Abstract
We have previously generated human monoclonal anti-human immunodeficiency virus type 1 (anti-HIV-1) antibodies 2F5IgG and 2G12IgG with an exceptional cross-clade neutralizing potential. 2F5IgG and 2G12IgG passively administrated to macaques were able to confer complete protection from both intravenous and mucosal challenge with pathogenic HIV-simian immunodeficiency virus chimeric strains and have shown beneficial effects in a phase-1 clinical trial. We now class-switched 2F5 and 2G12 to the immunoglobulin M (IgM) or IgA isotype, to enforce features like avidity, complement activation, or the potential to neutralize mucosal transmission. For this purpose we expressed functional polymeric 2F5 and 2G12 antibodies in CHO cells and evaluated their anti-HIV-1 activity in vitro. The class switch had a strong impact on the protective potential of 2F5 and 2G12. 2G12IgM inhibited HIV-1 infection of peripheral blood mononuclear cell cultures up to 28-fold-more efficiently than the corresponding IgG and neutralized all of the primary isolates tested. The 2F5 and 2G12 antibodies of all isotypes were able to interact with active human serum to inhibit viral infection. Furthermore, we demonstrated that polymeric 2F5 and 2G12 antibodies but not the corresponding IgGs could interfere with HIV-1 entry across a mucosal epithelial layer in vitro. Although polymeric 2F5 antibodies had only limited potential in the standard neutralization assay, the results from the mucosal assay suggest that 2F5 and 2G12 antibodies may have a high potential to prevent natural HIV-1 transmission in vivo.
Various attempts have been made to identify antigenic sites on human immunodeficiency virus type 1 (HIV-1) that are able to elicit a neutralizing immune response. Neutralizing antibodies against HIV-1 recognize mostly epitopes on the envelope glycoproteins, which are responsible for virus attachment and entry into the target cells. Primary isolates, however, are hardly neutralized by antibodies, since the conserved regions (including the CD4-binding site) of the HIV-1 envelope glycoproteins (gp120 and gp41) are mostly masked by the variable loops, resulting in a high resistance to neutralization. Furthermore, heavy glycosylation is assumed to contribute to hiding functionally important, conserved regions from antibody binding. It has been suggested that antibodies that are capable of recognizing epitopes in the functional complex of gp120-gp41 on the viral surface can neutralize the virus (45).
Human monoclonal anti-HIV-1 antibodies 2F5IgG and 2G12IgG directed against rare, nonimmunodominant epitopes with an exceptional protective potential against HIV-1 infection have been previously generated (9). 2F5 and 2G12 neutralize a variety of T-cell-line-adapted (TCLA) strains (39), primary isolates (including clades A, B, C, and E) (30, 38, 51, 53), and HIV-simian immunodeficiency virus chimeric strains (SHIV) (28, 27) in vitro. In vivo, 2G12 and 2F5 alone or in combination with other monoclonal antibodies (b12 and F105) and HIV immunoglobulin (Ig) act highly synergistic and are able to confer protection against both an intravenous and a mucosal challenge with SHIV in macaques (2, 22, 32, 31). Moreover, in the first clinical trial of passive immunization, 2F5IgG and 2G12IgG showed beneficial antiviral activity in humans chronically infected with HIV-1 (48).
It has been postulated that a neutralizing antibody response at the initial stages of HIV-1 entry into the body might be a critical protective determinant (37). Polymeric IgA is regarded as the major immunological barrier at mucosal sites and has been associated with the neutralization of virus entry and limitation of the local spread of viruses (34). Additionally, evidence for the importance of 2F5-like antibodies in the mucosal defense against HIV-1 was presented by Bomsel et al. (7), who demonstrated that polymeric antibodies against a sequence overlapping with the 2F5 epitope can block HIV-1 entry across a mucosal layer in vitro. We therefore assumed that polymeric 2F5 and 2G12 antibodies of an IgA or IgM isotype should provide a potent agent to inhibit the first steps of virus entry across a mucosal barrier.
