Abstract
Antibody levels specific for capsular polysaccharides of Streptococcus pneumoniae and Haemophilus influenzae type b (Hib) and for tetanus toxoid were measured in serum samples of 386 age-stratified subjects. The study group consists of healthy adult blood donors and hospitalized children undergoing elective surgery, excluding individuals with a history of infection. In children, anti-tetanus toxoid antibody levels displayed two peaks of 1.20 IU/ml (20.4 mg/liter) and 1.65 IU/ml (28.1 mg/liter) related to the schedule of routine childhood immunization in the first year and at 8 years of age. Eighty percent of the antibodies are of the immunoglobulin G1 (IgG1) isotype. For pneumococcal capsular polysaccharide (PCP), the specific antibody levels represent the acquisition of natural immunity. The initial concentration of 9.2 mg/liter was low in infancy (0.5 to 1 years of age) and remained low until 3 to 4 years of age (14.6 mg/liter). During this period PCP antibodies were almost 100% of the IgG2 subclass. Thereafter, IgG anti-PCP antibody titers increased steadily to adult levels (59.5 mg/liter). The data are intended to provide reference ranges to aid in the interpretation of specific antibody determinations in the clinical setting.
Serum-specific antibody levels are widely used as indicators of immune competence (25, 31). Interpretation of the results may be difficult. Although patients with congenital immunodeficiencies, such as common variable immunodeficiency (1, 7), selective immunoglobulin G (IgG) subclass deficiency (2, 8), and selective antibody deficiency with normal immunoglobulins (2), often have low levels of serum antibodies, many subjects with normal immune function also have low levels of serum-specific antibodies. A physiological delay of the immune response, especially to polysaccharide capsular antigens, a lack of immunization and, in addition, a decrease in specific antibody titers with time (11, 18) determine the outcome of serum-specific antibody measurements. Furthermore, in recent years the routine vaccination schedule has changed. In particular, Haemophilus influenzae type b (Hib) conjugate was introduced (36). Moreover, routine enzyme-linked immunosorbent assay (ELISA) test kits became available that allow specific antibodies to be assigned to IgG subclasses. The present study was designed to establish the pattern of specific antibody responses to polysaccharide and protein antigens in a large cohort of healthy subjects from Germany.
MATERIALS AND METHODS
Subjects.
The subjects of the present study were 313 clinically healthy children (214 males and 99 females) from 6 months to 18 years of age who were admitted to the hospital for minor surgery. Informed consent was obtained from the parents. The adult subjects were 73 healthy blood donors (36 males and 37 females) ranging from 20 to 61 years of age. Only subjects who were free of recurrent infections or inflammation, as assessed by a standardized questionnaire, and whose C-reactive protein concentrations were within the normal limit were included in the study. Peripheral venous blood was drawn after an overnight fast; sera were separated by centrifugation and then stored in aliquots at −20°C until use. In addition, the numbers of immunizations with tetanus toxoid, Hib conjugate, or pneumococcal polysaccharide were recorded.
ELISA.
For the measurement of specific antibodies, commercially available test kits (The Binding Site, Heidelberg, Germany) with precoated microtiter plates were used according to the manufacturer's instructions. Throughout the procedures pyrogen-free water was used to avoid nonspecific background binding (21).
Tetanus toxoid.
The antigen coupled to the microtiter plate was tetanus toxoid from Clostridium tetani.
For calibration, prediluted human sera at concentrations of 7.00, 2.33, 0.78, 0.26, 0.09, 0.03, and 0.01 IU/ml were used. These sera themselves were calibrated against the human tetanus anti-toxin 76/589 obtained from the National Institute for Biological Standards and Control, Potters Bar, United Kingdom. Wells were incubated for 30 min with prediluted (1/100) test sera or calibrators, washed three times, and subsequently incubated with affinity-purified sheep anti-human IgG or anti-human IgG1 antibody coupled to horseradish peroxidase. The optical density generated after incubation of the wells with H2O2 and 3,3′,5,5′-tetramethylbenzidine for 10 min and sulfonic acid was measured in a spectrophotometer at 450 nm. The concentrations of IgG or IgG1 tetanus toxoid-specific antibodies were determined from the calibration curve. Conversion of IU to milligrams was done by using the following formula: 1 IU/ml = 17 mg/liter.
