Abstract
Cyclin-dependent kinases (Cdk) are essential for promoting the initiation of DNA replication, presumably by phosphorylating key regulatory proteins that are involved in triggering the G1/S transition. Human Cdc6 (HsCdc6), a protein required for initiation of DNA replication, is phosphorylated by Cdk in vitro and in vivo. Here we report that HsCdc6 with mutations at potential Cdk phosphorylation sites was poorly phosphorylated in vitro by Cdk, but retained all other biochemical activities of the wild-type protein tested. Microinjection of mutant HsCdc6 proteins into human cells blocked initiation of DNA replication or slowed S phase progression. The inhibitory effect of mutant HsCdc6 was lost at the G1/S transition, indicating that phosphorylation of HsCdc6 by Cdk is critical for a late step in initiation of DNA replication in human cells.
INTRODUCTION
In eukaryotes, the cell division cycle is regulated by the periodic activation and inactivation of cyclin-dependent kinases (Cdk), which are thought to phosphorylate protein substrates that are involved in driving cell cycle transitions (reviewed in Sherr, 1996; Roberts, 1999). The transition from G1 to S phase of the mammalian cell cycle requires the activity of cyclin E/Cdk2 and cyclin A/Cdk2. Cyclin E is expressed in middle to late G1 (Dulic et al., 1992, Koff et al., 1992), whereas cyclin A is first expressed at the G1/S transition of the cell cycle (Girard et al., 1991; Pagano et al., 1992; Zindy et al., 1992). Microinjection of anticyclin E or anticyclin A antibodies into human cells or expression of antisense cyclin A RNA, inhibits initiation of DNA replication (Girard et al., 1991; Pagano et al., 1992; Zindy et al., 1992; Tsai et al., 1993; Ohtsubo et al., 1995). Furthermore, enhanced levels of cyclins A and E accelerate the G1/S transition in vivo (Resnitzky et al., 1994, 1995) and in vitro (Krude et al., 1997).
The initiation of DNA replication in eukaryotes requires the stepwise assembly of a multiprotein complex, called the prereplicative complex (pre-RC), on replicator elements in the chromatin (reviewed in Dutta and Bell, 1997; Newlon, 1997). Studies in yeast and in Xenopus have determined that a six-subunit complex, called origin recognition complex (ORC), serves to nucleate this assembly. In G1 of the cell cycle, the Cdc6 protein promotes the loading of the minichromosome maintenance proteins (MCMs) onto chromatin (Coleman et al., 1996; Aparicio et al., 1997; Donovan et al., 1997; Tanaka et al., 1997). This reaction requires an intact Walker A or Walker B motif in Cdc6 (Perkins and Diffley, 1998; Wang et al., 1999; Weinreich et al., 1999) and presumably involves direct physical interaction between Cdc6 and ORC (Li and Herskowitz, 1993; Liang et al., 1995; Mizushima et al., 2000). Although the mechanism of pre-RC assembly in human cells has not yet been characterized, all of the components of the pre-RC identified so far in yeast appear to be conserved in humans.
A growing body of evidence indicates that components of the pre-RC are Cdk substrates whose modification is likely to regulate the initiation of DNA replication. All Cdc6-related proteins identified so far contain several potential sites for phosphorylation by Cdk (see Figure 1A for a schematic representation of human Cdc6). Yeast and human Cdc6 physically interact with and become phosphorylated by cyclin/Cdk complexes in vitro and in vivo (Elsasser et al., 1996, 1999; Piatti et al., 1996; Brown et al. 1997; Jallepalli et al., 1997; Saha et al., 1998; Fujita et al., 1999; Jiang et al., 1999; Petersen et al., 1999; Calzada et al., 2000). Analysis of Saccharomyces cerevisiae Cdc6 and the Schizosaccharomyces pombe homologue Cdc18 revealed that phosphorylation of these proteins by Cdk targets them for ubiquitin-mediated degradation, presumably before DNA replication initiates but after the prereplicative complexes have formed (Jallepalli et al., 1997; Kominami and Toda, 1997; Baum et al., 1998; Elsasser et al., 1999; Sanchez et al., 1999; Drury et al., 2000). When Cdc18 degradation was inhibited by mutating the five N-terminal Cdk phosphorylation sites, an overreplication phenotype was observed, demonstrating that phosphorylation of Cdc18 by Cdk is not required to initiate but rather to prevent reinitiation of DNA replication by targeting Cdc18 for proteolytic degradation (Jallepalli et al., 1997; Lopez-Girona et al., 1998). In contrast, when multiple potential Cdk phosphorylation sites in S. cerevisiae Cdc6 were mutated, the mutant proteins were stabilized compared with wild-type Cdc6, but they retained the ability to support growth of cdc6 mutant yeast strains (Elsasser et al., 1999; Calzada et al., 2000; Drury et al., 2000). In addition, an overexpressed, truncated Cdc6 lacking N-terminal Cdk phosphorylation sites became resistant to degradation by the ubiquitin pathway but retained its ability to initiate and to prevent rereplication (Drury et al., 1997). Therefore, neither yeast requires phosphorylation of Cdc6/Cdc18 to initiate replication. Moreover, the regulatory mechanism that limits DNA replication to only once per cell cycle in S. pombe appears to differ from that in S. cerevisiae.
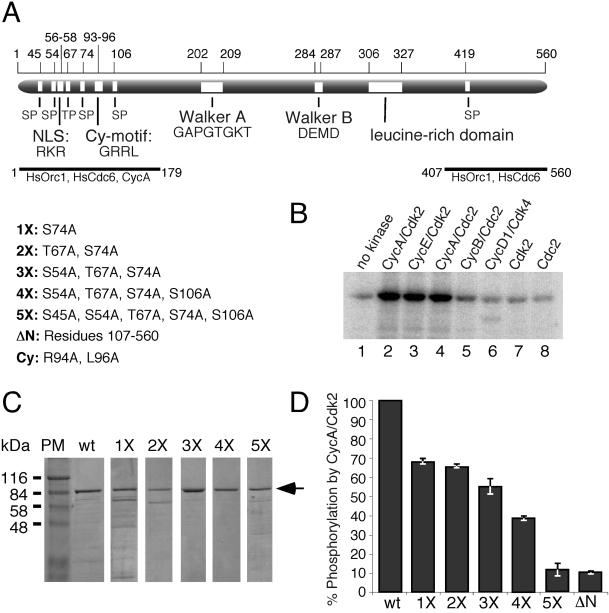
Figure 1.
HsCdc6 is specifically phosphorylated at N-terminal Cdk phosphorylation sites in vitro. (A) Schematic representation of the HsCdc6 protein illustrating six potential phosphorylation sites for Cdk (SP or TP), the cyclin-binding motif (Cy-motif), the nuclear localization signal (NLS; Takei et al., 1999), the ATP binding motif (P-loop), the Mg2+ binding motif (DEAD-box), a leucine-rich domain, and peptides shown to bind to HsOrc1, HsCdc6, and cyclin A (Saha et al., 1998). The numbers above the protein indicate the position of the illustrated motifs in the amino acid sequence. Five mutant forms of HsCdc6 with amino acid substitutions at potential Cdk phosphorylation sites and combinations of sites, as indicated, were prepared and named 1X-5X; ΔN represents HsCdc6 lacking the N-terminal 106 amino acids; Cy, two alanine substitutions in the cyclin-binding motif determined previously (Petersen et al., 1999). (B) GST-HsCdc6 (3 μg) was incubated with 1 U of purified baculovirus-expressed cyclin A/Cdk2 (lane 2), cyclin E/Cdk2 (lane 3), cyclin A/Cdc2 (lane 4), cyclin B/Cdc2 (lane 6), and 1.35 U of cyclin D1/Cdk4 in the presence of [γ-32P]ATP. As a control, GST-HsCdc6 was treated similarly without kinase (lane 1) or with 0.5 μg Cdk2 alone (lane 7) or 0.5 μg Cdc2 alone (lane 8). After 10% SDS-PAGE, phosphorylated GST-HsCdc6 was visualized by PhosphorImaging. (C) Purified wild-type HsCdc6 fused to GST (wt) and GST-HsCdc6 mutants with single and multiple amino acid substitutions at potential Cdk phosphorylation sites were separated by 10% SDS-PAGE and stained with Coomassie Brilliant Blue. PM, protein marker. (D) Equal amounts of wild-type and the indicated mutant forms of GST-HsCdc6 were incubated with 6 U of purified baculovirus-expressed cyclin A/Cdk2 in the presence of [γ-32P]ATP. The proteins were resolved by 10% SDS-PAGE and visualized by Coomassie Brilliant Blue staining and PhosphorImaging (not shown). The relative amount of phosphorylation of mutant proteins compared with wild type, defined as 100%, was determined using IPLabgel software. Error bars, average error of the mean as determined from two separate experiments.
In human cells, the N-terminal consensus Cdk phosphorylation sites of HsCdc6 are specifically phosphorylated in vitro by cyclin E/Cdk2 and cyclin A/Cdk2 and in vivo at the G1/S transition (Jiang et al., 1999; Petersen et al., 1999). Nevertheless, in actively dividing human cells, the total level of Cdc6 appears to remain fairly stable throughout the cell cycle (Williams et al., 1997; Saha et al., 1998; Fujita et al., 1999; Jiang et al., 1999). Interestingly, a major fraction of HsCdc6 translocates into the cytoplasm in early S phase, and alanine substitutions at N-terminal Cdk phosphorylation sites block nuclear export of overexpressed HsCdc6, whereas acidic amino acid substitutions facilitate this export (Saha et al., 1998; Fujita et al., 1999; Jiang et al., 1999; Petersen et al., 1999). Deletion of the cyclin-binding site in HsCdc6 prevented its phosphorylation by Cdk and its export to the cytoplasm in early S phase (Petersen et al., 1999). Moreover, ectopic expression of cyclin A/Cdk2 during G1 caused Cdc6 to be prematurely relocalized from the nucleus to the cytoplasm (Petersen et al., 1999). Thus, it seems likely that phosphorylation of HsCdc6 by Cdk regulates its nuclear export and that this mechanism may play a role in preventing reinitiation of DNA replication in S phase. However, it is less clear whether phosphorylation of HsCdc6 and its concomitant nuclear export are required for initiation of DNA replication in human cells.
In this study we have sought to determine whether phosphorylation of HsCdc6 by Cdk is essential to initiate DNA replication in human cells. We have purified and biochemically characterized a panel of mutant HsCdc6 proteins with amino acid substitutions at potential Cdk phosphorylation sites. The mutations reduced phosphorylation of HsCdc6 by recombinant cyclin/Cdk complexes to near background levels. However, these mutants retained their ability to bind to human Orc1, HsCdc6, and cyclins; to hydrolyze ATP; and to alter their conformation in the presence of ADP, suggesting that the mutant proteins retained their biochemical activities and were not grossly misfolded. When purified HsCdc6 protein containing mutations within the Cdk phosphorylation sites was microinjected into human cells during G1, it blocked DNA replication. However, mutant Cdc6 protein injected at the G1/S transition was unable to prevent replication. The ability of the mutant proteins to interfere with the activity of endogenous wild-type HsCdc6 did not require a stable physical interaction between mutant HsCdc6 and cyclin/Cdk complexes. These data indicate that phosphorylation of human Cdc6 by Cdk is necessary for initiation of DNA replication in human cells.