Furthermore, polymeric antibodies are expected to be more effective due to higher avidity and/or steric hindrance (11, 43). We have found before that 2G12IgG dimers neutralize more potently in vitro than the regular monomers (M. Purtscher, Eur. Conf. Exp. AIDS Res., poster no. 82-P2, 1998). This confirmed our assumption of a probable correlation between valence antiviral efficacy of the 2G12 antibody. A biological feature that has further been assigned to IgM is a strong complement-mediated cytolytic potential. Although the role of complement in HIV infection is quite controversial, it has been reported that HIV-specific monoclonal antibodies can mediate complement-dependent clearance of viral particles (47).
In the present study, we switched the class of 2F5IgG to the IgM or IgA isotype and that of 2G12IgG to the IgM isotype to combine their high affinity to neutralizing epitopes with the functional properties of human polymeric antibodies. For that purpose, we produced functionally assembled polymeric 2F5 and 2G12 antibodies in CHO cells and assessed their activity in assays representing relevant steps of HIV infection in vivo. In addition to regular in vitro neutralization assays, we assessed transepithelial neutralization of HIV-1 across a cellular barrier of intestinal or cervical origin in vitro.
MATERIALS AND METHODS
Generation of antibody sequences.
The generation of 2F5IgG and 2G12IgG have been described before (9). The coding sequences for the 2F5 and 2G12 variable regions have been published (26). The sequence for the human J-chain (33) and the murine dihydrofolate reductase (DHFR) (14) (National Center for Biotechnology Information [NCBI] accession no. NM010049) have also been published before. The human μ-chain constant region (secretory form) sequence was published in reference 19 (NCBI accession no. X67301 S50847), and the human α1-chain sequence was published in reference 18 (NCBI accession no. J00220). RNA from a human hybridoma cell line was used as a source for the amplification of the IgM heavy-chain constant region and human J-chain, and a human Epstein-Barr virus-transformed cell line producing IgA was used to amplify the IgA1 heavy-chain constant region. The heavy-chain isotype switch was performed by overlap extension PCR (23).
Antibody expression vectors.
For expression of the heavy chain, light chain, J-chain, and DHFR, two bicistronic vectors, pIRES/light-chain/J-chain and pIRES/heavy-chain/DHFR, were generated, each containing two of the coding sequences. The plasmid pIRES/neo (Clontech) has been modified by replacing the cytomegalovirus immediate-early enhancer/promoter with a simian virus 40 early enhancer/promoter from the Promega pSI vector cloned into the EcoRV cloning site. The J-chain or DHFR was next ligated into the SmaI/XbaI site and either light chains (2F5 or 2G12) or hybrid heavy chains (2F5-α-chain, 2F5-μ-chain, or 2G12-μ-chain) were blunt-end inserted into the multiple cloning site.
Cell lines and transfection.
One day before transfection, CHO DHFR cells (ATCC CRL-9096) were subcultured in 96-well tissue culture plates in culture medium (Dulbecco's modified Eagle's medium [DMEM] supplemented with 4 mM l-glutamine, 1× HT [8 mM hypoxanthine, 0.8 mM thymidine], and 10% fetal calf serum [FCS]). For the generation of polymeric-antibody-producing cell lines, the cells were cotransfected with the two expression vectors pIRES/light-chain/J-chain and pIRES/heavy-chain/DHFR at a 1:1 ratio with Lipofectin reagent (Invitrogen). Cells were kept for 2 weeks in DMEM plus 4 mM l-glutamine and 10% dialyzed FCS until nonproducers had disappeared and clonal growth was visible. Supernatants from the clone cultivations were screened for antibody productivity by enzyme-linked immunosorbent assay (ELISA), and expression was amplified by methotrexate pressure. 2F5IgA-, 2F5IgM-, and 2G12IgM-producing clones were expanded and subcloned.
ELISA.
Clones were analyzed for the expression of complete molecules with a light- and heavy-chain ELISA. Immunosorbent plates (maxisorp 96-well; Nunc) were coated with goat anti-human IgA (α-specific) (Sigma) or goat anti-human IgM (μ-specific) (Sigma) at a concentration of 2 μg/ml in coating buffer (0.1 N NaHCO3 buffer, pH 9.6 to 9.8). For quantitative ELISA, the samples were serially diluted in dilution buffer (phosphate-buffered saline [PBS] [pH 7.2 to 7.4], 0.1% Tween 20, 1% bovine serum albumin) together with human secretory IgA (sIgA) (Sigma) and human IgM (Sigma). Plates were then washed (PBS [pH 7.2 to 7.4], 0.1% Tween 20) and incubated with the sample dilutions. Plates were washed after 90 min, incubated with goat anti-human κ light chain conjugated with alkaline phosphatase (AP) (1:1,000) (Sigma) for 1 h, and detected by pNPP substrate in coating buffer (pH 9.7). Antibody productivity was calculated as micrograms of antibody per 106 cells per day.