Hib.
The antigen used for detection of Hib capsular polysaccharide-specific antibodies was composed of Hib polyribosyslribitolphosphate oligosaccharides conjugated to human serum albumin. Standard sera were calibrated against human anti-H. influenzae capsular protein polysaccharide (serum lot 1983) obtained from the U.S. Food and Drug Administration. The samples were prediluted 1/50. Affinity-purified sheep anti-human IgG coupled to horseradish peroxidase was used detect bound antibodies as described above.
Pneumococcus capsular polysaccharide.
The antigen used in the assay for pneumococcus-specific antibodies was composed of a mixture of capsular polysaccharide serotypes 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19F, 19A, 20, 22F, 23F, and 33F, which is the same composition as that of the licensed 23-valent vaccines. Standard sera were calibrated against human affinity-purified antipneumococcal capsular polysaccharide. Interfering antibodies were absorbed by prediluting the serum (1/100) in a buffer containing pneumococcal cellular wall antigen. After incubation for 30 min and three washing steps, affinity-purified sheep anti-human IgG or anti-human IgG2 antibody coupled to horseradish peroxidase was added. After a washing step, 100 μl in H2O2 and 3,3′,5,5′-tetramethylbenzidine substrate buffer was added to all wells. Absorbance readings and antibody concentrations were determined as described above.
Statistics.
Antibody titers did not conform to a Gaussian distribution. Therefore, values were log transformed. The geometric mean values and normal ranges (3rd and 97th percentile) were determined. Comparisons between males and females in each age group were accomplished by a Student t test of the log-transformed data.
RESULTS
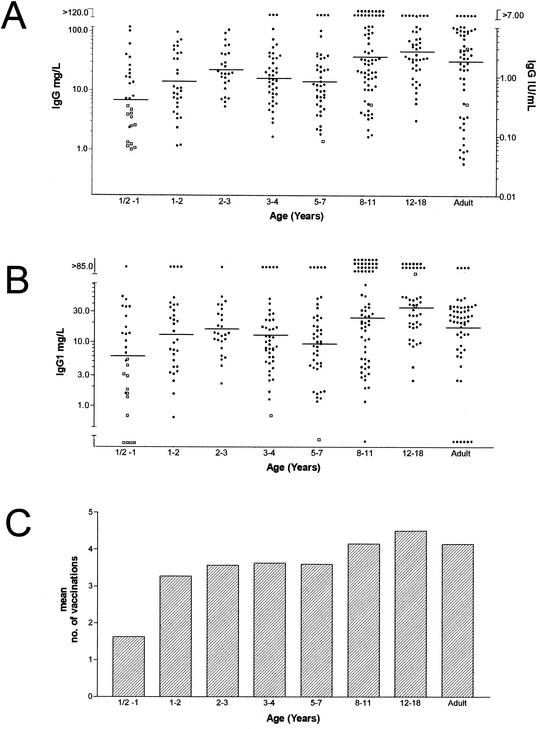
The anti-tetanus toxoid titers are shown in Fig. 1A, whereas the range of values obtained is detailed in Table 1. Two peaks of anti-tetanus toxoid antibody levels, 1.20 IU/ml (20.4 mg/liter) in children at 2 years of age and 1.65 IU/ml (28.1 mg/liter) in children at 8 years of age, were seen. The minimum protective level is 0.15 IU/ml (32). This means that 14% of the adult sera tested had levels below this limit. About 80% of the antibodies are of the IgG1 isotype in all age groups (Fig. 1B). Figure 1C indicates the number of tetanus vaccinations.
