Abstract
Helicobacter pylori vacuolating toxin (VacA) causes vacuolation in a variety of cultured cell lines, sensitivity to VacA differing greatly, however, among the different cell types. We found that the high sensitivity of HEp-2 cells to VacA was impaired by treating the cells with phosphatidylinositol-specific phospholipase C (PI-PLC) which removes glycosylphosphatidylinositol (GPI)-anchored proteins from the cell surface. Incubation of cells with a cholesterol-sequestering agent, that impairs both structure and function of sphingolipid-cholesterol-rich membrane microdomains (“lipid rafts”), also impaired VacA-induced cell vacuolation. Overexpression into HEp-2 cells of proteins inhibiting clathrin-dependent endocytosis (i.e., a dominant-negative mutant of Eps15, the five tandem Src-homology-3 domains of intersectin, and the K44A dominant-negative mutant of dynamin II) did not affect vacuolation induced by VacA. Nevertheless, F-actin depolymerization, known to block the different types of endocytic mechanisms, strongly impaired VacA vacuolating activity. Taken together, our data suggest that the high cell sensitivity to VacA depends on the presence of one or several GPI-anchored protein(s), intact membrane lipid rafts, and an uptake mechanism via a clathrin-independent endocytic pathway.
INTRODUCTION
Helicobacter pylori is a microaerophilic, spiral-shaped, Gram-negative bacterium that colonizes the gastric mucosa of ∼ 50 to 60% of the world's population (Taylor and Blaser, 1991; Blaser, 1992; Cave, 1997; Goodwin et al., 1997). It is now recognized that H. pylori plays a major role in the development of chronic gastritis, peptic ulcer, and gastric cancer (Nomura et al., 1991; Parsonnet et al., 1991; NIH, 1994; WHO, 1994; Zarrilli et al., 1999). This bacterium has developed properties that allow its survival and growth in a hostile environment like the human stomach where it causes an inflammatory reaction and epithelial damage with cellular swelling, cytoplasmic vacuolation, and expansion of endosomal compartments (Tricottet et al., 1986; Fiocca et al., 1994; Ernst et al., 1997). Bacterial extracts from H. pylori induce cytoplasmic vacuolar degeneration in cultured cells (Leunk et al., 1988; Cover et al., 1991, 1992; Ricci et al., 1993; Papini et al., 1993). A pivotal role in cell damage induced by H. pylori seems to be played by the vacuolating toxin (VacA) which is produced in an active form by ∼ 50% of H. pylori clinical isolates (Telford et al., 1994b; Ghiara et al., 1995; Cover, 1996; Atherton, 1997). VacA (thus far the only protein toxin known to be produced by H. pylori) causes vacuolation in a variety of cultured cell lines, although sensitivity to VacA greatly differs among the different cell types tested (Leunk et al., 1988; de Bernard et al., 1998; Massari et al., 1998). When given to mice, VacA causes gastric epithelial damage closely resembling that found in H. pylori-colonized patients (Telford et al., 1994b). The structure of the vacA gene varies, especially in the region encoding the signal sequence (which may be type s1a, s1b, or s2) and in the midregion (which may be type m1 or m2) (Atherton et al., 1995). VacA is synthesized by H. pylori as a 140-kDa protoxin (Telford et al., 1994b; Cover, 1996), which is processed to a 90-kDa protein forming the mature toxin released in the extracellular environment (Cover and Blaser, 1992). VacA monomers may be further processed to produce a 34- to 37-kDa N-terminal fragment and a 58-kDa C-terminal fragment, these fragments remaining associated after cleavage (Telford et al., 1994a,b; Cover, 1996). Purified toxin forms high molecular mass (∼ 1,000 kDa) oligomers (Cover and Blaser, 1992; Cover, 1996) which require to be disassembled in monomers by acid or alkaline treatment to become active (Cover et al., 1997, Montecucco et al., 1999, Yahiro et al., 1999). Nevertheless, the findings that both bacterium-associated toxin and bacterial broth culture filtrates are constitutively very active without requiring acid/alkaline pretreatment (Leunk et al., 1988; Cover et al., 1991, 1992; Ricci et al., 1993; Pelicic et al., 1999) suggest that VacA may be present in the extracellular microenvironment of human gastric epithelium in vivo in its active monomeric form. VacA has been reported to bind to specific, high-affinity cell surface receptors (Yahiro et al., 1997, 1999; Massari et al., 1998; Seto et al., 1998) and to be internalized by cells via a temperature-dependent process (Garner and Cover, 1996). After internalization, VacA localizes in the endocytic-endosomal compartment from which vacuoles originate (Ricci et al., 1993, 1997). Vacuole development is strictly dependent on the presence in the incubation medium of weak bases like ammonia (which can be generated by H. pylori urease) (Cover et al., 1992; Ricci et al., 1997). The finding of VacA in both endosomal tubulovesicles and vacuoles (Ricci et al., 1997; Sommi et al., 1998) further supports the hypothesis that the origin of vacuoles is endosomal. By using a panel of markers for varying intracellular compartments, Montecucco and coworkers (Papini et al., 1994; Molinari et al., 1997) demonstrated that vacuoles originate from late endosomal compartments and proposed that VacA induces the accumulation of a hybrid compartment resembling both late endosomes and lysosomes, but with a reduced proteolytic activity. Nevertheless, it has been suggested that VacA could cross the plasma membrane, directly penetrating the cytosol where it might exert its vacuolating action (Molinari et al., 1998). Recently, it has been reported that VacA may act as a channel-forming toxin (Czajkowsky et al., 1999; Szabo et al., 1999; Tombola et al., 1999), and that the formation of VacA membrane channels may involve oligomerization of membrane-bound monomers (Vinion-Dubiel et al., 1999). It has also been proposed that VacA channels play a direct role in cell vacuolation: endocytosed VacA channels could stimulate the turnover of endosomal V-ATPase by increasing the permeability of the endosomal membrane to anions (Szabo et al., 1999). This would lead to the accumulation of osmotically active species causing an osmotic imbalance of late endosomes with subsequent vacuole formation.
While an intracellular site of VacA action in causing cell vacuolation is widely accepted (Cover, 1998; Montecucco et al., 1999; Szabo et al., 1999; McClain et al., 2000), neither the reason for the different sensitivity of cell lines to VacA nor the molecular mechanisms of VacA binding, internalization, and intracellular trafficking have been clarified.
The present study was designed to investigate, by using different cell lines, the mechanisms of VacA binding and internalization required to enable this toxin to cause vacuolation in cultured cells. We found that 1) the high sensitivity of HEp-2 cells to low doses of VacA is impaired by a cell treatment with phosphatidylinositol-specific phospholipase C (PI-PLC), 2) VacA vacuolating activity can be abolished by the cholesterol-sequestering drug nystatin, and 3) VacA internalization occurs via a clathrin-independent endocytic pathway which is sensitive to the microfilament-disrupting agent cytochalasin D (CD).
MATERIALS AND METHODS
Cell Lines
HEp-2 (from a human larynx carcinoma) and HeLa (from a human cervix carcinoma) cells were cultured in DMEM (Bio-Withaker, Verviers, Belgium) supplemented with 7% fetal calf serum (FCS, from Bio-Media, Boussens, France) and 2 mM L-glutamine (Life Technologies, Paisley, UK). BHK 21 cells (from canine kidney tubular epithelium) were grown in Glasgow minimal essential medium (Sigma Chemical, St. Louis, MO) supplemented with 5% FCS and 2 mM L-glutamine (Abrami et al., 1998). CHO (from Chinese hamster ovary) and MKN 28 (from a human gastric adenocarcinoma) cells were cultured in DMEM-F12 (1:1) (Life Technologies) with 10% FCS and 2 mM L-glutamine. All the cells were maintained at 37°C in a humidified atmosphere of 5% CO2 in air and used at 30% to 40% confluency except for 125I-VacA binding experiments where we used confluent monolayers.
Bacterial Strains
Two well-characterized VacA-producing H. pylori strains (both with a type s1a/m1 vacA genotype) were used: CCUG 17874 (from Culture Collection University of Göteborg, Göteborg, Sweden) and 60190 (ATCC 49503).
Broth Culture Filtrate Production
VacA+ broth culture filtrate (BCF) was produced as described by Ricci et al. (1996, 1997). Briefly, bacteria were grown in Brucella broth (Difco, Detroit, MI) supplemented with 1% Vitox (Oxoid, Basingstoke, UK) and 5% FCS (Life Technologies) for 24 to 36 h at 37°C under microaerophilic conditions and continuous shaking. When bacterial suspensions reached 1.2 optical density units at 450 nm (corresponding to a bacterial concentration of 5 × 108 CFU/ml), bacteria were removed by centrifugation (12,000 g for 10 min) and the supernatant sterilized by passage through a 0.22 μm cellulose acetate filter. BCF was then concentrated 50-fold by using centrifugal filter devices (Millipore, Bedford, MA). Concentrated VacA+ BCF was stored at − 20°C and used for cell intoxication. VacA prepared by this procedure does not require activation by acid/alkaline treatment.
VacA Purification, Iodination, and Activation
Purified VacA, obtained from cultures of H. pylori strain 60190 (Cover et al., 1997), was kindly provided by T.L. Cover, Nashville, TN. Purified VacA was labeled with 125I using the Iodo-Beads iodination reagent (Pierce Chemical Co., Rockford, IL) for 1 min at 4°C. The radiolabeled toxin was separated from free iodine by gel filtration on a PD10-G25 column (Pharmacia Biotech, Uppsala, Sweden). Immediately before use, purified VacA (iodinated or not) was activated by dropwise acidification to pH 3.0 with 0.2 N HCl. Each preparation of 125I-VacA was tested for vacuolating activity on HEp-2 cells. No decrease in VacA vacuolating activity was found after iodination.
