Abstract
Induction of heparin-binding epidermal growth factor-like growth factor (HB-EGF) mRNA in mouse skin organ culture was blocked by two pan-ErbB receptor tyrosine kinase (RTK) inhibitors but not by genetic ablation of ErbB1, suggesting involvement of multiple ErbB species in skin physiology. Human skin, cultured normal keratinocytes, and A431 skin carcinoma cells expressed ErbB1, ErbB2, and ErbB3, but not ErbB4. Skin and A431 cells expressed more ErbB3 than did keratinocytes. Despite strong expression of ErbB2 and ErbB3, heregulin was inactive in stimulating tyrosine phosphorylation in A431 cells. In contrast, it was highly active in MDA-MB-453 breast carcinoma cells. ErbB2 displayed punctate cytoplasmic staining in A431 and keratinocytes, compared to strong cell surface staining in MDA-MB-453. In skin, ErbB2 was cytoplasmic in basal keratinocytes, assuming a cell surface pattern in the upper suprabasal layers. In contrast, ErbB1 retained a cell surface distribution in all epidermal layers. Keratinocyte proliferation in culture was found to be ErbB1-RTK-dependent, using a selective inhibitor. These results suggest that in skin keratinocytes, ErbB2 transduces ligand-dependent differentiation signals, whereas ErbB1 transduces ligand-dependent proliferation/survival signals. Intracellular sequestration of ErbB2 may contribute to the malignant phenotype of A431 cells, by allowing them to respond to ErbB1-dependent growth/survival signals, while evading ErbB2-dependent differentiation signals.
Keywords: ErbB receptors, skin organ culture, keratinocytes, heparin-binding EGF-like growth factor, receptor tyrosine kinase
Introduction
The mammalian c-erbB family is comprised of four closely related transmembrane proteins. ErbB1, ErbB2, and ErbB4 possess receptor tyrosine kinase (RTK) activity, and ErbB1, ErbB3, and ErbB4 bind multiple ligands of the epidermal growth factor (EGF) family [1,2]. These ligands can be classified based on their receptor affinities: EGF, transforming growth factor-α (TGF-α), amphiregulin (AR), and vaccinia growth factor preferentially bind to ErbB1, and epiregulin, betacellulin, and heparin-binding EGF-like growth factor (HB-EGF) bind to ErbB4 in addition to ErbB1. Finally, the heregulins (also known as neuregulins) bind to ErbB3 and/or ErbB4, but not to ErbB1 [3]. Ligand binding to the extracellular domain of ErbB receptors is believed to cause receptor homo- and heteroassociations, followed by trans-phosphorylation of tyrosine residues in the activation loop of the kinase domain. Tyrosine phosphorylation is thought to allow access of Mg-ATP to the catalytic site, overcoming autoinhibition of the kinase [4]. Other tyrosine phosphorylation events, also executed in trans, lead to the recruitment of multiple proteins bearing phosphotyrosine binding domains [5]. Signal diversification results from differential action of different ErbB ligands through a given receptor [6], and from differential utilization of downstream pathways by different ErbB receptor combinations [7]. Many of these combinations preferentially involve ErbB2, a receptor for which no direct ligand has been identified. Instead, ErbB2 is activated by heterodimerization and/or multimerization with other ErbB receptors [8–10]. Interestingly, ErbB2-ErbB3 heterodimers constitute a potent mitogenic combination [11], despite the fact that ErbB2 has no known ligand and the RTK activity of ErbB3 is severely limited [12].
The physiological significance of ErbB receptors has been established by studies of mice lacking various components of the ErbB network (reviewed in [1]). These studies indicate that ErbB1 is more important for normal development of the skin, lungs, mammary glands, and gastrointestinal tract, while ErbB2, ErbB3, and ErbB4 are more important for the development of the heart and central nervous system. Moreover, deregulated ErbB signaling has been implicated in various malignancies, including tumors of the breast, lung, ovary, and brain [13].
ErbB receptors play a prominent role in the epidermis, which is also capable of producing several ErbB1-selective ligands including TGF-α, AR, and HB-EGF [14]. Addition of EGF or TGF-α to skin wounds also significantly accelerates skin wound healing [15,16]. We have previously shown that the heparin-binding EGF-like growth factors AR and HB-EGF are rapidly and selectively induced in human skin organ culture, an ex vivo model system that faithfully recapitulates several early events in wound healing [17]. Overexpression of multiple ErbB ligands is also observed in psoriasis [18,19], a skin disease characterized by marked keratinocyte hyperplasia in the context of immunologically mediated skin inflammation [20]. Forced overexpression of AR in the epidermis of transgenic mice results in a markedly psoriasiform phenotype, including a prominent arthritis [21]. Substantial evidence also implicates ErbB signaling as an important factor in the pathogenesis of nonmelanoma skin cancer [22,23]. Therefore, a thorough understanding of ErbB signaling in the skin is important for improving our understanding of normal and abnormal skin physiology.
Thus far, most studies of ErbB signaling in skin have focused on the “traditional” EGF receptor, ErbB1. However, the fact that epidermal development proceeds in ErbB1 knockout mice [24–26] suggests that members of the ErbB family other than ErbB1 may also be involved in skin development and physiology. Indeed, ErbB2 and ErbB3 immunoreactivity has been reported in normal human skin [27–29], and in mouse skin and cultured murine keratinocytes [30,31]. Recently, the immortalized human keratinocyte cell line HaCaT has been shown to express ErbB2 and ErbB3 in addition to ErbB1 [32]. However, none of the prior human in vivo studies provided biochemical evidence for expression of the various ErbBs in skin, nor did they address whether the non-ErbB1 receptors identified were functional.
The objectives of this study were to define the expression of ErbB species in human skin by biochemical means, and to determine whether members of the ErbB family other than ErbB1 are required for various aspects of skin physiology. Our findings demonstrate expression and function of multiple ErbBs in human skin, and suggest that proliferating normal keratinocytes and malignant A431 cells maintain a state of ErbB1 dominance, at least in part, by restricting cell surface expression of ErbB2.
Materials and Methods
Reagents
Human recombinant EGF was purchased from Sigma (St. Louis, MO) or from Becton Dickinson/Collaborative Biomedical Products (Franklin Lakes, NJ). Human recombinant heregulin (heregulin-β1176–246) was from R&D Systems (Minneapolis, MN). Monoclonal antibodies directed against ErbB1, ErbB2, ErbB3, and ErbB4 were from Labvision (Fremont, CA), Transduction Laboratories (Franklin Lakes, NJ) and Santa Cruz Biotechnology (Santa Cruz, CA) (Table 1). The mouse monoclonal antiphosphotyrosine antibody 4G10 and horseradish peroxidase-conjugated goat antimouse IgG were from Upstate Biotechnology (Lake Placid, NY). Complete protease inhibitor tablets were from Boehringer Mannheim Biochemicals (Indianapolis, IN). All other biochemicals were purchased from Sigma (St. Louis, MO).
Table 1.
Anti-ErbB Antibodies Used in This Study.
| Receptor | Supplier | Antibody Name | Application | Concentration (−g/mL) | Epitope | Isotype |
| ErbB1 | Transduction Laboratories | Clone 13 | WB | 0.5 | aa 996–1022 | IgG1 |
| ErbB1 | Neo Markers | Ab 13 | IP | 2 | extracellular | IgG2a |
| ErbB1 | Neo Markers | Ab 14 | IF | 0.4 | extracellular | IgG1/IgG2a |
| ErbB1 | Neo Markers | Ab 15 | IF | 2 | intracellular | IgG1 |
| ErbB2 | Neo Markers | Ab 2 | IP/IF | 2 | extracellular | IgG1 |
| ErbB2 | Neo Markers | Ab 17 | IF/WB | 0.5 | intracellular | IgG1 |
| ErbB3 | Upstate Biotechnology | 2F12 | WB | 1 | aa 1295–1323 | IgG2a |
| ErbB3 | Neo Markers | Ab 4 | IP | 2 | extracellular | IgG2a |
| ErbB4 | Santa Cruz Biotechnology | C7 | WB | 4 | intracellular | IgG2a |
| ErbB4 | Neo Markers | Ab 1 | IP | 2 | extracellular | IgG1 |
Organ Culture
After sacrifice by CO2 inhalation, skin from adult wild-type C57BL6 mice (Jackson Laboratories, Bar Harbor, ME) was dissected down to the level of the panniculus carnosus, cut into ≈ 1 cm2 pieces, then incubated at 37°C in basal MCDB153 medium without growth factors just as previously described for human skin [17]. Organ cultures were maintained for various times (0h - 24h) in the presence or absence of various concentrations of the ErbB tyrosine kinase inhibitors PD153035 and PD158780. The same procedure was followed for two strains of ErbB (+/+), (+/-), or (-/-) mice, except that the mice were less than 1 month old at the time of sacrifice. One strain was engineered to lack exon 2, which encodes an amino-terminal segment of ErbB1 [24]. The second strain was engineered to lack exon 1, which encodes the promoter region and 5'-untranslated region of ErbB1 mRNA [25]. Skin samples were processed for RNA isolation and analyzed by northern blotting as described below.
