Abstract
Posttranslational modifications of p53 induced by two widely used anticancer agents, cisplatinum (DDP) and taxol were investigated in two human cancer cell lines. Although both drugs were able to induce phosphorylation at serine 20 (Ser20), only DDP treatment induced p53 phosphorylation at serine 15 (Ser15). Moreover, both drug treatments were able to increase p53 levels and consequently the transcription of waf1 and mdm-2 genes, although DDP treatment resulted in a stronger inducer of both genes. Using two ataxia telangiectasia mutated (ATM) cell lines, the role of ATM in drug-induced p53 phosphorylations was investigated. No differences in drug-induced p53 phosphorylation could be observed, indicating that ATM is not the kinase involved in these phosphorylation events. In addition, inhibition of DNA-dependent protein kinase activity by wortmannin did not abolish p53 phosphorylation at Ser15 and Ser20, again indicating that DNA-PK is unlikely to be the kinase involved. After both taxol and DDP treatments, an activation of hCHK2 was found and this is likely to be responsible for phosphorylation at Ser20. In contrast, only DDP was able to activate ATR, which is the candidate kinase for phosphorylation of Ser15 by this drug. This data clearly suggests that differential mechanisms are involved in phosphorylation and activation of p53 depending on the drug type.
Keywords: phosphorylation, cell cycle, checkpoints, anticancer agents, p53
Introduction
The product of the tumor suppressor gene p53 plays a crucial role in determining cellular response to various stress conditions [1–4]. In cancer cells, p53 has been reported to modulate the cytotoxicity of some drugs either by increasing or decreasing their activity. Tumor cell lines expressing wild-type (wt) p53 were reported to be more sensitive to cisdichloro-diammine platinum (DDP) and other anticancer drugs and less sensitive to tubulin interactive drugs such as taxanes than cells not expressing p53 or expressing mutated p53 [5,6]. The results of the screening program at the NCI on 60 cell lines reported that taxol had a tendency to show higher activity in cells not expressing p53 compared to those expressing p53 [7]. Opposite results were obtained with DDP [7], although recently an increased DDP cytotoxicity was found to be associated with loss of p53 function [8].
Taxol and DDP are two widely used anticancer agents that have different mechanisms of action; DDP reacts with DNA inducing inter-, intrastrand crosslinks [9], whereas taxol binds to tubulin, causing an inhibition of its depolymerization [10]. However, both compounds are able to activate p53 in different cell systems to a comparable extent [11–13]. For both drugs, it has been shown that increased p53 levels are able to activate at least one of the downstream targets of p53, the WAF1 gene encoding for p21 [11,13].
The pathway that leads to p53 activation after damage is complex and many proteins have been shown to be involved in this process [14–16]. It appears that different kinds of damage, particularly at the DNA level, use different “sensors” to obtain p53 activation [17–19]. The proteins reported to play a role in signalling mechanisms resulting in p53 activation following DNA damage include DNA-dependent protein kinase (DNA-PK), ataxia telangiectasia mutated (ATM), ATM-Rad3 related (ATR), hCHK1, hCHK2, and histone acetyl transferases [19–30]. Whereas the former protein kinases have been shown to specifically phosphorylate p53 at its amino or carboxy terminus, the latter histone acetyl transferases have been demonstrated to acetylate the p53 carboxy terminus [26,27]. Recent evidence suggests that after DNA damage, both the amino and carboxy terminus of p53 can be modified by phosphorylations and acetylations [17,18,24–26] and that these posttranslation modifications seem to be specifically associated with the kind of damage produced. Moreover, it appears that these posttranslational modifications of p53 could represent a crucial step in determining the specificity of downstream gene activation and thus be an important determinant in the decision between p53-induced cell cycle arrest or apoptosis.
Because DDP and taxol are very active anticancer drugs with different mechanisms of action, it was of interest to investigate if activation of different kinases induced different posttranslational modifications of p53. In this article, taxol and DDP induced accumulation of p53 in human ovarian cancer cells and different patterns of p53 phosphorylation are reported. The ability of p53 to bind DNA and to activate downstream genes has also been evaluated.
Materials and Methods
Cells and Drugs
The human ovarian cancer cell line A2780 was grown in RPMI1640 supplemented with 10% FCS. The human colocarcinoma cell line HCT-116 was maintained in Iscove's modified Dulbecco medium, supplemented with 10% FCS. The Epstein-Barr virus transformed lymphoblastoid cell lines IARC1663 (normal cell line), AT11 and AT13 (AT homozygote cell lines) were kindly provided by Dr. J. Hill (IARC, Lyon, France) and cultured in RPMI 1640 medium supplemented with 20% heat-inactivated FCS. DDP and taxol (Bristol-Myers Squibb, Syracuse, NY, USA) were dissolved in medium immediately before use; acetyl-Leu-Leu-norleucinal (LLnL) (Sigma, Milan, Italy) was dissolved just before use. Wortmannin (Sigma, Milan, Italy) was stored in DMSO at -20°C and diluted in medium just before use. DDP and taxol concentration inhibiting the growth by 50% (IC50) in the two different cell lines were determined by growth inhibition assay (data not shown).