MATERIALS AND METHODS
Plasmids
Point mutations at potential Cdk phosphorylation sites and the cyclin-binding motif of HsCdc6 were created in pBS-Cdc6-3 (Herbig et al., 1999) by overlap extension PCR (Voitenleitner et al., 1999). The mutation S74A (pBS-GST-Cdc6-1X) was generated using the forward primer 5′-CCTCCTTGTGCTCCACCAAAGC-3′ together with the backward primer 5′-GGTAGAATTCTATCTGTGAGATCC-3′ (Eco-R), the forward primer 5′-CCGGATCCATGCCTCAAACCCGATCCC-3′ (ATG-Cdc6) together with the backward primer 5′-GCTTTGGTGGAGCACAAGGAGG-3′, and pBS-Cdc6-3 as a template (Herbig et al., 1999). The mutation T67A (pBS-GST-Cdc6-T67A) was generated using the forward primer 5′-CCTATGCAACGCGCCCCATTTACC-3′ together with the backward primer Eco-R, the forward primer ATG-Cdc6 together with the backward primer 5′-GGTAAATGGGGCGCGTTGCATAGG -3′, and pBS-Cdc6-3 as a template. The mutation T67A/S74A (pBS-Cdc6-2X) was generated using the same primers as for the S74A construct and pBS-GST-Cdc6-T67A as a template. The mutation S54A/T67A/S74A (pBS-GST-Cdc6-3X) was generated using the forward primer 5′-CCTGCCTCTCGCCCCCAGGAAACG-3′ together with the backward primer Eco-R, the forward primer ATG-Cdc6 together with the backward primer 5′-CGTTTCCTGGGGGCGAGAGGCAGG -3′, and pBS-Cdc6-2X as a template. The mutation S54A/T67A/S74A/S106A (pBS-GST-Cdc6-4X) was generated using the forward primer 5′-GACAATTAAGGCTCCTAGCAAAAGAG-3′ together with the backward primer Eco-R, the forward primer ATG-Cdc6 together with the backward primer 5′-CTCTTTTGCTAGGAGCCTTAATTGTC-3′, and pBS-Cdc6-3X as a template. The mutation S45A/S54A/T67A/S74A/S106A (pBS-GST-Cdc6-5X) was generated using the forward primer 5′-CGTAACCTGTGCTCCTCGTG-3′ together with the backward primer Eco-R, the forward primer ATG-Cdc6 together with the backward primer 5′-CACGAGGAGCACAGGTTACG-3′, and pBS-Cdc6-4X as a template. The mutations R94A and L96A were generated in wild-type HsCdc6 using the forward primer 5′-CACATACACTTAAGGGAGCAAGAGCGGTATTTGAC-3′ together with the backward primer Eco-R, the forward primer ATG-Cdc6 together with the backward primer 5′-GTCAAATACCGCTCTTGCTCCCTTAAGTGTATGTG-3′, and pBS-Cdc6-3 as a template. The mutations R94A and L96A were generated in HsCdc6-5X using the same primers as for wild-type HsCdc6-Cy and pBS-Cdc6-5X as a template. All PCR amplification products were digested with BamHI/NdeI, and the resulting fragments were used to replace the BamHI/NdeI fragment in pBS-GST-Cdc6 (Herbig et al., 1999). The N-terminal deletion mutant of GST-Cdc6.107-560 (ΔN) was generated by PCR using the forward primer 5′-CCGGATCCAGCAAAAGAGAACTAGCCAAAG-3′, the reverse primer 5′-AGAACCTGATCTCTAGATACC-3′, and pBS-Cdc6-3 as a template. The PCR amplification product was digested with BamHI/XbaI, and the resulting fragment was used to replace the BamHI/XbaI fragment in pBS-GST-Cdc6. The wild-type and mutated cDNA was transferred as HincII/NotI fragments from the pBS plasmids into pVL1393 (Invitrogen, San Diego, CA), which had been digested with SmaI/NotI, to generate the corresponding baculovirus transfer vectors.
Protein Purification
All GST-HsCdc6 proteins were expressed and purified from baculovirus-infected insect cells as described previously (Herbig et al., 1999). Except for cyclin D1/Cdk4, the purification of hemagglutinin epitope (HA)-tagged Cdk2 and Cdc2 and the cyclin/Cdk complexes was performed essentially as described (Voitenleitner et al., 1999). Briefly, Hi-5 insect cells (Invitrogen) were coinfected with recombinant baculoviruses for 48 h at 27°C. Cells were disrupted by dounce homogenizing in 10 ml lysis buffer (50 mM HEPES-KOH [pH 7.5], 100 mM NaCl, 5 mM KCl, 1 mM MgCl2, 5 mM NaF, 5 mM EGTA, 2 mM EDTA, 1 mM DTT, 0.2% [vol/vol] Nonidet P-40, 1 mM PMSF, and 1 μg/ml each aprotinin and leupeptin). Cell debris was removed by centrifugation, and the supernatant was mixed gently with 0.4 ml 12CA5-Sepharose prepared by covalently coupling 12CA5 antibody to CNBr-activated Sepharose 4B (Pharmacia, Piscataway, NJ). After washing with PBS, the cyclin/Cdk complexes were eluted in a batch with 0.2 ml elution buffer (50 mM Tris [pH 7.5], 200 mM NaCl, 1 mM EDTA, 4 mg/ml HA-peptide [YPYDVPDYA]). The eluted fractions were dialyzed overnight against two liters of dialysis buffer (50 mM KPi [pH 7.5], 10% glycerol, 1 mM EDTA). Cyclin D1 and GST-Cdk4 coexpressed from recombinant baculoviruses was purified as described previously (Voitenleitner et al., 1999).
Kinase Assay
Reactions were performed in kinase buffer (20 mM HEPES-KOH [pH 7.5], 10 mM MgCl2, 1 mM DTT, 4 mM EGTA, 1 mM EDTA, 5 mM NaF, 0.1 mg/ml bovine serum albumin, 0.1 mM [γ-32P]ATP [1 Ci/mmol]) at 37°C for 15 min using 0.5–1 μg cyclin/Cdk complex and 1 μg of histone H1, or pRb for cyclin D1/Cdk4, as a substrate. The reaction was stopped by boiling in sample buffer, and the proteins were separated by SDS-PAGE on 10% gels. Protein bands were visualized by Coomassie Brilliant Blue staining and excised from the gel, and the amount of phosphate incorporated was determined by scintillation counting. One kinase unit was defined as the amount of kinase needed to incorporate 1 pmol phosphate into 1 μg of substrate in 15 min. Phosphorylation of GST-HsCdc6 was performed in a similar manner using the indicated amounts of kinase complexes.
Protein Interaction Assays
Interactions of GST-HsCdc6 with untagged radiolabeled HsOrc1 and HsCdc6 produced by in vitro translation were assayed as described previously (Herbig et al., 1999).
To assay protein interactions in vivo, 1 × 107 Hi-5 insect cells were coinfected with recombinant baculoviruses, as indicated in the figure legends, for 48 h at 27°C. Cells were lysed in 1 ml lysis buffer (50 mM HEPES-KOH [pH 7.5], 100 mM NaCl, 5 mM KCl, 1 mM MgCl2, 5 mM NaF, 5 mM EGTA, 2 mM EDTA, 1 mM DTT, 0.2% [vol/vol] Nonidet P-40, 1 mM PMSF, and 1 μg/ml each aprotinin and leupeptin), and insoluble proteins were removed by centrifugation. The supernatant was gently mixed with 50 μl glutathione agarose for 1 h at 4°C. The resin was washed six times with 1 ml PBS, and bound proteins were eluted by boiling in SDS sample buffer. Half of the eluate was analyzed by 12.5% SDS-PAGE and western blotting using the monoclonal anticyclin A antibody C160 (Giordano et al., 1989), the monoclonal anticyclin E antibody HE111 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and the anti HA-antibody 12CA5 (Wilson et al., 1984), to detect an N-terminal HA-tag on Cdk2. The other half of the eluate was analyzed by 12.5% SDS-PAGE followed by Coomassie staining to ensure that equal amounts of GST-fusion proteins were precipitated (our unpublished results).
ATPase Assay
Hydrolysis of ATP was measured in ATPase buffer (20 mM Tris-HCl [pH 7.5], 0.1 mg/ml bovine serum albumin, 0.5 mM DTT, 10 mM MgCl2, 2.5–250 μM [γ-32P]ATP [1 Ci/mmol]) with 0.25 pmol of GST-HsCdc6 or GST-HsCdc6 mutants as described previously (Herbig et al., 1999). The reaction products were separated by TLC on polyethyleneimine-cellulose and developed in 1 M LiCl and 0.5 M formic acid, and the amounts of [γ-32P]ATP hydrolyzed to [32P]orthophosphate were quantified using a PhosphorImager (Molecular Dynamics, Inc., Sunnyvale, CA). ATP hydrolysis was performed in the linear range of reaction time and protein concentration.
Partial Tryptic Digest
Partial tryptic digestion of 0.5 μg GST-HsCdc6-5X or GST-HsCdc6-5X.Cy was performed in 20 μl of buffer D (20 mM Tris-HCl [pH 7.5], 0.5 mM EDTA, 2 mM DTT, 8 mM MgCl2) with 2.5 ng/μl trypsin in the absence or presence of 2 mM NTP as described previously (Herbig et al., 1999). The proteolytic products were resolved by 12.5% SDS-PAGE and visualized by silver staining.
Cell Culture, Microinjection, and Immunostaining
HeLa-S3 cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS) and antibiotics in a humidified incubator at 37°C and 10% CO2. Cells were synchronized in G1/S by incubation in medium containing 2.5 mM thymidine (Sigma Chemical, St. Louis, MO) for 24 h. To release the cells into S phase, the medium was aspirated, and the cells were washed three times with DMEM containing 10% FBS and incubated for 8 h in thymidine-free media. A G2/M arrest was achieved by incubating exponentially growing cells with 50 ng/ml nocodazole for 16 h. The cells were released into G1 by gently shaking off mitotic cells and washing them three times with DMEM/10% FBS before plating them in nocodazole-free media. In some experiments, cells were released from the nocodazole block into medium containing 1 μg/ml aphidicolin, 2.5 mM thymidine, 2.5 mM hydroxyurea, or 0.5 mM mimosine. For bromodeoxyuridine (BrdU) labeling, the medium was supplemented with 10 μM BrdU (Sigma).