Binding Assay
Binding assay was performed in duplicate on 12-well microplates at 4°C on confluent HEp-2 (1.2 × 106 cells/well) or CHO (1.6 × 106 cells/well) cell monolayers in Hanks' balanced salt solution (HBSS) in accordance with Moya et al. (1985). Displacement of cell-associated radioactivity was performed by addition (30 min before 125I-VacA) of one or other of a 50-fold excess of nonradioactive VacA, several unrelated proteins (final concentration, 8 μg/ml), and 7% FCS.
Cell sensitivity to VacA
To test the sensitivity to VacA, cells were washed twice with HBSS and then treated as follows: 1) for 1 h at 4°C with either VacA+ BCF (at dilution 1:100) or purified VacA (final concentration, 0.6 μg/ml) in HBSS and, after a triple rinsing with ice-cold HBSS, incubated for 5 h or 16 h at 37°C in HBSS containing 5 mM NH4Cl; or 2) incubated for 5 h or 16 h at 37°C in HBSS containing 5 mM NH4Cl plus either VacA+ BCF (at dilution 1:100) or purified VacA (final concentration, 0.6 μg/ml). Cell vacuolation was quantitated by means of neutral red uptake.
PI-PLC Treatment
PI-PLC (from Sigma) treatment was performed in accordance with Abrami et al. (1998). Briefly, HEp-2 cells were incubated with HBSS containing 10 μg/ml cycloheximide (C-HBSS) and 5 U/ml PI-PLC for 1 h at 37°C. After extensive washing with C-HBSS, cells were incubated with VacA+ BCF (at dilution 1:300) or purified VacA (final concentration, 0.2 μg/ml) in C-HBSS for 10 min at 37°C. After triple rinsing, cells were incubated in HBSS containing 5 mM NH4Cl for 3 h at 37°C to allow development of vacuoles. To verify the effect of PI-PLC treatment on cells previously loaded with VacA, cells were incubated with VacA+ BCF or purified VacA as above, maintained in HBSS for 2 h at 37°C, then treated with PI-PLC and incubated for 3 h at 37°C with HBSS containing 5 mM NH4Cl. Cell vacuolation was then quantitated by means of neutral red uptake, while paired monolayers were processed for electron microscopy (see below). The efficiency of PI-PLC treatment on HEp-2 cells was monitored by measuring the release of alkaline phosphatase in the cell medium at the end of PI-PLC digestion step in accordance with Murer et al. (1976).
Nystatin and CD Treatments
To test the effect of nystatin treatment, cells were preincubated for 1 h with 25 μg/ml nystatin (Sigma) dissolved in DMSO or with DMSO alone (at dilution 1:1000), then VacA+ BCF (at dilution 1:300) was added and cells were incubated for 10 min at 37°C. After triple rinsing with HBSS containing nystatin or DMSO, cells were incubated for 5 h at 37°C in HBSS/NH4Cl containing nystatin or DMSO. To verify the effect of nystatin on cells previously loaded with VacA, cells were intoxicated as above, maintained in HBSS for 2 h at 37°C, and then incubated for 5 h at 37°C with nystatin or DMSO in HBSS/NH4Cl.
To test the effect of CD treatment, HEp-2 cells were preincubated for 2 h at 37°C with 0.5 μg/ml CD (Sigma) dissolved in DMSO or with DMSO alone (at dilution 1:2000), then VacA+ BCF (at dilution 1:300) was added and cells were incubated for 10 min at 37°C. After triple rinsing with HBSS containing CD or DMSO, cells were incubated for 5 h at 37°C in HBSS/NH4Cl containing CD or DMSO. To verify the effect of CD on cells previously loaded with VacA, cells were intoxicated as above, maintained in HBSS for 2 h at 37°C, and then incubated for 5 h at 37°C with CD or DMSO in HBSS/NH4Cl.
At the end of each experiment, cell vacuolation was quantitated by means of neutral red uptake.
Neutral Red Dye Uptake Assay
The degree of cell vacuolation was quantitated by means of neutral red dye uptake in accordance with Cover et al. (1991), and was expressed as micrograms of neutral red per micrograms of cell protein (Ricci et al., 1993). Protein content of cell monolayers was measured in accordance with Lowry et al. (1951). Neutral red is an acidotropic, membrane-permeate amine that accumulates in the vacuolar lumen (Ohkuma and Poole, 1981; Cover et al., 1991). Neutral red uptake is a widely accepted in vitro assay for H. pylori-induced cell vacuolation (Cover et al., 1991, 1992; Mégraud et al., 1992; Papini et al., 1993, 1994; Ricci et al., 1993).
Electron Microscopy
HEp-2 cell monolayers, either incubated or nonincubated with VacA+ BCF, were washed twice with cacodylate buffer (0.2 M (CH3)2AsO2Na3H2O, pH 7.3 with HCl) and fixed with a freshly prepared mixture of one part 2.5% glutaraldehyde and two parts 1% osmium tetroxide in cacodylate buffer for 40 min at 4°C. Fixed monolayers were dehydrated and then embedded in Epon-Araldite mixture directly in the culture well. Uranyl-lead stained ultrathin sections were viewed with a Zeiss EM 902 electron microscope (Oberkochen, Germany).
For the ultrastructural immunolocalization of VacA, we used the colloidal gold-labeling technique as previously described (Ricci et al., 1997). Briefly, ultrathin sections were collected on 300 mesh nickel grids, washed with buffer A (0.45 M NaCl, 1% Triton X-100, 0.05 M Tris-HCl, pH 7.4), and incubated in nonimmune goat serum at room temperature for 1 h, to prevent nonspecific binding of immunoglobulins. Some sections were then incubated overnight at 4°C with polyclonal rabbit anti-VacA serum (serum 123; kindly given by T.L. Cover), diluted 1:600 in buffer B (0.45 M NaCl, 1% BSA, 0.5% sodium azide, 0.05 M Tris-HCl, pH 7.4), while other (control) sections were incubated with nonimmune or unrelated immune rabbit serum under the same conditions. After further washing in buffer B, primary immunoglobulin binding was revealed by gold-labeled goat antirabbit IgG (EM GAR 20, British BioCell, Cardiff, UK) diluted 1:20 in buffer B. The sections were stained with uranyl and lead before electron microscopy investigation. Quantitative evaluation of gold labeling and of endosomal/vacuolar and cytoplasmic areas was performed by means of an IBAS 2 image analyzer (Zeiss).
Cell Transfection
Cells were grown on coverslip and transfected using the DOTAP transfection kit (Boehringer Mannheim, Mannheim, Germany) according to the manufacturer's instructions. Cells were transfected with eukaryotic expression vectors allowing the overexpression of one or other of the following fusion proteins: 1) a dominant-negative mutant of the Eps15 protein (Edelta95/295), tagged with the green fluorescent protein (GFP) (Benmerah et al., 1999), 2) the five tandem Src-homology-3 (SH3) domains of intersectin, tagged with GFP (Simpson et al., 1999) (kindly given by P.S. McPherson, Montreal, Canada), and 3) the K44A dominant-negative mutant of dynamin II, tagged with a hemagglutinin (HA) epitope (kindly given by C. Lamaze and S. Schmid, La Jolla, CA).
To test the sensitivity to VacA in transfected cells, cells were washed as above, incubated for 10 min at 37°C with VacA+ BCF (at dilution 1:300), rinsed three times, and then incubated for 16 h at 37°C in HBSS containing 3 mM NH4Cl to allow vacuole development.
Transferrin Uptake
Endocytosis of Texas Red-conjugated transferrin (TxR-Tf) (Molecular Probes, Eugene, OR) was performed on cells grown on coverslip and variously transfected. Cells, treated with VacA+ BCF and incubated for 16 h with HBSS containing 3 mM NH4Cl as described above, were incubated at 37°C for 1 h with 25 nM TxR-Tf in HBSS containing 1 mg/ml BSA and 3 mM NH4Cl. After incubation, cells were quickly cooled to 4°C, washed three times with ice-cold PBS, and then fixed with paraformaldehyde as described below.
Immunofluorescence
After triple washing in PBS, cells grown on coverslip were fixed with 4% paraformaldehyde in PBS (pH 7.4) for 15 min. After fixation, cells were washed three times in PBS and free aldehyde groups were quenched with 50 mM NH4Cl in PBS for 10 min. After two washes with PBS, cells were washed with PBS containing 0.5% BSA and 0.5% saponin (both from Sigma) for 5 min and then incubated with primary antibody in PBS/BSA/saponin for 30 min at RT. After triple rinsing of the cells with PBS/BSA/saponin, primary antibody binding was visualized with Cy5-labeled donkey anti-rabbit (from Jackson ImmunoResearch Laboratories, West Grove, PA) or FITC-labeled goat anti-mouse (from Dako, Glostrup, Denmark) secondary antibodies diluted in PBS/BSA/saponin for 30 min at room temperature. After triple rinsing in PBS, the coverslips were mounted on glass slides in Mowiol (Calbiochem, La Jolla, CA) and analyzed by confocal microscopy. For double immunofluorescence, cells were incubated with a mixture of the two primary antibodies and then with a mixture of the two secondary antibodies. As primary antibodies, in this study we used 1) anti-Rab7 rabbit polyclonal serum (at dilution 1:200; kindly given by C. Bucci, Naples, Italy), and 2) anti-hemagglutinin mouse monoclonal antibody 12CA5 (at dilution 1:400; kindly given by E. Van Obberghen-Schilling, Nice, France).