To determine antibody specificity, plates were coated with HIV gp160 or HIV gp120 (used at concentrations of 5 ng/μl), with the 2F5 epitope (ELDKWA as a fusion protein with glutathione S-transferase), or with M1G1, an anti-2G12 idiotype antibody (both generated in our own laboratory) in coating buffer. The supernatants were compared with 2F5IgG and 2G12IgG standards. The bound antibodies were detected by anti-human κ light chain conjugated with AP (1:1,000) and developed with pNPP substrate. A405/620 was measured with a plate reader.
Sodium dodecyl sulfate-PAGE and Western blots.
The degree of polymerization of the recombinant antibodies was specified by polyacrylamide gel electrophoresis (PAGE) (NuPAGE Tris-7% acetate or Tris-3 to 8% acetate precast gels; Novex) by using denaturing nonreducing conditions according to the manufacturer's instructions. For detection, the gels were electroblotted to a polyvinylidene difluoride membrane (0.45 μm pore size; Millipore) and incubated with goat anti-human κ light chain (f + b) conjugated with AP (Sigma) (in PBS, 0.1% Tween, and 1% skim milk powder).
Antibody concentration.
A lab-scale tangential flow filter system (Millipore XX42LS11 [115 V], XX42LSS12 [230 V], XX42LSS13 [kit 230 V]) was used for ultrafiltration and ultradiafiltration of the recombinant antibody supernatant. Antibodies were ultradiafiltrated against PBS with a cross-flow filter with a 300-kDa pore size for IgM and a 100-kDa pore size for IgA (cross-flow filter PLCMK 300 Pellicon XL or 100 Pellicon XL cartridge; Millipore). The filtration resulted in an antibody concentrate, with a reduced low-molecular-weight fraction.
Syncytium-inhibition assay (SIA) with and without complement.
The inhibiting concentration of antibodies against TCLA strain infection of AA-2 cells in vitro has been described previously (39). The AA-2 cells (human B lymphoblastoma expressing CD4) (AIDS Research and Reference Reagent Program, #135, 7/15/88) were maintained in RPMI 1640 supplemented with 4 mM l-glutamine and 10% FCS at a split ratio of 1:10 twice a week. To obtain normal human serum, whole human blood was coagulated at room temperature for 2 h and centrifuged and supernatants were stored at −80°C.
For the assay, anti-HIV antibodies were serially diluted in 4 parallels in 96-well culture plates in culture medium supplemented with 2 μg of Polybrene/ml. Next 50 μl of free virus stocks in medium at 102 to 103 50% tissue culture infective doses of MN (HIVHTLV-III MN) and RF (HIVHTLV-III RF) (AIDS Research and Reference Reagent Program) or NL4-3 (provided by H. Stoiber, Institute of Hygiene, Innsbruck, Austria)/ml was added. Next, 50 μl of either normal human serum (resulting in a final dilution of 1:8 to 1:16), heat-inactivated human plasma (56°C for 20 min), or regular assay medium was added per well. After 1 h at 37°C, 4 × 105 AA-2 cells were added per well. For each medium composition, we confirmed the virus titer by back titration. After 5 days, syncytium-negative wells were counted and the IC50 (the antibody concentration resulting in a 100% inhibition in 50% of the wells) was determined according to the method described in reference 41.
Neutralization assay.
The in vitro neutralizing activity of antibodies against HIV infection of phytohemagglutinin-stimulated human peripheral blood mononuclear cells (PBMCs) with primary strains was determined by measuring the levels of HIV-1 p24 as published before (39). The virus isolates S2/04, P2/71, P3/366, BR92/30, and UG92/37 were M-tropic, P7/366 was dualtropic, and UG92/29 was T-tropic. UG92/29, UG92/37, and BR92/30 were received from AIDS Research and Reference Reagent Program no. 1650, 1743, and 1774, respectively. The others were isolated from patients of the Social Medicine Centre, Baumgartner Höhe, Vienna, Austria.