HeLa-S3 cells, which had been released from a nocodazole block, were plated on glass coverslips for 6–8 h, at which time the majority of the cells had attached to the coverslips. Cells to be synchronized and injected in G1/S were grown on the glass coverslips. Except for GST, all fusion proteins were used at a concentration of 16–61 μg/ml and supplemented with GST at a concentration of 0.5 mg/ml for easier detection of injected cells. GST alone was also used at a concentration of 0.5 mg/ml. Before microinjection all samples were centrifuged for 30 min at 14,000 × g. Needles used for microinjection were pulled from glass capillaries (Clark Electromedical Instruments, Reading, United Kingdom) on an automatic pipette puller (Zeitz Instruments, Augsburg, Germany). The samples were delivered using a microinjector (model 5246; Eppendorf Scientific, Inc., Madison, WI) and a manipulator (model 5171; Eppendorf Scientific, Inc.) mounted on an inverted microscope (model IM35; Carl Zeiss, Oberkochen, Germany).
For immunofluorescent staining, the cells were washed with PBS, fixed with 3% formaldehyde in PBS for 20 min, permeabilized for 20 min using 0.2% Triton X-100, and incubated with 10% FBS in PBS for 1 h. GST was visualized by staining with a rabbit polyclonal anti-GST antibody (provided by R. Weber, Institute for Biochemistry, University of Munich, Germany) at a dilution of 1:100 in PBS/10% FBS for 2 h at room temperature, followed by FITC-conjugated goat anti-rabbit secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) at a dilution of 1:50 in PBS/10% FBS for 1 h at room temperature. BrdU incorporated into the DNA was visualized by staining with a mouse monoclonal anti-BrdU antibody (Amersham, Arlington Heights, IL) at a dilution of 1:100 in PBS/10% FBS containing 125 U/ml benzon nuclease (EM Science, Gibbstown, NJ) for 2 h at room temperature, followed by a Cy3-conjugated goat anti-mouse secondary antibody (Jackson ImmunoResearch Laboratories) at a dilution of 1:100 in PBS/10% FBS for 1 h at room temperature. The cells were washed and incubated for 15 min with Hoechst 33258 fluorochrome (Hoechst, Frankfurt, Germany) at a concentration of 2 μM in PBS. The coverslips were mounted in 90% glycerol containing 0.1 mg/ml paraphenylene-diamine in PBS (Johnson and Nogueira Araujo, 1981) and analyzed at a 63× or 100× magnification using a fluorescence microscope (model Axiovert 135; Carl Zeiss).
Quantification of Nuclear DNA
To quantify the nuclear DNA content, cells that had been stained with rabbit polyclonal anti-GST antibody and the FITC-conjugated goat anti-rabbit secondary antibody (see above) were incubated for 1 h in 10 mM Tris-HCl (pH 7.5) containing 1 M NaCl and 2 μM Hoechst 33258 fluorochrome at room temperature (Araki et al., 1987). Images of fluorescent cells were captured at 63× magnification using a digital camera (CCD camera, model C 4880; Hamamatsu Phototonics, Bridgewater, NJ). The amount of fluorescence emitted at 460 nm in the nucleus of the cell was measured using the Image-/MetaMorph Imaging System (Universal Imaging Corp., West Chester, PA). Fluorescence per nucleus was evaluated for injected and uninjected cells in each field of vision. The nuclear DNA content of injected cells was expressed as a percentage of the nuclear DNA content of uninjected cells in the same field of vision, which was set to 100%.
RESULTS
HsCdc6 Is Phosphorylated by Cdk In Vitro
Like its yeast and Xenopus counterparts, the human Cdc6 protein contains several potential phosphorylation sites for Cdk clustered in the N-terminus (Figure 1A). To confirm that our purified HsCdc6 protein is a substrate for Cdk in vitro, GST-HsCdc6 was incubated with various purified cyclin/Cdk complexes in the presence of [γ-32P]ATP. The kinases were compared by using equal activities, which were determined as described in MATERIALS AND METHODS (our unpublished results). Cyclin E/Cdk2, cyclin A/Cdk2, and cyclin A/Cdc2 all efficiently phosphorylated GST-HsCdc6 (Figure 1B), but not GST alone (our unpublished results). Cyclin B/Cdc2 phosphorylated HsCdc6 to a lesser extent, whereas cyclin D1/Cdk4, Cdk2, and Cdc2 did not phosphorylate HsCdc6 above background (compare Figure 1B, lanes 6, 7, and 8 with lane 1). Kinetic experiments revealed that CycA/Cdk2 phosphorylated purified HsCdc6 1.5-fold more efficiently than CycE/Cdk2 or CycA/Cdc2 and 5-fold more efficiently than CycB/Cdc2 (our unpublished results).
To assess the biological significance of Cdk phosphorylation of HsCdc6, we changed single or multiple serine or threonine residues at these sites to alanine by site-directed mutagenesis. The five mutant forms of HsCdc6 generated had Ser74 (1X); Thr67 and Ser74 (2X); Ser54, Thr67, and Ser74 (3X); Ser54, Thr67, Ser74, and Ser106 (4X); or Ser45, Ser54, Thr67, Ser74, and Ser106 (5X) changed to alanine (Figure 1A). All HsCdc6 mutants were expressed in insect cells as GST fusion proteins and purified in a soluble form that was comparable in size and yield to wild-type GST-HsCdc6 (Figure 1C). These proteins were then characterized in vitro and in vivo.
Of the six potential Cdk phosphorylation sites within HsCdc6, three represent the true consensus sequence S/T-P-X-K/R (Ser54, Ser74, and Ser106), and three represent the more relaxed consensus sequence S/T-P (Ser45, Thr67, and Ser419). To test the ability of the mutant proteins to block Cdk-dependent phosphorylation in vitro, we used wild-type and five mutant forms of GST-HsCdc6 as substrates in a kinase assay with cyclin A/Cdk2 (Figure 1D). Wild-type GST-HsCdc6 was phosphorylated to a greater extent than any of the mutant forms of GST-HsCdc6. As the number of mutant Cdk phosphorylation sites increased, the amount of phosphate incorporated into HsCdc6 decreased (Figure 1D). The greatest differences in phosphorylation were observed between wild-type and 1X and between 4X and 5X. Smaller differences were observed when 2X and 3X or 3X and 4X were compared. Little or no difference was detected between 1X and 2X or 5X and ΔN, a mutant form of HsCdc6 lacking the N-terminal 106 amino acids and thus all five N-terminal Cdk phosphorylation sites. Similar results were observed using cyclin E/Cdk2 instead of cyclin A/Cdk2 (our unpublished results). The results are consistent with recent reports that multiple N-terminal Cdk phosphorylation sites were targeted by cyclin A/Cdk2 and cyclin E/Cdk2 in vitro and in vivo (Jiang et al., 1999; Petersen et al., 1999).
Biochemical Activities of the Phosphorylation Site Mutants
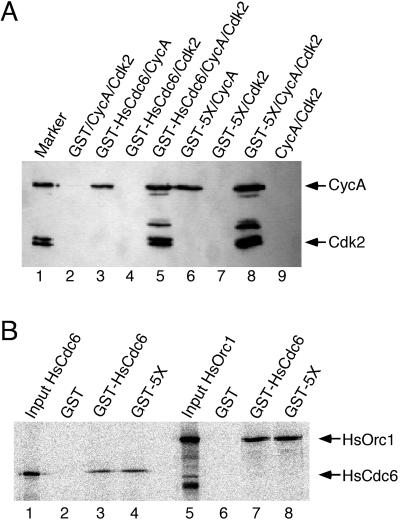
Recent studies of yeast and human Cdc6 homologs have demonstrated that they physically interact with components of the prereplicative complex (Liang et al., 1995; Saha et al., 1998, Herbig et al., 1999; Wang et al., 1999) and with Cdk (Elsasser et al., 1996; Piatti et al., 1996; Brown et al., 1997; Lopez-Girona et al., 1998; Saha et al., 1998; Petersen et al., 1999). Thus, the reduced phosphorylation observed with the mutant proteins could reflect changes in their ability to interact with the cyclin/Cdk complexes. To test this possibility, insect cells were coinfected with baculoviruses encoding GST-HsCdc6 or GST-HsCdc6 (5X) and viruses encoding either cyclin A and Cdk2, or cyclin A or Cdk2 alone. Kinase or cyclin that bound to GST-HsCdc6 was coprecipitated on glutathione agarose and analyzed by Western blotting. As shown in Figure 2A, cyclin A alone (lane 3) and in complex with Cdk2 (lane 5) associated with wild-type GST-HsCdc6, but Cdk2 alone was not coprecipitated with GST-HsCdc6 (lane 4), indicating that the interaction between HsCdc6 and the kinase subunit was mediated by cyclin A. The interactions observed appeared to be specific because GST did not coprecipitate either cyclin A or Cdk2 (lane 2). GST-HsCdc6 (5X) interacted with cyclin A alone (lane 6) and with the CycA/Cdk2 complex (lane 8), but not with Cdk2 alone (lane 7), demonstrating that the 5X mutant resembled the wild type in its ability to interact with cyclin/Cdk.
Figure 2.
Wild-type and mutant forms of HsCdc6 bind to CycA,
HsOrc1, and HsCdc6. (A) Insect cells were coinfected with recombinant
baculoviruses encoding the indicated proteins. Cell extracts were
prepared and incubated with glutathione agarose. After washing, the
beads were boiled in sample buffer, and the eluted proteins were
resolved by 10% SDS-PAGE. Cyclin A or Cdk2 that coprecipitated with
the GST-fusion proteins was detected by immunoblotting
using anti-CycA antibody C160 or anti-HA antibody 12CA5 to recognize
the an N-terminal HA tag on Cdk2. Lane 1 contained 0.5 μg purified
baculovirus-expressed CycA/Cdk2 as a marker. (B) Glutathione agarose
beads containing equal amounts of GST (lanes 2 and 6), GST-HsCdc6
(lanes 3 and 7), or GST-HsCdc6-5X (GST-5X; lanes 4 and 8) were
incubated with radiolabeled, in vitro–translated HsCdc6 protein (lanes
2–4) or HsOrc1 protein (lanes 6–8). After washing, the beads were
boiled in sample buffer, and the eluted proteins were resolved by 10%
SDS-PAGE. Bound proteins were visualized by PhosphorImaging. Lanes 1
and 5 contained  of the radiolabeled input protein.
of the radiolabeled input protein.