Statistics
The statistical significance of the differences was evaluated by the Student's t test and by analysis of variance followed by Newman-Keuls' Q test
RESULTS
HEp-2 Cells Are Highly Sensitive to VacA, Whereas CHO Cells Exhibit a Low Sensitivity to the Toxin.
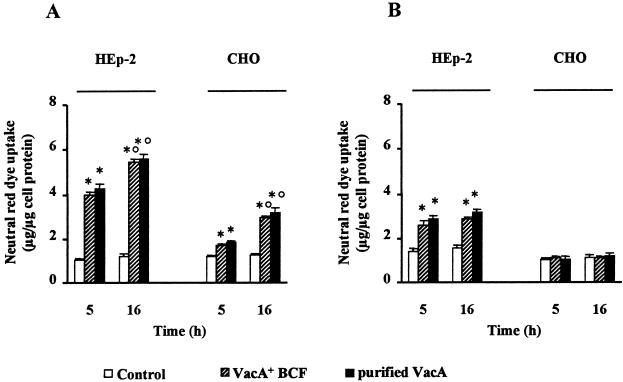
We found that HEp-2 cells exhibited a statistically significant (p < 0.05) neutral red uptake after continuous exposure to a low dose of VacA for 5 h or 16 h (Figure 1A) as well as after exposure to VacA for 1 h at 4°C followed by 5 h or 16 h incubation at 37°C in HBSS/NH4Cl to allow vacuole development (Figure 1B). After intoxication for 1 h at 4°C, the ratio between the neutral red uptake of VacA-treated and untreated control cells evaluated at 5 h was 1.85 and 2.03 for VacA+ BCF and purified VacA, respectively. These values were virtually identical to those obtained at 16 h (1.90 and 2.08, respectively). When HEp-2 cells were continuously exposed to VacA, the ratio between neutral red uptake of VacA-treated and control cells at 5 h was 3.80 and 4.04 for VacA+ BCF and purified VacA, respectively. After 16-h incubation, this ratio was slightly higher (4.50 and 4.65 for VacA+ BCF and purified VacA, respectively). MKN 28 and HeLa cells, tested in the same conditions, exhibited the same behavior shown by HEp-2 cells (not shown). CHO cells also exhibited a statistically significant (p < 0.05) neutral red uptake after continuous exposure to a low dose of VacA for several hours at 37°C (Figure 1A). However, unlike HEp-2 cells, they were completely insensitive to VacA when exposed to the toxin for 1 h at 4°C (Figure 1B). It is noteworthy that after continuous exposure to VacA for 16 h at 37°C, the ratio between neutral red uptake of VacA-treated and control cells (2.35 and 2.52 for VacA+ BCF and purified VacA, respectively) was about twice that observed after 5-h incubation (1.40 and 1.55). BHK 21 cells, tested in the same conditions, exhibited the same behavior shown by CHO cells (not shown).
Figure 1.
Neutral red uptake by HEp-2 and CHO cells variously exposed to VacA. (A) Cells were incubated for either 5 h or 16 h at 37°C with VacA+ BCF or purified VacA in HBSS containing 5 mM NH4Cl (see Methods). (B) Cells were exposed to VacA+ BCF or purified VacA in HBSS for 1 h at 4°C and then, after extensive washing, incubated for either 5 h or 16 h in HBSS containing 5 mM NH4Cl (see Methods). Controls were paired monolayers not exposed to VacA. Means ± SEM of three independent experiments. * = p < 0.05 versus paired Control. ° = p < 0.05 versus the same condition at 5 h.
A Specific Receptor for VacA Cannot Be Shown by 125I-VacA Binding Experiments
The above results raise the possibility that HEp-2 cells exhibit a specific receptor which could be lacking in CHO cells. To investigate this possibility, we studied the binding of 125I-VacA to HEp-2 cells. In time-course experiments, we found no convincing plateau for binding of 125I-VacA (concentration, 2 × 10−9 M) even after 3 h (not shown). Moreover, we found no saturation of the binding by using different concentrations of 125I-VacA (concentration range from 5 × 10−10 M to 3 × 10−8 M) (not shown). As shown in Table 1, we found a displacement of 34% of cell-associated radioactivity by using a 50-fold excess of nonradioactive VacA. Nevertheless, a comparable displacement was also obtained by using, instead of nonradioactive VacA, comparable amounts of other unrelated proteins like diphtheria toxin and cytotoxic necrotizing factor 1 from Escherichia coli (Table 1). The most effective competitor was 7% FCS, which gave a 91% displacement of cell radioactivity (Table 1). Binding experiments on CHO cells gave virtually the same behavior shown with HEp-2 cells (Table 1).
Table 1.
Displacement of 125I-VacA binding to HEp-2 or CHO cells by several competitors
| Percent of bindinga
|
||
|---|---|---|
| HEp-2 | CHO | |
| No competitorb | 100 ± 8 | 100 ± 6 |
| Nonradioactive VacAc | 66 ± 4 | 71 ± 5 |
| BSAd | 89 ± 7 | 89 ± 8 |
| Heparind | 82 ± 5 | 80 ± 6 |
| Cytotoxic necrotizing factor 1d | 56 ± 3 | 61 ± 4 |
| Diphtheria toxind | 66 ± 5 | 82 ± 7 |
| FCSe | 9 ± 1 | 10 ± 1 |
Normalized vs absence of competitor. Means ± SEM (n = 3).
Confluent cells were incubated with HBSS in the absence or presence of competitors for 30 min at 4°C and then 125I-VacA (final concentration, 2 × 10−9 M) was added for 2 min.
Final concentration, 10−8 M.
Final concentration, 8 μg/ml.
Final concentration, 7%.
PI-PLC Treatment Inhibits VacA-induced Vacuolation of HEp-2 Cells
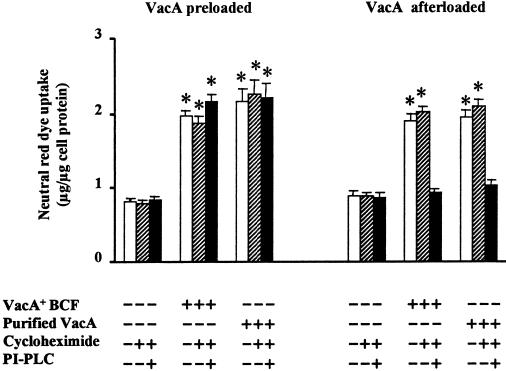
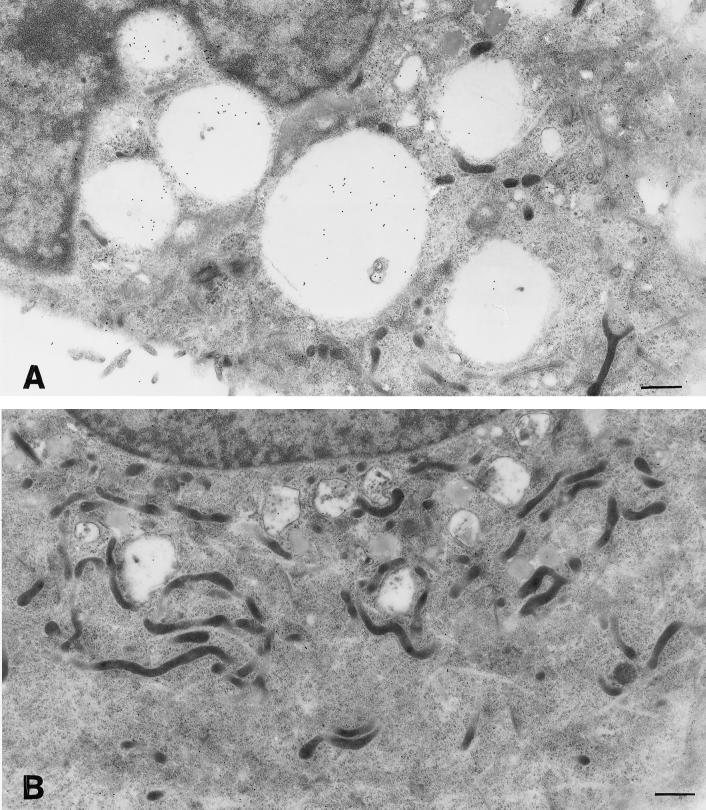
Even if binding data clearly demonstrated that most VacA molecules are probably nonspecifically adsorbed onto the cell membrane, this does not necessarily rule out the possibility that there could be a specific receptor in small amounts. In this respect, we tested whether VacA interaction with plasma membrane could exhibit some similarity with that of other bacterial channel-forming toxins like aerolysin from Aeromonas hydrophila and alpha toxin from Clostridium septicum, which are known to bind to GPI-anchored receptors that can be clustered in sphingolipid-cholesterol-rich microdomains (Abrami et al., 1998; Abrami and van der Goot, 1999; Gordon et al., 1999). To explore this possibility, we tested the effect of PI-PLC treatment (a well-established approach to remove GPI-anchored molecules from the cell surface), in the presence of cycloheximide to prevent further synthesis of GPI-anchored proteins, followed by brief exposure (10 min) of HEp-2 cells to a very low dose of VacA (both as VacA+ BCF and as purified VacA). Note that CHO cells were completely insensitive to this intoxication protocol (not shown). Figure 2 shows that PI-PLC treatment virtually abolished VacA-induced neutral red uptake in HEp-2 cells. In parallel electron microscopy investigations (Figure 3), PI-PLC treated, VacA incubated HEp-2 cells showed few dilated endosomes, only occasional cellular vacuoles, and markedly reduced intracellular endosomal/vacuolar accumulation of VacA immunoreactivity. The latter was found to be prominent in PI-PLC untreated, VacA incubated cells, mostly within cytoplasmic vacuoles and dilated endosomes (Figure 3). Quantitative analysis of 20 cell profiles (randomly selected) for each experimental condition showed that in PI-PLC untreated, VacA incubated cells the total endosomal/vacuolar area (4,060 μm2) was ∼ 45% of the total cytoplasmic area (8,971 μm2) and contained 2,240 gold grains, whereas in PI-PLC treated, VacA incubated cells the total endosomal/vacuolar area (526 μm2) was ∼ 6% of the total cytoplasmic area (8,615 μm2) and contained 163 gold grains. The mean gold particles per micrometers squared of endosomal/vacuolar area were 0.54 ± 0.08 for PI-PLC untreated, VacA incubated cells and 0.35 ± 0.07 for PI-PLC treated, VacA incubated cells (means ± SEM; p = 0.082). These findings suggest that when HEp-2 cells are exposed to very low concentrations of VacA, a GPI-anchored protein could play a pivotal role in cell binding and internalization of VacA leading to cell vacuolation. To rule out the possibility that the PI-PLC effect we observed could be due to a toxic effect or to interference with vacuole development rather than to selective cleavage of a GPI-anchored protein required for high sensitivity to VacA, we treated VacA preloaded cells with PI-PLC. Figure 2 shows that under this condition PI-PLC treatment was completely ineffective and cells exhibited the same neutral red uptake as untreated cells.
