Abstract
Tumor metastases are extremely rare in striated muscles. Lately, we have found that muscle cell conditioned medium (MCM) inhibits the proliferation of various tumor cells while maintaining the growth of normal murine bone marrow cells. This dual activity was confirmed in vivo when the MCM was administered orally, i.e., it inhibited the development of tumor growth in mice and prevented the myelotoxic effects of chemotherapy. Adenosine was found to be one of the active components of MCM, inhibiting tumor cell growth while maintaining bone marrow cell proliferation in vitro. Adenosine is known to act as an important regulatory molecule through its binding to specific G-protein-associated A1, A2a, A2b and A3 cell surface receptors. In distinction from MCM, adenosine did not suppress tumor development in mice and was not active as a chemoprotective agent when administered orally or intravenously. Thus, the in vivo activity of MCM could not be attributed to adenosine. In this study, MCM from which adenosine was enzymatically removed still retained its dual activity that was also found to be mediated through the A3 adenosine receptor (A3AR). This result led to the conclusion that natural agonists to A3AR were responsible for the activity of MCM. We further tested synthetic agonist to the A3AR and demonstrated that it possessed the same in vitro and in vivo activity profile as MCM. Taken together, muscle cells, in addition to adenosine, secrete natural agonists to A3AR. These agonists are stable nondegradable molecules and may contribute to the systemic anticancer and chemoprotective activity exerted by MCM. This group of molecules may account for the rarity of tumor metastases in muscle.
Keywords: muscle, tumor, A3 adenosine receptor, agonists
Introduction
The resistance of muscle tissue to the development of tumor metastases is a well-recognized clinical phenomenon [1–3]. Several mediators released by muscle cells may account for the rarity of tumor metastases in this tissue, including cytokines with anticancer activity such as TNFα, TGFβ, lymphocyte infiltrating factor, interferon γ, lactic acid, and proteolytic enzymes such as plasminogen activator inhibitor [4–10]. Moreover, muscle sarcolemma serves as a physical barrier against tumor cell invasion [11,12].
In our previous studies, we demonstrated that small molecules (<800 Da) present in muscle cell conditioned medium (MCM1) exerted an inhibitory effect on the growth of various tumor cell lines and simultaneously stimulate the proliferation of normal bone marrow cells [13]. MCM was found to be water soluble, heat stable and resistant to the activity of proteolytic enzymes. When administered orally to mice, it inhibited the development of melanoma and sarcoma lung metastases, while protecting against the myelotoxic effects of chemotherapy [14].
We have recently found that one of the active components of MCM is adenosine that exerts a differential effect on tumor and normal cell growth in vitro [15]. Adenosine, an ubiquitous nucleoside, is released into the extracellular environment from metabolically active or stressed cells. It is known to act as an important regulatory molecule by binding to specific G-protein-associated A1, A2a, A2b and A3 cell surface receptors [16,17]. Our recent studies, using antagonists to the adenosine receptors, revealed that adenosine exerted its in vitro inhibitory effect as well as its stimulatory activity through the activation of the A3 adenosine receptor (A3AR) [18,19].
However, in contrast to MCM, adenosine did not suppress tumor development or act as a chemoprotective agent when administered orally to mice.
In this study, we defined additional molecules in MCM, namely, agonists to the A3AR. These agonists inhibit tumor cell growth and stimulate bone marrow cell proliferation. Moreover, synthetic A3AR agonists, known as stable bioavailable molecules, were shown to exert antitumor and chemoprotective effects in vivo.
Materials and Methods
Preparation of MCM
MCM was obtained from the L-8 cell line (consisting of proliferating myoblasts) purchased from the American Type Tissue Culture Collection, Rockville, MD (ATCC). The cells were routinely maintained in DMEM containing 4.5 gr% glucose and 15% fetal bovine serum (FBS) (Biological Industries, Beit Haemek, Israel).
To prepare MCM, cultures were grown until confluence, medium discarded, cells washed twice with phosphate-buffered saline (PBS), and then incubated for an additional 20 hours in PBS. At the end of the incubation period, the supernatant was collected, centrifuged and filtered through a 0.22 µ filter. Because our previous work [15] showed that the inhibitory activity is detected in the molecular weight fraction lower than 3 kDa, MCM was subjected to ultrafiltration through an Amicon membrane with a molecular cut-off of 3 kDa.
Tumor and Normal Cells
The B16-F10 murine melanoma cell line was used in the in vitro and in vivo experiments. Cells were maintained in RPMI medium containing 10% FBS, penicillin and streptomycin. They were transferred twice weekly to a freshly prepared medium.