Western Blotting Analysis
Cell extracts were prepared by lysing cells in 50 mM Tris-HCI pH 7.4, 250 mM NaCI, 0.1% Nonidet np-40, 5 mM EDTA, 50 mM NaF in the presence of aprotinin, leupeptine, and PMSF as proteases inhibitors, for 30 minutes on ice. Insoluble material was pelleted at 13,000xg for 10 minutes at 4°C and the protein concentration was determined using a Biorad assay kit (BioRad, Milan, Italy). Forty micrograms of total cellular proteins was separated on SDS-PAGE and electrotransferred to nitrocellulose. Immunoblotting was carried out with anti-p53 monoclonal antibodies (DO-1, Santa Cruz Biotechnology, Santa Cruz, CA), anti-p21, and anti-DNA-PK polyclonal antibodies (Santa Cruz Biotechnology), anti-mdm-2 and anti-ATR polyclonal antibodies (Calbiochem, Milan, Italy), and the polyclonal anti-hCHK2 antibody kindly provided by Dr. M. Foiani (University of Milan, Italy). Antibody binding was revealed by peroxidase secondary antibodies and visualised using enhanced chemiluminescence (ECL) (Amersham, Milan, Italy).
Immunoblot Analysis of p53 Phosphorylation
Antibodies specifically recognizing phosphorylated p53 at Ser15 and Ser20 were generated as previously described [20]. Different replicates of blots obtained as described above were incubated with these specific antibodies and the presence of phosphorylated p53 revealed with the ECL (Amersham). The blots were re-probed with anti-p53 antibodies (DO-1) to verify the presence of p53 protein.
Nucleic Extracts and EMSA
Cells (106) were lysed in ice in buffer containing 10 mM Hepes pH7.9, 10 mM KCI, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, 0.5 mM PMSF, and 0.8% Nonidet NP-40. Nuclei were pelleted, extracted for 1 hour in extraction buffer (20 mM Hepes pH 7.9, 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 1 mM PMSF), and cleared by centrifugation at 12,000xg for 15 minutes. Ten micrograms of nucleic extracts was incubated on ice for 1 hour in 15 µl of buffer containing 20 mM Hepes pH 7.5, 100 mM NaCI, 1 mM MgCl2, 0.1% NP40, 5% sucrose, 1 µg poly(dldC), 2 µl of pAb421 hybridoma supernatant, and 1 ng of 32P-end-labeled oligonucleotide CON (containing the p53 binding site 5′-GGACATGCCCGGGCATGTCG-3′) or p21 (containing the P53 binding site 5′-CAACATGTTGGGACATGTTC-3′). DNA-protein complexes were separated by electrophoresis through a 5% native polyacrylamide gel, dried, and visualized.
DNA-PK and ATR Activities
Cell extracts for DNA-PK activity were obtained according to procedures described previously [31]. DNA-PK activity was assayed using a kit according to the manufacturer (SigmaTECT DNA-PK Assay System, Promega, Madison, Wl) with the modifications described by Woo et al. [23]. Assays were performed in triplicate and the mean values±SD are shown.
ATR activity was determined after immunoprecipitation with anti-ATR antibody as recently described [32] using recombinant PHAS-I (Stratagene, La Jolla, CA) in the presence of 32P-ATP, resolved in SDS-PAGE, and autoradiographed.
Results
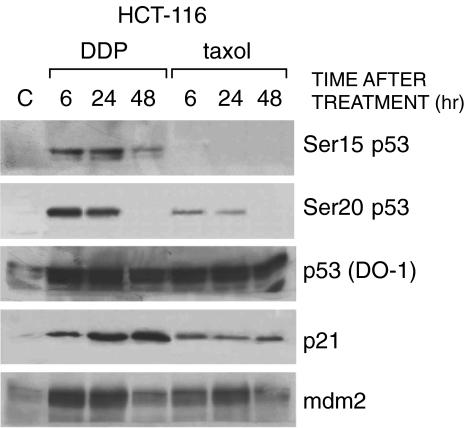
The human colocarcinoma cell line HCT-116 and the human ovarian carcinoma cell line A2780, both expressing wt p53, were treated for 24 hours with concentrations of taxol and DDP close to their respective IC50. Total cell extracts were then taken after 6, 24, and 48 hours of incubation in drug-free medium. A typical Western blot analysis, performed in HCT-116 cells, is reported in Figure 1A Both drugs induced an increase in the levels of p53. A marked phosphorylation at Ser15 of p53 was observed after DDP treatment but not after taxol treatment. Both drugs were able to induce phosphorylation at Ser20.