RNA Isolation and Northern Blotting
Total RNA was isolated by homogenization of full-thickness mouse skin in RNAzol (Tel-Test, Friendswood, TX) followed by cesium chloride gradient centrifugation as previously described [34]. Ten to thirty micrograms of RNA was separated on 1% formaldehyde agarose gels, transferred to derivitized nylon membranes (Zeta-Probe, BioRad, Richmond CA), and hybridized against 32P-labeled HB-EGF cDNA inserts as previously described [17]. As a control for RNA loading and intactness, the blots were stripped and rehybridized against 36B4, which encodes the human acidic ribosomal phosphoprotein PO [35]. Hybridization signals were quantitated by densitometer (Molecular Dynamics, Sunnyvale, CA), normalized against 36B4, and expressed either as fold changes relative to nonorgan-cultured skin or as percentages of untreated controls.
Cell Culture
A431 human epidermoid carcinoma cells [36] and MDA-MB-453, MDA-MB-468, and MCF-7 human breast carcinoma cells [37,38] were obtained from the American Type Culture Collection (Manassas, VA) and grown in DMEM (A431, MCF-7) or DMEM/F12 (1:1) (MDA-MB-453, MDA-MB-468), containing 10% fetal bovine serum and antibiotics. Normal human keratinocyte (NHK) cultures were established from sun-protected adult human skin as described [39] in serum-free medium optimized for high-density keratinocyte growth (Medium 154; Cascade Biologics, Portland, OR). NHKs were used for experiments in the second or third passage. All cells were plated at 5000 cells/cm2. For cell growth experiments, NHKs were allowed to attach overnight, after which two dishes were harvested by trypsinization and counted in triplicate using a hemacytometer (Reichert-Jung, Buffalo, NY). The remaining cultures were then maintained for 3 days in the presence or absence of 20 to 2500 nM of the ErbB tyrosine kinase inhibitors PD153035, PD158780, or PD166547, followed by trypsinization and counting. Standard deviations for counting were <20% of the mean. Duplicate plates were harvested for each data point; average cell counts obtained for duplicate cultures differed by less than 20%. IC50 values were determined from curves generated by linear interpolation of the average cell counts for duplicate cultures after subtracting the average cell count obtained from replicate dishes after overnight cell attachment.
Cell and Tissue Lysis and Western Blotting
All procedures involving human volunteers were approved by the University of Michigan Medical School Institutional Review Board. After obtaining informed consent, human skin keratomes were harvested from the buttocks of healthy volunteers as previously described [33] and 0.4 cm2 of tissue was extracted by boiling for 10 min in 0.5 ml of boiling, freshly-prepared, Laemmli sample buffer (62.5 mM Tris-HCI pH 6.8, 2% sodium dodecyl sulfate (SDS), 10% glycerol, 50 mM dithiothreitol) [40]. Cultured cells were grown in 100 mm dishes until 70–80% confluent, then lysed by scraping with a rubber policeman with 1 ml of boiling Laemmli sample buffer. After a 5 minute spin at 12,000g to remove insoluble material, equal amounts of lysate (as determined by OD280) were run on 4% to 20% Tris-glycine gels (Novex, San Diego, CA) and transferred electrophoretically to PVDF membranes according to the manufacturer's instructions. Western blots were decorated by rocking in Dulbecco's phosphate-buffered saline (PBS) without calcium or magnesium (PBS) containing 5% nonfat dry milk (NFDM) for 30 minutes and incubated overnight with the various antibodies shown in Table 1 at 4°C in PBS/5% NFDM. The membranes were washed twice for 30 minutes with PBS/5% NFDM and incubated with horseradish peroxidase-conjugated goat antimouse IgG (0.5 µg/mL; Upstate Biotechnology) in PBS/5% milk for 1 hour at room temperature. After two further 30-minute washes in PBS/NFDM and a 5-minute wash in PBS plus 0.1% Tween-20, bound antibodies were detected by chemiluminescence (ECL-Plus; Amersham Pharmacia, Piscataway, NJ) according to the manufacturer's instructions.
Tyrosine Phosphorylation Assay
NHK, A431, or MDA-MB-453 cells were grown until 40% to 50% confluent, then incubated for 48 hours in their own culture media without growth factors (NHK) or serum (A431, MDA-MB-453). After growth factor deprivation, cells were preincubated with 2 to 10,000 nM of the ErbB tyrosine kinase inhibitors PD153035, PD158780, PD166547, or DMSO vehicle for 2 hours, then stimulated with EGF (100 ng/mL) or heregulin (50 ng/mL) for 10 minutes. After stimulation, cells were harvested and processed for Western blotting as described above, except that equal aliquots were defined as equal proportions of the total lysate volume. Blots were decorated with the antiphosphotyrosine mAb 4G10 at 0.5 µg/ml. For determination of IC50 values for the various RTK inhibitors, the ligand-inducible phosphotyrosine signals in the 170 to 180 kDa range were quantitated using a densitometer (Molecular Dynamics). Data are expressed as a stimulation index (SI), calculated according to the following formula:
where a, b, and c are the densitometric intensities of the 170 to 180 kDa band obtained from inhibitor-pretreated, ligand-stimulated cultures, vehicle-pretreated, ligand-stimulated cultures, and unstimulated cultures, respectively. IC50 values were determined by linear interpolation of SI values.
Immunoprecipitations
NHKs were depleted of growth factors for 48 hours and treated with EGF as described above. The cells were washed with PBS then lysed in 1 mL ice-cold Buffer A per dish (10 mM PIPES 6.8, 250 mM sucrose, 3 mM MgCl2, 150 mM KCl, 5 mM EGTA, 100 mM sodium fluoride, 5 mM sodium orthovanadate, 10 mM sodium pyrophosphate, 10% glycerol, 1% Triton X-100, and 1x protease inhibitor cocktail). The cell lysates were precleared by centrifugation for 15 minutes at 3000g and 500 µg of protein, as determined by the Bradford assay (Bio-Rad, Hercules, CA), was rotated overnight at 4°C with one of the four anti-ErbB antibodies at the concentrations listed in Table 1. Controls contained the same concentration of an isotype-matched control mouse IgG (Sigma). The immune complexes were bound to protein A/G agarose (Santa Cruz Biotechnology) according to the manufacturer's instructions, washed three times with cold PBS, then pelleted by centrifugation for 5 minutes at 3000g. After boiling in Laemmli sample buffer for 5 minutes, immunoprecipitates were analyzed by Western blotting as described above.
Immunofluorescence Microscopy
NHK or A431 cells were seeded on glass coverslips at 5000 cells/cm2, grown to approximately 50% confluence in complete M154 or DMEM/10% FCS, respectively, and processed for immunofluorescence 24 hours after their last feeding. Normal human skin was obtained from sun-protected sites, snap-frozen in OCT compound, and cryosectioned. Fixation and immunostaining were performed exactly as previously described [41], using anti-ErbB1 and anti-ErbB2 antibodies at the concentrations given in Table 1. The secondary antibody was fluorescein isothiocyanate (FITC)-conjugated goat antimouse IgG (Santa Cruz Biotechnology), which was used at 15 µg/mL. The diluent was 1x PBS containing 1% BSA. Controls included equivalent molar amounts of the isotype control mAb and omission of primary antibody; all controls were negative. Slides were examined under a Zeiss Axioskop microscope equipped for fluorescence microscopy. Images were captured digitally on a 2.2 megapixel diode array camera (Optronics, Goleta, CA).
Results
Mouse Skin Organ Culture
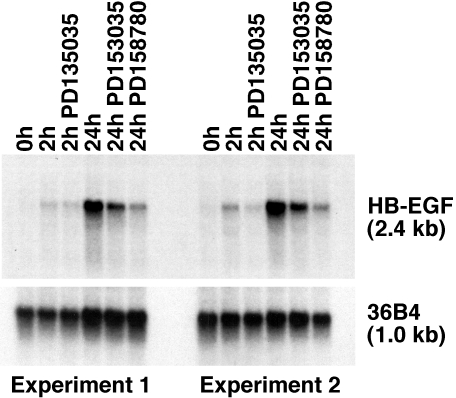
Expression of HB-EGF mRNA was assessed after organ culture of full-thickness dorsal mouse skin subjected to organ culture for 2 or 24 hours. As shown in Figure 1, induction of HB-EGF mRNA was detectable after 2 hours, and quite marked after 24 hours. Furthermore, two ErbB RTKIs active against all ErbB-RTKs (see below) markedly inhibited induction of HB-EGF mRNA. After 24 hours of organ culture, 1 µM PD158780 and 0.2 µM PD153035 inhibited HB-EGF mRNA to 22.6±6.5% and 45.7±9.1% of the vehicle controls, respectively (mean±range, n=2).
Figure 1.
Inhibition of inducible HB-EGF mRNA expression in mouse skin organ culture by ErbB-RTK inhibitors. Skin fragments were subjected to organ culture in the presence or absence of 0.2 µM PD153035 or 1 µM PD158780 for the times shown above the autoradiograms. Total RNA (30 µg/lane) was subjected to Northern blotting and hybridized against HB-EGF or 36B4 probes as described in Materials and Methods section. 36B4 is shown as control for RNA loading. Results for two different mice are shown.