FIG. 1.
Specific IgG (A) and IgG1 (B) anti-tetanus toxoid antibody concentrations as determined by ELISA in age-stratified sera and number of vaccinations with tetanus toxoid (C). The closed circles (•) denote values obtained from vaccinated individuals, and the open boxes (□) indicate values obtained from unvaccinated individuals. In panel A, the left y axis refers to results expressed as milligrams/liter, and the right y axis to results expressed as IU/milliliter.
TABLE 1.
Antibody titers (geometric means) and ranges with respect to agea
| IgG type | Mean antibody titer (range) at age:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 0.5-1 yr (n = 35) | 1-2 yr (n = 30) | 2-3 yr (n = 33) | 3-4 yr (n = 45) | 4-8 yr (n = 83) | 8-12 yr (n = 56) | 12-18 yr (n = 31) | Adult (n = 73) | |
| TT IgG (IU/ml) | 0.44 (0.02-3.12) | 0.69 (0.04-3.92) | 1.20 (0.16-7.87) | 0.98 (0.11-7.79) | 0.80 (0.09-12.87) | 1.65 (0.28-18.78) | 2.30 (0.26-15.44) | 1.24 (0.05-39.62) |
| TT IgG (mg/liter) | 7.48 (0.34-53.0) | 11.73 (0.68-66.6) | 20.4 (2.72-133.8) | 16.66 (1.87-132.4) | 13.6 (1.53-218.8) | 28.05 (4.76-319.3) | 39.1 (4.42-262.5) | 21.08 (0.85-673.5) |
| TT IgG1 (mg/liter) | 6.6 (0.3-126.4) | 17.1 (1.4-208.0) | 12.9 (1.7-99.8) | 11.5 (1.2-108.7) | 14.3 (0.9-228.5) | 29.2 (2.6-323.5) | 29.8 (4.9-180.0) | 13.2 (0.7-258.1) |
| Hib IgG (mg/liter) | 0.84 (0.08-9.2) | 2.54 (0.16-40.8) | 3.06 (0.22-42.8) | 2.05 (0.19-21.6) | 2.08 (0.15-29.5) | 2.48 (0.16-37.2) | 1.90 (0.10-34.5) | 1.32 (0.09-19.5) |
| PCP IgG (mg/liter) | 9.2 (0.9-93.0) | 4.6 (0.9-29.2) | 12.3 (1.4-110.4) | 14.6 (0.8-262.1) | 45.5 (9.2-225.9) | 59.5 (11.0-320.8) | 65.9 (8.7-502.6) | 43.8 (10.0-191.2) |
| PCP IgG2 (mg/liter) | 7.6 (0.5-117.3) | 6.5 (0.5-87.0) | 13.1 (1.2-142.8) | 11.7 (1.2-113.4) | 9.7 (0.8-122.4) | 11.6 (1.2-107.1) | 11.4 (1.9-69.2) | 20.5 (4.7-89.4) |
The IgG concentrations for tetanus toxoid in mg/liter were calculated based on 1 IU/ml = 17 mg/liter. Values in parentheses represent normal ranges (3rd and 97th percentiles). TT, tetanus toxoid; Hib, H. influenzae capsule polysaccharide; PCP, pneumococcus capsule polysaccharide; n, number of sera.