Transwell cultures.
The transcytosis assay was adapted for our purposes from reference 5. The Me180 (epidermoid cervix carcinoma [ATCC 33-HTB]) or HT-29 (colorectal adenocarcinoma [ATCC 38-HTB]) cell line was used to generate polarized monolayers. Me-180 cells were maintained in RPMI 1640 supplemented with 4 mM l-glutamine and 10% FCS and split with a ratio of 1:4 twice a week. HT-29 cells were cultured in DMEM-Ham's F-12 1:1 supplemented with 4 mM l-glutamine and 10% FCS and split at a ratio of 1:3 twice a week. For transwell assays, permeable supports (polycarbonate membrane or polyester membrane, 6.5-mm diameter, 0.4-μm pore size; Corning Scientific Products) were inoculated with 2 × 105 cells/well. Starting at day 3, culture medium was replaced daily. After every manipulation, the integrity of the monolayers was checked with a light microscope. Wells with damaged cell layers were discarded. Transwells were used at days 8 to 11. Before starting a transwell assay, the tightness of the cell layer was confirmed in at least two wells as published before (20).
Antibody transcytosis.
The transcytosis of the recombinant polymeric antibodies through the epithelial cells was visualized by scanning the cell layer for antibodies after loading the cells with 3 to 30 μg of antibody for 2 h from the basal side. Paraformaldehyde fixation was performed in two steps according to the method described in reference 6. Internalized antibody was detected by mouse anti-human κ light chain conjugated with fluorescein isothiocyanate (Sigma). The membranes were carefully excised, mounted onto a slide, and covered with 50% glycerin in PBS. The layer was scanned vertically by confocal microscopy for the fluorescein isothiocyanate fluorescence.
Transwell assay.
The inserts were moved to a new plate containing 3 to 30 μg of the anti-HIV antibodies in the basal chamber. Every antibody was tested at least in duplicate wells. Maximal virus transport was determined from duplicate wells incubated with an unspecific isotype control (polyclonal human IgM or human sIgA).
The plates were incubated for 20 min to allow antibody transcytosis. Then 105 to 106 HIV-1-infected PBMCs were applied to the apical chamber and incubated for 180 min before the contents of both sides of the insert were inactivated in 2% NP-40 for 1 h at 37°C. The levels of HIV-1 p24 antigen in the basal (and apical) supernatants were measured by ELISA (39) to determine the amount of transcellular virus penetration.
RESULTS
Recombinant polymeric antibodies.
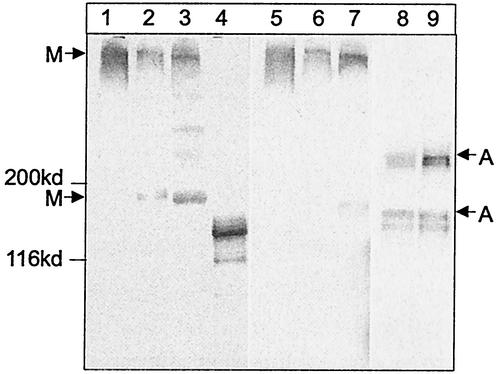
We have generated human polymeric class-switched 2F5 and 2G12 anti-HIV-1 antibodies with the mammalian CHO DHFR system. We confirmed the presence of complete, covalently linked, polymeric antibodies in the supernatants and the ultradiafiltrated concentrates by using denaturing, nonreducing sodium dodecyl sulfate-PAGE and Western blots. The immunoblots revealed a mainly pentameric and monomeric form for the IgM isotype at an estimated ratio of at least 1:2 for 2F5IgM and 3:1 for 2G12IgM (Fig. 1). The data presented herein were generated with ultradiafiltrated supernatants by using 100- and 300-kDa membranes. In previous experiments with our recombinant IgG1 versions of 2F5 and 2G12, we found that despite having the same Fc domain, the proteins behave differently concerning the formation of dimers. 2F5 always tends to disaggregate while 2G12 forms dimers. From our point of view, the IgM and IgA molecules have tendencies to aggregate and disaggregate similar to those of their IgG counterparts whether they are purified or not. Our data correspond with results published by others on the expression of polymeric IgM in lymphoid (40) and nonlymphoid (17, 54) cell lines. The 2F5IgA supernatants contained equal amounts of monomeric and dimeric antibodies (Fig. 1). The J-chain has been suggested to be crucial for pentamer formation of IgM (16) and dimer formation of IgA (4, 10). Since a suitable antibody to detect incorporated human J-chain was not available, we did not determine J-chain in the immunoblots. At the mRNA level, J-chain and light-chain amounts were equal (data not shown).