The ability of wild-type GST-HsCdc6 and the 5X mutant to oligomerize and to associate with HsOrc1 was also tested (Saha et al., 1998; Herbig et al., 1999). 35S-radiolabeled HsCdc6 or HsOrc1, produced by in vitro translation, was incubated with glutathione beads containing equal amounts of GST, GST-HsCdc6, and GST-HsCdc6 (5X). After washing the beads, proteins were analyzed by SDS-PAGE, followed by Coomassie Blue staining (our unpublished results) and PhosphorImaging. Wild-type HsCdc6 and the 5X mutant bound equally well to labeled HsCdc6 (Figure 2B, lanes 3 and 4) and HsOrc1 (lanes 7 and 8). Binding of HsOrc1 and HsCdc6 to GST was not detectable (lanes 2 and 6), confirming that the interaction was mediated by the HsCdc6 portion of the fusion protein. This result indicates that the amino acid substitutions at the five N-terminal Cdk phosphorylation sites did not affect the oligomerization or HsOrc1-binding activity of HsCdc6.
To address the role of cyclin binding in HsCdc6 functions and its relationship to Cdk phosphorylation of HsCdc6, we also created GST-HsCdc6 mutants in which the conserved Arg94 and Leu96 of the cyclin-binding motif of HsCdc6 had been substituted with alanines (Cy-motif; Figure 1A). The mutations were introduced into wild-type HsCdc6 (HsCdc6-Cy) and into HsCdc6-5X (HsCdc6-5X.Cy).
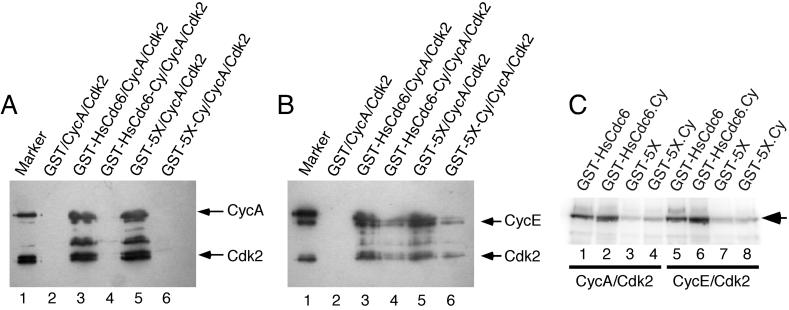
To test whether the generated mutations interfered with the ability of HsCdc6 to interact with the Cyc/Cdk2 complex in vivo, insect cells were coinfected with recombinant baculoviruses encoding wild-type or mutant GST-HsCdc6 as indicated, together with viruses encoding either cyclin A and Cdk2 (Figure 3A) or cyclin E and Cdk2 (Figure 3B). Kinase and cyclin, which associated with GST fusion proteins in the extracts, were precipitated on glutathione agarose and analyzed by Western blotting (Figure 3, A and B). No binding of cyclin A/Cdk2 to GST-HsCdc6-Cy (Figure 3A; lane 4), GST-HsCdc6-5X.Cy (lane 6), or to GST alone (lane 2) was detected under these conditions, whereas GST-HsCdc6 (lane 3) and GST-HsCdc6-5X (lane 5) efficiently bound to the kinase complex. These results demonstrate that the two point mutations in the cyclin-binding motif of HsCdc6 specifically interfered with the ability of the protein to interact stably with CycA/Cdk2. The association of GST-HsCdc6-Cy and GST-HsCdc6-5X.Cy with cyclin E/Cdk2 was reduced when compared with wild-type GST-HsCdc6 and GST-HsCdc6-5X, but not abolished (Figure 3B; compare lanes 4 and 6 with lanes 3 and 5). No interaction was observed between CycE/Cdk2 and GST alone (Figure 3B; lane 2). Thus, a small fraction of CycE/Cdk2 remained associated with the cyclin-binding site mutants under these conditions, suggesting that cyclin E may make contact with more residues in HsCdc6 than does cyclin A.
Figure 3.
HsCdc6 with mutations in the cyclin-binding motif is reduced in its affinity for cyclins, but is efficiently phosphorylated by Cdk2. (A and B) Insect cells were coinfected with recombinant baculoviruses encoding the indicated GST fusion proteins together with Cyclin A and Cdk2 (A) and Cyclin E and Cdk2 (B). Cell extracts were prepared and incubated with glutathione agarose. After washing, the beads were boiled in sample buffer, and the eluted proteins resolved by 10% SDS-PAGE. Cyclin A (A), cyclin E (B), or Cdk2 (A and B) that coprecipitated with the GST-fusion proteins was detected by immunoblotting using anti-CycA antibody C160 (A), anti-CycE antibody HE111 (B), or anti-HA antibody 12CA5 (A and B) to recognize the N-terminal HA tag on Cdk2. Lane 1 contained 0.5 μg purified baculovirus-expressed CycA/Cdk2 (A) or CycE/Cdk2 (B) as a marker. (C) Equal amounts of wild-type and the indicated mutant forms of GST-HsCdc6 were incubated with 6 U of purified baculovirus-expressed cyclin A/Cdk2 (lanes 1–4) or cyclin E/Cdk2 (lanes 5–8) in the presence of [γ-32P]ATP. The proteins were resolved by 12.5% SDS-PAGE and visualized by PhosphorImaging.
To determine whether the cyclin-binding site mutants were altered in their ability to be phosphorylated by the Cdk, purified wild-type and mutant GST-HsCdc6 fusion proteins were incubated with purified cyclin A/Cdk2 (Figure 3C; lanes 1–4) and cyclin E/Cdk2 (lanes 5–8) in the presence of [γ-32P]ATP. The reaction products were separated by SDS-PAGE and analyzed by PhosphorImaging (Figure 3C). Cyclin A/Cdk2 and cyclin E/Cdk2 efficiently phosphorylated wild-type GST-HsCdc6 (lanes 1 and 5) and GST-HsCdc6-Cy (lanes 2 and 6). Phosphorylation of GST-HsCdc6-5X and GST-HsCdc6-5X.Cy by cyclin A/Cdk2 (lanes 3 and 4) and cyclin E/Cdk2 (lanes 7 and 8) was reduced to the background level observed without added kinase (our unpublished results, but see Figure 1B, lane 1). These data suggest that the cyclin/Cdk complex need not stably interact with HsCdc6 to efficiently phosphorylate the protein.
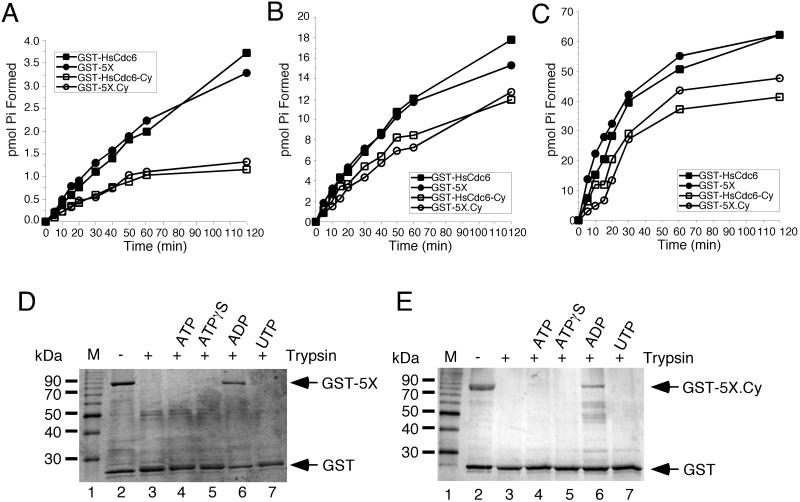
Human Cdc6 displays weak ATPase activity that is specifically disrupted by amino acid substitutions within the Walker A or B motifs (Herbig et al., 1999). To determine whether mutations in the N-terminal Cdk phosphorylation sites or in the cyclin-binding motif of HsCdc6 interfered with the ability of HsCdc6 to hydrolyze ATP, the ATPase activity of purified wild-type protein and mutant HsCdc6 was tested at ATP concentrations ranging from 2.5 to 250 μM (Figure 4, A–C). The ATPase activity of GST-HsCdc6-5X was essentially identical to that of wild-type GST-HsCdc6 at all ATP concentrations tested, indicating that the alanine substitutions at Cdk phosphorylation sites did not affect the ATPase activity of HsCdc6. At an ATP concentration of 2.5 μM, the ATPase activities of GST-HsCdc6-Cy and GST-5X.Cy were reduced by ∼60% compared with wild-type GST-HsCdc6 (Figure 4A). At higher ATP concentrations, this reduction was not as dramatic, and both Cy-mutant proteins displayed ATPase activities that were ∼35% less than that of wild-type HsCdc6 (Figure 4, B and C). A truncation mutant of HsCdc6 lacking the N-terminal 106 amino acids (ΔN, Figure 1A) did not display any detectable ATPase activity and therefore was not further analyzed (our unpublished results). These data indicate that in addition to the Walker A and B motifs (Herbig et al., 1999), residues within the N-terminus of HsCdc6 can also affect ATP hydrolysis.
Figure 4.
Nucleotide binding and hydrolysis properties of mutant GST-HsCdc6. (A–C) GST-HsCdc6 (0.25 pmol; ▪), GST-HsCdc6-5X (GST-5X; ●) GST-HsCdc6-Cy (□) and GST-HsCdc6-5X.Cy (GST-5X.Cy; ○) were incubated with (A) 2.5 μM, (B) 25 μM, or (C) 250 μM [γ-32P]ATP for the indicated times at 37°C. Hydrolysis products were separated by TLC, and the amount of phosphate formed was quantified by PhosphorImaging. GST-HsCdc6-5X (0.5 μg; D) or GST-HsCdc6-5X.Cy (E) bound to glutathione agarose was partially digested with trypsin in the absence of nucleotide (lane 3) or in the presence of 2 mM ATP (lane 4), ATPγS (lane 5), ADP (lane 6), or UTP (lane 7). The reaction products were analyzed by 12.5% SDS-PAGE and silver staining. No trypsin was added to the reaction shown in lane 2. M, 10-kDa marker protein ladder.
Studies in our laboratory have demonstrated that HsCdc6 binds specifically to ATP, ATPγS, and ADP, but not to UTP and that its conformation in the presence of ADP differs from that in the presence of ATP or ATPγS or that without nucleotide (Herbig et al., 1999). To test whether the mutations in the N-terminal Cdk phosphorylation sites affected the ability of HsCdc6 to undergo this ADP-induced switch in conformation, a partial tryptic digest was performed on GST-HsCdc6-5X in the absence and presence of ATP, ATPγS, ADP, and UTP (Figure 4D). As demonstrated in Figure 4D, GST-HsCdc6-5X was highly sensitive to partial tryptic digestion in the absence of nucleotide (lane 3) and in the presence of ATP (lane 4), ATPγS (lane 5), or UTP (lane 7). In contrast, when the mutant protein was bound to ADP, it became resistant to partial tryptic digestion (lane 6). These results are essentially identical to those observed with wild-type GST-HsCdc6 (Herbig et al., 1999), indicating that the amino acid substitutions at the N-terminal Cdk sites did not affect the interactions of HsCdc6 with nucleotides or its ATPase activity.
