B. (1995) Evidence that the FK506-binding immunophilin heat shock protein 56 is required for trafficking of the glucocorticoid receptor from the cytoplasm to the nucleus. Mol. Endocrinol., 9, 1549–1560. [DOI] [PubMed] [Google Scholar]
- Dean D.A., Urban,G., Aragon,I.V., Swingle,M., Miller,B., Rusconi,S., Bueno,M., Dean,N.M. and Honkanen,R.E. (2001) Serine/threonine protein phosphatase 5 (PP5) participates in the regulation of glucocorticoid receptor nucleocytoplasmic shuttling. BMC Cell Biol., 2, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCenzo M.T., Park,S.T., Jarrett,B.P., Aldape,R.A., Futer,O., Murcko,M.A. and Livingston,D.J. (1996) FK506-binding protein mutational analysis: defining the active-site residue contributions to catalysis and the stability of ligand complexes. Protein Eng., 9, 173–180. [DOI] [PubMed] [Google Scholar]
- Denny W.B., Valentine,D.L., Reynolds,P.D., Smith,D.F. and Scammell,J.G. (2000) Squirrel monkey immunophilin FKBP51 is a potent inhibitor of glucocorticoid receptor binding. Endocrinology, 141, 4107–4113. [DOI] [PubMed] [Google Scholar]
- Dittmar K.D. and Pratt,W.B. (1997) Folding of the glucocorticoid receptor by the reconstituted Hsp90-based chaperone machinery. The initial hsp90.p60.hsp70-dependent step is sufficient for creating the steroid binding conformation. J. Biol. Chem., 272, 13047–13054. [DOI] [PubMed] [Google Scholar]
- Dittmar K.D., Demady,D.R., Stancato,L.F., Krishna,P. and Pratt,W.B. (1997) Folding of the glucocorticoid receptor by the heat shock protein (hsp) 90-based chaperone machinery. The role of p23 is to stabilize receptor.hsp90 heterocomplexes formed by hsp90.p60.hsp70. J. Biol. Chem., 272, 21213–21220. [DOI] [PubMed] [Google Scholar]
- Dolinski K., Scholz,C., Muir,R.S., Rospert,S., Schmid,F.X., Cardenas,M.E. and Heitman,J. (1997) Functions of FKBP12 and mitochondrial cyclophilin active site residues in vitro and in vivo in Saccharomyces cerevisiae. Mol. Biol. Cell, 8, 2267–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duina A.A., Chang,H.C., Marsh,J.A., Lindquist,S. and Gaber,R.F. (1996) A cyclophilin function in Hsp90-dependent signal transduction. Science, 274, 1713–1715. [DOI] [PubMed] [Google Scholar]
- Eshel D., Urrestarazu,L.A., Vissers,S., Jauniaux,J.C., van Vliet-Reedijk,J.C., Planta,R.J. and Gibbons,I.R. (1993) Cytoplasmic dynein is required for normal nuclear segregation in yeast. Proc. Natl Acad. Sci. USA, 90, 11172–11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman B.C., Toft,D.O. and Morimoto,R.I. (1996) Molecular chaperone machines: chaperone activities of the cyclophilin Cyp-40 and the steroid aporeceptor-associated protein p23. Science, 274, 1718–1720. [DOI] [PubMed] [Google Scholar]
- Freeman B.C., Felts,S.J., Toft,D.O. and Yamamoto,K.R. (2000) The p23 molecular chaperones act at a late step in intracellular receptor action to differentially affect ligand efficacies. Genes Dev., 14, 422–434. [PMC free article] [PubMed] [Google Scholar]
- Galigniana M.D., Radanyi,C., Renoir,J.M., Housley,P.R. and Pratt,W.B. (2001) Evidence that the peptidylprolyl isomerase domain of the hsp90-binding immunophilin FKBP52 is involved in both dynein interaction and glucocorticoid receptor movement to the nucleus. J. Biol. Chem., 276, 14884–14889. [DOI] [PubMed] [Google Scholar]
- Garabedian M.J. and Yamamoto,K.R. (1992) Genetic dissection of the signaling domain of a mammalian steroid receptor in yeast. Mol. Biol. Cell, 3, 1245–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J.L. and Toft,D.O. (1995) Binding of p23 and hsp90 during assembly with the progesterone receptor. Mol. Endocrinol., 9, 670–678. [DOI] [PubMed] [Google Scholar]