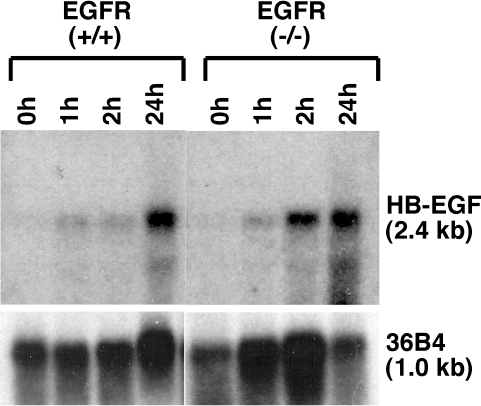
We next performed additional organ culture experiments with skin isolated from two different strains of ErbB1 knockout mice [24,25]. Substantial induction of HB-EGF mRNA was observed in both strains (Figure 2). After densitometric quantitation and normalization for the control gene 36B4, a 9.3±3.6-fold (mean±SEM, n=5) induction was obtained after 24 hours of organ culture in all ErbB (-/-) animals combined, as compared to a 3.9±0.7-fold induction in their ErbB (+/+) littermates (mean±SEM, n=5). This difference was not statistically significant. There was appreciable degradation of some of the RNA samples, probably due to prolonged storage of the tissue homogenates prior to final purification. Degradation happened to be more severe in the ErbB1 (+/+) group (data not shown). The mean normalized fold induction of HB-EGF mRNA in the ErbB1 (-/-) animals was comparable to that obtained in the experiments shown in Figure 1, in which RNA purification was carried out immediately after skin was harvested (10.8±3.6-fold, mean±range, n=2).
Figure 2.
Induction of HB-EGF mRNA is intact in skin organ cultures of ErbB1 knockout mice. Skin fragments from ErbB1 knockout (-/-) mice and their nontransgenic (+/+) littermates were placed in organ culture for various times, as indicated on top of the figure. Total RNA (30 µg/lane) was subjected to Northern blotting and hybridized against 32P-labeled HB-EGF and 36B4 cDNA probes as described in Materials and Methods section. 36B4 is shown as control for RNA loading. Quantitative densitometry revealed a 9.3±3.6-fold induction of HB-EGF after 24 hours of organ culture in all ErbB1 (-/-) animals combined (mean±SEM, n=5), compared to a 3.9±0.7-fold induction in their ErbB (+/+) littermates (mean±SEM, n=5). The differences between ErbB (+/+) and ErbB (-/-) animals were not statistically significant (P=.198 by Welch's unpaired t test assuming unequal variances and a two-tailed hypothesis).
The experiments depicted in Figures 1 and 2 suggested that the organ culture HB-EGF response was at least partially dependent upon ErbB-RTK activity, but not specifically dependent on the presence of ErbB1. This implied that ErbB species other than ErbB1 must be available to carry out the required signaling events, at least in ErbB1 (-/-) mice. We proceeded to address this question further in human skin because (i) we have a dermatological interest in human skin problems, (ii) mouse and human skin behave similarly in organ culture (see Discussion section), and (iii) a comprehensive panel of appropriate antibodies was available for humans but not for mice.
Expression of ErbB Receptors in Human Skin, Carcinoma Cell Lines and NHK
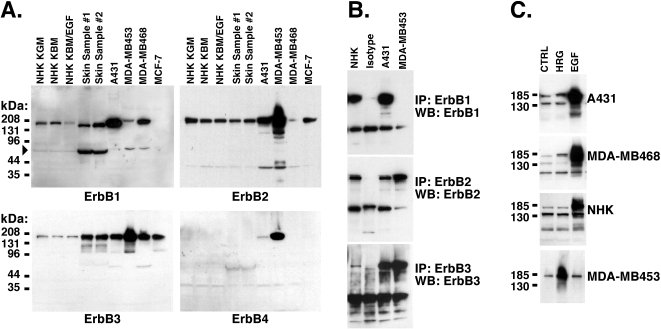
We detected ErbB1, ErbB2, ErbB3, and ErbB4 in ionic detergent lysates by Western blotting, using antibodies specifically directed against the four ErbB family members. The sources and concentrations of antibodies used are given in Table 1. The specificity and sensitivity of these antibodies were assessed by analysis of A431, MDA-MB453, MDA-MB-468, and MCF-7 cells (Figure 3). As shown in Figure 3A, A431 cells expressed substantial amounts of ErbB1, as expected given the ∼30-fold amplification of the ErbB1 gene in these cells [42]. However, they also expressed substantial amounts of ErbB2 and ErbB3, but little ErbB4. MDA-MB-453 cells yielded very strong expression of ErbB2, and readily detectable amounts of ErbB3 and ErbB4. The anti-ErbB1 antibody detected a faint 180 to 185 kDa band in MD-AMB-453 cells. However, this band could not be immunoprecipitated by a second antibody directed against a different epitope of ErbB1 (Figure 3B). In MDA-MB-468 cells, we detected large amounts of ErbB1, moderate amounts of ErbB3, and low levels of ErbB2 (Figure 3A). Finally, MCF-7 cells expressed little or no ErbB1 or ErbB4, but readily detectable ErbB2 and ErbB3. These results were in good agreement with the available literature (see Discussion section).
Figure 3.
Presence of multiple ErbB receptors in human keratinocytes and skin. (A) Ionic detergent lysates were prepared from normal human skin as well as NHK, A431, MDA-MB-453, MDA-MB-468, and MCF-7 cells as described in Materials and Methods section. All cell cultures except those labeled “NHK KBM” and “NHK KBM/EGF” were grown in complete medium and harvested at approximately 80% confluence. The cells shown in lanes “NHK KBM” and “NHK KBM/EGF” were grown in complete medium until 40% to 50% confluent, growth factor-depleted for 48 hours, then treated with 100 ng/mL EGF or PBS vehicle for 10 minutes. Equal amounts of protein lysate were analyzed by Western blotting. Replicate blots were decorated with ErbB1, ErbB2, ErbB3, and ErbB4 antibodies. See Table 1 for antibody concentrations and isotypes. The band indicated by the filled triangle co-migrated with a prominent band on amido black-stained filters, and was not seen using different anti-ErbB1 primary antibodies, or by immunoprecipitation (data not shown). Also, this band partitioned into the insoluble fraction of the homogenate when skin homogenates were prepared in the absence of detergents (data not shown). Therefore, this band probably represents nonspecific cross reactivity of this particular anti-ErbB1 antibody with keratin. Keratinocyte cultures prepared from three different donors all demonstrated comparably low expression of ErbB3 (data not shown). (B) Immunoprecipitation of ErbB1, ErbB2, and ErbB3 in NHK and carcinoma cell lines. Nonionic detergent lysates of the indicated cell lines were immunoprecipitated, then subjected to Western blotting for detection of the same ErbB species. The ErbB3 panel is a 10-minute exposure, whereas the ErbB1 and ErbB2 panels are 1-minute exposures. No ErbB1 was detectable in MDA-MB-453 cells, even after 10 minutes of exposure (not shown). ErbB4 results are not shown because none of the antibodies we tested immunoprecipitated ErbB4 from MDA-MB-453 cell lysates. (C) Ligand-stimulated tyrosine phosphorylation in NHK and carcinoma cell lines. Cells were growth factor-depleted and stimulated with ligand, followed by ionic detergent extraction and Western blotting for phosphotyrosine. Note that the ability to be stimulated by EGF correlates with the presence of ErbB1, but that the ability to be stimulated by heregulin does not correlate with the presence of ErbB2 and ErbB3.
Having validated our assays, we proceeded to analyze ionic detergent lysates from human skin and cultured keratinocytes. ErbB1, ErbB2, and ErbB3 were consistently detected by Western blotting in normal human skin and NHK. ErbB3 expression was substantially lower in normal keratinocytes than in human skin on a protein basis; A431 cells also expressed higher levels of ErbB3 than did NHK (Figure 3A). Across a large number of experiments involving three different antibodies, ErbB4 immunoreactivity was very low to undetectable in keratinocytes and skin (Figure 3A, data not shown).
We next compared the ligand specificity of total cell tyrosine phosphorylation after a 10-minute stimulation with EGF or heregulin. As shown in Figure 3C, A431 cells, MDA-MB-468 cells, and NHK displayed robust tyrosine phosphorylation in response to EGF, but not in response to heregulin. Conversely, MDA-MB-453 cells displayed robust tyrosine phosphorylation in response to heregulin, but not in response to EGF. For the most part, these patterns of ligand-induced tyrosine phosphorylation correlated well with the receptor profiles shown in Figure 3A and B, given the established ligand specificities of each receptor [1,2]. However, the failure of A431 and NHK cells to respond to heregulin was puzzling, given that A431 cells expressed large amounts of ErbB2 and ErbB3, and NHK expressed substantial amounts of ErbB2 and at least some ErbB3.