The mutant form of HsCdc6 in which all N-terminal Cdk phosphorylation sites and the cyclin-binding motif had been mutated (GST-HsCdc6-5X.Cy) also became partially resistant to tryptic digestion when the protein was complexed to ADP (Figure 4E, lane 6). When GST-HsCdc6-5X.Cy was bound to ATP (lane 4) or ATPγS (lane 5) or in the presence of UTP (lane 7), the tryptic digestion pattern was the same as that observed with protein in the absence of nucleotide (lane 3). Therefore, the mutant protein was still able to undergo a conformational change when bound to ADP, although the ATPase activity of HsCdc6 was reduced by the amino acid substitutions in the cyclin-binding motif.
Taken together, the biochemical characterization indicates that mutations in potential Cdk phosphorylation sites of HsCdc6 reduced its ability to be phosphorylated by cyclin/Cdk but did not detectably alter its ability to bind to Cdk, HsCdc6, or HsOrc1, to hydrolyze ATP, or to undergo an ADP-dependent change in conformation. These results strongly suggest that these mutant proteins are suitable reagents to probe the role of cyclin/Cdk phosphorylation in regulating the functions of HsCdc6 in DNA replication. Mutations in the cyclin-binding motif reduced or abolished stable cyclin binding without markedly affecting HsCdc6 phosphorylation. On the other hand, the cyclin-binding site mutations also reduced the ATPase activity moderately, indicating that the N-terminus of HsCdc6 may affect multiple functions of the protein.
HsCdc6 Phosphorylation Site Mutants Block Chromosomal DNA Replication in Vivo
If phosphorylation of HsCdc6 by Cdk is required for DNA replication in human cells, then a nonphosphorylatable mutant form of HsCdc6 that is able to associate with prereplicative complexes should prevent the initiation of DNA replication by interfering with the functions of endogenous wild-type protein. To test this prediction, we microinjected purified wild-type and the five phosphorylation site mutants of HsCdc6 into HeLa-S3 cells. Cells to be injected were either synchronized in early G1, before HsCdc6 function was required, or as a control, in very early S phase. Early G1 cells were obtained by releasing HeLa-S3 cells blocked in G2/M with nocodazole into drug-free medium for 6 to 8 h, and G1/S phase cells were obtained by using a thymidine block (Herbig et al., 1999). After the cells were microinjected, the medium was supplemented with BrdU, and the cells were allowed to grow for an additional 17 h (injected in G1) or 12 h (injected in G1/S), at which time uninjected cells had reached G2/M (our unpublished results).
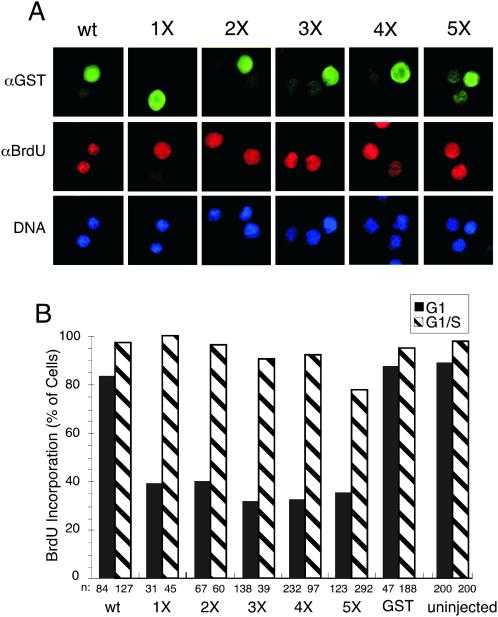
The effects of the phosphorylation site mutants on DNA replication were then evaluated by immunofluorescent microscopy. Cells that had been microinjected with the indicated GST-fusion proteins were detected by immunofluorescent staining against GST (Figure 5A, top row). Cells were analyzed for BrdU incorporation into the DNA (middle row) and stained with Hoechst dye (bottom row) to detect bulk nuclear DNA. Microinjection of wild-type GST-HsCdc6 had no effect on the ability of cells to enter and complete S-phase (wt). BrdU incorporation was strongly reduced in cells that had been microinjected with GST-HsCdc6-1X (Figure 5A, our unpublished results). Cells that had been microinjected with GST-HsCdc6-2X, GST-HsCdc6-3X, GST-HsCdc6-4X, and GST-HsCdc6-5X apparently failed to incorporate BrdU.
Figure 5.
HsCdc6 with mutations at Cdk phosphorylation sites inhibits DNA replication in human cells. (A) HeLa-S3 cells were arrested for 16 h in G2/M using nocodazole. At 6–8 h after release into nocodazole-free medium, the following proteins were injected into the nucleus of the cells: GST-HsCdc6 (column 1), GST-HsCdc6-1X (1X; column 2), GST-HsCdc6-2X (2X; column 3), GST-HsCdc6-3X (3X; column 4), GST-HsCdc6-4X (4X; column 5), and GST-HsCdc6-5X (5X; column 6). The injected cells were grown for 17 h in medium containing bromodeoxyuridine (BrdU). The cells were stained with anti-GST polyclonal antibody and FITC-conjugated goat anti-rabbit secondary antibody (top row), anti-BrdU monoclonal antibody and Cy3-conjugated goat anti-mouse secondary antibody (middle row), and Hoechst 33258 fluorochrome (bottom row). Micrographs were taken at a 100× magnification with a digital camera mounted on a fluorescence microscope. (B) The following proteins at the indicated concentrations were injected into the nuclei of HeLa-S3 cells in G1 or G1/S: GST (500 ng/μl), GST-HsCdc6 (52 ng/μl), GST-HsCdc6 (1X; 23 ng/μl), GST-HsCdc6 (2X; 16.9 ng/μl), GST-HsCdc6 (3X; 53 ng/μl), GST-HsCdc6 (4X; 51 ng/μl), and GST-HsCdc6 (5X; 55.8 ng/μl). The proteins were injected either at 6–8 h after release from nocodazole (black bars) or in G1/S, immediately after release from a thymidine block (hatched bars). After injection of G1 cells, growth was continued for 17 h in medium containing BrdU. Cells injected in G1/S were grown for an additional 12 h in medium containing BrdU. Cells were fixed, stained with antibodies against GST and against BrdU, and analyzed by indirect immunofluorescence microscopy. n represents the number of cells that were successfully injected and analyzed for BrdU incorporation. Cells that incorporated BrdU as efficiently as uninjected cells were defined as BrdU-positive, whereas BrdU-negative cells were those not stained or stained weakly.
A quantitative evaluation of DNA replication in microinjected and uninjected cells is shown in Figure 5B. More than 90% of the uninjected cells analyzed 23 h after release from a nocodazole block (black bars) or 12 h after release from a thymidine block (hatched bars) had replicated their DNA. GST and GST-HsCdc6 (wt) had no effect on DNA replication when injected in G1 or G1/S. The percentage of cells injected with 1X mutant protein that were BrdU positive but incorporated significantly less BrdU than wild-type protein was >60% (Figure 5B, black bars). Microinjection of GST-HsCdc6-2X, GST-HsCdc6-3X, GST-HsCdc6-4X, and GST-HsCdc6-5X completely blocked DNA replication in 60–70% of the cells when injected in G1 (Figure 5B, black bars). None of the phosphorylation site mutants interfered with chromosomal DNA replication when injected in G1/S just after DNA replication had initiated (hatched bars). These results demonstrate that HsCdc6 deficient in its ability to become phosphorylated by Cdk specifically inhibited DNA replication when present during G1, but not when introduced after S phase had begun.
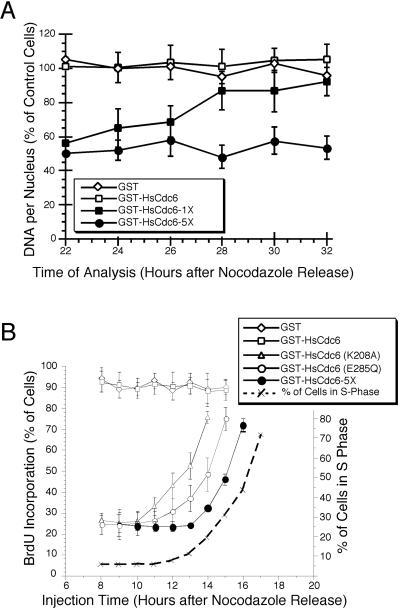
For the experiments shown in Figure 5, microinjected cells were analyzed 23 h after they had been released from a nocodazole block. At this time, uninjected cells would have reached the G2 phase of the cell cycle as determined by flow cytometry (our unpublished results). Thus, the failure of cells microinjected with GST-HsCdc6-5X to incorporate BrdU could reflect either a delay in initiation of DNA replication or a block. The reduced BrdU incorporation in cells injected with the 1X mutant could indicate that the S phase entry was delayed compared with uninjected cells, but progressed at the normal rate; that S phase progression was slowed, but eventually completed; or that DNA replication was not completed. To distinguish among these possibilities, HeLa-S3 cells were injected with GST-HsCdc6-5X and with GST-HsCdc6-1X at 6 to 9 h after a release from a nocodazole block. At 12 h after the release, the medium was again supplemented with nocodazole to prevent cells from passing thorough mitosis into the following G1 phase. At 22 h after the release, the injected cells were identified by immunofluorescent staining against GST and their nuclear DNA content was quantitatively evaluated by comparing nuclear Hoechst 33258 staining with that in uninjected cells (Araki et al., 1987).
The nuclear DNA content of cells that had been microinjected with GST or wild-type GST-HsCdc6 was similar to that of uninjected cells at all times tested (Figure 6A, open symbols). The nuclear DNA content of cells that had been microinjected with GST-HsCdc6-5X was approximately one half that of uninjected cells at each time point tested, indicating that the mutant form of HsCdc6 lacking the N-terminal Cdk phosphorylation sites prevented the G1/S transition (Figure 6A, filled circles). In contrast, the nuclear DNA content of cells that had been microinjected with GST-HsCdc6-1X increased steadily between 22 and 32 h after the release from a nocodazole block, eventually reaching the same level as GST-injected control cells (filled squares). These data indicate that in cells that had been injected with the single phosphorylation site mutant of HsCdc6, S phase progression was significantly slower than that in cells injected with wild-type GST-HsCdc6 or GST, but did eventually continue to completion.
Figure 6.