Figure 1.
Western blot analysis in HCT-116 cells treated with DDP or taxol. Extracts were obtained at different times after treatment. Blots were hybridized with antibodies recognizing p53 phosphorylated at Ser15, Ser20, p53 (DO-1), p21, and mdm-2.
Tests were then performed to see whether the different phosphorylations of p53 induced by DDP and taxol could result in a differential activation of downstream genes. Levels of p21 and mdm-2 proteins following treatment with the two drugs were tested by Western blotting. Both DDP and taxol induced an increase in the levels of p21 and mdm-2 in HCT-116 cells, with DDP giving a higher p21 signal at 24 and 48 hours compared to taxol.
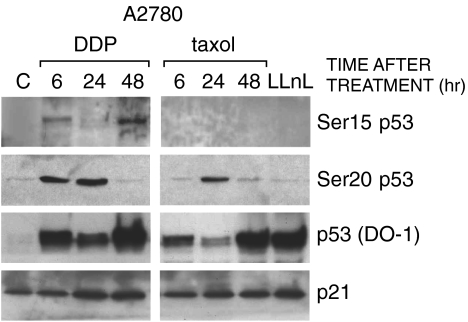
These findings were then extended to another cell system, the human ovarian cancer cell line A2780. Again, Ser15 of p53 was clearly phosphorylated after treatment with DDP but not after taxol treatment, under conditions in which a comparable level of phosphorylation at Ser20 and increased p53 levels were found after both drug treatments (Figure 2). A2780 cells were treated with the proteosome inhibitor LLnL, which stabilizes p53 protein levels by inhibiting degradation. In these conditions, no bands could be observed after incubation with both Ser15 and Ser20 phosphoantibodies, despite p53 amounts comparable to those induced after DDP or taxol treatment. As in the HCT-116 cell line, increased levels of p21 were observed after treatment with both drugs, and again DDP treatment induced a greater accumulation; mdm-2 protein could not be detected in this cell line by Western blot analysis, even after immunoprecipitation followed by Western blot analysis (data not shown).
Figure 2.
Western blot analysis in A2780 cells treated with DDP or taxol. Experimental conditions were as reported in Figure 1. Cells were also treated with 50 µM of LLnL for 3 hours and cellular extracts taken at the end of treatment.
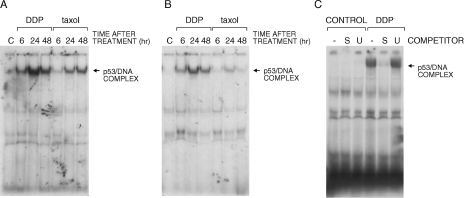
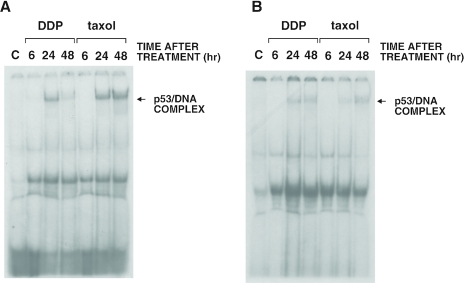
Using the same treatment conditions, we further analyzed the ability of p53 to bind DNA in gel shift experiments using two different oligonucleotides containing the p53 binding site as probes. With both the CON and p21 oligonucleotides, a retarded band was observed from extracts of HCT-116 cells treated with DDP or taxol (Figure 3A and B). This band was observable after the addition of the monoclonal antibody against p53 pAb421 and designated as p53/DNA complex. This complex was competed by an excess of unlabeled CON or p21 oligonucleotide but not with an excess of an unrelated oligonucleotide (Figure 3C). The binding of p53 to DNA was observed in extracts from both DDP- and taxol-treated cells, although a stronger band was observed with both the CON and p21 oligonucleotides when using extracts from DDP-treated cells (Figure 3A and B). When testing A2780 extracts from control and DDP- or taxol-treated cells, a retarded band was observed, although the overall complex seemed less than observed when using HCT-116-treated cell extracts (Figure 4A and B).
Figure 3.
Gel shift assay with extracts obtained from HCT-116 cells treated with DDP or taxol and performed with two different oligonucleotides (CON, panel A and p21, panel B), both containing a p53 binding site. Panel C reports the analysis with the CON oligonucleotide performed in the presence of a 50-fold molar excess of unlabeled specific (S) or unspecific (U) oligonucleotide.
Figure 4.
Gel shift assay with extracts obtained from human ovarian cancer A2780 cells treated with DDP or taxol. Experiments were performed with two different oligonucleotides (CON, panel A, and p21, panel B).