Bone marrow cells were obtained from the femur of C57BL/6J mice. Cells were disaggregated by passing through a 25G needle.
In addition, the HCT-116 human colon carcinoma tumor cell line was used in the in vivo studies.
Cell Proliferation Assays
[3H]Thymidine incorporation assay was used to evaluate cell growth. A total of 1.5x104/ml B16-F10 melanoma or 3x105/ml bone marrow cells were cultured in RPMI medium containing 10% FBS in 96-well microtiter plates. These cultures were incubated in the presence of MCM at a concentration of 50%. Because MCM was prepared in PBS, cultures containing tumor or bone marrow cells suspended in 50% PBS served as controls. During the last 18 hours of incubation, each well was pulsed with 1 µCi [3H]thymidine. The cells were harvested and the [3H]thymidine uptake was determined in an LKB liquid scintillation counter (LKB, Piscataway, NJ).
Results are expressed as percentage of cell proliferation inhibition or stimulation, calculated according to the following equations:
where A=cell count of sample and B=cell count of control. According to this calculation control values will be 0% of inhibition or stimulation.
One activity unit was defined as the amount of MCM exerting 50% proliferation inhibition of the B16-F10 melanoma cells.
Elimination of Adenosine from MCM by Treatment with Adenosine Deaminase (ADA)
To eliminate adenosine from MCM preparations, adenosine deaminase (ADA) (Sigma, St. Louis, MO) was added for 1 hour. To remove the enzyme, the preparation was ultrafiltrated through a 3000 dalton Amicon membrane. This sample was designated as MCM+ADA and its effect on the proliferation of tumor or bone marrow cells was examined as described above.
Effect of A3AR Antagonist on the Activity of MCM+ADA
In this set of experiments, we examined whether the effect of MCM+ADA on tumor or normal cells was mediated through A3AR. A total of 1.5x104/ml B16-F10 melanoma or 3x105/ml bone marrow cells were cultured in RPMI medium containing 10% FBS in 96-well microtiter plates. These cultures were preincubated for 30 minutes in the presence of 0.1, 0.05, and 0.001 µM of the A3AR antagonist 9-chloro-2-(2-furanyl)-5-[(phenylacetyl)amino] [1,2,4]-triazolo[1,5-c] quinazoline (MRS-1220) (RBI, MA). At the end of this incubation period, MCM+ADA, at a final concentration of 50%, was added to the cultures for an additional 48 hours. Cells were preincubated with MRS-1220 for 30 minutes and then 50% PBS+ADA added for 48 hours served as controls. During the last 18 hours of incubation, each well was pulsed with 1 µCi [3H]thymidine. [3H]Thymidine uptake was determined as described above.
Effect of Synthetic A3AR Agonist on the Proliferation of Tumor and Bone Marrow Cells
A synthetic agonist to the A3AR, 1-deoxy-1-[6-[[(3-iodophenyl)methyl]amino]-9H-purine-9-yl]-N-methyl-β-d-ribofuranuronamide (IB-MECA) (Sigma) was used to examine its effect on B16-F10 melanoma and murine bone marrow cells. A stock solution was prepared by dissolving 5 mg IB-MECA in 1 ml DMSO. Further dilutions were performed in RPMI for in vitro studies and PBS for in vivo experiments. B16-F10 melanoma (1.5x104/ml) or bone marrow cells (3x105/ml) were cultured in RPMI medium containing 10% FBS in 96-well microtiter plates. IB-MECA at concentrations of 0.1, 0.01, and 0.001 µM was added to these cell cultures for 48 hours. [3H]Thymidine uptake was determined as described above.
In Vivo Studies
Experiments were performed in accordance with the guidelines established by the Institutional Animal Care and Use Committee at the Rabin Medical Center, Petach Tikva, Israel.
To examine the effect of the different preparations on tumor cell growth, B16-F10 (2.5x105) melanoma cells were intravenously inoculated to C57BL/6J mice. Each group contained 20 mice and experiments were repeated at least four times. Mice were treated orally according to the following protocol starting 1 day after tumor inoculation:
Adenosine — 268 µg/kg body weight.
MCM, 4AU twice daily.
MCM+ADA 4AU twice daily.
IB-MECA — 3µg/kg body weight or 6 µg/kg body weight once daily.
Vehicle — twice daily.
An additional group of mice was treated intraperitoneally with adenosine (268 µg/kg body weight) each day.
Mice were sacrificed after 15 days, lungs removed, and black metastatic foci were counted using a dissecting microscope.