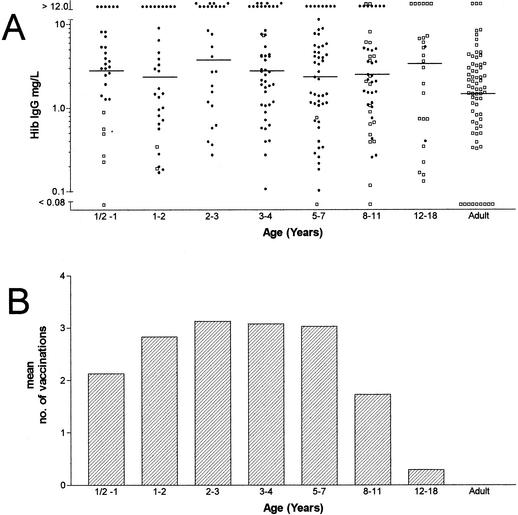
The acquisition of anti-Hib polysaccharide antibody can be seen in Fig. 2A. The antibody concentrations showed a level of ca. 2.5 mg/liter for the vaccinated pediatric population. The unimmunized infants below 1 year of age displayed concentrations of anti-Hib polysaccharide antibodies that were <1.0 mg/ml. For the adults, the geometric mean level was 1.28 mg/liter. Thus, in the adult group, 34% of individuals studied had anti-Hib PRP antibody concentrations that were <1.0 mg/liter, and 12% were found to have levels of <0.15 μg/liter. Figure 2B shows the number of vaccinations with Hib conjugate vaccine.
FIG. 2.
Specific IgG anti-H. influenzae antibody concentrations as determined by ELISA in age-stratified sera (A) and number of vaccinations with conjugated Hib vaccine (B). Closed circles (•) indicate values obtained from vaccinated individuals, and open boxes (□) denote values obtained from unvaccinated individuals.
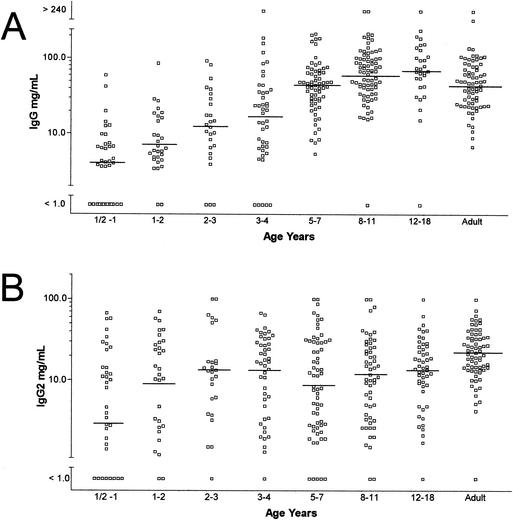
The distribution of IgG anti-PCP antibody concentrations in relation to age is shown in Fig. 3A. Geometric mean concentrations were low at age 6 months to 1 year (9.2 mg/liter) and increased steadily to reach adult levels (59.5 mg/liter) at age 8 to 11 years. Anti-PCP antibodies of the IgG2 isotype increased with age (Fig. 3B). Plateau levels were reached by 3 years of age (13.1 mg/liter) and maintained thereafter. In the young children the antipneumococcal antibodies belonged to the IgG2 subclass, whereas thereafter the proportion of IgG2 antipneumococcal antibodies declines to values of as low as 20%.
FIG. 3.
Specific IgG (A) and IgG2 (B) antipneumococcal capsular polysaccharide antibody concentrations as determined by ELISA in age-stratified sera.
Geometric mean concentrations and the putative normal ranges for each of the assays are shown in Table 1. For all specific antibodies measured there were no differences between male and female participants.
DISCUSSION
Measurement of specific antibodies is an indispensable part of the immunological assessment of patients with suspected immunodeficiency (5, 25). These measurements can be accomplished with modern, commercially available ELISAs. However, reference ranges are not readily available. The present study was designed to establish the pattern of specific antibody responses to polysaccharide and protein antigens in a large cohort of age-stratified healthy subjects.