Kinetics of replication interference by mutant HsCdc6. (A) HeLa-S3 cells were arrested for 16 h in G2/M with nocodazole and released into nocodazole-free medium. At the indicated times, GST (500 ng/μl; ⋄), GST-HsCdc6 (61 ng/μl; □), GST-HsCdc6 (K208A; 20 ng/μl; ▵), GST-HsCdc6 (E285Q; 30 ng/μl; ○), and GST-HsCdc6; 5X; 55.8 ng/μl; ●) were microinjected into the nuclei of the cells. Immediately after microinjection, the medium was supplemented with BrdU, and growth was continued until 22 h after the nocodazole release. Cells were then stained with anti-GST and anti-BrdU antibodies and evaluated by indirect immunofluorescence microscopy. For each time point, the average value obtained from at least 40 injected cells and the SD of the mean are shown. The dashed line indicates the percentage of uninjected cells in S phase at the indicated times after release from a nocodazole block, as determined by flow cytometry of HeLa-S3 cells blocked and released in the same manner. (B) HeLa-S3 cells that had been arrested in G2/M with nocodazole for 16 h were released into nocodazole-free medium for 6–8 h. The following proteins at the indicated concentrations were microinjected into the nuclei of the cells: GST (500 ng/μl; ⋄), GST-HsCdc6 (61 ng/μl; □), GST-HsCdc6 (1X; 17 ng/μl; □), and GST-HsCdc6 (5X; 34 ng/μl; ○). At 12 h after the release, the medium was again supplemented with nocodazole to prevent progression through mitosis. At the indicated times, the cells were stained with anti-GST polyclonal antibody and FITC-conjugated goat anti-rabbit secondary antibody, and Hoechst 33258 fluorochrome. Nuclear DNA content of injected cells, measured by fluorescence microscopy, is expressed as a percentage of the nuclear DNA content of uninjected cells in the same field of vision, which was set to 100%. For each time point, the average value obtained from at least 10 cells is shown. Error bars, the SD of the mean.
To investigate more precisely when in G1 the phosphorylation site mutants interfered with the activity of endogenous HsCdc6, HeLa-S3 were microinjected with GST-HsCdc6-5X at different times after the release from a nocodazole block. To monitor DNA replication, BrdU was added to the medium after injection, and incorporation was evaluated by immunofluorescence at 22 h after release from the block. As shown in Figure 6B, microinjection of GST-HsCdc6-5X at 9 to 13 h after release from a nocodazole block prevented BrdU incorporation in 75% of the cells (filled circles). However, cells that were injected at 14 h after release or later did incorporate BrdU. The fraction of BrdU-positive cells increased steadily with the time of injection. Significantly, this increase was delayed by 1–1.5 h compared with that in cells injected with GST-HsCdc6 (E285Q) and 2–2.5 h compared with that in cells injected with GST-HsCdc6 (K208A; Herbig et al., 1999). The drop in inhibitory activity of GST-HsCdc6-5X almost coincided temporally with the initiation of DNA replication as determined by flow cytometry (dashed line). These findings suggest that phosphorylation of HsCdc6 by Cdk was required after nucleotide binding and hydrolysis by HsCdc6 and very close to the time when DNA replication initiated.
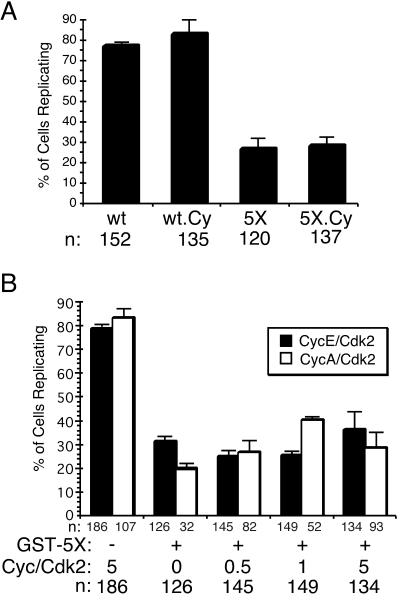
An alternative explanation of these results might be that binding of the multiple phosphorylation site mutants to the G1s cyclin/Cdk2 complexes may be stronger than that of the wild-type protein, causing them to sequester Cdk2 and thereby preventing phosphorylation of other key substrates late in the initiation process. If this were the case, then GST-HsCdc6-5X.Cy should allow DNA replication when injected into G1 HeLa cells, because its affinity for cyclin A/Cdk2 and cyclin E/Cdk2 was strongly reduced (Figures 3, A and B). We therefore microinjected purified wild-type and mutant proteins into HeLa-S3 cells that had been released from a nocodazole block into early G1 of the cell cycle. DNA synthesis in the microinjected cells was analyzed as in Figure 5, and a quantitative evaluation of the results is shown in Figure 7A. Microinjection of wild-type GST-HsCdc6 (wt) and GST-HsCdc6-Cy (wt-Cy) had no detectable effect on the ability of cells to replicate nuclear DNA. This result indicates that the reduced ATPase of the Cy mutant had no effect on the replication activity of the endogenous HsCdc6. However, >70% of the cells that had been microinjected with either GST-HsCdc6-5X (5X) or GST-HsCdc6-5X.Cy (5X.Cy) failed to incorporate BrdU. Because a small fraction of cyclin E/Cdk2 remained associated with the cyclin-binding site mutants of HsCdc6 (see Figure 3B), it could also be argued that even the 5X.Cy mutant was able to sequester endogenous CycE/Cdk2 and thereby block DNA replication in microinjected cells. To assess this possibility, we microinjected GST-HsCdc6-5X (Figure 7, 5X) together with increasing amounts of purified recombinant cyclin E/Cdk2 (black bars) or cyclin A/Cdk2 (white bars) into HeLa G1 cells as described above. Neither cyclin E/Cdk2 nor cyclin A/Cdk2 was able to rescue the DNA replication defect caused by GST-HsCdc6-5X. These data indicate that the multiple phosphorylation site mutants of HsCdc6 inhibit DNA replication by a mechanism that is independent of their association with cyclin/Cdk2 complexes.
Figure 7.
Inhibition of DNA replication by HsCdc6-5X does not require stable protein interactions between HsCdc6-5X and G1 cyclins. (A) HeLa-S3 cells were arrested for 16 h in G2/M using nocodazole. At 6–8 h after release into nocodazole-free medium, the following proteins were injected into the nucleus of the cells: GST-HsCdc6 (wt), GST-HsCdc6-Cy (wt Cy), GST-HsCdc6-5X (5X), and GST-HsCdc6-5X.Cy (5X-Cy). Cells were fixed, stained with antibodies against GST and against BrdU, and analyzed by indirect immunofluorescence microscopy. n represents the number of cells that were successfully injected and analyzed for BrdU incorporation. Cells that visibly incorporated BrdU were defined as BrdU-positive cells, whereas BrdU-negative cells were those not stained. Error bars, the average error of the mean as determined from two separate experiments. (B) HeLa-S3 cells that had been synchronized as described in (A) were injected with GST-HsCdc6-5X (GST-5X) together with indicated amounts of cyclin/Cdk complexes, shown as molar excess over GST-HsCdc6-5X. For the first column pair, cells were injected only with cyclin/Cdk. Error bars, the average error of the mean as determined from two separate experiments.
DISCUSSION
Consistent with two recent reports that Cdk phosphorylates multiple N-terminal sites in HsCdc6 in vitro and in vivo (Jiang et al., 1999; Petersen et al., 1999), we have shown that purified, biochemically active HsCdc6 is specifically phosphorylated by CycE/Cdk2, CycA/Cdk2, and CycA/Cdc2 in vitro (Figure 1B) and that sequential mutagenesis of potential Cdk sites in HsCdc6 resulted in stepwise reduction of HsCdc6 phosphorylation by CycA/Cdk2 (Figure 1D) and CycE/Cdk2 (our unpublished results). Biochemical characterization of the 5X phosphorylation site mutant revealed that it retained wild-type activity in binding to cyclin A, HsOrc1, and HsCdc6; in hydrolysis of ATP; and in its ability to assume a protease-resistant conformation in the presence of ADP (Figures 2 and 4). These data provide strong evidence that the phosphorylation site mutant proteins are poor substrates for Cdk, but are functional and probably not grossly distorted in structure. In contrast, the N-terminal truncation mutant ΔN displayed no detectable ATPase activity, suggesting that it was improperly folded and nonfunctional (our unpublished results). HsCdc6 with mutations in the cyclin-binding motif displayed reduced affinity for cyclins, but was phosphorylated by cyclin/Cdk2 to levels similar to that of wild-type HsCdc6 (Figure 3). Although the ATPase activity of the cyclin-binding mutants was reduced (Figure 4A–C), GST-HsCdc6-5X.Cy was able to undergo the ADP-dependent conformational change (Figure 4E), indicating that nucleotide binding was not compromised by these mutations.
Microinjection of the HsCdc6 phosphorylation site mutant proteins into HeLa cell nuclei in G1 interfered with the DNA replication activity of endogenous wild-type HsCdc6 (Figures 5 and 6). In contrast, injection of the ΔN mutant protein in G1 did not affect DNA replication (our unpublished results), consistent with the idea that it was structurally aberrant and nonfunctional. Cells microinjected with mutant HsCdc6 lacking a single Cdk phosphorylation site at Ser74 (GST-HsCdc6-1X) entered S phase, but the duration of S phase was at least doubled compared with that in cells injected with wild-type GST-HsCdc6 (Figure 6A). A superficial interpretation of these observations would be that phosphorylation of HsCdc6 is required for S phase progression. However, because the replication initiation functions of Cdc6 are thought to be restricted to G1 phase and the G1/S transition (Dutta and Bell, 1997) and cyclin A/Cdk2 appears to be required for the G1/S transition but not S phase progression (Pagano et al., 1992), it seems unlikely that phosphorylation of HsCdc6 by cyclin A/Cdk2 is necessary for S phase progression. An alternative interpretation is that the single site mutant is leaky and allows a small number of origins to fire at the G1/S transition under the control of the endogenous wild-type HsCdc6. If too few origins were fired and hence too few replication forks were assembled, this could explain the observed delay in completion of S phase. We are currently investigating whether other single Cdk phosphorylation site mutants of HsCdc6 display a phenotype similar to GST-HsCdc6-1X.
If this latter interpretation is correct, it would imply that phosphorylation of multiple Cdk sites in HsCdc6 is required to achieve wild-type levels of initiation of DNA replication. In support of this notion, cells injected with mutants lacking two or more Cdk phosphorylation sites failed to initiate DNA replication, even at 18 h after control cells had entered S phase (Figures 5 and 6; our unpublished results). Because Thr67 was poorly phosphorylated by cyclin A/Cdk2 and cyclin E/Cdk2 in vitro and in vivo compared with the serine sites (Figure 1D, compare 1X and 2X; Jiang et al., 1999; Petersen et al., 1999), it is probably not targeted by Cdk. However, microinjection of the HsCdc6 mutant with alanine substitutions at positions 74 and 67 blocked S phase entry more efficiently than the single mutant with alanine only at serine 74 (Figure 5, A and B). This observation suggests that the mutation at Thr67 may affect the same biochemical activity of HsCdc6 that requires phosphorylation of serine Cdk sites to initiate DNA replication. So far, the only other activity of HsCdc6 known to require Cdk phosphorylation is nuclear export of HsCdc6 at the onset of S phase (Jiang et al., 1999; Petersen et al., 1999). Thus, it will be of interest to test whether Thr67 plays a role in HsCdc6 nuclear export independent of Cdk phosphorylation of nearby sites.