In a different set of experiments, we tested the effect of IB-MECA on the growth of HCT-116 human colon carcinoma cells. Cells (1.2x106) were subcutaneously injected to the flank of nude/BalbC mice. Mice were treated orally on alternate days with IB-MECA (6 µg/kg), starting 1 day following tumor inoculation. Mice treated with vehicle only served as controls. Tumor size (width [W] and length [L]) was measured every 4 days and was calculated according to the following formula:
The myeloprotective effect of the various preparations were examined by injecting mice intraperitoneally with 50 mg/kg body weight of cyclophosphamide. Each group contained 10 mice and experiments were repeated at least four times. Adenosine, MCM (4 activity units) or MCM+ADA (4 activity units) were each orally administered 48 and 72 hours following the chemotherapy. After 120 hours blood samples were withdrawn. Number of leukocytes and percentage of neutrophils were evaluated.
In another set of experiments, mice were injected with cyclophosphamide as described above. IB-MECA (6 µg/kg body weight) was orally administered 48 and 72 hours following the chemotherapy. Blood samples were withdrawn 96, 120, 144, and 168 hours following chemotherapy and the number of leukocytes and percentage of neutrophils evaluated.
Statistical Analysis
The efficacy of the various agents in vitro and in vivo was evaluated using the student's t-test. The criterion for statistical significance was P<0.05.
Results
Adenosine Is Not Responsible for the In Vivo Activity of MCM
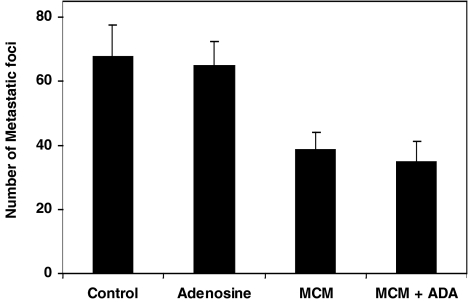
MCM or MCM+ADA were tested for their efficacy as inhibitors of melanoma lung metastatic foci growth in mice. All the preparations were administered to the mice through an oral route. MCM and MCM+ADA induced an inhibitory effect on tumor growth (42.7±5.8%, P<0.001; 49±3.7%, P<0.001, respectively, Figure 1).
Figure 1.
The effect of adenosine, MCM, and MCM+ADA on the development of lung metastases in mice inoculated with B16-F10 melanoma cells. The number of melanoma foci was inhibited (P<0.001) following treatment with 4 activity units of MCM or MCM+ADA. All the preparations were administered to mice through an oral route. Adenosine failed to inhibit tumor growth.
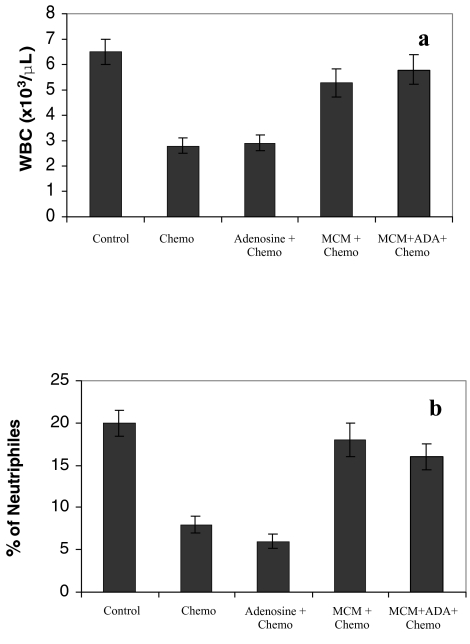
Administration of MCM or MCM+ADA to mice following chemotherapy, prevented the myelotoxic effects of the cytotoxic drug, i.e., induced an increase in the number of leukocytes (Figure 2a) and percentage of neutrophils (Figure 2b). Adenosine failed to inhibit tumor growth or act as a chemoprotective agent when administered orally or intraperitoneally.
Figure 2.
The in vivo effect of adenosine, MCM, and MCM+ADA on the number of WBC and percentage of neutrophils in mice treated with 50 mg/kg body weight cyclophosphamide. Chemotherapy alone decreased the number of WBC and percentage of neutrophils. MCM or MCM+ADA, administered after chemotherapy, increased the number of WBC (a) and percentage of neutrophils (b) to almost normal values.