The results have to be interpreted with regard to the use of the antigen as vaccines for primary prophylaxis and with regard to the nature of the immunological response elicited by the antigen. Tetanus toxoid is widely used as a vaccine. Two peaks in anti-tetanus toxoid antibody levels were seen in children of 1.20 and 1.65 IU/ml, values corresponding to the schedule of routine childhood immunization in the second year and at 9 years of age (36). In the adult group, there were a considerable number of individuals who displayed titers below the protective threshold of 0.15 IU/ml (32). Approximately 80% of the total anti-tetanus toxoid activity was IgG1, as described before (28). The ability to respond to antigenic challenge is dependent upon both host and antigen factors (1, 9, 14, 25). To elicit an antibody response to tetanus toxoid, both cellular and humoral arms of the immune system are involved. The cellular pathway requires antigen processing and presentation, recognition of foreign antigen in association with major histocompatibility complex molecules by T cells, and the synthesis of cytokines. B-cell activation requires antigen recognition by the appropriate B-cell clones, cell division, and differentiation into plasma and memory cells driven by the presence of antigen, cytokines, and T cells. The inability to respond adequately to booster vaccination suggests an immune deficiency that may occur at several different sites in the pathway leading to antibody production (14, 17, 25). Patients with common variable immunodeficiency were found to respond poorly to tetanus toxoid secondary to defects in T-cell activation and antigen recognition (10, 35). Although serum-specific antibodies are markers of the humoral arm of the immune response, defects in cellular immunity may be reflected by low antibody titers.
Both Hib and Pneumococcus spp. possess a polysaccharide capsule that impairs opsonization and phagocytosis and is responsible for the virulence of the organisms (23). The ability to produce antibodies to polysaccharide antigens has been correlated with the appearance of a subset of CD21+ B lymphocytes in the splenic marginal zone, which is not seen before the third year of life (26). This physiological inability to produce antibodies to capsular polysaccharide antigens is associated with an increased risk of infection with encapsulated bacteria. Yet in our study the few unimmunized infants below 1 year of age displayed a low but detectable concentration of anti-Hib polysaccharide antibodies. It is unlikely that these results are due to a low specificity of the assay since precautions were taken to avoid factors lowering assay specificity as endotoxin contamination of reagents or a low dilution of the serum samples (20, 21). The specificity of the assay is stressed by the finding of a considerable proportion of unimmunized adults with low unprotective anti-Hib antibody concentrations. The low but presumably protective titers found in the unimmunized infants might be explained by persisting maternal antibodies on the one hand and emerging cross-reactive protective antibodies on the other hand (19). In contrast to the native Hib polysaccharide, a protein-conjugated Hib vaccine behaves physiologically like proteins such as tetanus toxoid (29) and confers an effective protection against Hib to infants. A protein-conjugated Hib vaccine was introduced in Germany in 1990 (36). As with tetanus toxoid, there was a good correlation between the number of vaccinations and the level of Hib-specific antibodies. Nearly all of the children exhibit a protective titer of ≥0.15 mg/liter, whereas a considerable number of the unvaccinated adults displayed low, unprotective titers. The production of antibodies to conjugated Hib vaccines in patients with common variable immunodeficiency is undetectable (27). In contrast, some patients diagnosed with more selective immunodeficiencies, such as the selective deficiency of IgG2, particularly in the young, may respond to the conjugated vaccine but not to the polysaccharide capsule itself (15, 16).
In contrast to H. influenzae, there is at present no routine vaccination against Streptococcus pneumoniae in Germany. Since the assay described here detects 23 serotypes of pneumococcal capsular polysaccharides, the results reflect the natural acquisition of antipneumococcal immunity. However, a value in the normal range in our assay does not necessarily indicate an effective protection against every given strain of S. pneumoniae. First, there are 90 pneumococcal serotypes identified thus far (4). Second, it is known from vaccination studies that the response to the pneumococcal serotypes is heterogeneous between individuals even of the same age group (34) and third our assay might detect nonopsonic antibodies. The main proportion of nonopsonic and hence nonprotective antibodies are directed against the cell wall of S. pneumoniae (6). In our assay, preincubation of the serum eliminates the cell wall-specific antibodies by absorption. However, it was recently demonstrated that the capsular polysaccharides of different serotypes share common epitopes which induce nonprotective antibodies. These antibodies can be excluded by preabsorption with one of the polysaccharides, for instance, serotype 22F (6, 33). However, there is strong evidence that the epitopes responsible for a cross-reaction between capsular polysaccharides of different serotypes are also carbohydrate antigens in nature (6). Given the purpose of our assay to discriminate patients who are able to respond to carbohydrate antigens from those with an immunodeficiency, the limitations mentioned above will not restrict the usefulness of the assay.