Subcellular Localization of ErbB1 and ErbB2
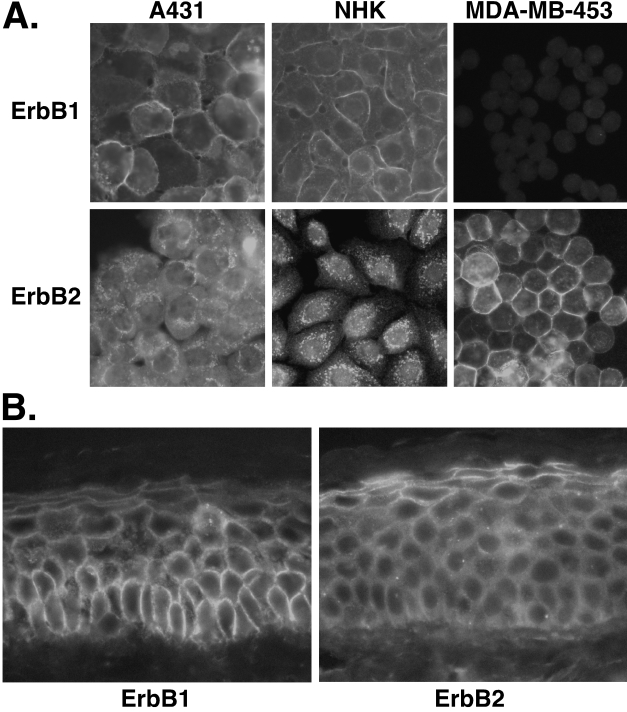
One possible explanation for the failure of A431 cells and NHK to respond to heregulin would be intracellular compartmentalization of ErbB2. Indeed, immunofluorescence microscopy revealed markedly different patterns of intracellular distribution for ErbB1 and ErbB2 in proliferating A431 cells and NHK, compared to MDA-MB-453 cells. In A431 and NHK, ErbB1 displayed a well-defined cell surface pattern, together will low levels of punctate intracytoplasmic staining. In contrast, ErbB2 displayed only the punctate cytoplasmic pattern, with no evidence of membrane staining (Figure 4A). Similar patterns were observed with two other anti-ErbB2 antibodies (data not shown). In MDA-MB-453 cells, ErbB1 was not detectable, consistent with the Western blotting results. However, unlike A431 and NHK, MDA-MB-453 cells showed a strong pattern of cell surface ErbB2 staining (Figure 4A). Cells in dense keratinocyte cultures often became large and flattened, and express makers of epidermal differentiation. These large, flattened cells often displayed a cell surface staining pattern for ErbB2, whereas A431 cells remained rhomboidal in shape in dense cultures and did not display cell surface expression of ErbB2 (data not shown).
Figure 4.
Immunofluorescence microscopy of ErbB1 and ErbB2 in actively growing NHK, MDA-MB-453, and A431 cells (A) and normal human skin (B). Immunofluorescence was carried out as described in Materials and Methods section, using anti-ErbB1 and ErbB2 antibodies at the concentrations indicated in Table 1. (A) Cultured cells. Note differential compartmentalization of ErbB1 and ErbB2 in A431 cells and NHK. Similar results were observed for at least two anti-ErbB1 and anti-ErbB2 antibodies (data not shown). (B) Normal human skin. Photomicrographs taken through the 40 objective are shown. Note the plasma membrane (“chicken wire”) staining pattern throughout all epidermal layers for ErbB1, whereas a discrete plasma membrane staining pattern is only observed in the uppermost epidermal layers for ErbB2. The results shown are representative of those obtained for at least three normal individuals. Similar results were obtained with at least two different antibodies each for ErbB1 and ErbB2 (data not shown).
Next, cryostat sections of normal human skin were immunostained for ErbB1 and ErbB2 (Figure 4B, left panel). The ErbB1 antibody revealed a strong “chicken wire” staining pattern in all epidermal layers, consistent with cell membrane staining. Higher power views through the 100x oil immersion objective (not shown) reveal that this “chicken wire” pattern is comprised of linear plasma membrane staining together with punctate staining in the cortical cytoplasm just beneath the plasma membrane. Scattered punctate staining was also seen at low levels throughout the cytoplasm. In contrast, ErbB2 staining was diffusely cytoplasmic and punctate in the lower epidermal layers, transitioning to an increasingly cortical submembranous location in the upper suprabasal layers, and finally to a sharp “chicken wire” pattern in the stratum granulosum, the uppermost viable keratinocyte layers just below the stratum corneum (Figure 4B, right panel).
Validation of ErbB-RTK Inhibitors
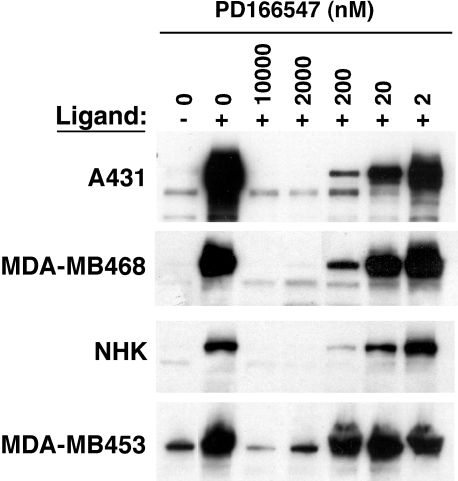
We next utilized ErbB-RTK inhibitors of varying specificity to explore the functional contribution of different ErbB receptors to keratinocyte growth in culture. Three inhibitors were selected for study. Two of them, PD158780 and PD153035, were previously shown to have similar inhibitory potencies against ErbB1 versus non-ErbB1-RTKs, whereas a third, PD166547 (“compound 5k” in Ref. [43]), was approximately 40-fold more potent against ErbB1-RTK than against non-ErbB1-RTKs (Table 2) [43–45]. Because the specificity of inhibitors is a critical issue, we repeated these dose-response experiments under low-calcium, serum-free conditions known to be optimal for EGF-stimulated proliferation and gene responses in NHK [46,47]. PD158780 and PD153035 markedly and potently inhibited ligand-stimulated tyrosine phosphorylation under these conditions in EGF-stimulated A431 cells and in heregulin-stimulated MDA-MB-453 cells (Table 2). In contrast, and consistent with previous reports [43], PD166547 was clearly less potent in heregulin-stimulated MDA-MB-453 cells than in EGF-stimulated A431 cells, MDA-MB-468 cells, and NHK (Figure 5, Table 2). Table 2 also demonstrates that all of these compounds were more potent in our hands than previously reported [43–45], possibly due to the use of culture conditions known to be optimized for ErbB signaling [46,47].
Table 2.
Relative Potencies of ErbB-RTK Inhibitors.
| Compound | IC50 Values for Inhibition of Ligand-Induced Tyrosine Phosphorylation | IC50 Values for Inhibition of NHK Growth in Complete Medium | ||||
| EGF-Stimulated A431 Cells | Heregulin-Stimulated MDA-MB-453 Cells | EGF-Stimulated NHK | ||||
| Previous Reports* (nM) | This Report (nM) | Previous Reports* (nM) | This Report (nM) | This Report (nM) | This Report (nM) | |
| PD153035 | 13 | <2 | 195 | <2 | <2 | 68 |
| PD158780 | 14 | <2 | 52 | <2 | <2 | 95 |
| PD166547 | 28 | 8 | 1100 | 365 | 72 | 490 |
Refs. [43–45].
Figure 5.
Inhibition of ligand-stimulated tyrosine phosphorylation in carcinoma cell lines and NHK by PD166547. Cells were cultured, deprived of growth factors, preincubated for 2 hours with inhibitors or DMSO vehicle, then stimulated for 10 minutes with heregulin or EGF as detailed in Materials and Methods section. Western blots were decorated with the antiphosphotyrosine antibody 4G10. Bands shown illustrate the areas of the gels used for quantitation of tyrosine phosphorylation in Table 2. Note the lower potency of PD166547 in MDA-MB-453 cells.
Effects of ErbB-RTK Inhibitors on NHK Growth
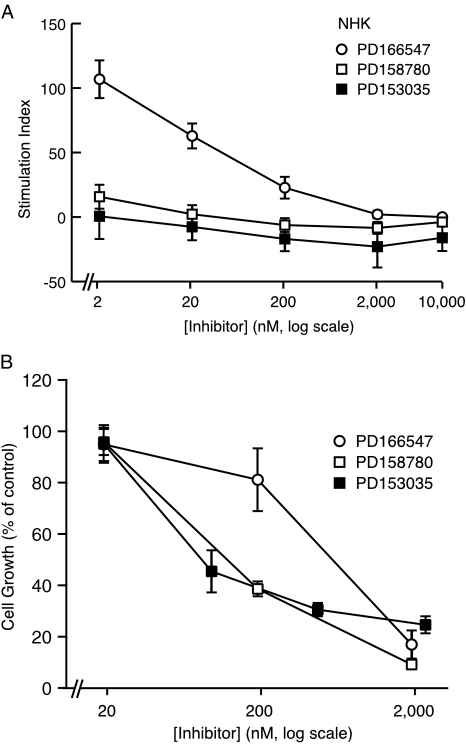
We next assessed the effects of the same three inhibitors on NHK cell number in a 3-day growth assay. In this assay, the growth medium contained insulin and bovine pituitary extract in addition to EGF. Under these conditions, all three compounds were substantially (10 to 50 times) less active as inhibitors of NHK growth than they were as inhibitors of EGF-stimulated tyrosine phosphorylation (Figure 6, Table 2, also see Discussion section). PD153035 and PD158780 were similarly potent in their ability to inhibit NHK growth, whereas PD166547 was five- to seven-fold less potent in this regard (Figure 6, Table 2). PD158780 and PD153035 were also at least four times more potent as inhibitors of EGF-stimulated tyrosine phosphorylation in A431 cells, compared to PD166547 (Table 2). Taken together, these results indicated that relative to their intrinsic potencies as RTK inhibitors, all three compounds were approximately equally efficacious as inhibitors of NHK growth despite large differences in their selectivity for ErbB1.