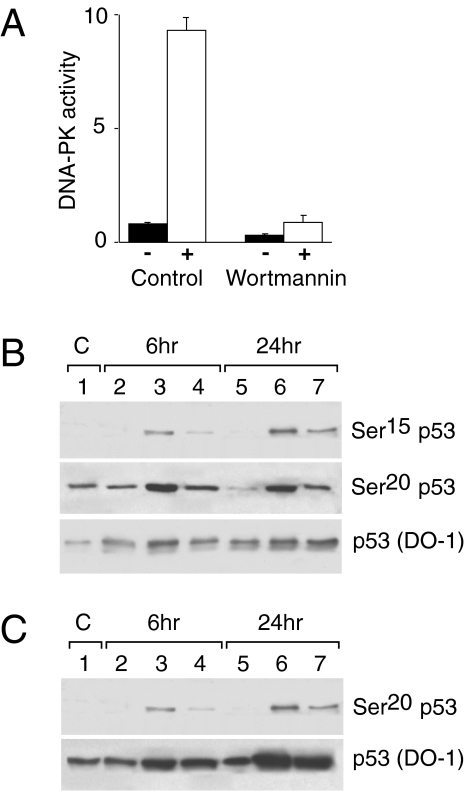
To gain insights into the pathways leading to treatment-induced phosphorylation at Ser15 and Ser20 of p53, we investigated the possible role of the kinases reportedly involved in these phosphorylation events. Possible candidates are the phosphoinositide 3-kinase-related kinases (PIKs) (DNA-PK, ATM, ATR) hCHK1 and hCHK2, all proteins involved in cell cycle checkpoints and cellular response to DNA damage [33–35]. We treated HCT-116 cell lines with wortmannin, a well-known inhibitor of the PIKs, as described in the Materials and Methods section. Despite the almost complete inhibition of DNA-PK activity in cellular extracts (Figure 5, panel A), phosphorylation at Ser15, although quantitatively slightly lower, could be observed after the concomitant treatment of DDP and wortmannin (Figure 5, panel B). Under the same treatment conditions, Ser20 phosphorylation after DDP and taxol treatment was reduced but still present.
Figure 5.
HCT-116 cells were pretreated with wortmannin 35.5 µM for two hours before treatment with DDP or taxol. At the end of DDP or taxol treatment cells were washed in PBS and medium containing wortmannin (35.5 µM) was added. Panel A: Extracts for DNA-PK activity were obtained 8 hours after treatment with and without wortmannin and processed as described in Materials and Methods. Values are the means±S.E. of pmolATP incorporated per minute per microgram protein in the biotinylated substrate in the absence (black columns) or in the presence of double-strand DNA (white columns). Panel B: Total cellular extracts were taken at 6 and 24 hours after treatment with wortmannin (lanes 2 and 5), with DDP (lanes 3 and 6), and with wortmannin and DDP (lanes 4 and 7). Blots were hybridized with antibodies recognizing p53 phosphorylated at Ser15, Ser20 and p53 (DO-1). Panel C: Total cellular extracts were taken at 6 and 24 hours after treatment with wortmannin (lanes 2 and 5), with taxol (lanes 3 and 6), and with wortmannin and taxol (lanes 4 and 7). Blots were hybridized with antibodies recognizing p53 phosphorylated at Ser20 and p53 (DO-1).
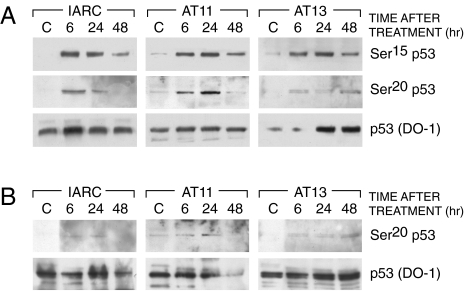
We then asked whether ATM-defective cells were able to phosphorylate p53 at Ser15 in response to DDP treatment. Normal B lymphoblasts (IARC1663) and ataxia telangiectasia (AT) lymphoblasts (AT11 and AT13 cell lines) were treated with 10 µM of DDP for two hours, a dose close to their IC50S. In response to DDP, p53 was phosphorylated at Ser15 both in normal and AT cell lines; this event was correlated with the induction of p53 (Figure 6). Similarly, phosphorylation at Ser20 was observable, either in wt IARC1663 cells or in AT-defective cells after both DDP and taxol treatment.
Figure 6.
Western blot analysis in IARC1663 and AT cell lines (AT11 and AT13) treated with DDP (Panel A) or taxol (Panel B). Extracts were taken at different times after treatment. Blots were hybridized with antibodies recognizing p53 phosphorylated at Ser15, Ser20 and p53 (DO-1).