Figure 2.
Effect of PI-PLC treatment on VacA-induced vacuolation of HEp-2 cells. Neutral red uptake was measured either 1) in cells previously exposed to VacA pulse (both as VacA+ BCF and as purified VacA) and then treated or not with PI-PLC (VacA preloaded), or 2) in cells treated or not with PI-PLC and then exposed or not to VacA pulse (VacA afterloaded) (see Methods). Incubation for vacuole development: 3 h at 37°C in HBSS containing 5 mM NH4Cl. Means ± SEM of three independent experiments.* = p < 0.05 versus paired monolayers not exposed to VacA.
Figure 3.
Ultrastuctural features of HEp-2 cells treated or not with PI-PLC and then exposed to VacA. Cells untreated (A) or treated with PI-PLC (B) were incubated for 10 min with VacA+ BCF and then for 3 h with HBSS containing 5 mM NH4Cl (see Methods). Note large vacuoles filled with intense VacA immunoreactivity in A, to be compared with the small vacuoles and scarce VacA immunoreactivity in B. Aldehyde-osmium fixation, VacA immunogold, uranyl-lead counterstaining. Bars = 0.85 μm.
In addition, to rule out the possibility that our results might be biased by the specific batch or type of PI-PLC preparation used, we tested the effect of different batches of PI-PLC from Sigma and of PI-PLC from another supplier (Oxford GlycoSciences, Abingdon, UK). We found no difference in the results obtained using different Sigma batches (not shown). Using 10 U/ml PI-PLC from Oxford GlycoSciences (so as to obtain a release of alkaline phosphatase comparable with that of the Sigma preparations) we obtained a statistically significant (p < 0.05) inhibition of neutral red uptake in HEp-2 cells pretreated with PI-PLC, whereas neutral red uptake by cells preloaded with VacA and then treated with PI-PLC was virtually identical to that of cells not treated with PI-PLC (not shown). The finding that PI-PLC treatment also impaired neutral red uptake in HeLa cells exposed to very low doses of VacA (data not shown) suggests that the sensitivity to PI-PLC treatment we observed is not unique to HEp-2 cells.
To test whether PI-PLC treatment has any effect on 125I-VacA binding, we performed binding experiments on HEp-2 cells treated with PI-PLC. We found that PI-PLC treatment had no significant effect on 125I-VacA binding (not shown).
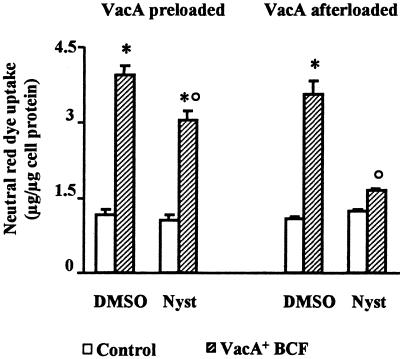
Nystatin Treatment Inhibits VacA-induced Vacuolation of HEp-2 Cells
To determine whether sphingolipid-cholesterol-rich microdomains play some role in cell intoxication by VacA, we studied VacA vacuolating activity in HEp-2 cells treated with nystatin. This drug complexes cholesterol altering structure and function of glycolipid microdomains and caveolae (Rothberg et al., 1992; Deckert et al., 1996; Skretting et al., 1999). Nystatin is reported to exhibit no effect on clathrin-coated pits, actin cables, or other membranous structures (Rothberg et al., 1992). HEp-2 cells treated with nystatin before VacA pulse exhibited no statistically significant neutral red uptake in comparison to control cells (i.e., cells not exposed to VacA pulse) (Figure 4). On the contrary, cells treated with nystatin, after VacA pulse, showed statistically significant (p < 0.05) neutral red uptake compared with control cells, even if less (p < 0.05) than that exhibited by cells preloaded with VacA and treated with DMSO alone (Figure 4).
Figure 4.
Effect of nystatin (Nyst) treatment on VacA-induced vacuolation of HEp-2 cells. Neutral red uptake was measured either 1) in cells previously exposed to VacA+ BCF pulse, or in control cells and then treated with Nyst dissolved in DMSO or with DMSO alone (VacA preloaded); or 2) in cells treated with Nyst dissolved in DMSO, or with DMSO alone and then exposed or not (Control) to VacA+ BCF pulse (VacA afterloaded) (see Methods). Incubation for vacuole development: 5 h at 37°C in HBSS containing 5 mM NH4Cl. Means = SEM of three independent experiments.* = p < 0.05 versus paired Control. ° = p < 0.05 versus the same condition but DMSO-treated.
VacA Internalization in HEp-2 Cells is Insensitive to the Inhibition of Clathrin-dependent Endocytosis, Whereas It Is Inhibited by F-actin Depolymerization
Results obtained with PI-PLC treatment suggested that a receptorial role could be played by GPI-anchored molecules, known to be internalized mainly via clathrin-independent endocytosis (Parton et al., 1994; Stahl and Mueller, 1995; Deckert et al., 1996; Skretting et al., 1999). Therefore the possibility arises that a clathrin-independent pathway of endocytosis might play a role in VacA cell uptake. To investigate this possibility, we performed transient tranfections of HEp-2 cells with vectors allowing overexpression of proteins specifically inhibiting the formation of clathrin-coated vesicles. We induced overexpression of 1) a GFP-linked dominant-negative mutant (Edelta95/295) of the Eps15, a protein recently identified as a constituent of plasma membrane clathrin-coated pits and required for the earliest steps of clathrin-dependent endocytosis (Benmerah et al., 1998, 1999). The expression of Edelta95/295 mutant protein is known to induce an inhibition of coated pit assembly at a very early step (i.e., coat protein recruitment onto the plasma membrane) causing specific inhibition of clathrin-dependent receptor-mediated endocytosis, whereas fluid phase endocytosis is not inhibited (Benmerah et al., 1999); 2) GFP-linked five tandem SH3 domains of intersectin, causing an inhibition of intermediate events leading to the formation of constricted coated pits (Simpson et al., 1999); and 3) the K44A mutant of dynamin II (HA-tagged). Overexpression of this mutant protein (Lys44→Ala) potently and specifically inhibits the clathrin-dependent endocytosis, its inhibiting action being accounted for by its deficiency in GTP binding and hydrolysis which impairs the fission of invaginated coated pits (Lamaze and Schmid, 1995).
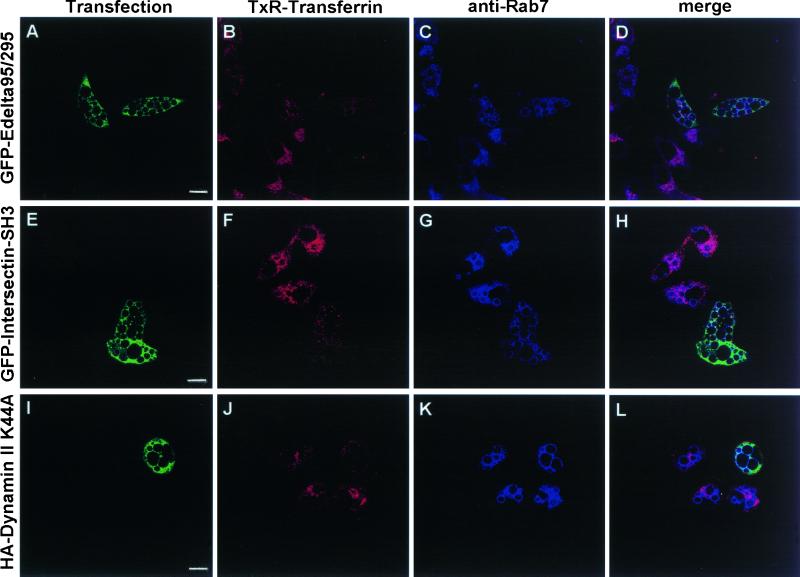
Cells overexpressing proteins able to block clathrin-dependent endocytosis exhibited virtually no uptake of TxR-Tf (a bona fide marker of this kind of endocytosis), whereas VacA-dependent cell vacuolation (evaluated as typical, Rab 7-decorated, cytoplasmic vacuoles) was virtually identical to that of the neighboring, nontransfected cells (Figure 5). These data clearly suggest that VacA internalization sufficient to give full biological activity occurred even if the clathrin-dependent pathway was inhibited.