Figure 6.
Inhibition of EGF-stimulated tyrosine phosphorylation and growth of NHK in response to the ErbB tyrosine kinase inhibitors PD158780, PD153035, and PD166547. (A) Tyrosine phosphorylation assay. NHK were growth factor-depleted, pretreated with inhibitor, and stimulated with EGF for 10 minutes as described in Materials and Methods section. After Western blotting and decoration with antiphosphotyrosine mAb 4G10, the prominent ligand-inducible band at 170 to 180 kDa was quantitated by densitometric scanning of autoradiograms. Stimulation indices were calculated as described in Materials and Methods section and expressed as a percentage of the response obtained for ligand-treated cells in the absence of inhibitor. Negative stimulation indices indicate that tyrosine phosphorylation was blocked to below basal levels. PD166547, open circles. PD158780, open squares. PD153035, solid squares. Number of replicates: PD153035, n=3; PD158780, n=5, PD166547, n=3. Error bars represent SEM. (B) Growth assay. NHKs were grown in complete medium in the presence of the various inhibitors or diluent for 72 hours without change of medium, then harvested by trypsinization and counted as described in Materials and Methods section. The data are expressed as percent of untreated controls. Error bars indicate mean±SEM. The number of replicates: PD153035, n=4; PD158780, n=6; PD166547, n=3.
Discussion
Historically, substantial evidence has accumulated to implicate ErbB1, the cardinal member of the ErbB family, in the control of epidermal growth and differentiation. However, much of the earlier data made use of EGF binding assays that would not detect ErbB1 specifically [48]. The discovery of multiple additional members of the ErbB family [1,2] raises the question of whether ErbB1 is the sole mediator of these processes in the skin. We have addressed this question via a combination of approaches, including knockout mice, immunoblotting, pharmacologic inhibitors, and immunolocalization experiments.
As shown in Figure 1, both PD153035 and PD158780 markedly inhibited the HB-EGF mRNA response in organ cultures prepared from wild type mice. Both compounds inhibited the mouse skin response with the same potency they displayed in human skin organ culture (Ref. [17] and data not shown). This suggests that ErbB signaling plays a similar role in the skin organ culture response in man and mouse. However, two strains of ErbB1 (-/-) animals demonstrated very similar induction of HB-EGF mRNA compared to their wild-type littermates (Figure 2) or to other wild-type animals (Figure 1). This would not be expected if ErbB1 were the only relevant ErbB species involved in the skin organ culture response. These results cannot be explained by nonspecificity of the inhibitors, as we utilized PD153035 and PD158780 at 0.2 to 1 µM, concentrations well below their thresholds of activity against non-ErbB RTKs (≈50 µM) [43–45]. Alternatively, it could be argued that the exon 2 knockout construct might express the cytoplasmic domain of ErbB1 due to alternative splicing. Indeed, a minor ErbB1-immunoreactive band has been reported in keratinocytes from another ErbB1 exon 2 knockout strain [49]. However, the ErbB1 exon 1 knockout we tested has been shown to be totally lacking in ErbB1 mRNA expression [25], and the only ErbB1 mRNA expressed by the exon 2 knockout we tested contains multiple stop codons (Z.W., unpublished data).
It could also be argued that ErbB family members other than ErbB1 come into play only when ErbB1 is genetically ablated. However, our own earlier studies of human skin organ culture [17] argue against this interpretation. In those studies, mAb 225 IgG was utilized as a reagent to block inducible HB-EGF expression in organ culture. 225 IgG is known to specifically inhibit ligand activation of ErbB1-RTK in A431 cells [50]. Despite use of 225 IgG concentrations 16 times higher than required to completely inhibit ligand-induced ErbB tyrosine phosphorylation, and >50 times higher than that required to maximally inhibit EGF-inducible TGF-α expression [47], 225 IgG inhibition was substantially less complete than that produced by nontoxic doses of the pan-ErbB inhibitor PD153035 (Table 2 of this report, and Figure 2 of [17]). As discussed in more detail below, Figure 3 documents robust expression of ErbB1, ErbB2, and ErbB3 in human skin. Taken together, these results strongly suggest that multiple ErbB species participate in the human skin organ culture HB-EGF response, even when ErbB1 is present.
In order to identify sensitive and specific reagents for detection of each ErbB species, we screened a total of 15 commercially available antibodies. To validate the specificity and sensitivity of these antibodies, we tested them against a panel of commonly used tumor cell lines. The results provide a uniform comparison of ErbB expression in these lines for the first time. MDA-MB-468 cells expressed large amounts of ErbB1, moderate amounts of ErbB3, and a small amount of ErbB2, as previously reported [51,52]. In our hands, MCF-7 cells expressed ErbB2 and ErbB3, but no ErbB1 and little or no ErbB4 (Figure 3). It has long been established that ErbB2 and ErbB3 are expressed by MCF-7 cells [53]. While one recent report found expression of ErbB4 in this cell type [54], two other reports found little or no ErbB4 unless it was ectopically expressed [55,56]. A431 cells expressed substantial amounts of ErbB1, ErbB2, and ErbB3, but very little ErbB4 (Figure 3A). These findings were confirmed by immunoprecipitation using antibodies directed against different epitopes of ErbB1, ErbB2, and ErbB3 (Figure 3B). This pattern is similar to previous reports [57,58] and is of interest because earlier studies emphasized gene amplification and overexpression of ErbB1 in A431 cells [42] without considering the other ErbB species. We found that MDA-MB-453 cells expressed large amounts of ErbB2, lesser but substantial amounts of ErbB3 and ErbB4, and small amounts of a 180- to 185-kDa band detected by anti-ErbB1. This band probably represents cross reactivity against ErbB2 because it co-migrated with ErbB2 and because we could not detect it by immunoprecipitation using a different anti-ErbB1 antibody (Figure 3B). This expression pattern is also in good agreement with previous reports [45,52]. However, we found more ErbB4 in MDA-MB-453 cells than reported by others [52]. Substantial amounts of all four ErbB mRNAs have been detected in MDA-MB-453 cells by RT-PCR [59]. Taken together, these results validate the specificity of the antibodies used, and demonstrate that these frequently used tumor cell lines display characteristic patterns of ErbB protein expression. However, they also suggest that these lines probably undergo stochastic or adaptive changes in ErbB expression as they are maintained in culture.
Despite the fact that A431 cells, NHK, and MDA-MB-453 cells all expressed substantial amounts of ErbB2 and ErbB3, very different patterns of ligand-stimulated tyrosine phosphorylation were observed between ErbB1-expressing A431 cells and NHK on one hand, and ErbB1-nonexpressing MDA-MB-453 cells on the other (Figure 3C). Some of these differences are explicable in light of current theories of receptor activation [4], known patterns of ligand-receptor binding and intrinsic RTK activity [1,2], and the ErbB expression patterns shown in Figure 3. MDA-MB-453 cells showed only a slight increase in tyrosine phosphorylation in response to EGF, as compared to a 20- to 100-fold induction in response to heregulin (Figure 3C). Similar observations have been reported recently [52]. This response probably reflects the lack of ErbB1 in MDA-MB-453 cells, coupled with the fact that EGF does not bind efficiently to any of the other ErbB species [1,2]. In contrast, A431 cells and NHK showed only a slight increase in protein tyrosine phosphorylation in response to heregulin, as compared to a 20- to 100-fold increase in response to EGF (Figure 3C).
This lack of heregulin responsiveness is only partially explained by recalling the very low affinity of heregulin for ErbB1 [60] coupled with lack of ErbB4 (Figure 3A). Coexpression of ErbB2 and ErbB3 in cells that normally lack ErbB receptors leads to robust tyrosine phosphorylation of ErbB2 and ErbB3 [61,62]. NHK expressed only low levels of ErbB3 (Figure 3A and B), meaning that these cells could only form limited amounts of ErbB2-ErbB3 heterodimers to serve as a functional heregulin receptor. This could explain why NHKs were unresponsive to heregulin. However, low ErbB3 levels cannot explain why heregulin-dependent tyrosine phosphorylation was not observed in A431 cells, as both of these receptors are well expressed in A431 (Figure 3A). This paradox can be explained by the observation that ErbB2 was confined to the cytoplasm of A431 cells (Figure 4A), suggesting that ErbB2 and ErbB3 are unable to form a functional heregulin receptor on the surface of A431 cells. Interestingly, a similar phenomenon was observed in intact skin. ErbB1 retains a predominantly peripheral plasma membrane and cortical submembranous location throughout the epidermis, forming a “chicken wire” pattern (Figure 4B). In contrast, ErbB2 assumes a diffuse punctate distribution throughout the cytoplasm in the basal and lower suprabasal keratinocytes that comprise the proliferative compartment of the epidermis, but transitions to a discrete “chicken wire” pattern in the highly differentiated keratinocytes of the upper epidermal layers (Figure 4B).