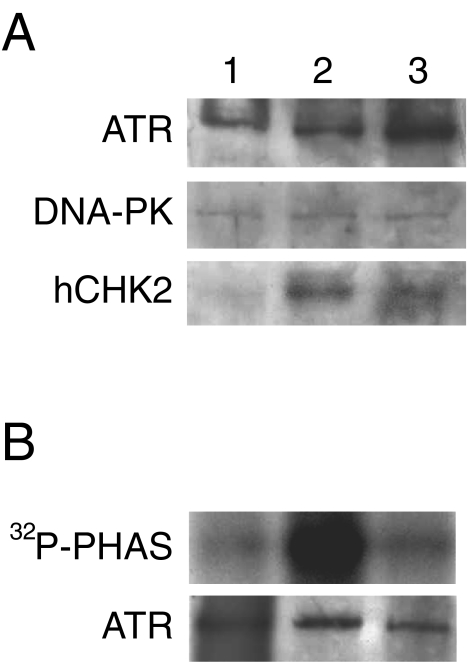
We finally investigated the effect of DDP and taxol on the levels and/or the activity of the kinases involved in Ser15 and Ser20 phosphorylation of p53. Figure 7A reports the Western blot analysis of HCT-116 cells treated with taxol or DDP. Whereas the levels of ATR and DNA-PK did not change after treatment with either drug, only DDP was able to induce ATR kinase activity (Figure 7B). Both DDP and taxol induced activation of hCHK2, as shown by a change in its intensity and electrophoretic migration (Figure 7A).
Figure 7.
Panel A: Western blotting analysis in HCT-116 cells untreated (lane 1) and treated with DDP (lane 2) or taxol (lane 3). Extracts were obtained 24 hours after treatment. Panel B: Activation of ATR kinase activity by DDP (lane 2) but not by taxol (lane 3) 24 hours after treatment in HCT-116 cells (lane 1 reports untreated cells). ATP, was immunoprecipitated and kinase activity was assessed using PHAS substrate as described in the Materials and Methods section. The amounts of ATP, present in each reaction were determined by Western blot and are shown at the bottom of the figure.
Discussion
p53 is a critical mediator of cellular response to different stress conditions [1,2]. Even through p53's role in mediating cellular sensitivity and resistance to anticancer agents has been extensively studied, the results obtained so far in different cell systems are still quite controversial. This is particularly true for two widely used and effective anticancer agents, DDP and taxol. The cellular targets of DDP and taxol are very different, DNA for the former and tubulin for the latter [9,10]. Besides their different mechanisms of action, both drugs have been shown not only to activate p53 in different systems to a comparable level, but also to activate p53 downstream genes such as p21 and bax.
The amount of data regarding the signal transduction pathways leading to activation of p53 after cellular damage is increasing. It is now clear that posttranslational modification of p53, either phosphorylation or acetylation at its amino and carboxy-terminal domains are important for the activation of p53 as a transcriptional factor [20,36–38]. These posttranslational modifications are thought to play a crucial role in determining the specificity of the p53 downstream genes activated and could represent an important determinant of the cellular decision between the p53-dependent cell cycle arrest or apoptosis. Interestingly enough, it seems that different types of damage utilize different sensors to achieve activation of p53. For example, the signalling mechanisms to p53 after IR damage are ATM dependent, but not after UV damage [24,25].
In the present study we focused on the Ser15 and Ser20 phosphorylation of p53 after treatment with two different anticancer agents DDP and taxol. Ser15 has been the first p53 site shown to be phosphorylated after DNA damage and was reported to be important for inhibition of p53 degradation by mdm-2 [19,20]. Ser20 phosphorylation has been reported to directly affect the binding between p53 and mdm-2 [39].
As already described for IR and UV damage, it was also found that treatment with DDP is able to activate a kinase that phosphorylates Ser15, whereas treatment with taxol is not. The reason why taxol failed to induce Ser15 phosphorylation in two different cellular systems is most likely due to its mechanism of action. This mechanism, not involving direct DNA damage (as in the case of DDP) is therefore not able to activate the putative kinases (particularly ATR) involved in the phosphorylation at this site. Ser15 of p53 has, in fact, been shown to be phosphorylated in vitro by the DNA-PK, ATM, and ATR kinases, all belonging to the PI3K family [33,40,41]. ATM is unlikely to be the kinase activated by DDP as judged by the Ser15 phosphorylation obtained in AT cell lines. Similarly, for DNA-PK there was evidence that, in spite of an almost complete inhibition of its activity by wortmannin, phosphorylation of Ser15 could be observed.
The results show that ATR kinase activity is clearly enhanced by DDP but not by taxol and this supports that the Ser15 phosphorylation is induced by DDP but not by taxol. These data are in concordance with recent observations that ATR effectively phosphorylates Ser15 of p53 in vivo after DNA damage [33,42].