Our observations that Cdk phosphorylation site mutants of HsCdc6 interfere with DNA replication in human cells (Figures 5 and 6) confirm and extend those reported by Jiang et al. (1999) while this work was in preparation. In contrast, another recent study found that two different phosphorylation-deficient HsCdc6 mutants had no effect on DNA replication in human cells (Petersen et al., 1999). In one mutant, Ser54, Ser74, and Ser106 were substituted by alanines, the same substitutions tested by Jiang et al., (1999), whereas the other mutant lacked the cyclin A binding site (amino acids 93–100 deleted). Because our HsCdc6 mutants with two amino acid substitutions in the cyclin-binding motif displayed reduced ATPase activity (Figure 4, A–C) and our ΔN truncation mutant had no detectable ATPase activity (our unpublished results), it is likely that the N-terminus of the protein contributes to nucleotide binding, to hydrolysis, and/or to proper folding of HsCdc6. If so, the Cy-motif deletion mutant used by Petersen et al. (1999) may have been nonfunctional. It is unlikely that the differences in the phenotypes of HsCdc6 phosphorylation mutants are due to the cell type used, because these mutants interfered with DNA replication not only in HeLa cells, but also in normal human fibroblasts and U2OS cells (Jiang et al., 1999; Figures 5 and 6; our unpublished results). Moreover, the ability of phosphorylation site mutants to interfere with replication is not specific to the constructs used to express HsCdc6, because mutants fused to either GST (Figures 5 and 6) or GFP (Jiang et al., 1999) had a dominant negative phenotype. The dominant negative phenotype was also independent of the protocol used to introduce the mutant proteins (Figures 5 and 6; Jiang et al., 1999). Other experimental differences that could account for the discrepancy in results are the level of overexpressed mutant protein in the cells and the possibility that some N-terminal fusions could render the mutant proteins nonfunctional.
Three lines of evidence indicate that the replication defect caused by the multiple phosphorylation site mutants is probably not due to sequestration of the cyclin/Cdk complexes and the consequent failure to sufficiently phosphorylate other replication initiation proteins. First, the phosphorylation site mutants bound the same amounts of cyclin A/Cdk2 and cyclin E/Cdk2 as the wild-type protein, at least in vitro (Figures 2A, 3A, and 3B; Petersen et al., 1999). Second, microinjection of a HsCdc6 mutant that was reduced in its affinity for G1 cyclin/Cdk and lacked the N-terminal Cdk phosphorylation sites was able to block replication when injected in G1 of the cell cycle, whereas the cyclin-binding mutant that could be phosphorylated by Cdk2 did not block replication (Figure 7A). Third, microinjection of GST-HsCdc6-5X together with cyclin A/Cdk2 or cyclin E/Cdk2 did not rescue the replication defect caused by the phosphorylation mutant, even when the kinases were in fivefold molar excess over mutant HsCdc6 (Figure 7B).
The DNA replication-interfering activity of GST-HsCdc6-5X was lost very late in G1 close to the G1/S transition (Figure 6B). This is significantly later in the cell cycle than the loss of replication-interfering activity observed for the Walker A and the Walker B mutant forms of HsCdc6 (Figure 6B; Herbig et al., 1999), suggesting that phosphorylation of HsCdc6 by Cdk is a separate event that occurs after HsCdc6 has bound to and hydrolyzed ATP. The simplest explanation for our results and those of Jiang et al. (1999) is that phosphorylation of Cdc6 by Cdk is an obligatory step late in initiation of human DNA replication.
At least two biochemical roles for HsCdc6 phosphorylation in the initiation process can be postulated. Phosphorylation of HsCdc6 by Cdk is a prerequisite for its export from the nucleus in early S phase (Saha et al., 1998; Jiang et al., 1999; Petersen et al., 1999), and the data currently available are consistent with the possibility that nuclear export of HsCdc6 is necessary for initiation of replication in human somatic cells. Additional work will be required to test this correlation directly. Alternatively, phosphorylation of HsCdc6 may be required to remodel the prereplicative complex late in the initiation process. For example, if HsCdc6 retention in the pre-RC inhibited an activity or an event required late in the initiation process, phosphorylation of HsCdc6 might be necessary to induce its dissociation from the pre-RC. Consistent with this interpretation, it was recently demonstrated that overexpression of PR48, a regulatory subunit of the protein phosphatase 2A (PP2A) that binds to HsCdc6, caused a G1 arrest in human cells (Yan et al., 2000). It was suggested that dephosphorylation of HsCdc6 by PP2A prevents the dissociation of HsCdc6 from the prereplicative complex, thereby inhibiting origin firing (Yan et al., 2000). Also, consistent with a role for Cdk phosphorylation in remodeling the metazoan prereplicative complex, high Cdk activity has been shown to promote the displacement of Xenopus Cdc6 from chromatin after assembly of MCM proteins on chromatin (Hua and Newport, 1998). However, it remains unclear whether phosphorylation of Xenopus Cdc6 and its displacement from chromatin are required to initiate DNA replication in egg extracts in vitro.
Our results and those of Jiang et al. (1999) strongly suggest that HsCdc6 phosphorylation is essential for subsequent steps in initiation in human somatic cells. Because phosphorylation of Cdc6/Cdc18 is dispensable for initiation of DNA replication in yeast (Drury et al., 1997; Jallepalli et al., 1997; Elsasser et al., 1999; Calzada et al., 2000; Drury et al., 2000), this interpretation would imply that initiation of replication in somatic human cells and perhaps other metazoan cells is subject to an additional mechanism of positive control by Cdk that is not found in yeast. Although many DNA replication initiation factors are conserved between yeast, Xenopus, and humans, it seems plausible that additional levels of control evolved in multicellular organisms. The replication initiation protein Cdt1 is essential for S phase entry in Xenopus and is conserved in humans but not in budding yeast (Maiorano et al., 2000; Nishitani et al., 2000). In addition, geminin has been shown to negatively regulate DNA replication in Xenopus, but is apparently not found in budding or fission yeast (McGarry and Kirschner, 1998). These differences, as well as the requirement for Cdc6 phosphorylation, imply that the regulation of replication initiation in human somatic cells is probably more complex than that in yeast or in the early stages of metazoan development.
ACKNOWLEDGMENTS
We thank R. Weber for providing the polyclonal antibodies against GST, J. Schneider-Mergener for the HA-peptide, E. Harlow and H. Chu for the GST-Cdk4 and CycD1 baculovirus, and A. H. Lin and M. Zayas for assistance with the experiments. We are grateful to V. Podust for critical reading of this manuscript. This work was supported by the National Institutes of Health (GM52948), a Pfizer Undergraduate Research Fellowship, a Shared Equipment grant from the National Science Foundation (BIR-9419667), and Vanderbilt University.
Note added in proof.
C. Pelizon, M.A. Madine, P. Romanowski, and R.A. Laskey (Genes Dev. [2000]; 14, 2526–2533) recently reported that phosphorylation site mutants of Xenopus Cdc6 did not inhibit DNA replication in egg extracts and that the mutant proteins could replace wild-type Cdc6 in replication assays. These results, taken together with our results and those of Jiang et al., (1999), reveal clear differences between human and Xenopus Cdc6 in regulating metazoan DNA replication.
REFERENCES
- Aparicio OM, Weinstein DM, Bell. SP. Components and dynamics of DNA replication complexes in S. cerevisiae: redistribution of MCM proteins and cdc45p during S phase. Cell. 1997;91:59–69. doi: 10.1016/s0092-8674(01)80009-x. [DOI] [PubMed] [Google Scholar]
- Araki T, Yamamoto A, Yamada M. Accurate determination of DNA content in single cell nuclei stained with Hoechst 33258 fluorochrome at high salt concentration. Histochemistry. 1987;87:331–338. doi: 10.1007/BF00492587. [DOI] [PubMed] [Google Scholar]
- Baum B, Nishitani H, Yanow S, Nurse P. Cdc18 transcription and proteolysis couple S phase to passage through mitosis. EMBO J. 1998;17:5689–5698. doi: 10.1093/emboj/17.19.5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GW, Jallepalli PV, Huneycutt BJ, Kelly TJ. Interaction of the S phase regulator cdc18 with cyclin-dependent kinase in fission yeast. Proc Natl Acad Sci USA. 1997;94:6142–6147. doi: 10.1073/pnas.94.12.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzada A, Sánchez M, Sánchez E, Bueno A. The stability of the Cdc6 protein is regulated by cyclin-dependent kinase/cyclin B complexes in Saccharomyces cerevisiae. J Biol Chem. 2000;275:9734–9741. doi: 10.1074/jbc.275.13.9734. [DOI] [PubMed] [Google Scholar]
- Coleman TR, Carpenter PB, Dunphy WG. The Xenopus Cdc6 protein is essential for the initiation of a single round of DNA replication in cell-free extracts. Cell. 1996;87:53–63. doi: 10.1016/s0092-8674(00)81322-7. [DOI] [PubMed] [Google Scholar]
- Donovan S, Harwood J, Drury LS, Diffley JF. Cdc6p-dependent loading of Mcm proteins onto pre-replicative chromatin in budding yeast. Proc Natl Acad Sci USA. 1997;94:5611–5616. doi: 10.1073/pnas.94.11.5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury LS, Perkins G, Diffley JF. The Cdc4/34/53 pathway targets Cdc6p for proteolysis in budding yeast. EMBO J. 1997;16:5966–5976. doi: 10.1093/emboj/16.19.5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury LS, Perkins G, Diffley JF. The cyclin-dependent kinase Cdc28p regulates distinct modes of Cdc6p proteolysis during the budding yeast cell cycle. Curr Biol. 2000;10:231–240. doi: 10.1016/s0960-9822(00)00355-9. [DOI] [PubMed] [Google Scholar]