Inhibition of Tumor Cell Growth and Stimulation of Bone Marrow Cell Proliferation Is Mediated through the A3AR
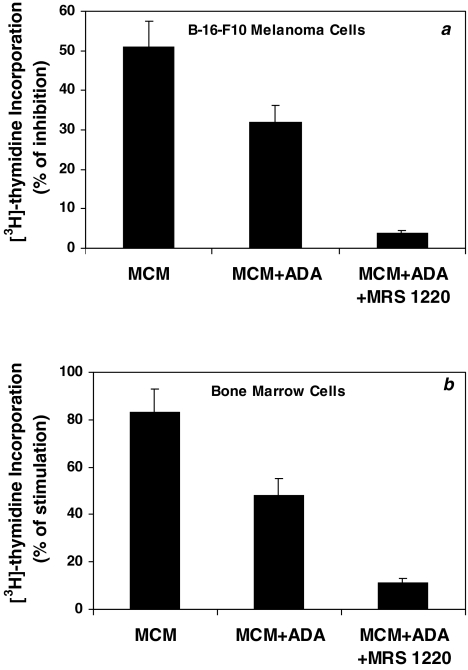
The above results demonstrate that the active component in MCM responsible for the dual activity in vivo, is not adenosine. However, because the MCM+ADA preparation still retained this same dual activity, we presumed that both effects were mediated through the A3AR. To explore this assumption, B16-F10 melanoma cells or murine bone marrow cells were incubated with MCM+ADA in the presence or absence of an antagonist to the A3AR. A statistically significant inhibition of [3H]thymidine uptake following incubation with the MCM or MCM+ADA was observed. Because adenosine was removed from MCM+ADA, this preparation induced a decreased inhibitory effect. However, incubation of cells with MCM+ADA in the presence of MRS-1220, canceled most of the inhibitory effect (Figure 3a). Similarly, MCM and to a lesser extent MCM+ADA, stimulated the proliferation of bone marrow cells in vitro (83±9.2% and 48±7.1%, respectively). Most of the stimulatory effect was lost following the incubation of bone marrow cells with MCM+ADA, in the presence of MRS-1220 (Figure 3b). MRS-1220, by itself, had no effect on the proliferation of the B16-F10 or bone marrow cells. No differences were observed between the various concentrations of the antagonist. Figure 3a and b, using a concentration of 0.001) µM of MRS-1220, are representative of these results.
Figure 3.
MCM and to a lesser extent MCM+ADA induced the inhibitory effect on the growth of B16-F10 melanoma cells (a) or stimulatory effect on the proliferation of murine bone marrow cells (b). Cell proliferation was measured by [3H]thymidine incorporation assay. The introduction of MRS-1220, canceled most of the inhibitory (a) or stimulatory (b) effect that was exerted by MCM+ADA.
These results demonstrated that the inhibitory and the stimulatory activity of MCM were mediated through A3AR, the active component being an agonist to this receptor.
IB-MECA (A3AR Agonist) Inhibits Tumor Cell Growth and Stimulates Bone Marrow Cell Proliferation In Vitro
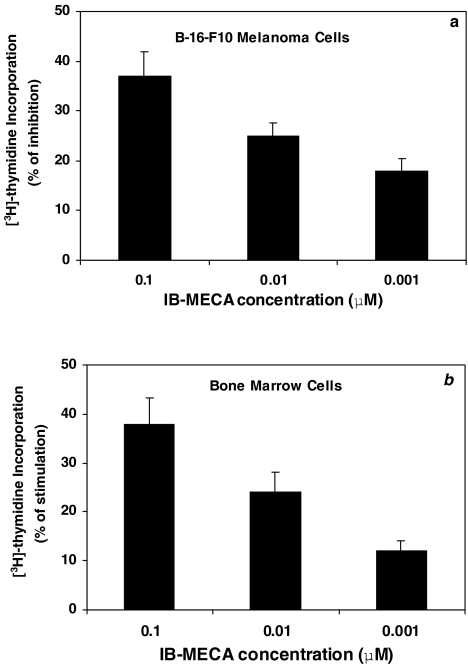
The above results, depicting the role of A3AR activation in the inhibition of tumor cell growth and stimulation of bone marrow cell proliferation, led us to test the effect of IB-MECA on these activities. Indeed, IB-MECA exerted a dose-dependent inhibitory effect on the proliferation of B16-F10 cells (Figure 4a) while stimulating bone marrow cell proliferation (Figure 4b).
Figure 4.
IB-MECA, a synthetic A3AR agonist, at different concentrations, inhibitedthe proliferation of B16-F10 melanoma cells (a) and stimulated bone marrow cell proliferation (b), measured by [3H]thymidine incorporation assay.
IB-MECA Inhibits Tumor Cell Growth In Vivo and Protects against Myelotoxicity Following Chemotherapy
In this set of experiments, we evaluated IB-MECA's ability to inhibit the growth of lung metastases in mice inoculated with B16-F10 melanoma cells and in nude mice with HCT-116 colon carcinoma tumors.