In accordance with previously published data (3, 12, 13, 30) IgG pneumococcal capsular polysaccharide-specific antibody levels show a slight decline during the first year of life, presumably due to the loss of maternal antibodies. They remained low until 3 to 4 years. Interestingly, in this age group antipneumococcal antibodies belong to the IgG2 subclass, whereas thereafter the proportion of IgG2 antipneumococcal antibodies declines to values as low as 20%. The reason for this observation is not clear; it cannot be excluded, however, that activation requirements and thus the secreted IgG isotype of long-lived memory B cells differs from naive B cells (24).
The calculated normal ranges allow individuals who have very low levels to be identified for further study. However, a significant number of healthy individuals have low levels of specific antibodies. Vaccination should be considered in individuals with low levels of specific antibodies. In normal individuals, specific antibodies should increase by at least threefold in response to the polyvalent pure pneumococcal vaccine and the protein-conjugated Hib vaccine (27) and 20-fold in response to tetanus toxoid (22).
Acknowledgments
We thank C. Süsal, Institut für Immunologie, Universität Heidelberg, for collecting blood from healthy blood donors.
This work was supported by The Binding Site.
REFERENCES
- 1.Anonymous. 1999. Primary immunodeficiency diseases. Report of an IUIS Scientific Committee. Int. Union Immunol. Soc. Clin. Exp. Immunol. 118(Suppl. 1):1-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambrosino, D. M., D. T. Umetsu, G. R. Siber, G. Howie, T. A. Goularte, R. Michaels, P. Martin, P. H. Schur, J. Noyes, G. Schiffman, et al. 1988. Selective defect in the antibody response to Haemophilus influenzae type b in children with recurrent infections and normal serum IgG subclass levels. J. Allergy Clin. Immunol. 81:1175-1179. [DOI] [PubMed] [Google Scholar]
- 3.Austrian, R. 1989. Pneumococcal polysaccharide vaccines. Rev. Infect. Dis. 11(Suppl. 3):S598-S602. [DOI] [PubMed] [Google Scholar]
- 4.Butler, J. C., R. F. Breiman, H. B. Lipman, J. Hofmann, and R. R. Facklam. 1995. Serotype distribution of Streptococcus pneumoniae infections among preschool children in the United States, 1978-1994: implications for development of a conjugate vaccine. J. Infect. Dis. 171:885-889. [DOI] [PubMed] [Google Scholar]
- 5.Chapel, H. M. 1994. Consensus on diagnosis and management of primary antibody deficiencies. BMJ 308:581-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Concepcion, N. F., and C. E. Frasch. 2001. Pneumococcal type 22F polysaccharide absorption improves the specificity of a pneumococcal-polysaccharide enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 8:266-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunningham-Rundles, C. 1989. Clinical and immunologic analyses of 103 patients with common variable immunodeficiency. J. Clin. Immunol. 9:22-33. [DOI] [PubMed] [Google Scholar]
- 8.De Gracia, J., M. J. Rodrigo, F. Morell, M. Vendrell, M. Miravitlles, M. J. Cruz, R. Codina, and J. M. Bofill. 1996. IgG subclass deficiencies associated with bronchiectasis. Am. J. Respir. Crit. Care Med. 153:650-655. [DOI] [PubMed] [Google Scholar]
- 9.Edwards, K. M. 1993. Pediatric immunizations. Curr. Probl. Pediatr. 23:186-209. [DOI] [PubMed] [Google Scholar]