- Kimura Y., Yahara,I. and Lindquist,S. (1995) Role of the protein chaperone YDJ1 in establishing Hsp90-mediated signal transduction pathways. Science, 268, 1362–1365. [DOI] [PubMed] [Google Scholar]
- Kosano H., Stensgard,B., Charlesworth,M.C., McMahon,N. and Toft,D. (1998) The assembly of progesterone receptor–hsp90 complexes using purified proteins. J. Biol. Chem., 273, 32973–32979. [DOI] [PubMed] [Google Scholar]
- Kralli A., Bohen,S.P. and Yamamoto,K.R. (1995) LEM1, an ATP-binding-cassette transporter, selectively modulates the biological potency of steroid hormones. Proc. Natl Acad. Sci. USA, 92, 4701–4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberts S.W. (2001) Hereditary glucocorticoid resistance. Ann. Endocrinol. (Paris), 62, 164–167. [PubMed] [Google Scholar]
- Liu J.W. and Picard,D. (1998) Bioactive steroids as contaminants of the common carbon source galactose. FEMS Microbiol. Lett., 159, 167–171. [DOI] [PubMed] [Google Scholar]
- Louvion J.-F., Warth,R. and Picard,D. (1996) Two eukaryote-specific regions of Hsp82 are dispensable for its viability and signal transduction functions in yeast. Proc. Natl Acad. Sci. USA, 93, 13937–13942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q. and Whitlock,J.P.,Jr (1997) A novel cytoplasmic protein that interacts with the Ah receptor, contains tetratricopeptide repeat motifs and augments the transcriptional response to 2,3,7,8-tetrachlorodibenzo-p-dioxin. J. Biol. Chem., 272, 8878–8884. [PubMed] [Google Scholar]
- Mamane Y., Sharma,S., Petropoulos,L., Lin,R. and Hiscott,J. (2000) Posttranslational regulation of IRF-4 activity by the immunophilin FKBP52. Immunity, 12, 129–140. [DOI] [PubMed] [Google Scholar]
- McLaughlin S.H., Smith,H.W. and Jackson,S.E. (2002) Stimulation of the weak ATPase activity of human hsp90 by a client protein. J. Mol. Biol., 315, 787–798. [DOI] [PubMed] [Google Scholar]
- Meyer B.K., Pray-Grant,M.G., Vanden Heuvel,J.P. and Perdew,G.H. (1998) Hepatitis B virus X-associated protein 2 is a subunit of the unliganded aryl hydrocarbon receptor core complex and exhibits transcriptional enhancer activity. Mol. Cell. Biol., 18, 978–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumberg D., Muller,R. and Funk,M. (1995) Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene, 156, 119–122. [DOI] [PubMed] [Google Scholar]
- Nair S.C., Rimerman,R.A., Toran,E.J., Chen,S., Prapapanich,V., Butts,R.N. and Smith,D.F. (1997) Molecular cloning of human FKBP51 and comparisons of immunophilin interactions with Hsp90 and progesterone receptor. Mol. Cell. Biol., 17, 594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan D. and Lindquist,S. (1995) Mutational analysis of Hsp90 function: interactions with a steroid receptor. Mol. Cell. Biol., 15, 3917–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peattie D.A., Harding,M.W., Fleming,M.A., DeCenzo,M.T., Lippke,J.A., Livingston,D.J. and Benasutti,M. (1992) Expression and characterization of human FKBP52, an immunophilin that associates with the 90-kDa heat shock protein and is a component of steroid receptor complexes. Proc. Natl Acad. Sci. USA, 89, 10974–10978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard D., Khursheed,B., Garabedian,M.J., Fortin,M.G., Lindquist,S. and Yamamoto,K.R. (1990a) Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature, 348, 166–168. [DOI] [PubMed] [Google Scholar]
- Picard D., Schena,M. and Yamamoto,K.R. (1990b) An inducible expression vector for both fission and budding yeast. Gene, 86, 257–261. [DOI] [PubMed] [Google Scholar]