- Dulic V, Lees E, Reed SI. Association of human cyclin E with a periodic G1-S phase protein kinase. Science. 1992;257:1958–1961. doi: 10.1126/science.1329201. [DOI] [PubMed] [Google Scholar]
- Dutta A, Bell SP. Initiation of DNA replication in eukaryotic cells. Annu Rev Cell Dev Biol. 1997;13:293–332. doi: 10.1146/annurev.cellbio.13.1.293. [DOI] [PubMed] [Google Scholar]
- Elsasser S, Lou F, Wang B, Campbell JL, Jong A. Interaction between yeast Cdc6 protein and B-type cyclin/Cdc28 kinases. Mol Biol Cell. 1996;7:1723–1735. doi: 10.1091/mbc.7.11.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsasser S, Chi Y, Campbell JL. Phosphorylation controls timing of Cdc6p destruction: a biochemical analysis. Mol Biol Cell. 1999;10:3263–3277. doi: 10.1091/mbc.10.10.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Yamada C, Goto H, Yokoyama N, Kuzushima K, Inagaki M, Tsurumi T. Cell cycle regulation of human CDC6 protein. Intracellular localization, interaction with the human MCM complex, and Cdc2 kinase-mediated hyperphosphorylation. J Biol Chem. 1999;274:25927–25932. doi: 10.1074/jbc.274.36.25927. [DOI] [PubMed] [Google Scholar]
- Giordano A, Whyte P, Harlow E, Franza BR, Beach D, Draetta G. A 60 kd Cdc2-associated polypeptide complexes with the E1A proteins in adenovirus-infected cells. Cell. 1989;85:981–990. doi: 10.1016/0092-8674(89)90949-5. [DOI] [PubMed] [Google Scholar]
- Girard F, Strausfeld U, Fernandez A, Lamb NJ. Cyclin A is required for the onset of DNA replication in mammalian fibroblasts. Cell. 1991;67:1169–1179. doi: 10.1016/0092-8674(91)90293-8. [DOI] [PubMed] [Google Scholar]
- Herbig U, Marlar CA, Fanning E. The cdc6 nucleotide-binding site regulates its activity in DNA replication in human cells. Mol Biol Cell. 1999;10:2631–2645. doi: 10.1091/mbc.10.8.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua XH, Newport J. Identification of a preinitiation step in DNA replication that is independent of origin recognition complex and cdc6, but dependent on Cdk2. J Cell Biol. 1998;137:183–192. doi: 10.1083/jcb.140.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jallepalli PV, Brown GW, Muzi-Falconi M, Tien D, Kelly TJ. Regulation of the replication initiator protein p65cdc18 by CDK phosphorylation. Genes Dev. 1997;11:2767–2779. doi: 10.1101/gad.11.21.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Wells NJ, Hunter T. Multistep regulation of DNA replication by Cdk phosphorylation of HsCdc6. Proc Natl Acad Sci USA. 1999;96:6193–6198. doi: 10.1073/pnas.96.11.6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DG, Nogueira Araujo GM. A simple method of reducing the fading of immunofluorescence during microscopy. J Immunol Methods. 1981;43:349–350. doi: 10.1016/0022-1759(81)90183-6. [DOI] [PubMed] [Google Scholar]
- Koff A, Giordano A, Desai D, Yamashita K, Harper JW, Elledge S, Nishimoto T, Morgan DO, Franza BR, Roberts JM. Formation and activation of a cyclin E-Cdk2 complex during the G1 phase of the human cell cycle. Science. 1992;257:1689–1694. doi: 10.1126/science.1388288. [DOI] [PubMed] [Google Scholar]
- Kominami K, Toda T. Fission yeast WD-repeat protein pop1 regulates genome ploidy through ubiquitin-proteasome-mediated degradation of the CDK inhibitor Rum1 and the S-phase initiator Cdc18. Genes Dev. 1997;11:1548–1560. doi: 10.1101/gad.11.12.1548. [DOI] [PubMed] [Google Scholar]
- Krude T, Jackman M, Pines J, Laskey RA. Cyclin/Cdk-dependent initiation of DNA replication in a human cell-free system. Cell. 1997;88:109–119. doi: 10.1016/s0092-8674(00)81863-2. [DOI] [PubMed] [Google Scholar]
- Li JJ, Herskowitz I. Isolation of ORC6, a component of the yeast origin recognition complex by a one-hybrid system. Science. 1993;262:1870–1874. doi: 10.1126/science.8266075. [DOI] [PubMed] [Google Scholar]
- Liang C, Weinreich M, Stillman B. ORC and Cdc6p interact and determine the frequency of initiation of DNA replication in the genome. Cell. 1995;81:667–676. doi: 10.1016/0092-8674(95)90528-6. [DOI] [PubMed] [Google Scholar]
- Lopez-Girona A, Mondesert O, Leatherwood J, Russell P. Negative regulation of Cdc18 DNA replication protein by Cdc2. Mol Biol Cell. 1998;9:63–73. doi: 10.1091/mbc.9.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiorano D, Moreau J, Mechaeli M. XCDT1 is required for the assembly of pre-replicative complexes in Xenopus laevis. Nature. 2000;404:622–625. doi: 10.1038/35007104. [DOI] [PubMed] [Google Scholar]
- McGarry TJ, Kirschner MW. Geminin, an inhibitor of DNA replication, is degraded during mitosis. Cell. 1998;93:1043–1053. doi: 10.1016/s0092-8674(00)81209-x. [DOI] [PubMed] [Google Scholar]
- Mizushima T, Takahashi N, Stillman B. Cdc6p modulates the structure and DNA binding activity of the origin recognition complex in vitro. Genes Dev. 2000;14:1631–1641. [PMC free article] [PubMed] [Google Scholar]
- Newlon CS. Putting it all together: building a prereplicative complex. Cell. 1997;91:717–720. doi: 10.1016/s0092-8674(00)80459-6. [DOI] [PubMed] [Google Scholar]
- Nishitani H, Lygerou Z, Nishimoto T, Nurse P. The Cdt1 protein is required to license DNA for replication in fission yeast. Nature. 2000;404:625–628. doi: 10.1038/35007110. [DOI] [PubMed] [Google Scholar]
- Ohtsubo M, Theodoras AM, Schumacher J, Roberts JM, Pagano M. Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol Cell Biol. 1995;15:2612–2624. doi: 10.1128/mcb.15.5.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano M, Pepperkok R, Verde F, Ansorge W, Draetta G. Cyclin A is required at two points in the human cell cycle. EMBO J. 1992;11:961–971. doi: 10.1002/j.1460-2075.1992.tb05135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins G, Diffley JF. Nucleotide-dependent prereplicative complex assembly by Cdc6p, a homolog of eukaryotic and prokaryotic clamp-loaders. Mol Cell. 1998;2:23–32. doi: 10.1016/s1097-2765(00)80110-0. [DOI] [PubMed] [Google Scholar]
- Petersen BO, Lukas J, Sorensen CS, Bartek J, Helin K. Phosphorylation of mammalian CDC6 by cyclin A/CDK2 regulates its subcellular localization. EMBO J. 1999;18:396–410. doi: 10.1093/emboj/18.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatti S, Bohm T, Cocker JH, Diffley JF, Nasmyth K. Activation of S-phase-promoting CDKs in late G1 defines a “point of no return” after which Cdc6 synthesis cannot promote DNA replication in yeast. Genes Dev. 1996;10:1516–1531. doi: 10.1101/gad.10.12.1516. [DOI] [PubMed] [Google Scholar]
- Resnitzky D, Gossen M, Bujard H, Reed S. Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol Cell Biol. 1994;14:1669–1679. doi: 10.1128/mcb.14.3.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnitzky D, Hengst L, Reed. S. Cyclin A-associated. kinase activity is rate limiting for entrance into S phase and is negatively regulated in G1 by p27Kip1. Mol Cell Biol. 1995;15:4347–4352. doi: 10.1128/mcb.15.8.4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JM. Evolving ideas about cyclins. Cell. 1999;98:129–132. doi: 10.1016/s0092-8674(00)81007-7. [DOI] [PubMed] [Google Scholar]
- Sanchez M, Calzada A, Bueno A. The Cdc6 protein is ubiquitinated in vivo for proteolysis in Saccharomyces cerevisiae. J Biol Chem. 1999;274:9092–9097. doi: 10.1074/jbc.274.13.9092. [DOI] [PubMed] [Google Scholar]
- Saha P, Chen J, Thome KC, Lawlis SJ, Hou ZH, Hendricks M, Parvin JD, Dutta A. Human CDC6/Cdc18 associates with Orc1 and cyclin-Cdk and is selectively eliminated from the nucleus at the onset of S phase. Mol Cell Biol. 1998;18:2758–2767. doi: 10.1128/mcb.18.5.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- Takei Y, Yamamoto K, Tsujimoto G. Identification of the sequence responsible for the nuclear localization of human Cdc6. FEBS Lett. 1999;447:292–296. doi: 10.1016/s0014-5793(99)00306-3. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Knapp D, Nasmyth K. Loading of an MCM protein onto DNA replication origins is regulated by Cdc6p and CDKs. Cell. 1997;90:649–660. doi: 10.1016/s0092-8674(00)80526-7. [DOI] [PubMed] [Google Scholar]
- Tsai LH, Lees E, Faha B, Harlow E, Riabowol K. The Cdk2 kinase is required for the G1-to-S transition in mammalian cells. Oncogene. 1993;8:1593–1602. [PubMed] [Google Scholar]
- Voitenleitner C, Rehfuess C, Hilmes M, O'Rear L, Liao PC, Gauge DA, Ott R, Nasheuer HP, Fanning E. Cell cycle-dependent regulation of human DNA polymerase α-primase activity by phosphorylation. Mol Cell Biol. 1999;19:646–656. doi: 10.1128/mcb.19.1.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Feng L, Hu Y, Huang SH, Reynolds CP, Wu L, Jong AY. The essential role of Saccharomyces cerevisiae CDC6 nucleotide-binding site in cell growth, DNA synthesis, and Orc1 association. J Biol Chem. 1999;274:8291–8218. doi: 10.1074/jbc.274.12.8291. [DOI] [PubMed] [Google Scholar]
- Weinreich M, Liang C, Stillman B. The Cdc6p nucleotide-binding motif is required for loading mcm proteins onto chromatin. Proc Natl Acad Sci USA. 1999;96:441–446. doi: 10.1073/pnas.96.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RS, Shohet RV, Stillman B. A human protein related to yeast Cdc6p. Proc Natl Acad Sci USA. 1997;94:142–147. doi: 10.1073/pnas.94.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson IA, Niman HL, Houghten RA, Cherenson AR, Connolly ML, Lerner RA. The structure of an antigenic determinant in a protein. Cell. 1984;37:767–778. doi: 10.1016/0092-8674(84)90412-4. [DOI] [PubMed] [Google Scholar]
- Yan Z, Fedorov SA, Mumby MC, Williams RS. PR48, a novel regulatory subunit of protein phosphatase 2A, interacts with Cdc6 and modulates DNA replication in human cells. Mol Cell Biol. 2000;20:1021–1029. doi: 10.1128/mcb.20.3.1021-1029.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zindy F, Lamas E, Chenivesse X, Sobczak J, Wang J, Fesquet D, Henglein B, Brechot C. Cyclin A is required in S phase in normal epithelial cells. Biochem Biophys Res Commun. 1992;182:1144–1154. doi: 10.1016/0006-291x(92)91851-g. [DOI] [PubMed] [Google Scholar]