Figure 5.
VacA-induced vacuolation in HEp-2 cells overexpressing proteins specifically inhibiting the formation of clathrin-coated vesicles. HEp-2 cells grown on coverslip were transiently transfected with vectors allowing overexpression of 1) a dominant-negative mutant of the Eps15 protein, tagged with GFP (GFP-Edelta95/295) (A-D); 2) the five tandem SH3 domains of intersectin, tagged with GFP (GFP-Intersectin-SH3) (E-H); and 3) the K44A dominant-negative mutant of dynamin II, tagged with a HA epitope (HA-Dynamin II K44A) (I-L). Transfected monolayers were exposed to VacA+ BCF pulse, incubated for 16 h at 37°C in HBSS containing 3 mM NH4Cl, and then tested for TxR-Transferrin endocytosis as described in Methods. After fixations, cells were processed for immunofluorescence to detect Rab7-positive vacuoles and, where required, HA tag. (A, E, I): transfected cells (green). (B, F, J): TxR-Transferrin (red). (C, G, K): Rab7 localization (cyan). (D, H, L): merge images showing several Rab7-positive vacuoles in transfected cells where there was virtually no uptake of TxR-Transferrin. Bars = 10 μm.
We next studied whether VacA internalization was actin-dependent by using CD, a well-known microfilament-disrupting agent. It has been reported that the actin cytoskeleton plays a role in different types of endocytic mechanisms: clathrin-dependent endocytosis (Lamaze et al., 1997), clathrin-independent endocytosis (Sandvig and van Deurs, 1990; van Deurs et al., 1995), macropinocytosis (Poussin et al., 1998), and caveolae-mediated internalization (Parton et al., 1994; Lamaze and Schmid, 1995). We found that while CD treatment was only scantly effective in cells preloaded with VacA, when cells were allowed to internalize VacA in the presence of CD there was no statistically significant neutral red uptake in comparison to control cells (Figure 6).
Figure 6.
Effect of cytochalasin D (CD) treatment on VacA-induced vacuolation of HEp-2 cells. Neutral red uptake was measured either 1) in cells previously exposed to VacA+ BCF pulse, or in control cells and then treated with CD dissolved in DMSO or with DMSO alone (VacA preloaded); or 2) in cells treated with CD dissolved in DMSO, or with DMSO alone and then exposed or not (Control) to VacA+ BCF pulse (VacA afterloaded) (see Methods). Incubation for vacuole development: 5 h at 37°C in HBSS containing 5 mM NH4Cl. Means = SEM of three independent experiments. * = p < 0.05 versus paired Control. ° = p < 0.05 versus the same condition but DMSO-treated.
DISCUSSION
Right from the first paper showing the existence of VacA (Leunk et al., 1988), it was clear that sensitivity to this toxin varies greatly among different cell lines tested, CHO cells being classified as insensitive. Further studies confirmed this cell type-specific sensitivity to VacA and proposed that it could be due to the absence or presence (with different levels of expression) of a specific plasma membrane receptor (Yahiro et al., 1997; de Bernard et al., 1998; Pagliaccia et al., 1998; Padilla et al., 2000). Interestingly, Pagliaccia et al. (1998) proposed that VacA may have two different receptor binding sites, one specific for the m1 variant and the other specific for the m2 variant. This could partly account for the different sensitivity among different cell lines tested. Differently to previous investigators, in the present study we evaluated cell sensitivity to VacA by using a single, low concentration of a single type of VacA (s1a/m1) but two different protocols of intoxication: 1) continuous exposure for several hours at 37°C so as to allow both specific and nonspecific binding and internalization; and 2) exposure for 1 h at 4°C so as to block endocytosis allowing only binding to membrane receptors (if any), followed by several hours at 37°C in the presence of NH4Cl to allow internalization of bound toxin and vacuole development. This approach enabled us to classify the cell lines tested into two groups in respect to their sensitivity to VacA. The first group was represented by cells (HEp-2, MKN 28, HeLa) with high sensitivity, defined as the capacity to develop vacuoles not only after continuous exposure to the toxin for several hours, but also after a pulse of VacA at 4°C. It is worth noting that substantial neutral red uptake can also be obtained in HEp-2 by using a 1-min pulse (V. Ricci and P. Boquet, unpublished data). On the contrary, the cell lines of the second group (CHO and BHK 21) resulted to be sensitive to VacA only when VacA was continuously present in the cell medium for several hours at 37°C. Interestingly, whereas in CHO cells there was an important difference (about two times) between neutral red uptake after 5 h and 16 h of continuous exposure to VacA, this difference was sharply reduced in HEp-2. This suggests that the intoxication kinetics for CHO cells is very slow in comparison to that of HEp-2 cells, which also exhibited a much higher amount of neutral red uptake.
A possible explanation for these data is that the high sensitivity of cell lines of the I group was due to the presence of a specific receptor which is lacking on cell lines of the II group. By using indirect immunofluorescence, flow cytometry, and immunoprecipitation, some authors presented data suggesting the existence of a specific, high-affinity cell surface receptor for VacA, although they failed to agree on its molecular characteristics (Yahiro et al., 1997, 1999; Massari et al., 1998; Seto et al., 1998; Padilla et al., 2000). However, none of these studies used the classic iodinated-ligand binding approach for the characterization of the putative receptor for VacA. Surprisingly, by using this approach, we found no evidence of a specific receptor in HEp-2 cells. The displacement achieved using an excess of nonradioactive VacA was unspecific since it was also obtained by using unrelated proteins (like diphtheria toxin and cytotoxic necrotizing factor 1) or, even more, by using 7% FCS. In addition, we found virtually the same displacement behavior in CHO cells. Our data confirm and extend recent observations (Vinion-Dubiel et al., 1999; McClain et al., 2000) that HeLa cells exhibit a high level of noncompetable (nonspecific) binding for VacA with only a small reduction in the presence of a 100-fold excess of unlabeled VacA. Our evidence of strong competition between VacA and FCS in binding experiments also fits very well with the data of de Bernard et al. (1998) showing an increased sensitivity to VacA with diminishing FCS concentration in the incubation medium, particularly relevant at low toxin concentration (< 10 nM). The previously reported binding of VacA to multiple cell-surface proteins (Yahiro et al., 1997, 1999; Seto et al., 1998) might explain, at least in part, our failure to observe saturable and specific binding for VacA. In addition, the previously reported binding of VacA to lipid membranes (Moll et al., 1995; Molinari et al., 1998; Czajkowsky et al., 1999) might have contributed to the “nonspecific” binding we observed.
In any case, the lack of evidence of a specific receptor in radioactive binding experiments does not rule out the possibility that there could be a functionally important, though quantitatively small, specific receptor in addition to the nonspecific binding. This is, for example, the case of diphtheria toxin on HeLa cells. HeLa cells are sensitive to diphtheria toxin because they have its specific receptor (now identified as heparin-binding EGF-like precursor); nevertheless, by using the 125I-toxin binding approach, no specific binding has been measured, at variance with the case of other cell lines (like Vero) which exhibit larger numbers of binding sites (Middlebrook et al., 1978; Skretting et al., 1999).
Since mounting evidence suggests a channel-forming action for VacA, an intriguing hypothesis may be that VacA interaction with plasma membrane has some similarities with that of other well-known bacterial channel-forming toxins. Aerolysin from Aeromonas hydrophila binds to GPI-anchored receptors and is clustered in sphingolipid-cholesterol-rich microdomains or “lipid rafts,” which act as concentration platforms favoring toxin oligomerization and channel formation (Abrami et al., 1998b; Abrami and van der Goot, 1999). Alpha toxin from Clostridium septicum is another channel-forming toxin which exploits GPI-anchored receptors to intoxicate cells, cells defective in GPI-anchored proteins being insensitive to low concentrations of this toxin (Gordon et al., 1999). Our findings that PI-PLC pretreatment (to remove GPI-anchored proteins from plasma membrane) inhibited VacA internalization as well as VacA-induced cell vacuolation in HEp-2 cells suggest that one or several still unidentified GPI-anchored protein(s) could play a pivotal role in cell sensitivity to VacA. Although not all GPI anchors are PI-PLC sensitive, PI-PLC treatment is widely accepted as a standard for the presence of GPI-anchored proteins (Ferguson, 1999). The fact that HEp-2 cells preloaded with VacA and then treated with PI-PLC exhibited neutral red uptake virtually identical to that of cells not treated with PI-PLC strongly supports the specificity of PI-PLC action at the level of GPI-anchored proteins required for high sensitivity to VacA. However, PI-PLC treatment had no substantial effect on 125I-VacA binding to HEp-2 cells, further demonstrating that VacA binding is for the most part nonspecific and suggesting that the number of specific receptors, if any, should be very low.
Although VacA interaction with plasma membrane seems to share some properties with other channel-forming toxins, it also exhibits some peculiarities. Aerolysin binds to a broad subset of GPI-anchored proteins, the anchor being itself an essential part of the toxin binding determinant (Diep et al., 1998; Abrami et al., 2000). Both CHO and BHK 21 cells are highly sensitive to aerolysin since they exhibit GPI-anchored proteins suitable as receptors (Abrami et al., 1998a,b). The fact that both CHO and BHK 21 cells were insensitive to a pulse of a low dose of VacA at 4°C suggests that GPI-anchored proteins with highly specific features are required for high cell sensitivity to VacA.