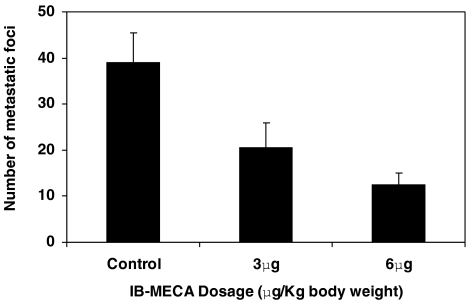
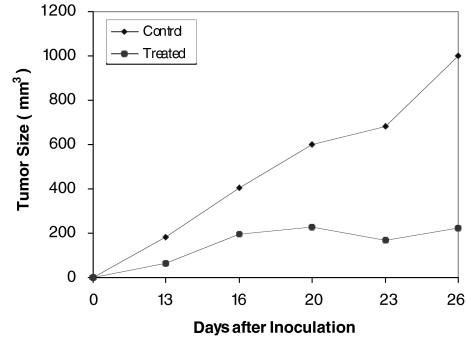
A dose-dependent, statistically significant inhibition (P<0.001) of the number of melanoma lung foci was observed (Figure 5). Tumor size in the nude mice was markedly inhibited following treatment with IB-MECA (Figure 6).
Figure 5.
The effect of IB-MECA on the development of lung metastases in mice inoculated with B16-F10 melanoma cells. IB-MECA was administered orally daily at a dosage of 3 or 6 µg/kg body weight. A dose-dependent inhibition of lung metastatic foci is depicted.
Figure 6.
The effect of IB-MECA on the growth of HCT-116 human colon carcinoma cells in nude mice. Tumor cells (1.2x106) were subcutaneously injected to the flank of nude mice. One group was treated every other day with 6 µg/kg IB-MECA and the other, treated with vehicle only, served as control. Tumor size was measured every 4 days. Tumor size in the IB-MECA treated mice was smaller than controls.
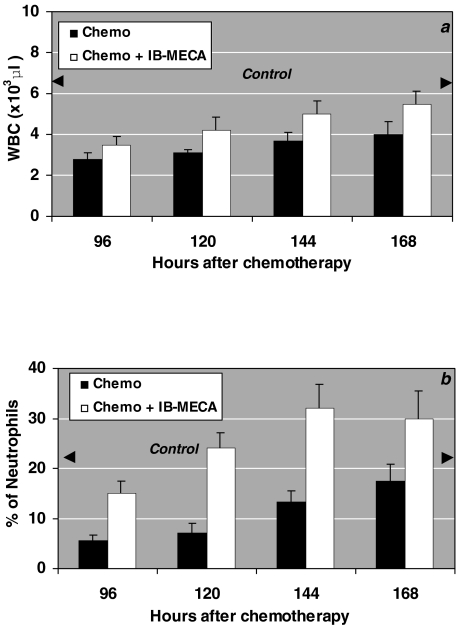
Mice treated with cyclophosphamide exhibited a decline in the number of peripheral blood leukocytes and neutrophils. Administration of IB-MECA following chemotherapy, increased the number of white blood cells (Figure 7a) and restored the percentage of neutrophils (Figure 7b).
Figure 7.
The effect of IB-MECA on the myeloid system in mice treated with 50 mg/kg body weight cyclophosphamide. Chemotherapy alone decreased the number of WBC and percentage of neutrophils. IB-MECA, administered 48 and 72 hours after chemotherapy, increased the number of total WBC (a) and restored the percentage of neutrophils (b).
Discussion
The results of the present study show that natural agonists to the A3AR are present in MCM and may be responsible for its anticancer and chemoprotective activity.
The resistance of muscle tissue to the development of metastases has been previously investigated [20–22]. Our studies demonstrated that the low-molecular-weight fraction of MCM exhibited a unique characteristic of differentiating between tumor and normal cells. It inhibited tumor cell growth and maintained bone marrow cell proliferation in vitro [13]. Similar data was reproduced in vivo when MCM was orally administered to mice with melanoma or sarcoma. It suppressed tumor development and induced a prolongation of survival time in the treated mice. Moreover, it acted as a chemoprotective agent by preventing the myelotoxic effects of cyclophosphamide [14].
At a later stage, we found that adenosine was one of the active ingredients within MCM and, in vitro, it exhibited a dual activity similar to that of MCM [15]. Adenosine is known to act as a signal transduction regulatory molecule through its interaction with specific cell surface receptors. The binding of adenosine or its agonist to A1 or A3 adenosine receptors (associated with the Gi proteins) inhibits the activity of the second messenger adenylyl cyclase and leads to a decrease in the intracellular cAMP level [16,17]. cAMP is known to act as a cell growth regulator and affect differentially the proliferation of various cell types [23–25].