Taken together, the absence of ErbB4, the lack of cell surface ErbB2, and the inactive nature of the ErbB3 RTK lead us to conclude that addition of EGF to rapidly proliferating A431 cells or NHK must primarily stimulate ErbB1-RTK activity (although later activation of intracellular ErbB2 cannot be ruled out). These findings support the use of A431 cells stimulated briefly with EGF as a selective assay for ErbB1- RTK activity. Because we and others have found no ErbB1 in MDA-MB-453 cells, we can also justify the use of MDA-MB-453 cells stimulated briefly with heregulin as an assay for ErbB-RTKs other than ErbB1. Using these assays (Table 2), we found that the RTK inhibitor PD166547 was about 45 times more potent in EGF-stimulated A431 cells than it was in heregulin-stimulated MDA-MB-453 cells, confirming earlier reports of a 40-fold ErbB1 selectivity for this compound [43]. In contrast, PD153035 and PD158780 were of very similar potency in the two assays and therefore represented “pan-ErbB” inhibitors. By comparing the potency of the three inhibitors in EGF-stimulated A431 cells, we could obtain an estimate of their intrinsic potencies. As shown in Table 2, PD166547 was at least four times less potent than either PD153035 or PD158780. PD166547 was also five to seven times less potent as a growth inhibitor for NHK, compared to the two pan-ErbB inhibitors (Figure 6). These differences in growth-inhibitory potency approximately match the differences in intrinsic potency, despite the fact that PD166547 is 40 to 45 times more potent against ErbB1-RTK than against the other ErbB species. Taken together, these results strongly suggest that ErbB1-RTK plays a predominant role in the regulation of NHK growth in culture.
NHK growth inhibition required 50 to 100 times more RTK inhibitor than was required to inhibit ligand-stimulated ErbB tyrosine phosphorylation (Table 2). Similar observations have been made previously [44,45]. Given the presence of the non-EGF mitogens insulin and bovine pituitary extract in the keratinocyte growth medium, this difference is most likely due to the contributions of non-ErbB signaling pathways to the process of keratinocyte growth. Indeed, we have observed that ErbB-RTKIs are at least an order of magnitude more potent as growth inhibitors when non-EGF mitogens are omitted from the growth medium [70]. This phenomenon probably also explains the lower potencies of these compounds in the skin organ culture system (Figure 1), in which a variety of signaling pathways in addition to ErbB are likely to be active.
These results are consistent with our previous work demonstrating that the mAb 225 IgG, which specifically blocks the ligand-inducible activation of ErbB1-RTK [50], is a potent inhibitor of NHK growth [47,63]. NHKs express high levels of the ErbB1-selective, EGF-like ligands HB-EGF, AR, and TGF-µ in an autocrine fashion [46,47,64]. In contrast, heregulin expression appears to be limited relative to TGF-µ, AR, and HB-EGF in cultured keratinocytes [65], and heregulins are not potent mitogens for keratinocytes relative to EGF [30,66]. Moreover, heregulin produced only a slight increase in tyrosine phosphorylation in NHK (Figure 3) due to low expression of ErbB3 (Figure 3) and/or to intracellular compartmentalization of ErbB2 (Figure 4). Taken together, these findings suggest that ligand-dependent ErbB-mediated keratinocyte proliferative responses pass primarily through ErbB1, whereas ligands of the heregulin family interacting with ErbB2 on the cell surface of upper-layer keratinocytes may play an important role in terminal differentiation. Recent studies (Predd H, Underwood R, Peterson T, Cook P, and Piepkorn M. Society for Investigative Dermatology 2001 Annual Meeting, Abstract 104) demonstrate that differentiating keratinocytes in culture and in intact human skin express heregulin in the same distribution we have observed for ErbB2. As ErbB1 expression is maintained throughout the epidermis (Figure 4B), ErbB1-selective ligands may also interact with ErbB2 in differentiated keratinocytes. Indeed, AR, which binds exclusively to ErbB1 [1,2], has been implicated in the control of what is arguably the most highly differentiated response of keratinocytes: maintenance of the stratum corneum permeability barrier [67].
This conclusion returns us to the original observations that initiated this work: mouse skin organ cultures appear to be highly dependent on ErbB signaling (Figure 1), yet independent of ErbB1 (Figure 2). Early epidermal wound healing responses are dominated by specialized and accelerated differentiation responses, which proceed for 1 to 3 days prior to the onset of significant epidermal proliferation in vivo [68] and in organ culture [17]. The primary function of these early responses, during which keratinocytes flatten and rapidly migrate to cover the wound, is to restore epidermal barrier function as rapidly as possible. Based on our results, we would speculate that signaling through ErbB family members other than ErbB1 in differentiated keratinocytes of the upper epidermal layers may be critical for these early wound healing responses, including induction of HB-EGF.
Finally, our data suggest that intracellular sequestration of ErbB2 may be an important mechanism by which malignant A431 cells limit their responsiveness to heregulin. If the interactions of ligands such as AR and heregulin with cell surface ErbB2 lead to growth arrest and terminal differentiation, as has been suggested for skin [67] and mammary epithelial cells [69], then the ability of A431 cells to limit cell surface expression of ErbB2 may be an important mechanism by which A431 and other skin carcinomas evade terminal differentiation signals in vivo. The intermingling of differentiation and proliferation has long been a puzzling feature of squamous cell carcinomas. Unraveling the mechanisms by which A431 cells limit cell surface expression of ErbB2 may provide new avenues for differentiation therapy of epithelial cancers, which in aggregate account for over 90% of all human malignancies.
Abbreviations
- AR
amphiregulin
- EGF
epidermal growth factor
- FITC
fluorescein isothiocyanate
- HB-EGF
heparin-binding EGF-like growth factor
- NHKs
normal human keratinocytes
- PBS
phosphate-buffered saline
- RTK
receptor tyrosine kinase
- TGF-α
transforming growth factor-α
Footnotes
This work was supported by an award (R29-AR40016) from the National Institute for Arthritis, Musculoskeletal and Skin Diseases, National Institute of Health, a University of Michigan Clinical Research Partnership Grant, and the Babcock Memorial Trust. S. W. S. is supported by a Chesebrough Pond's Lever Brothers Dermatology Foundation Research Career Development Award and a Dermatology Foundation Research Grant. S. K. and T. Z. are supported by an National Research Service Award from the National Institute for Arthritis, Musculoskeletal and Skin Diseases, National Institutes of Health (T32-AR07197). J. T. E. is supported by the Ann Arbor Veterans Affairs Hospital.
References
- 1.Klapper LN, Kirschbaum MH, Sela M, Yarden Y. Biochemical and clinical implications of the ErbB/HER signaling network of growth factor receptors. Adv Cancer Res. 2000;77:25–79. [PubMed] [Google Scholar]
- 2.Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harari D, Tzahar E, Romano J, Shelly M, Pierce JH, Andrews GC, Yarden Y. Neuregulin-4: a novel growth factor that acts through the ErbB-4 receptor tyrosine kinase. Oncogene. 1999;18:2681–2689. doi: 10.1038/sj.onc.1202631. [DOI] [PubMed] [Google Scholar]
- 4.Hubbard SR, Mohammadi M, Schlessinger J. Autoregulatory mechanisms in protein tyrosine kinases. J Biol Chem. 1998;273:11987–11990. doi: 10.1074/jbc.273.20.11987. [DOI] [PubMed] [Google Scholar]
- 5.Hackel PO, Zwick E, Prenzel N, Ullrich A. Epidermal growth factor receptors: critical mediators of multiple receptor pathways. Curr Opin Cell Biol. 1999;11:184–189. doi: 10.1016/s0955-0674(99)80024-6. [DOI] [PubMed] [Google Scholar]
- 6.Sweeney C, Lai C, Riese DJ, II, Diamonti AJ, Cantley LC, Carraway KL., III Ligand discrimination in signaling through an ErbB4 receptor homodimer. J Biol Chem. 2000;275:19803–19807. doi: 10.1074/jbc.C901015199. [DOI] [PubMed] [Google Scholar]