- 10.Fischer, M. B., H. M. Wolf, I. Hauber, H. Eggenbauer, V. Thon, M. Sasgary, and M. M. Eibl. 1996. Activation via the antigen receptor is impaired in T cells, but not in B cells from patients with common variable immunodeficiency. Eur. J. Immunol. 26:231-237. [DOI] [PubMed] [Google Scholar]
- 11.Galazka, A., and J. Keja. 1988. Diphtheria: incidence trends and age-wise changes of immunity. Scand. J. Infect. Dis. 20:355-356. [DOI] [PubMed] [Google Scholar]
- 12.Hammarstrom, L., and C. I. Smith. 1986. IgG subclass changes in response to vaccination. Monogr. Allergy 19:241-252. [PubMed] [Google Scholar]
- 13.Hazlewood, M. A., D. S. Kumararatne, A. D. Webster, M. Goodall, P. Bird, and M. Daha. 1992. An association between homozygous C3 deficiency and low levels of anti-pneumococcal capsular polysaccharide antibodies. Clin. Exp. Immunol. 87:404-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huston, D. P., A. F. Kavanaugh, P. W. Rohane, and M. M. Huston. 1991. Immunoglobulin deficiency syndromes and therapy J. Allergy Clin. Immunol. 87:1-17. [DOI] [PubMed] [Google Scholar]
- 15.Insel, R. A., and P. W. Anderson. 1986. Oligosaccharide-protein conjugate vaccines induce and prime for oligoclonal IgG antibody responses to the Haemophilus influenzae b capsular polysaccharide in human infants. J. Exp. Med. 163:262-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Insel, R. A., and P. W. Anderson. 1986. Response to oligosaccharide-protein conjugate vaccine against Haemophilus influenzae b in two patients with IgG2 deficiency unresponsive to capsular polysaccharide vaccine. N. Engl. J. Med. 315:499-503. [DOI] [PubMed] [Google Scholar]
- 17.Iseki, M., and D. C. Heiner. 1993. Immunodeficiency disorders. Pediatr. Rev. 14:226-236. [DOI] [PubMed] [Google Scholar]
- 18.Lau, R. C., K. A. Bettelheim, and A. C. Patel. 1988. The 1985 national immunization survey: diphtheria, tetanus, and pertussis (whooping cough). N. Z. Med. J. 101:797-800. [PubMed] [Google Scholar]
- 19.Leino, T., K. Auranen, P. H. Makela, H. Kayhty, M. Ramsay, M. Slack, and A. K. Takala. 2002. Haemophilus influenzae type b and cross-reactive antigens in natural Hib infection dynamics: modeling in two populations Epidemiol. Infect. 129:73-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madore, D. V., P. Anderson, B. D. Baxter, G. M. Carlone, K. M. Edwards, R. G. Hamilton, P. Holder, H. Kayhty, D. C. Phipps, C. C. A. Peeters, R. Schneerson, G. R. Siber, J. I. Ward, and C. E. Frasch. 1996. Interlaboratory study evaluating quantitation of antibodies to Haemophilus influenzae type b polysaccharide by enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 3:84-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madore, D. V., S. A. Quataert, M. Mariani, and G. A. M. Berbers. 1999. Interlaboratory reproducibility of an enzyme-linked immunosorbent assay for quantitation of antibodies for Haemophilus influenzae type b polysaccharide. Clin. Diagn. Lab. Immunol. 6:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCusker, C., W. Somerville, V. Grey, and B. Mazer. 1997. Specific antibody responses to diphtheria/tetanus revaccination in children evaluated for immunodeficiency. Ann. Allergy Asthma Immunol. 79:145-150. [DOI] [PubMed] [Google Scholar]
- 23.Moxon, E. R., and J. S. Kroll. 1990. The role of bacterial polysaccharide capsules as virulence factors. Curr. Top. Microbiol. Immunol. 150:65-85. [DOI] [PubMed] [Google Scholar]