Both DDP and taxol are able to induce phosphorylation of Ser20, which likely is responsible for the release of mdm-2 from p53 and the subsequent p53 stabilization observed after treatment with both drugs. The kinase responsible for the phosphorylation of Ser20 in vivo has been recently characterized as the human homologue of the yeast cdsl/chk2 (hCHK2).
When checked whether the different p53 phosphorylations induced by DDP and taxol could result in different activations of p53 downstream genes, it was found that both drugs are able to induce increased levels of p21 and that DDP treatment was a better p21 inducer than taxol. The results show that both drugs are able to activate hCHK2, which phosphorylates p53 at Ser20, an event necessary for p53 stabilization [28–30], but that only DDP is able to activate ATR and to induce Ser15 phosphorylation. This is likely to be responsible for the stronger p21 downstream activation induced by DDP, because Ser15 phosphorylation has been reported to stabilize p53/DNA binding [43,44].
Although it is plausible that, in vivo, different kinases could participate in phosphorylation of p53 (particularly at the sites investigated here) and that it could therefore be difficult to identify a single kinase activated by a stress signal, knowledge of the different signal transduction pathways and mechanisms of p53 activation used by anticancer agents with different mechanisms of action might help in designing not only critical and specific modulators of p53 activity, but also in identifying new therapeutic targets.
Acknowledgements
The generous contributions of the Italian Association for Cancer Research (AIRC) and of the Nerina and Mario Mattioli Foundation are gratefully acknowledged. Faina Vikhanskaya is a visiting scientist from Institute of Cytology (Russian Academy of Sciences), St. Petersburg, Russia. We would like to thank A. Levine for the generous gift of pAb421 antibody.
Abbreviations
- AT
ataxia telangiectasia
- ATM
ataxia telangiectasia mutated
- ATR
ATM-Rad3 related
- DDP
cis-dichloro-diammineplatinum
- DNA-PK
DNA-dependent protein kinase
- Ser15
serine 15
- Ser20
serine 20
- IC50
concentration inhibiting the growth by 50%
- PI3Ks
phosphoinositide 3-kinase related kinases
- wt
wild type
References
- 1.KoL J, Prives C. p53: puzzle and paradigm. GenesDev. 1996;56:2649–2654. doi: 10.1101/gad.10.9.1054. [DOI] [PubMed] [Google Scholar]
- 2.Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 3.Chen X, Ko LJ, Jayaraman L, Prives C. p53 levels, functional domains, and DNA damage determine the extent of the apoptotic response of tumor cells. Genes Dev. 1996;10:2438–2451. doi: 10.1101/gad.10.19.2438. [DOI] [PubMed] [Google Scholar]
- 4.Ullrich SJ, Anderson CW, Mercer WE, Appella E. The p53 tumor suppressor protein, a modulator of cell proliferation. J Biol Chem. 1992;267:15259–15262. [PubMed] [Google Scholar]
- 5.Wahl AF, Donaldson KL, Fairchild C, Lee FY, Foster SA, Demers GW, Galloway DA. Loss of normal p53 function confers sensitization to Taxol by increasing G2/M arrest and apoptosis. Nat Med. 1996;2:72–79. doi: 10.1038/nm0196-72. [DOI] [PubMed] [Google Scholar]
- 6.Vikhanskaya F, Vignati S, Beccaglia P, Ottoboni C, Russo P, D'Incalci M, Broggini M. Inactivation of p53 in a human ovarian cancer cell line increases the sensitivity to paclitaxel by inducing G2/M arrest and apoptosis. Exp Cell Res. 1998;241:96–101. doi: 10.1006/excr.1998.4018. [DOI] [PubMed] [Google Scholar]
- 7.O'Connor PM, Jackman J, Bae I, Myers TG, Fan S, Mutoh M, Scudiero DA, Sausville EA, Weinstein JN, Friend S, et al. Characterization of the p53 tumor suppressor pathway in cell lines of the National Cancer Institute anticancer drug screen and correlations with the growth-inhibitory potency of 123 anticancer agents. Cancer Res. 1997;57:4285–4300. [PubMed] [Google Scholar]
- 8.Fan S, Smith ML, Rivet DJ, Duba D, Zhan Q, Kohn KW, Fornace AJJ, O'Connor PM. Disruption of p53 function sensitizes breast cancer MCF-7 cells to cisplatin and pentoxifylline. Cancer Res. 1995;55:1649–1654. [PubMed] [Google Scholar]