- 7.Pinkas Kramarski R, Shelly M, Glathe S, Ratzkin BJ, Yarden Y. Neu differentiation factor/neuregulin isoforms activate distinct receptor combinations. J Biol Chem. 1996;271:19029–19032. doi: 10.1074/jbc.271.32.19029. [DOI] [PubMed] [Google Scholar]
- 8.Graus Porta D, Beerli RR, Daly JM, Hynes NE. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 1997;16:1647–1655. doi: 10.1093/emboj/16.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang GC, Ouyang X, Epstein RJ. Proxy activation of protein ErbB2 by heterologous ligands implies a heterotetrameric mode of receptor tyrosine kinase interaction. Biochem J. 1998;331:113–119. doi: 10.1042/bj3310113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gamett DC, Pearson G, Cerione RA, Friedberg I. Secondary dimerization between members of the epidermal growth factor receptor family. J Biol Chem. 1997;272:12052–12056. doi: 10.1074/jbc.272.18.12052. [DOI] [PubMed] [Google Scholar]
- 11.Lenferink AE, Pinkas-Kramarski R, van de Poll ML, van Vugt MJ, Klapper LN, Tzahar E, Waterman H, Sela M, van Zoelen EJ, Yarden Y. Differential endocytic routing of homo- and heterodimeric ErbB tyrosine kinases confers signaling superiority to receptor heterodimers. EMBO J. 1998;17:3385–3397. doi: 10.1093/emboj/17.12.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guy PM, Platko JV, Cantley LC, Cerione RA, Carraway KL. Insect-cell-expressed p180erbB3 possesses an impaired tyrosine kinase activity. Proc Natl Acad Sci USA. 1994;91:8132–8136. doi: 10.1073/pnas.91.17.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woodburn JR. The epidermal growth factor receptor and its inhibition in cancer therapy. Pharmacol Ther. 1999;82:241–250. doi: 10.1016/s0163-7258(98)00045-x. [DOI] [PubMed] [Google Scholar]
- 14.Piepkorn M, Pittelkow MR, Cook PW. Autocrine regulation of keratinocytes: the emerging role of heparin-binding, epidermal growth factor-related growth factors. J Invest Dermatol. 1998;111:715–721. doi: 10.1046/j.1523-1747.1998.00390.x. [DOI] [PubMed] [Google Scholar]
- 15.Brown RL, Breeden MP, Greenhalgh DG. PDGF and TGF-alpha act synergistically to improve wound healing in the genetically diabetic mouse. J Surg Res. 1994;56:562–570. doi: 10.1006/jsre.1994.1090. [DOI] [PubMed] [Google Scholar]
- 16.Nanney LB. Epidermal and dermal effects of epidermal growth factor during wound repair. J Invest Dermatol. 1990;94:624–629. doi: 10.1111/1523-1747.ep12876204. [DOI] [PubMed] [Google Scholar]
- 17.Stoll S, Garner W, Elder J. Heparin-binding ligands mediate autocrine EGF receptor activation in skin organ culture. J Clin Invest. 1997;100:1271–1281. doi: 10.1172/JCI119641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elder JT, Fisher GJ, Lindquist PB, Bennett GL, Pittelkow MR, Coffey R, Jr, Ellingsworth L, Derynck R, Voorhees JJ. Overexpression of transforming growth factor alpha in psoriatic epidermis. Science. 1989;243:811–814. doi: 10.1126/science.2916128. [DOI] [PubMed] [Google Scholar]
- 19.Stoll SW, Elder JT. Retinoid regulation of heparin-binding EGF-like growth factor expression in human keratinocytes and skin. Exp Dermatol. 1998;7:391–397. doi: 10.1111/j.1600-0625.1998.tb00339.x. [DOI] [PubMed] [Google Scholar]
- 20.Elder JT, Voorhees JJ. Psoriasis. In: Jameson JL, editor. Principles of Molecular Medicine. Totowa, NJ: Humana; 1998. pp. 793–800. [Google Scholar]
- 21.Cook PW, Piepkorn M, Clegg CH, Plowman GD, DeMay JM, Brown JR, Pittelkow MR. Transgenic expression of the human amphiregulin gene induces a psoriasis-like phenotype. J Clin Invest. 1997;100:2286–2294. doi: 10.1172/JCI119766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen LA, Woodson RL, II, Holbus S, Strain K, Lo YC, Yuspa SH. The epidermal growth factor receptor is required to maintain the proliferative population in the basal compartment of epidermal tumors. Cancer Res. 2000;60:3328–3332. [PubMed] [Google Scholar]
- 23.Sibilia M, Fleischmann A, Behrens A, Stingl L, Carroll J, Watt FM, Schlessinger J, Wagner EF. The EGF receptor provides an essential survival signal for SOS-dependent skin tumor development. Cell. 2000;102:211–220. doi: 10.1016/s0092-8674(00)00026-x. [DOI] [PubMed] [Google Scholar]
- 24.Miettinen PJ, Berger JE, Meneses J, Phung Y, Pedersen RA, Werb Z, Derynck R. Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature. 1995;376:337–341. doi: 10.1038/376337a0. [DOI] [PubMed] [Google Scholar]
- 25.Sibilia M, Wagner EF. Strain-dependent epithelial defects in mice lacking the EGF receptor [published erratum appears in Science 1995; 269(5226), 909] Science. 1995;269:234–238. doi: 10.1126/science.7618085. [DOI] [PubMed] [Google Scholar]
- 26.Threadgill DW, Dlugosz AA, Hansen LA, Tennenbaum T, Lichti U, Yee VD, LaMantia C, Mourton T, Herrup K, Harris RC, Barnard JA, Yuspa SH, Coffey RJ, Magnuson T. Targeted isruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science. 1995;269:230–234. doi: 10.1126/science.7618084. [DOI] [PubMed] [Google Scholar]
- 27.Press MF, Cordon-Cardo C, Slamon DJ. Expression of the HER-2/neu proto-oncogene in normal human adult and fetal tissues. Oncogene. 1990;5:953–962. [PubMed] [Google Scholar]
- 28.Prigent SA, Lemoine NR, Hughes CM, Plowman GD, Selden C, Gullick WJ. Expression of the c-erbB-3 protein in normal human adult and fetal tissues. Oncogene. 1992;7:1273–1278. [PubMed] [Google Scholar]
- 29.Danilenko DM, Ring BD, Lu JZ, Tarpley JE, Chang D, Liu N, Wen D, Pierce GF. Neu differentiation factor upregulates epidermal migration and integrin expression in excisional wounds. J Clin Invest. 1995;95:842–851. doi: 10.1172/JCI117734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marikovsky M, Lavi S, Pinkas-Kramarski R, Karunagaran D, Liu N, Wen D, Yarden Y. ErbB-3 mediates differential mitogenic effects of NDF/heregulin isoforms on mouse keratinocytes. Oncogene. 1995;10:1403–1411. [PubMed] [Google Scholar]
- 31.Xian W, Rosenberg MP, DiGiovanni J. Activation of erbB2 and c-src in phorbol ester-treated mouse epidermis: possible role in mouse skin tumor promotion. Oncogene. 1997;14:1435–1444. doi: 10.1038/sj.onc.1200980. [DOI] [PubMed] [Google Scholar]
- 32.Marques MM, Martinez N, Rodriguez-Garcia I, Alonso A. EGFR family-mediated signal transduction in the human keratinocyte cell line HaCaT. Exp Cell Res. 1999;252:432–438. doi: 10.1006/excr.1999.4634. [DOI] [PubMed] [Google Scholar]
- 33.Voorhees JJ, Duell EA, Bass LJ, Powell JA, Harrell ER. Decreased cyclic AMP in the epidermis of lesions of psoriasis. Arch Dermatol. 1972;105:695–701. [PubMed] [Google Scholar]
- 34.Gendimenico GJ, Mallon JP, Cromie MA, Mezick JA, Astrom A, Elder JT. Regulation of cellular retinoic acid binding protein expression in rhino mouse skin by all-trans retinoic acid. Skin Pharmacol. 1995;8:167–172. doi: 10.1159/000211342. [DOI] [PubMed] [Google Scholar]
- 35.Laborda J. 36B4 cDNA used as an estradiol-independent mRNA control is the cDNA for human acidic ribosomal phosphoprotein PO. Nucleic Acids Res. 1991;19 doi: 10.1093/nar/19.14.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giard DJ, Aaronson SA, Todaro GJ, Arnstein P, Kersey JH, Dosik H, Parks WP. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst. 1973;51:1417–1423. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- 37.Cailleau R, Olive M, Cruciger QV. Long-term human breast carcinoma cell lines of metastatic origin: preliminary characterization. In Vitro. 1978;14:911–915. doi: 10.1007/BF02616120. [DOI] [PubMed] [Google Scholar]
- 38.McGrath CM, Grant PM, Soule HD, Glancy T, Rich MA. Replication of oncornavirus-like particle in human breast carcinoma cell line, MCF-7. Nature. 1974;252:247–250. doi: 10.1038/252247a0. [DOI] [PubMed] [Google Scholar]
- 39.Elder JT, Fisher GJ, Zhang QY, Eisen D, Krust A, Kastner P, Chambon P, Voorhees JJ. Retinoic acid receptor gene expression in human skin. J Invest Dermatol. 1991;96:425–433. doi: 10.1111/1523-1747.ep12469889. [DOI] [PubMed] [Google Scholar]
- 40.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 41.Deshpande R, Woods TL, Fu J, Zhang T, Stoll SW, Elder JT. Biochemical characterization of S100A2 in human keratinocytes: subcellular localization, dimerization, and oxidative cross-linking. J Invest Dermatol. 2000;115:477–485. doi: 10.1046/j.1523-1747.2000.00078.x. [DOI] [PubMed] [Google Scholar]
- 42.Merlino GT, Xu YH, Ishii S, Clark AJ, Semba K, Toyoshima K, Yamamoto T, Pastan I. Amplification and enhanced expression of the epidermal growth factor receptor gene in A431 human carcinoma cells. Science. 1984;224:417–419. doi: 10.1126/science.6200934. [DOI] [PubMed] [Google Scholar]