- Pirkl F. and Buchner,J. (2001) Functional analysis of the Hsp90-associated human peptidyl prolyl cis/trans isomerases FKBP51, FKBP52 and Cyp40. J. Mol. Biol., 308, 795–806. [DOI] [PubMed] [Google Scholar]
- Pratt W.B. and Toft,D.O. (1997) Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr. Rev., 18, 306–360. [DOI] [PubMed] [Google Scholar]
- Pratt W.B., Silverstein,A.M. and Galigniana,M.D. (1999) A model for the cytoplasmic trafficking of signalling proteins involving the hsp90-binding immunophilins and p50cdc37. Cell Signal., 11, 839–851. [DOI] [PubMed] [Google Scholar]
- Prodromou C., Siligardi,G., O’Brien,R., Woolfson,D.N., Regan,L., Panaretou,B., Ladbury,J.E., Piper,P.W. and Pearl,L.H. (1999) Regulation of Hsp90 ATPase activity by tetratricopeptide repeat (TPR) domain co-chaperones. EMBO J., 18, 754–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renoir J.M., Radanyi,C., Faber,L.E. and Baulieu,E.E. (1990) The non-DNA-binding heterooligomeric form of mammalian steroid hormone receptors contains a hsp90-bound 59-kilodalton protein. J. Biol. Chem., 265, 10740–10745. [PubMed] [Google Scholar]
- Reynolds P.D., Ruan,Y., Smith,D.F. and Scammell,J.G. (1999) Glucocorticoid resistance in the squirrel monkey is associated with overexpression of the immunophilin FKBP51. J. Clin. Endocrinol. Metab., 84, 663–669. [DOI] [PubMed] [Google Scholar]
- Russell L.C., Whitt,S.R., Chen,M.S. and Chinkers,M. (1999) Identification of conserved residues required for the binding of a tetratricopeptide repeat domain to heat shock protein 90. J. Biol. Chem., 274, 20060–20063. [DOI] [PubMed] [Google Scholar]
- Saunders W.S., Koshland,D., Eshel,D., Gibbons,I.R. and Hoyt,M.A. (1995) Saccharomyces cerevisiae kinesin- and dynein-related proteins required for anaphase chromosome segregation. J. Cell Biol., 128, 617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scammell J.G. (2000) Steroid resistance in the squirrel monkey: an old subject revisited. ILAR J., 41, 19–25. [DOI] [PubMed] [Google Scholar]
- Scammell J.G., Denny,W.B., Valentine,D.L. and Smith,D.F. (2001) Overexpression of the FK506-binding immunophilin FKBP51 is the common cause of glucocorticoid resistance in three New World primates. Gen. Comp. Endocrinol., 124, 152–165. [DOI] [PubMed] [Google Scholar]
- Schena M. and Yamamoto,K.R. (1988) Mammalian glucocorticoid receptor derivatives enhance transcription in yeast. Science, 241, 965–967. [DOI] [PubMed] [Google Scholar]
- Sinars C.R., Cheung-Flynn,J., Rimerman,R.A., Scammell,J.G., Smith,D.F. and Clardy,J. (2003) Structure of the large FK506-binding protein FKBP51, an Hsp90-binding protein and a component of steroid receptor complexes. Proc. Natl Acad. Sci. USA, 100, 868–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilardell J. and Warner,J.R. (1997) Ribosomal protein L32 of Saccharomyces cerevisiae influences both the splicing of its own transcript and the processing of rRNA. Mol. Cell. Biol., 17, 1959–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H.B., Rogers,K., Qi,Y., Kanematsu,T. and Fleischer,S. (1999) Three amino acid residues determine selective binding of FK506-binding protein 12.6 to the cardiac ryanodine receptor. J. Biol. Chem., 274, 15315–15319. [DOI] [PubMed] [Google Scholar]
- Yoshida N.L., Miyashita,T., U,M., Yamada,M., Reed,J.C., Sugita,Y. and Oshida,T. (2002) Analysis of gene expression patterns during glucocorticoid-induced apoptosis using oligonucleotide arrays. Biochem. Biophys. Res. Commun., 293, 1254–1261. [DOI] [PubMed] [Google Scholar]