The results of the present study show that adenosine was not responsible for the in vivo efficacy of MCM. This was concluded following an experiment in which adenosine failed to exert anticancer or chemoprotective effects when given orally or intraperitoneally. Moreover, when adenosine was eliminated from MCM (this preparation was designated MCM+ADA), the latter still retained its dual effect. In vivo, MCM+ADA inhibited the development of melanoma lung metastatic foci and acted as a chemoprotective agent, similarly to the MCM. In vitro, MCM+ADA inhibited the proliferation of melanoma cells while inducing the proliferation of bone marrow cells. These activities were blocked by the MRS-1220 A3AR antagonist, indicating the role of this receptor in mediating both activities. MRS-1220 is known to possess a high affinity to A3AR [26] and it blocked the inhibitory/stimulatory effect at all three concentrations used, even at 0.001 µM. The data demonstrating that the dual activity of MCM+ADA, in vitro, was mediated through the A3AR, led us to conclude that the active component in MCM is a natural agonist to A3AR. In previous studies we showed that the A2 and A1 adenosine receptors played a role in the inhibitory/stimulatory activity of adenosine, respectively. Activation of the A1 adenosine receptor by specific agonist induced chemoprotective effects [18,19]. In the present study, MRS-1220 blocked most of the inhibitory/stimulatory activities, thus we presume that A1 or A2 adenosine receptor agonists are not present in the MCM preparation.
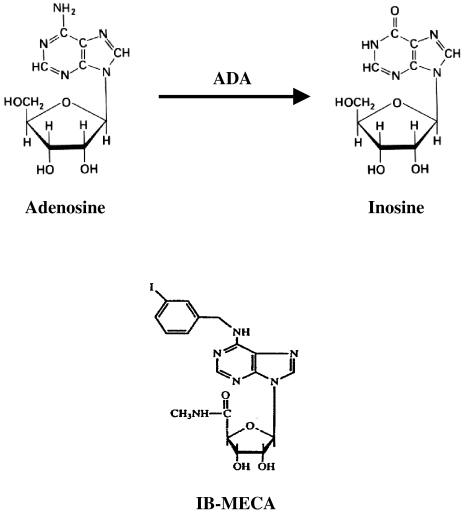
These findings were further confirmed by demonstrating that a synthetic agonist to the A3AR, namely, IB-MECA, exerted a similar anticancer and chemoprotective effect in mice when given orally. IB-MECA is resistant to degradation by the enzyme adenosine deaminase, in contrast to adenosine, which metabolizes rapidly in vivo to inosine (Figure 8). IB-MECA contains modifications at two sites of the adenosine structure, namely, substitution at the 2-position of adenosine in combination with modifications at N6 and 5′-positions. This agonist exerted similar activity to that of adenosine in vitro, with the advantage of being stable and orally available [27,28]. Two types of in vivo models were tested in the present study, i.e., a metastatic melanoma and primary colon carcinoma. IB-MECA suppressed the development of both tumor types, indicating that it affects tumor growth rather than metastatic invasion.
Figure 8.
Metabolization of adenosine to inosine occurred rapidly in vivo mainly by the enzyme adenosine deaminase. The A3AR agonist, IB-MECA, resists degradation by adenosine deaminase, due to substitution, at the 2-position of adenosine, combined with modifications at N6 and 5′-positions [26,27].
In two previous studies [18,19], we showed that activation of A3AR by nanomolar adenosine concentrations induced a differential effect on tumor and normal cell growth. A cytostatic effect of adenosine on the growth of Nb2-11C lymphoma cells was shown by the inhibition of [3H]thymidine incorporation, a decrease in cell count, a cell cycle arrest in the G0/G1 phase and a reduction of the telomeric signal in these cells. At the same time, adenosine induced bone marrow cell proliferation in vitro and its subcutaneous administration to mice following chemotherapy and prevented the myelotoxic effects of the cytotoxic drug, i.e., induced an increase in the number of leukocytes and percentage of neutrophils. The mechanism involved was the induction of granulocyte-colony stimulating factor (G-CSF) production by adenosine, through the activation of A3AR [18]. G-CSF, a myeloid growth factor, has become a standard therapy for cancer patients with neutropenia. It stimulates the proliferation and differentiation of myeloid progenitors, leading to a reduction in the incidence of febrile neutropenia and acceleration of neutrophil recovery after chemotherapy. It may be suggested that in this study, the inhibition of melanoma growth by IB-MECA and its chemoprotective effects in vivo were mediated through similar mechanisms, namely, cytostatic effects toward tumor cells and induction of G-CSF production.