An increasing body of evidence suggests that GPI-anchored proteins are clustered in lipid rafts (Ferguson, 1999; Muniz and Riezman, 2000) and that lipid rafts play a pivotal role in cell intoxication by several bacterial toxins (Orlandi and Fishman, 1998; Abrami and van der Goot, 1999; Fivaz et al., 1999). In this respect, the possibility that a GPI-anchored protein could play a role as VacA receptor prompted us to verify whether alteration of structure/function of lipid rafts could inhibit VacA-induced vacuolation in HEp-2 cells. This was indeed the case. We found that nystatin, a cholesterol-binding drug known to impair both structure and function of lipid rafts and caveolae (Rothberg et al., 1992; Deckert et al., 1996; Skretting et al., 1999), induced almost complete inhibition of VacA-induced cell vacuolation in HEp-2 cells. This finding suggests that lipid rafts could play an important role in cell intoxication by VacA. However, it is noteworthy that nystatin also caused a slight, but statistically significant, inhibition of neutral red uptake in cells preloaded with VacA and then treated with the drug, suggesting that nystatin-induced alteration of lipid rafts may also interfere with vacuole genesis.
Although the fact that VacA needs to be internalized to exert its vacuolating activity is widely accepted (Garner and Cover, 1996; Ricci et al., 1997; Pagliaccia et al., 1998; Montecucco et al., 1999; Vinion-Dubiel et al., 1999; McClain et al., 2000), the mechanisms of VacA internalization are still largely unknown. The fact that high cell sensitivity to VacA depends on a GPI-anchored protein of the cell surface and that GPI-anchored molecules are known to be internalized mainly via clathrin-independent endocytosis (Parton et al., 1994; Stahl and Mueller, 1995; Deckert et al., 1996; Skretting et al., 1999) raises the possibility that clathrin-independent endocytosis may play a major role in VacA internalization. Our data show that VacA internalization sufficient to give full biological activity did occur even if the clathrin-dependent pathway was virtually completely inhibited. However these data did not rule out the possibility that VacA could simply cross the plasma membrane to penetrate directly into the cytosol where it could exert its vacuolating action (Molinari et al., 1998). Since actin cytoskeleton plays a role in different forms of vesicle-mediated internalization, our finding that pretreatment with the microfilament-disrupting agent CD blocked VacA internalization (evaluated as inhibition of VacA-induced cell vacuolation) supports a vesicle-mediated internalization of VacA. CD is known to affect both clathrin-dependent endocytosis (Lamaze et al., 1997) and clathrin-independent endocytosis (Sandvig and van Deurs, 1990; van Deurs et al., 1995) as well as macropinocytosis (Poussin et al., 1998) and caveolae-mediated internalization (Parton et al., 1994; Lamaze and Schmid, 1995). CD inhibits cytotoxicity both of ricin toxin (which exploits both clathrin-dependent and clathrin-independent endocytosis to enter cells) and of diphtheria toxin (which exploits only the clathrin-dependent pathway) (Sandvig and van Deurs, 1990; P. Boquet and V. Ricci, unpublished data). Taken together, our results suggest that clathrin-independent endocytosis plays a major role in VacA internalization. The recent observation that VacA binds to the surface of rabbit erythrocytes but is not able to enter these cells (McClain et al., 2000) further suggests that VacA internalization does not occur spontaneously following binding to membranes.
In the first paper dealing with VacA binding and internalization, Garner and Cover (1996) reported that VacA exhibits a very slow rate of internalization in comparison to other bacterial toxins like diphtheria toxin, Pseudomonas exotoxin A, and botulinum C2 toxin. Interestingly, Skretting et al. (1999) found that internalization of diphtheria toxin bound to a mutated receptor, where the transmembrane and cytoplasmic domains had been replaced by a GPI-moiety, was clathrin-independent and occurred at a low rate compared with clathrin-dependent internalization of the same toxin bound to wild-type receptors. These observations well fit with our findings of clathrin-independent internalization of VacA after binding to a GPI-anchored protein.
Nevertheless, our finding that also CHO and BHK 21 cells developed vacuoles when exposed to VacA for several hours at 37°C and the fact that the PI-PLC-induced protective action in HEp-2 cells seemed to disappear when either VacA concentration or the time of cell exposure to the toxin were increased (our unpublished results) raise the possibility that multiple binding sites and/or multiple internalization pathways might contribute to cell intoxication by VacA, especially when high doses of, or long time of exposure to, VacA are used.
Recently, Montecucco et al. (1999) proposed a model for cell intoxication by VacA. Briefly, monomeric VacA binds, via its carboxy-terminal domain, to an unidentified cell receptor and inserts into the plasma membrane via hydrophobic protein-lipid interactions. The formation of anion-selective channels results from assembling of monomers into an oligomeric structure. VacA endocytosis and transport to late endosomes could increase the anionic permeability of this compartment, which in turn would enhance the V-ATPase proton pumping activity leading, in the presence of weak bases like ammonia, to an increased accumulation of osmotically active ions (like NH4+). This leads to water influx and vesicle swelling, an essential step in vacuole formation.
Our data fit very well with Montecucco's model. A possible scenario might be the following: 1) in highly sensitive cells exposed to a low dose of the toxin, VacA monomers bind to one or several GPI-anchored protein(s); 2) subsequent interaction between VacA monomers, required for the oligomerization step and channel formation, are favored both by the high lateral mobility of GPI-anchored proteins within the phospholipid bilayer and by the capacity of GPI-anchored proteins to associate with lipid rafts; 3) lipid rafts can thus act as concentration platforms enabling VacA to concentrate locally and, therefore, to oligomerize efficiently; and 4) subsequent clathrin-independent endocytosis and transport to late endosomes of VacA could lead to vacuole development, if weak bases are present.
ACKNOWLEDGMENTS
We are deeply indebted to T.L. Cover (Nashville, TN) for helpful discussions and suggestions, and for providing us with purified VacA and anti-VacA serum. We thank E. Montini for the art work. This work was supported by grants from the Institut National de la Santé et de la Recherche Médicale (INSERM), France, and the Italian Ministry of Health to IRCCS Policlinico San Matteo. V.R. was a visiting scientist (Poste Vert INSERM) from the University of Pavia Medical School (Pavia, Italy).
Abbreviations used:
- BCF
broth culture filtrate
- CD
cytochalasin D
- C-HBSS
cycloheximide-containing HBSS
- FCS
fetal calf serum
- GFP
green fluorescent protein
- GPI
glycosylphosphatidylinositol
- HA
hemagglutinin
- HBSS
Hanks' balanced salt solution
- PI-PLC
phosphatidylinositol-specific phospholipase C
- SH3
Src-homology-3
- TxR-Tf
Texas Red-conjugated transferrin
REFERENCES
- Abrami L, Fivaz M, Decroly E, Seidah NG, Jean F, Thomas G, Leppla SH, Buckley JT, van der Goot FG. The pore-forming toxin proaerolysin is activated by furin. J Biol Chem. 1998a;273:32656–32661. doi: 10.1074/jbc.273.49.32656. [DOI] [PubMed] [Google Scholar]
- Abrami L, Fivaz M, Glauser P-E, Parton RJ, van der Goot FG. A pore-forming toxin interacts with a GPI-anchored protein and causes vacuolation of the endoplasmic reticulum. J Cell Biol. 1998b;140:525–540. doi: 10.1083/jcb.140.3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrami L, Fivaz M, van der Goot FG. Adventures of a pore-forming toxin at the target cell surface. Trends Microbiol. 2000;8:168–172. doi: 10.1016/s0966-842x(00)01722-4. [DOI] [PubMed] [Google Scholar]
- Abrami L, van der Goot FG. Plasma membrane microdomains act as concentration platforms to facilitate intoxication by aerolysin. J Cell Biol. 1999;147:175–184. doi: 10.1083/jcb.147.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherton JC. The clinical relevance of strain types of Helicobacter pylori. Gut. 1997;40:701–703. doi: 10.1136/gut.40.6.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherton JC, Cao P, Peek RM, Tummuru MKR, Blaser MJ, Cover TL. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori: association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem. 1995;270:17771–17777. doi: 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- Benmerah A, Bayrou M, Cerf-Bensussan N, Dautry-Varsat A. Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J Cell Sci. 1999;112:1303–1311. doi: 10.1242/jcs.112.9.1303. [DOI] [PubMed] [Google Scholar]