- 9.Eastman A. Characterization of the adducts produced in DNA by cis-diamminedichloroplatinum (II) and cis-dichloro(ethylenediamine)platinum. Biochemistry. 1983;22:3927–3933. doi: 10.1021/bi00285a031. [DOI] [PubMed] [Google Scholar]
- 10.Horwitz SB. Mechanism of action of taxol. Trends Pharmacol Sci. 1992;13:134–136. doi: 10.1016/0165-6147(92)90048-b. [DOI] [PubMed] [Google Scholar]
- 11.Debernardis D, Sire EG, De Feudis P, Vikhanskaya F, Valenti M, Russo P, Parodi S, D'Incalci M, Broggini M. p53 status does not affect sensitivity of human ovarian cancer cell lines to paclitaxel. Cancer Res. 1997;57:870–874. [PubMed] [Google Scholar]
- 12.Tishler RB, Lamppu DM, Park S, Price BD. Microtubule-active drugs taxol, vinblastine, and nocodazole increase the levels of transcriptionally active p53. Cancer Res. 1995;55:6021–6025. [PubMed] [Google Scholar]
- 13.De Feudis P, Debernardis D, Beccaglia P, Valenti M, Graniela Siré EA, Arzani D, Stanzione S, Parodi S, D'Incalci M, Russo P, et al. DDP-induced cytotoxicity is not influenced by p53 in nine human ovarian cancer cell lines with different p53 status. Br J Cancer. 1997;7B:474–479. doi: 10.1038/bjc.1997.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meek DW. Multisite phosphorylation and the integration of stress signals at p53. Cell Signal. 1998;10:159–166. doi: 10.1016/s0898-6568(97)00119-8. [DOI] [PubMed] [Google Scholar]
- 15.Lane D. Awakening angels. Nature. 1998;394:616–617. doi: 10.1038/29166. [DOI] [PubMed] [Google Scholar]
- 16.Giaccia AJ, Kastan MB. The complexity of p53 modulation: emerging patterns from divergent signals. Genes Dev. 1998;12:2973–2983. doi: 10.1101/gad.12.19.2973. [DOI] [PubMed] [Google Scholar]
- 17.Kapoor M, Lozano G. Functional activation of p53 via phosphorylation following DNA damage by UV but not gamma radiation. Proc Natl Acad Sci USA. 1998;95:2834–2837. doi: 10.1073/pnas.95.6.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu H, Taya Y, Ikeda M, Levine AJ. Ultraviolet radiation, but not gamma radiation or etoposide-induced DNA damage, results in the phosphorylation of the murine p53 protein at serine-389. Proc Natl Acad Sci USA. 1998;95:6399–6402. doi: 10.1073/pnas.95.11.6399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siliciano JD, Canman CE, Taya Y, Sakaguchi K, Appella E, Kastan MB. DNA damage induces phosphorylation of the amino terminus of p53. Genes Dev. 1997;11:3471–3481. doi: 10.1101/gad.11.24.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shieh SY, Ikeda M, Taya Y, Prives C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell. 1997;91:325–334. doi: 10.1016/s0092-8674(00)80416-x. [DOI] [PubMed] [Google Scholar]
- 21.Meek DW, Campbell LE, Jardine LJ, Knippschild U, McKendrick L, Milne DM. Multi-site phosphorylation of p53 by protein kinases inducible by p53 and DNA damage. Biochem Soc Trans. 1997;25:416–419. doi: 10.1042/bst0250416. [DOI] [PubMed] [Google Scholar]
- 22.Anderson CW. DNA damage and the DNA-activated protein kinase. Trends Biochem Sci. 1993;18:433–437. doi: 10.1016/0968-0004(93)90144-c. [DOI] [PubMed] [Google Scholar]
- 23.Woo RA, McLure KG, Lees-Miller SP, Rancourt DE, Lee PW. DNA-dependent protein kinase acts upstream of p53 in response to DNA damage. Nature. 1998;394:700–704. doi: 10.1038/29343. [DOI] [PubMed] [Google Scholar]
- 24.Banin S, Moyal L, Shieh S, Taya Y, Anderson CW, Chessa L, Smorodinsky Nl, Prives C, Reiss Y, Shiloh Y, et al. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science. 1998;281:1674–1677. doi: 10.1126/science.281.5383.1674. [DOI] [PubMed] [Google Scholar]
- 25.Canman CE, Lim DS, Cimprich KA, Taya Y, Tamai K, Sakaguchi K, Appella E, Kastan MB, Siliciano JD. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science. 1998;281:1677–1679. doi: 10.1126/science.281.5383.1677. [DOI] [PubMed] [Google Scholar]