Cytoprotective effects toward cardiac myocytes or brain cells have previously been attributed to A3AR agonists [29,30]. These effects may be beneficial in overcoming the adverse events of chemotherapy.
Thus, it may be concluded that the active component in MCM is a natural agonist to the A3AR. The high bioavailability of agonists to A3AR and their efficacy in vivo when given orally, suggest that this family of compounds may be applied for use in cancer therapy.
Abbreviations
- MCM
muscle cell conditioned medium
- A3AR
A3 adenosine receptor
- PBS
phosphate-buffered saline
- FBS
fetal bovine serum
- ADA
adenosine deaminase
References
- 1.Sarma DP, Weilbaccher TG, Love GL. Intramyofiber metastasis in skeletal muscle. J Surg Oncol. 1985;30:103–105. doi: 10.1002/jso.2930300208. [DOI] [PubMed] [Google Scholar]
- 2.Pellegrini AE. Carcinoma of the lung occurring as a skeletal muscle mass. Arch Surg. 1979;114:550–555. doi: 10.1001/archsurg.1979.01370280204040. [DOI] [PubMed] [Google Scholar]
- 3.Merimsky O, Levin T, Chaitchik S. Recurrent solitary metastasis of renal cell carcinoma in skeletal muscles. Tumori. 1990;76:407–409. doi: 10.1177/030089169007600422. [DOI] [PubMed] [Google Scholar]
- 4.Husmann I, Soulet L, Gautron J, Martelly I, Barritault D. Growth factors in skeletal muscle regeneration. Cytokine Growth Factor Rev. 1996;7:249–258. doi: 10.1016/s1359-6101(96)00029-9. [DOI] [PubMed] [Google Scholar]
- 5.Birnbaum M, Trink B, Shainberg A, Salzberg S. Activation of the interferon system during myogenesis in vitro. Differentiation. 1990;45:138–145. doi: 10.1111/j.1432-0436.1990.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 6.Rosztoczy I, Megyeri K, Papos M. Interferon production by normal mouse tissues in organ cultures. Interferon Res. 1989;9:509–515. doi: 10.1089/jir.1989.9.509. [DOI] [PubMed] [Google Scholar]
- 7.Kurek JB, Nouri S, Kannourakis G, Murphy M, Austin L. Leukemia inhibitory factor and interleukin-6 are produced by diseased and regenerating skeletal muscle. Muscle Nerve. 1996;19:1291–1301. doi: 10.1002/(SICI)1097-4598(199610)19:10<1291::AID-MUS6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 8.Lafyatis R, Lechleider R, Roberts AB, Sporn MB. Secretion of transcriptional regulation of transforming growth factor-beta 3 during myogenesis. Mol Cell Biol. 1991;7:3795–3803. doi: 10.1128/mcb.11.7.3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takada S, Nagato Y, Yamamura M. Effect of cyclic polylactates on tumor cells and tumor bearing mice. Biochem Mol Biol Int. 1997;43:9–17. doi: 10.1080/15216549700203761. [DOI] [PubMed] [Google Scholar]
- 10.Lee E, Vaughan DE, Parikh SH. Regulation of matrix metalloproteinases and plasminogen activator inhibitor-1 synthesis by plasminogen in cultured human vascular smooth muscle cells. Circ Res. 1996;78:44–49. doi: 10.1161/01.res.78.1.44. [DOI] [PubMed] [Google Scholar]
- 11.Zacks SI, Sheff MF, Saito A. Structure and staining characteristics of myofiber external lamina. J Histochem Cytochem. 1973;21:703–714. doi: 10.1177/21.8.703. [DOI] [PubMed] [Google Scholar]
- 12.Zacks SI, Vandenburgh HH, Sheff MF. Cytochemical and physical properties of myofiber external lamina. J Histochem Cytochem. 1973;21:895–901. doi: 10.1177/21.10.895. [DOI] [PubMed] [Google Scholar]
- 13.Djaldetti M, Sredni B, Zigelman R, Verber M, Fishman P. Muscle cells produce a low molecular weight factor with anti-cancer activity. Clin Exp Metastasis. 1996;14:189–196. doi: 10.1007/BF00053891. [DOI] [PubMed] [Google Scholar]
- 14.Bar-Yehuda S, Farbstein T, Barer F, Ohana G, Fishman P. Oral administration of muscle derived small molecules inhibits tumor spread while promoting normal cell growth in mice. Clin Exp Metastasis. 1999;17:531–535. doi: 10.1023/a:1006649617918. [DOI] [PubMed] [Google Scholar]