- Benmerah A, Lamaze C, Begue B, Schmid SL, Dautry-Varsat A, Cerf-Bensussan N. AP-2/Eps15 interaction is required for receptor-mediated endocytosis. J Cell Biol. 1998;140:1055–1062. doi: 10.1083/jcb.140.5.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser MJ. Helicobacter pylori: its role in disease. Clin Infect Dis. 1992;15:386–393. doi: 10.1093/clind/15.3.386. [DOI] [PubMed] [Google Scholar]
- Cave RD. How is Helicobacter pylori transmitted? Gastroenterology. 1997;113:S9–S14. doi: 10.1016/s0016-5085(97)80004-2. [DOI] [PubMed] [Google Scholar]
- Cover TL. The vacuolating cytotoxin of Helicobacter pylori. Mol Microbiol. 1996;20:241–246. doi: 10.1111/j.1365-2958.1996.tb02612.x. [DOI] [PubMed] [Google Scholar]
- Cover TL. An intracellular target for Helicobacter pylori vacuolating toxin. Trends Microbiol. 1998;6:127–128. doi: 10.1016/s0966-842x(98)01231-1. [DOI] [PubMed] [Google Scholar]
- Cover TL, Blaser MJ. Purification and characterization of the vacuolating toxin from Helicobacter pylori. J Biol Chem. 1992;267:10570–10575. [PubMed] [Google Scholar]
- Cover TL, Hanson PI, Heuser JE. Acid-induced dissociation of VacA, the Helicobacter pylori cytotoxin, reveals its pattern of assembly. J Cell Biol. 1997;138:759–769. doi: 10.1083/jcb.138.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover TL, Puryear W, Perez-Perez GI, Blaser MJ. Effect of urease on HeLa cell vacuolation induced by Helicobacter pylori cytotoxin. Infect Immun. 1991;59:1264–1270. doi: 10.1128/iai.59.4.1264-1270.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover TL, Vaughn SG, Cao P, Blaser MJ. Potentiation of Helicobacter pylori vacuolating toxin activity by nicotine and other weak bases. J Infect Dis. 1992;166:1073–1078. doi: 10.1093/infdis/166.5.1073. [DOI] [PubMed] [Google Scholar]
- Czajkowsky DM, Iwamoto H, Cover TL, Shao Z. The vacuolating toxin from Helicobacter pylori forms hexameric pores in lipid bilayers at low pH. Proc Natl Acad Sci USA. 1999;96:2001–2006. doi: 10.1073/pnas.96.5.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bernard M, Moschioni M, Papini E, Telford J, Rappuoli R, Montecucco C. Cell vacuolization induced by Helicobacter pylori VacA toxin: cell line sensitivity and quantitative estimation. Toxicol Lett. 1998;99:109–115. doi: 10.1016/s0378-4274(98)00140-4. [DOI] [PubMed] [Google Scholar]
- Deckert M, Ticchioni M, Bernard A. Endocytosis of GPI-anchored proteins in human lymphocytes: role of glycolipid-based domains, actin cytoskeleton, and protein kinases. J Cell Biol. 1996;133:791–799. doi: 10.1083/jcb.133.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diep DB, Nelson KL, Raja SM, Pleshak EN, Buckley JT. Glycosylphosphatidylinositol anchors of membrane glycoproteins are binding determinants for the channel-forming toxin aerolysin. J Biol Chem. 1998;273:2355–2360. doi: 10.1074/jbc.273.4.2355. [DOI] [PubMed] [Google Scholar]
- Ernst PB, Crowe SE, Reyes VE. How does Helicobacter pylori cause mucosal damage? The inflammatory response. Gastroenterology. 1997;113:S35–S42. doi: 10.1016/s0016-5085(97)80009-1. [DOI] [PubMed] [Google Scholar]
- Ferguson MAJ. The structure, biosynthesis and functions of glycosylphosphatidylinositol anchors, and the contributions to trypanosome research. J Cell Sci. 1999;112:2799–2809. doi: 10.1242/jcs.112.17.2799. [DOI] [PubMed] [Google Scholar]
- Fiocca R, Luinetti O, Villani L, Chiaravalli AM, Capella C, Solcia E. Epithelial cytotoxicity, immune responses, and inflammatory components of Helicobacter pylori gastritis. Scand J Gastroenterol. 1994;29(Suppl 205):11–21. [PubMed] [Google Scholar]
- Fivaz M, Abrami L, van der Goot FG. Landing on lipid rafts. Trends Cell Biol. 1999;9:212–213. doi: 10.1016/s0962-8924(99)01567-6. [DOI] [PubMed] [Google Scholar]
- Gaidarov I, Santini F, Warren RA, Keen JH. Spatial control of coated-pit dynamics in living cells. Nat Cell Biol. 1999;1:1–7. doi: 10.1038/8971. [DOI] [PubMed] [Google Scholar]
- Garner JA, Cover TL. Binding and internalization of Helicobacter pylori vacuolating cytotoxin by epithelial cells. Infect Immun. 1996;64:4197–4203. doi: 10.1128/iai.64.10.4197-4203.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiara P, Marchetti M, Blaser MJ, Tummuru MKR, Cover TL, Segal ED, Tompkins LS, Rappuoli R. Role of the Helicobacter pylori virulence factors vacuolating cytotoxin, CagA, and urease in a mouse model of disease. Infect Immun. 1995;63:4154–4160. doi: 10.1128/iai.63.10.4154-4160.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin CS, Mendall MM, Northfield TC. Helicobacter pylori infection. Lancet. 1997;349:265–269. doi: 10.1016/S0140-6736(96)07023-7. [DOI] [PubMed] [Google Scholar]
- Gordon VM, Nelson KL, Buckley JT, Stevens VL, Tweten RK, Elwood PC, Leppla SH. Clostridium septicum alpha toxin uses glycosylphosphatidylinositol-anchored protein receptors. J Biol Chem. 1999;274:27274–27280. doi: 10.1074/jbc.274.38.27274. [DOI] [PubMed] [Google Scholar]
- Lamaze C, Fujimoto LM, Yin HL, Schmid SL. The actin cytoskeleton is required for receptor-mediated endocytosis in mammalian cells. J Biol Chem. 1997;272:20332–20335. doi: 10.1074/jbc.272.33.20332. [DOI] [PubMed] [Google Scholar]
- Lamaze C, Schmid SL. The emergence of clathrin-independent pinocytic pathways. Curr Opin Cell Biol. 1995;7:573–580. doi: 10.1016/0955-0674(95)80015-8. [DOI] [PubMed] [Google Scholar]
- Leunk RD, Johnson PT, David BC, Kraft WG, Morgan DR. Cytotoxic activity in broth-culture filtrates of Campylobacter pylori. J Med Microbiol. 1988;26:93–99. doi: 10.1099/00222615-26-2-93. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Massari P, Manetti R, Burroni D, Nuti S, Norais N, Rappuoli R, Telford JL. Binding of the Helicobacter pylori vacuolating cytotoxin to target cells. Infect Immun. 1998;66:3981–3984. doi: 10.1128/iai.66.8.3981-3984.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain MS, Schraw W, Ricci V, Boquet P, Cover TL. Acid activation of Helicobacter pylori vacuolating cytotoxin (VacA) results in toxin internalization by eukaryotic cells. Mol Microbiol. 2000;37:433–442. doi: 10.1046/j.1365-2958.2000.02013.x. [DOI] [PubMed] [Google Scholar]
- Mégraud F, Neman-Simha V, Brugmann D. Further evidence of the toxic effect of ammonia produced by Helicobacter pylori urease on human epithelial cells. Infect Immun. 1992;60:1858–1863. doi: 10.1128/iai.60.5.1858-1863.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrook JL, Dorland RB, Leppla SH. Association of diphtheria toxin with Vero cells. Demonstration of a receptor. J Biol Chem. 1978;253:7325–7330. [PubMed] [Google Scholar]
- Molinari M, Galli C, de Bernard M, Norais N, Ruysschaert J-M, Rappuoli R, Montecucco C. The acid activation of Helicobacter pylori toxin VacA: structural and membrane binding studies. Biochem Biophys Res Commun. 1998;248:334–340. doi: 10.1006/bbrc.1998.8808. [DOI] [PubMed] [Google Scholar]
- Molinari M, Galli C, Norais N, Telford JL, Rappuoli R, Luzio JP, Montecucco C. Vacuoles induced by Helicobacter pylori toxin contain both late endosomal and lysosomal markers. J Biol Chem. 1997;272:25339–25344. doi: 10.1074/jbc.272.40.25339. [DOI] [PubMed] [Google Scholar]
- Moll G, Papini E, Colonna R, Burroni D, Telford J, Rappuoli R, Montecucco C. Lipid interaction of the 37-kDa and 58-kDa fragments of the Helicobacter pylori cytotoxin. Eur J Biochem. 1995;234:947–952. doi: 10.1111/j.1432-1033.1995.947_a.x. [DOI] [PubMed] [Google Scholar]
- Montecucco C, Papini E, de Bernard M, Zoratti M. Molecular and cellular activities of Helicobacter pylori pathogenic factors. FEBS Lett. 1999;452:16–21. doi: 10.1016/s0014-5793(99)00652-3. [DOI] [PubMed] [Google Scholar]
- Moya M, Dautry-Varsat A, Goud B, Louvard D, Boquet P. Inhibition of coated pit formation in HEp-2 cells blocks the cytotoxicity of diphtheria toxin but not that of ricin toxin. J Cell Biol. 1985;101:548–559. doi: 10.1083/jcb.101.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniz M, Riezman H. Intracellular transport of GPI-anchored proteins. EMBO J. 2000;19:10–15. doi: 10.1093/emboj/19.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murer H, Ammann E, Biber J, Hopfer U. The surface membrane of the small intestinal epithelial cell. I. Localization of adenyl cyclase. Biochim Biophys Acta. 1976;433:509–519. doi: 10.1016/0005-2736(76)90277-7. [DOI] [PubMed] [Google Scholar]
- NIH. Consensus Development Panel on Helicobacter pylori in Peptic Ulcer Disease. Helicobacter pylori in peptic ulcer disease. J Am Med Assoc. 1994;272:65–69. [PubMed] [Google Scholar]