- 26.Liu L, Scolnick DM, Trievel RC, Zhang HB, Marmorstein R, Halazonetis TD, Berger SL. p53 sites acetylated in vitro by PCAF and p300 are acetylated in vivo in response to DNA damage. Mol Cell Biol. 1999;19:1202–1209. doi: 10.1128/mcb.19.2.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gu W, Roeder RG. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90:595–606. doi: 10.1016/s0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- 28.Hirao A, Kong Y-Y, Matsuoka S, Wakeham A, Ruland J, Yoshida H, Liu D, Elledge SJ, Mak TW. DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science. 2000;287:1824–1827. doi: 10.1126/science.287.5459.1824. [DOI] [PubMed] [Google Scholar]
- 29.Shieh SY, Ahn J, Tamai K, Taya Y, Prives C. The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Genes Dev. 2000;14:289–300. [PMC free article] [PubMed] [Google Scholar]
- 30.Chehab NH, Malikzay A, Appel M, Halazonetis TD. Chk2/hCdsl functions as a DNA damage checkpoint in G1 by stabilizing p53. Genes Dev. 2000;14:278–288. [PMC free article] [PubMed] [Google Scholar]
- 31.Allalunis-Turner MJ, Lintott LG, Barron GM, Day RS, Lees-Miller SP. Lack of correlation between DNA-dependent protein kinase activity and tumor cell radiosensitivity. Cancer Res. 1995;55:5200–5202. [PubMed] [Google Scholar]
- 32.Sarkaria JN, Tibbetts RS, Busby EC, Kennedy AP, Hill DE, Abraham RT. Inhibition of phosphoinositide 3-kinase related kinases by the radiosensitizing agent wortmannin. Cancer Res. 1998;58:4375–4382. [PubMed] [Google Scholar]
- 33.Tibbetts RS, Brumbaugh KM, Williams JM, Sarkaria JN, Cliby WA, Shieh SY, Taya Y, Prives C, Abraham RT. A role for ATR in the DNA damage-induced phosphorylation of p53. Genes Dev. 1999;13:152–157. doi: 10.1101/gad.13.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Westphal CH. Cell-cycle signaling: ATM displays its many talents. Curr Biol. 1997;7:R789–R792. doi: 10.1016/s0960-9822(06)00406-4. [DOI] [PubMed] [Google Scholar]
- 35.Smith GCM, Jackson SP. The DNA-dependent protein kinase. Genes Dev. 1999;13:916–934. doi: 10.1101/gad.13.8.916. [DOI] [PubMed] [Google Scholar]
- 36.Sakaguchi K, Sakamoto H, Lewis MS, Anderson CW, Erickson JW, Appella E, Xie D. Phosphorylation of serine 392 stabilizes the tetramer formation of tumor suppressor protein p53. Biochemistry. 1997;36:10117–10124. doi: 10.1021/bi970759w. [DOI] [PubMed] [Google Scholar]
- 37.Sakaguchi K, Herrera JE, Saito S, Miki T, Bustin M, Vassilev A, Anderson CW, Appella E. DNA damage activates p53 through a phosphorylation-acetylation cascade. Genes Dev. 1998;12:2831–2841. doi: 10.1101/gad.12.18.2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waterman MJ, Stavridi ES, Waterman JL, Halazonetis TD. ATM-dependent activation of p53 involves dephosphorylation and association with 14-3-3 proteins. Nat Genet. 1998;19:175–178. doi: 10.1038/542. [DOI] [PubMed] [Google Scholar]
- 39.Chehab NH, Malikzay A, Stavridi ES, Halazonetis TD. Phosphorylation of Ser-20 mediates stabilization of human p53 in response to DNA damage. Proc Natl Acad Sci USA. 1999;96:13777–13782. doi: 10.1073/pnas.96.24.13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lees-Miller SP, Sakaguchi K, Ullrich SJ, Appella E, Anderson CW. Human DNA-activated protein kinase phosphorylates serines 15 and 37 in the amino-terminal transactivation domain of human p53. Mol Cell Biol. 1992;12:5041–5049. doi: 10.1128/mcb.12.11.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khanna KK, Keating KE, Kozlov S, Scott S, Gatei M, Hobson K, Taya Y, Gabrielli B, Chan D, Lees-Miller SP, et al. ATM associates with and phosphorylates p53: mapping the region of interaction. Nat Genet. 1998;20:398–400. doi: 10.1038/3882. [DOI] [PubMed] [Google Scholar]
- 42.Prives C. Signaling to p53: breaking the MDM2-p53 circuit. Cell. 1998;95:5–8. doi: 10.1016/s0092-8674(00)81774-2. [DOI] [PubMed] [Google Scholar]
- 43.Lambert PF, Kashanchi F, Radonovich MF, Shiekhattar R, Brady JN. Phosphorylation of p53 serine 15 increases interaction with CBP. J Biol Chem. 1998;273:33048–33053. doi: 10.1074/jbc.273.49.33048. [DOI] [PubMed] [Google Scholar]
- 44.Dumaz N, Meek DW. Serine15 phosphorylation stimulates p53 transactivation but does not directly influence interaction with HDM2. EMBO J. 1999;18:7002–7010. doi: 10.1093/emboj/18.24.7002. [DOI] [PMC free article] [PubMed] [Google Scholar]