- 15.Fishman P, Bar-Yehuda S, Vagman L. Adenosine and other low molecular weight factors released by muscle cells inhibit tumor cell growth. Cancer Res. 1998;58:3181–3187. [PubMed] [Google Scholar]
- 16.Linden J. Structure and function of A1 adenosine receptors. FASEB J. 1991;5:2668–2676. doi: 10.1096/fasebj.5.12.1916091. [DOI] [PubMed] [Google Scholar]
- 17.Stiles G. Adenosine receptors and beyond: molecular mechanisms of physiological regulation. Clin Res. 1990;38:10–18. [PubMed] [Google Scholar]
- 18.Fishman P, Bar-Yehuda S, Farbstein T, Barer F, Ohana G. Adenosine acts as a chemoprotective agent by stimulating G-CSF production: a role for A1 and A3 adenosine receptors. J Cellular Physiol. 2000;183:393–398. doi: 10.1002/(SICI)1097-4652(200006)183:3<393::AID-JCP12>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 19.Fishman P, Bar-Yehuda S, Ohana G, Pathak S, Wasserman L, Multani AS, Barer F. Adenosine acts as an inhibitor of lymphoma cell growth: a major role for the A3 adenosine receptor. Eur J Cancer. 2000;36:1452–1458. doi: 10.1016/s0959-8049(00)00130-1. [DOI] [PubMed] [Google Scholar]
- 20.Lasser A, Zacks IS. Intraskeletal myofiber metastasis of breast carcinoma. Hum Pathol. 1982;13:1045–1046. doi: 10.1016/s0046-8177(82)80098-1. [DOI] [PubMed] [Google Scholar]
- 21.Slatkin DN, Pearson J. Intramyofiber metastases in skeletal muscle. Hum Pathol. 1976;7:347–349. doi: 10.1016/s0046-8177(76)80044-5. [DOI] [PubMed] [Google Scholar]
- 22.Ioachim HL. Tumor cells within skeletal muscle cells. Hum Pathol. 1983;14:924–929. doi: 10.1016/s0046-8177(83)80168-3. [DOI] [PubMed] [Google Scholar]
- 23.Kurland JI, Hadden JW, Moore MAS. Role of cyclic nucleotides in the proliferation of committed granulocyte-macrophage progenitor cells. Cancer Res. 1977;37:4534–4538. [PubMed] [Google Scholar]
- 24.Lee AW. Synergistic activation of mitogen-activated protein kinase by cyclic AMP and myeloid growth factors opposes cyclic AMP's growth-inhibitory effects. Blood. 1999;93:537–553. [PubMed] [Google Scholar]
- 25.Pastan IH, Johnson GS, Anderson WB. Role of cyclic nucleotides in growth control. Annu Rev Biochem. 1975;44:491–522. doi: 10.1146/annurev.bi.44.070175.002423. [DOI] [PubMed] [Google Scholar]
- 26.Jacobson KA, Mora S, Kim Y, Lee A. A3 adenosine receptors: protective vs. damaging effects identified using novel agonists and antagonists. Drug Dev Res. 1998;45:113–124. doi: 10.1002/(SICI)1098-2299(199811/12)45:3/4<113::AID-DDR5>3.0.CO;2-S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobson KA, Kim HO, Siddiqi SM, Olah ME, Stiles G, Von Lubitz DKJE. A3 adenosine receptors: design of selective ligands and therapeutic prospects. Drugs Future. 1995;20:689–699. doi: 10.1358/dof.1995.020.07.531583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobson KA, Yong-Chul K, An-Hu Li. A3 adenosine receptors: protective vs. damaging effects identified using novel agonists and antagonists. Drug Dev Res. 1998;45:113–124. doi: 10.1002/(SICI)1098-2299(199811/12)45:3/4<113::AID-DDR5>3.0.CO;2-S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu GS, Richards SC, Olsson RA, Mullane KH, Walsh RS, Downey JM. Evidence that the adenosine A3 receptor may mediate the protection afforded by preconditioning in the isolated rabbit heart. Cardiovasc Res. 1994;28:1057–1061. doi: 10.1093/cvr/28.7.1057. [DOI] [PubMed] [Google Scholar]
- 30.von Lubitz D, Lin RCS, Popik P, Carter MF, Jacobson KA. Adenosine A3 receptor stimulation and cerebral-ischemia. Eur Js Pharmacol. 1994;263:59–67. doi: 10.1016/0014-2999(94)90523-1. [DOI] [PMC free article] [PubMed] [Google Scholar]