- Nomura A, Stemmermann GN, Chyou P-H, Kato I, Perez-Perez GI, Blaser MJ. Helicobacter pylori infection and gastric carcinoma in a population of Japanese Americans in Hawaii. N Engl J Med. 1991;325:1132–1136. doi: 10.1056/NEJM199110173251604. [DOI] [PubMed] [Google Scholar]
- Ohkuma S, Poole B. Cytoplasmic vacuolation of mouse peritoneal macrophages and the uptake into lysosomes of weakly basic substances. J Cell Biol. 1981;90:656–664. doi: 10.1083/jcb.90.3.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlandi PA, Fishman PH. Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains. J Cell Biol. 1998;141:905–915. doi: 10.1083/jcb.141.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla PI, Wada A, Yahiro K, Kimura M, Niidome T, Aoyagi H, Kumatori A, Anami M, Hayashi T, Fujisawa J, Saito H, Moss J, Hirayama T. Morphologic differentiation of HL-60 cells is associated with appearance of RPTPβ and induction of Helicobacter pylori VacA sensitivity. J Biol Chem. 2000;275:15200–15206. doi: 10.1074/jbc.275.20.15200. [DOI] [PubMed] [Google Scholar]
- Pagliaccia C, de Bernard M, Lupetti P, Ji X, Burroni D, Cover TL, Papini E, Rappuoli R, Telford JL, Reyrat J-M. The m2 form of the Helicobacter pylori cytotoxin has cell type-specific vacuolating activity. Proc Natl Acad Sci USA. 1998;95:10212–10217. doi: 10.1073/pnas.95.17.10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papini E, Bugnoli M, de Bernard M, Figura N, Rappuoli R, Montecucco C. Bafilomycin A1 inhibits Helicobacter pylori-induced vacuolization of HeLa cells. Mol Microbiol. 1993;7:323–327. doi: 10.1111/j.1365-2958.1993.tb01123.x. [DOI] [PubMed] [Google Scholar]
- Papini E, de Bernard M, Milia E, Bugnoli M, Rappuoli R, Montecucco C. Cellular vacuoles induced by Helicobacter pylori originate from late endosomal compartments. Proc Natl Acad Sci USA. 1994;91:9720–9724. doi: 10.1073/pnas.91.21.9720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- Parton RG, Joggert B, Simons K. Regulated internalization of caveolae. J Cell Biol. 1994;127:1199–1215. doi: 10.1083/jcb.127.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelicic V, Reyrat J-M, Sartori L, Pagliaccia C, Rappuoli R, Telford JL, Montecucco C, Papini E. Helicobacter pylori VacA cytotoxin associated with the bacteria increases epithelial permeability independently of its vacuolating activity. Microbiology. 1999;145:2043–2050. doi: 10.1099/13500872-145-8-2043. [DOI] [PubMed] [Google Scholar]
- Poussin C, Foti M, Carpentier JL, Pugin J. CD14-dependent endotoxin internalization via a macropinocytic pathway. J Biol Chem. 1998;273:20285–20291. doi: 10.1074/jbc.273.32.20285. [DOI] [PubMed] [Google Scholar]
- Ricci V, Ciacci C, Zarrilli R, Sommi P, Tummuru MKR, Del Vecchio Blanco C, Bruni CB, Cover TL, Blaser MJ, Romano M. Effect of Helicobacter pylori on gastric epithelial cell migration and proliferation in vitro: role of VacA and CagA. Infect Immun. 1996;64:2829–2833. doi: 10.1128/iai.64.7.2829-2833.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci V, Sommi P, Fiocca R, Figura N, Romano M, Ivey KJ, Solcia E, Ventura U. Cytotoxicity of Helicobacter pylori on human gastric epithelial cells in vitro: role of cytotoxin(s) and ammonia. Eur J Gastroenterol Hepatol. 1993;5:687–694. [Google Scholar]
- Ricci V, Sommi P, Fiocca R, Romano M, Solcia E, Ventura U. Helicobacter pylori vacuolating toxin accumulates within the endosomal-vacuolar compartment of cultured gastric cells and potentiates the vacuolating activity of ammonia. J Pathol. 1997;183:453–459. doi: 10.1002/(SICI)1096-9896(199712)183:4<453::AID-PATH950>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Rothberg KG, Heuser JE, Donzell WC, Ying Y-S, Glenney JR, Anderson RGW. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- Sandvig K, van Deurs B. Selective modulation of the endocytic uptake of ricin and fluid phase markers without alteration in transferrin endocytosis. J Biol Chem. 1990;265:6382–6388. [PubMed] [Google Scholar]
- Seto K, Hayashi-Kuwabara Y, Yoneta T, Suda H, Tamaki H. Vacuolation induced by cytotoxin from Helicobacter pylori is mediated by the EGF receptor in HeLa cells. FEBS Lett. 1998;431:347–350. doi: 10.1016/s0014-5793(98)00788-1. [DOI] [PubMed] [Google Scholar]
- Simpson F, Hussain NK, Qualmann B, Kelly RB, Kay BK, McPherson PS, Schmid SL. SH3-domain-containing proteins function at distinct steps in clathrin-coated vesicle formation. Nat Cell Biol. 1999;1:119–124. doi: 10.1038/10091. [DOI] [PubMed] [Google Scholar]
- Skretting G, Torgersen ML, van Deurs B, Sandvig K. Endocytic mechanisms responsible for uptake of GPI-linked diphtheria toxin receptor. J Cell Sci. 1999;112:3899–3909. doi: 10.1242/jcs.112.22.3899. [DOI] [PubMed] [Google Scholar]
- Sommi P, Ricci V, Fiocca R, Necchi V, Romano M, Telford JL, Solcia E, Ventura U. Persistence of Helicobacter pylori VacA toxin and vacuolating potential in cultured gastric epithelial cells. Am J Physiol. 1998;275:G681–G688. doi: 10.1152/ajpgi.1998.275.4.G681. [DOI] [PubMed] [Google Scholar]
- Stahl A, Mueller BM. The urokinase-type plasminogen activator receptor, a GPI-linked protein, is localized in caveolae. J Cell Biol. 1995;129:335–344. doi: 10.1083/jcb.129.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo I, Brutsche S, Tombola F, Moschioni M, Satin B, Telford JL, Rappuoli R, Montecucco C, Papini E, Zoratti M. Formation of anion-selective channels in the cell plasma membrane by the toxin VacA of Helicobacter pylori is required for its biological activity. EMBO J. 1999;18:5517–5527. doi: 10.1093/emboj/18.20.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DN, Blaser MJ. The epidemiology of Helicobacter pylori infections. Epidemiol Rev. 1991;13:42–59. doi: 10.1093/oxfordjournals.epirev.a036078. [DOI] [PubMed] [Google Scholar]
- Telford JL, Covacci A, Ghiara P, Montecucco C, Rappuoli R. Unraveling the pathogenic role of Helicobacter pylori in peptic ulcer: potential new therapies and vaccine. Trends Biotechnol. 1994a;12:420–426. doi: 10.1016/0167-7799(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Telford JL, Ghiara P, Dell'Orco M, Comanducci M, Burroni D, Bugnoli M, Tecce MF, Censini S, Covacci C. Gene structure of the Helicobacter pylori cytotoxin and evidence of its key role in gastric disease. J Exp Med. 1994b;179:1653–1658. doi: 10.1084/jem.179.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombola F, Carlesso C, Szabo I, de Bernard M, Reyrat JM, Telford JL, Rappuoli R, Montecucco C, Papini E, Zoratti M. Helicobacter pylori vacuolating toxin forms anion-selective channels in planar lipid bilayers: possible implications for the mechanism of cellular vacuolation. Biophys J. 1999;76:1401–1409. doi: 10.1016/S0006-3495(99)77301-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricottet V, Bruneval P, Vire O, Camilleri JP, Bloch F, Bonte N, Roge J. Campylobacter-like organisms and surface epithelium abnormalities in active, chronic gastritis in humans: an ultrastructural study. Ultrastruct Pathol. 1986;10:113–122. doi: 10.3109/01913128609014587. [DOI] [PubMed] [Google Scholar]
- van Deurs B, Holm PK, Kayser L, Sandvig K. Delivery to lysosomes in the human carcinoma cell line HEp-2 involves an actin filament-facilitated fusion between mature endosomes and preexisting lysosomes. Eur J Cell Biol. 1995;66:309–323. [PubMed] [Google Scholar]
- Vinion-Dubiel AD, McClain MS, Czajkowsky DM, Iwamoto H, Ye D, Cao P, Schraw W, Szabo G, Blanke SR, Shao Z, Cover TL. A. dominant negative mutant of Helicobacter pylori vacuolating toxin (VacA) inhibits VacA-induced cell vacuolation. J Biol Chem. 1999;274:37736–37742. doi: 10.1074/jbc.274.53.37736. [DOI] [PubMed] [Google Scholar]
- WHO. Schistosomes, liver flukes and Helicobacter pylori. IARC Monogr Eval Carcinog Risk Hum. 1994;61:177–240. [PMC free article] [PubMed] [Google Scholar]
- Yahiro K, Niidome T, Hatakeyama T, Aoyagi H, Kurazono H, Padilla PI, Wada A, Hirayama T. Helicobacter pylori vacuolating cytotoxin binds to the 140-kDa protein in human gastric cancer cell lines, AZ-521 and AGS. Biochem Biophys Res Commun. 1997;238:629–632. doi: 10.1006/bbrc.1997.7345. [DOI] [PubMed] [Google Scholar]
- Yahiro K, Niidome T, Kimura M, Hatakeyama T, Aoyagi H, Kurazono H, Imagawa K, Wada A, Moss J, Hirayama T. Activation of Helicobacter pylori VacA toxin by alkaline or acid conditions increases its binding to a 250-kDa receptor protein-tyrosine phosphatase. J Biol Chem. 1999;274:36693–36699. doi: 10.1074/jbc.274.51.36693. [DOI] [PubMed] [Google Scholar]
- Zarrilli R, Ricci V, Romano M. Molecular response of gastric epithelial cells to Helicobacter pylori-induced cell damage. Cell Microbiol. 1999;1:93–99. doi: 10.1046/j.1462-5822.1999.00018.x. [DOI] [PubMed] [Google Scholar]