- 43.Rewcastle GW, Murray DK, Elliott WL, Fry DW, Howard CT, Nelson JM, Roberts BJ, Vincent PW, Showalter HD, Winters RT, Denny WA. Tyrosine kinase inhibitors: 14. Structure-activity relationships for methylamino-substituted derivatives of 4-[(3-bromophenyl)amino]-6-(methylamino)-pyrido[3,4-d]pyrimidine (PD 158780), a potent and specific inhibitor of the tyrosine kinase activity of receptors for the EGF family of growth factors. J Med Chem. 1998;41:742–751. doi: 10.1021/jm970641d. [DOI] [PubMed] [Google Scholar]
- 44.Fry DW, Kraker AJ, McMichael A, Ambroso LA, Nelson JM, Leopold WR, Connors RW, Bridges AJ. A specific inhibitor of the epidermal growth factor receptor tyrosine kinase. Science. 1994;265:1093–1095. doi: 10.1126/science.8066447. [DOI] [PubMed] [Google Scholar]
- 45.Fry DW, Nelson JM, Slintak V, Keller PR, Rewcastle GW, Denny WA, Zhou H, Bridges AJ. Biochemical and antiproliferative properties of 4-[ar(alk)ylamino]pyridopyrimidines, a new chemical class of potent and specific epidermal growth factor receptor tyrosine kinase inhibitor. Biochem Pharmacol. 1997;54:877–887. doi: 10.1016/s0006-2952(97)00242-6. [DOI] [PubMed] [Google Scholar]
- 46.Coffey RJ, Jr, Derynck R, Wilcox JN, Bringman TS, Goustin AS, Moses HL, Pittelkow MR. Production and autoinduction of transforming growth factor-alpha in human keratinocytes. Nature. 1987;328:817–820. doi: 10.1038/328817a0. [DOI] [PubMed] [Google Scholar]
- 47.Klein SB, Fisher GJ, Jensen TC, Mendelsohn J, Voorhees JJ, Elder JT. Regulation of TGF-alpha expression in human keratinocytes: PKC-dependent and -independent pathways. J Cell Physiol. 1992;151:326–336. doi: 10.1002/jcp.1041510214. [DOI] [PubMed] [Google Scholar]
- 48.Nanney LB, Stoscheck CM, Magid M, King L., Jr Altered [125I]epidermal growth factor binding and receptor distribution in psoriasis. J Invest Dermatol. 1986;86:260–265. doi: 10.1111/1523-1747.ep12285389. [DOI] [PubMed] [Google Scholar]
- 49.Dlugosz AA, Hansen L, Cheng C, Alexander N, Denning MF, Threadgill DW, Magnuson T, Coffey RJ, Jr, Yuspa SH. Targeted disruption of the epidermal growth factor receptor impairs growth of squamous papillomas expressing the v-ras(Ha) oncogene but does not block in vitro keratinocyte responses to oncogenic ras. Cancer Res. 1997;57:3180–3188. [PubMed] [Google Scholar]
- 50.Gill GN, Kawamoto T, Cochet C, Le A, Sato JD, Masui H, McLeod C, Mendelsohn J. Monoclonal antiepidermal growth factor receptor antibodies which are inhibitors of epidermal growth factor binding and antagonists of epidermal growth factor binding and antagonists of epidermal growth factor-stimulated tyrosine protein kinase activity. J Biol Chem. 1984;259:7755–7760. [PubMed] [Google Scholar]
- 51.Filmus J, Trent JM, Pollak MN, Buick RN. Epidermal growth factor receptor gene-amplified MDA-468 breast cancer cell line and its nonamplified variants. Mol Cell Biol. 1987;7:251–257. doi: 10.1128/mcb.7.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crovello CS, Lai C, Cantley LC, Carraway KL., III Differential signaling by the epidermal growth factor-like growth factors neuregulin-1 and neuregulin-2. J Biol Chem. 1998;273:26954–26961. doi: 10.1074/jbc.273.41.26954. [DOI] [PubMed] [Google Scholar]
- 53.Beerli RR, Graus Porta D, Woods Cook K, Chen X, Yarden Y, Hynes NE. Neu differentiation factor activation of ErbB-3 and ErbB-4 is cell-specific and displays a differential requirement for ErbB-2. Mol Cell Biol. 1995;15:6496–6505. doi: 10.1128/mcb.15.12.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang CK, Concepcion XZ, Milan M, Gong X, Montgomery E, Lippman ME. Ribozyme-mediated downregulation of ErbB-4 in estrogen receptor-positive breast cancer cells inhibits proliferation both in vitro and in vivo. Cancer Res. 1999;59:5315–5322. [PubMed] [Google Scholar]
- 55.Aguilar Z, Akita RW, Finn RS, Ramos BL, Pegram MD, Kabbinavar FF, Pietras RJ, Pisacane P, Sliwkowski MX, Slamon DJ. Biologic effects of heregulin/neu differentiation factor on normal and malignant human breast and ovarian epithelial cells. Oncogene. 1999;18:6050–6062. doi: 10.1038/sj.onc.1202993. [DOI] [PubMed] [Google Scholar]
- 56.Egeblad M, Jaattela M. Cell death induced by TNF or serum starvation is independent of ErbB receptor signaling in MCF-7 breast carcinoma cells. Int J Cancer. 2000;86:617–625. doi: 10.1002/(sici)1097-0215(20000601)86:5<617::aid-ijc3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 57.Arcaro A, Zvelebil MJ, Wallasch C, Ullrich A, Waterfield MD, Domin J. Class II phosphoinositide 3-kinases are downstream targets of activated polypeptide growth factor receptors. Mol Cell Biol. 2000;20:3817–3830. doi: 10.1128/mcb.20.11.3817-3830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Olayioye MA, Beuvink I, Horsch K, Daly JM, Hynes NE. ErbB receptor-induced activation of stat transcription factors is mediated by Src tyrosine kinases. J Biol Chem. 1999;274:17209–17218. doi: 10.1074/jbc.274.24.17209. [DOI] [PubMed] [Google Scholar]
- 59.Nakano N, Higashiyama S, Kajihara K, Endo T, Ishiguro H, Yamada K, Nagatsu T, Taniguchi N. NTAK-alpha and beta isoforms stimulate breast tumor cell growth by means of different receptor combinations. J Biochem (Tokyo) 2000;127:925–930. doi: 10.1093/oxfordjournals.jbchem.a022688. [DOI] [PubMed] [Google Scholar]
- 60.Jones GM, Sanford KK, Parshad R, Gantt R, Price FM, Tarone RE. Influence of added catalase on chromosome stability and neoplastic transformation of mouse cells in culture. Br J Cancer. 1985;52:583–590. doi: 10.1038/bjc.1985.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pinkas Kramarski R, Soussan L, Waterman H, Levkowitz G, Alroy I, Klapper L, Lavi S, Seger R, Ratzkin BJ, Sela M, Yarden Y. Diversification of Neu differentiation factor and epidermal growth factor signaling by combinatorial receptor interactions. EMBO J. 1996;15:2452–2467. [PMC free article] [PubMed] [Google Scholar]
- 62.Riese DJ, II, van Raaij TM, Plowman GD, Andrews GC, Stern DF. The cellular response to neuregulins is governed by complex interactions of the erbB receptor family. Mol Cell Biol. 1995;15:5770–5776. doi: 10.1128/mcb.15.10.5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stoll SW, Benedict M, Mitra R, Hiniker A, Elder JT, Nuñez G. EGF receptor signaling inhibits keratinocyte apoptosis: evidence for mediation by Bcl-XL. Oncogene. 1998;16:1493–1499. doi: 10.1038/sj.onc.1201657. [DOI] [PubMed] [Google Scholar]
- 64.Barnard JA, Graves Deal R, Pittelkow MR, DuBois R, Cook P, Ramsey GW, Bishop PR, Damstrup L, Coffey RJ. Auto- and cross-induction within the mammalian epidermal growth factor-related peptide family. J Biol Chem. 1994;269:22817–22822. [PubMed] [Google Scholar]
- 65.Schelfhout VR, Coene ED, Delaey B, Thys S, Page DL, De Potter CR. Pathogenesis of Paget's disease: epidermal heregulin-alpha, motility factor, and the HER receptor family. J Natl Cancer Inst. 2000;92:622–628. doi: 10.1093/jnci/92.8.622. [DOI] [PubMed] [Google Scholar]
- 66.Castagnino P, Lorenzi MV, Yeh J, Breckenridge D, Sakata H, Munz B, Werner S, Bottaro DP. Neu differentiation factor/heregulin induction by hepatocyte and keratinocyte growth factors. Oncogene. 2000;19:640–648. doi: 10.1038/sj.onc.1203357. [DOI] [PubMed] [Google Scholar]
- 67.Liou A, Elias PM, Grunfeld C, Feingold KR, Wood LC. Amphiregulin and nerve growth factor expression are regulated by barrier status in murine epidermis. J Invest Dermatol. 1997;108:73–77. doi: 10.1111/1523-1747.ep12285638. [DOI] [PubMed] [Google Scholar]
- 68.Woodley DT. Re-epithelialization. In: Clark RAF, editor. The Molecular and Cellular Biology of Wound Repair. New York: Plenum; 1996. pp. 339–354. [Google Scholar]
- 69.Peles E, Bacus SS, Koski RA, Lu HS, Wen D, Ogden SG, Levy RB, Yarden Y. Isolation of the neu/HER-2 stimulatory ligand: a 44-kD glycoprotein that induces differentiation of mammary tumor cells. Cell. 1992;69:205–216. doi: 10.1016/0092-8674(92)90131-u. [DOI] [PubMed] [Google Scholar]
- 70.Varani J, Ziegler M, Dame MK, Kang S, Fisher GJ, Voorhees JJ, Stoll SW, Elder JT. HB-EGF activation of keratinocyte ErbB receptors mediates epidermal hyperplasia, a prominent side effect of retinoid therapy. J Invest Dermatol. 2001 doi: 10.1046/j.0022-202x.2001.01564.x. in press. [DOI] [PubMed] [Google Scholar]