- 24.Ochsenbein, A. F., D. D. Pinschewer, S. Sierro, E. Horvath, H. Hengartner, and R. M. Zinkernagel. 2000. Protective long-term antibody memory by antigen-driven and T help-dependent differentiation of long-lived memory B cells to short-lived plasma cells independent of secondary lymphoid organs Proc. Natl. Acad. Sci. USA 97:13263-13268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pacheco, S. E., and W. T. Shearer. 1994. Laboratory aspects of immunology. Pediatr. Clin. N. Am. 41:623-655. [DOI] [PubMed] [Google Scholar]
- 26.Peset Llopis, M. J., G. Harms, M. J. Hardonk, and W. Timens. 1996. Human immune response to pneumococcal polysaccharides: complement-mediated localization preferentially on CD21-positive splenic marginal zone B cells and follicular dendritic cells. J. Allergy Clin. Immunol. 97:1015-1024. [DOI] [PubMed] [Google Scholar]
- 27.Rodrigo, M. J., M. Vendrell, M. J. Cruz, M. Miravitlles, C. Pascual, F. Morell, and J. De Gracia. 2000. Utility of the antibody response to a conjugated Haemophilus influenzae type B vaccine for diagnosis of primary humoral immunodeficiency. Am. J. Respir. Crit. Care Med. 162:1462-1465. [DOI] [PubMed] [Google Scholar]
- 28.Rubin, R. L., F. L. Tang, A. H. Lucas, H. L. Spiegelberg, and E. M. Tan. 1986. IgG subclasses of anti-tetanus toxoid antibodies in adult and newborn normal subjects and in patients with systemic lupus erythematosus, Sjogren's syndrome, and drug-induced autoimmunity. J. Immunol. 137:2522-2527. [PubMed] [Google Scholar]
- 29.Schneerson, R., O. Barrera, A. Sutton, and J. B. Robbins. 1980. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J. Exp. Med. 152:361-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schur, P. H., F. Rosen, and M. E. Norman. 1979. Immunoglobulin subclasses in normal children. Pediatr. Res. 13:181-183. [DOI] [PubMed] [Google Scholar]
- 31.Shearer, W. T., R. H. Buckley, R. J. Engler, A. F. Finn, Jr., T. A. Fleisher, T. M. Freeman, H. G. Herrod III, A. I. Levinson, M. Lopez, R. R. Rich, S. I. Rosenfeld, L. J. Rosenwasser, et al. 1996. Practice parameters for the diagnosis and management of immunodeficiency. Ann. Allergy Asthma Immunol. 76:282-294. [DOI] [PubMed] [Google Scholar]
- 32.Simonsen, O., M. W. Bentzon, and I. Heron. 1986. ELISA for the routine determination of antitoxic immunity to tetanus. J. Biol. Stand. 14:231-239. [DOI] [PubMed] [Google Scholar]
- 33.Soininen, A., M. Karpala, S. L. Wahlman, H. Lehtonen, and H. Kayhty. 2002. Specificities and opsonophagocytic activities of antibodies to pneumococcal capsular polysaccharides in sera of unimmunized young children. Clin. Diagn. Lab. Immunol. 9:1032-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sorensen, R. U., L. E. Leiva, F. C. Javier, D. M. Sacerdote, N. Bradford, B. Butler, P. A. Giangrosso, and C. Moore. 1998. Influence of age on the response to Streptococcus pneumoniae vaccine in patients with recurrent infections and normal immunoglobulin concentrations. J. Allergy Clin. Immunol. 102:215-221. [DOI] [PubMed] [Google Scholar]
- 35.Stagg, A. J., M. Funauchi, S. C. Knight, A. D. Webster, and J. Farrant. 1994. Failure in antigen responses by T cells from patients with common variable immunodeficiency (CVID). Clin. Exp. Immunol. 96:48-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.STIKO. 2001. Impfempfehlungen der Ständigen Impfkommission (STIKO) am Robert Koch-Institut. Epidemiol. Bull. 2001:203-218. [Google Scholar]