FIG. 1.
Molecular distribution of the polymeric anti-HIV-1 antibodies. Western blots of denaturating nonreducing Tris-3 to 8% acetate (for IgM) and Tris-7% acetate (for IgA) gels were stained with goat anti-human κ light chain. The blots compare supernatants and concentrates containing 2F5IgM (lanes 2 and 3), 2G12IgM (lanes 6 and 7), 2F5IgA (lanes 8 and 9), 2F5IgG (lane 4), and human IgM from serum (lanes 1 and 5). The arrows indicate the positions of pentameric and monomeric IgM (M) and dimeric and monomeric IgA (A), respectively. The position of the 200- and 116-kDa molecular mass marker proteins are indicated.
Specificity of the polymeric antibodies.
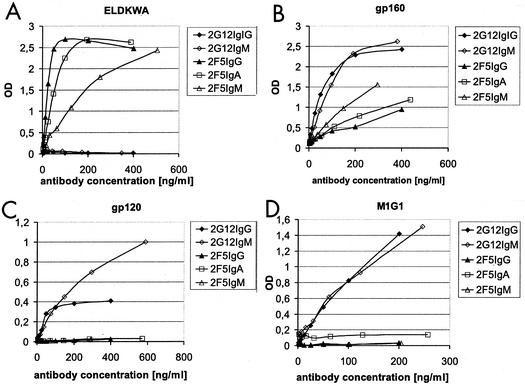
We assessed the reactivity of the anti-HIV antibodies with the 2F5 epitope (ELDKWA), an anti-2G12 idiotype antibody, and the HIV-1 envelope antigens gp120 and gp160, which is the uncleaved precursor of the HIV-1 envelope containing gp120 and gp41.
The antibodies were found to be of the desired specificity, and no cross-reactivity was observed. However, binding properties varied between the isotypes of a given idiotype (Fig. 2). 2F5IgM appeared to bind with a lower affinity than 2F5IgG in an ELDKWA ELISA, whereas the binding curves of 2F5IgA and 2F5IgG were almost identical (Fig. 2A). With gp160, 2F5IgM showed clearly enhanced binding compared to the other isotypes, which was probably due to higher avidity (Fig. 2B). In the soluble gp160 monomer, the 2F5 epitope is probably presented differently than in the ELDKWA ELISA. This may be the reason for the discrepancy between the two results.
FIG. 2.
Specificity ELISA. Comparison of 2F5IgA, 2F5IgM, and 2G12IgM CHO supernatants with 2F5IgG and 2G12IgG. Antibody concentrations (in nanograms/milliliter) are plotted against optical density (OD) values. For the specificity ELISA, we used the HIV-1 envelope antigens ELDKWA (amino acids 662 to 667) (A), gp160 (B), and gp120 (C) and a mouse anti-2G12 idiotype antibody (D). The samples were detected by anti-human κ light chain AP conjugate.
2G12IgG and 2G12IgM were equally recognized by the anti-2G12 anti-idiotype (M1G1) (Fig. 2D), indicating identical antigen binding sites. Both 2G12 antibodies bound the HIV gp160 and gp120 specifically; however, the saturation characteristics in the gp120 ELISA were diverse (Fig. 2C). The variations in binding properties probably depend on the higher avidity of IgM compared to that of the IgG monomer.
SIA.
TCLA HIV strains induce the formation of syncytia in a CD4+ cell line, which correlates with the degree of viral infection. Table 1 summarizes the effective inhibitory concentrations against syncytium formation for 2F5IgA/IgM and 2G12IgM compared to those of 2F5IgG and 2G12IgG. The 2G12IgM isotype clearly surpassed the corresponding IgG (by factors of 3.7 and 8) in effectiveness against the tested laboratory strains, whereas 2F5IgG was the most-potent isotype compared to IgM and IgA counterparts. Nevertheless, 2F5IgM did neutralize the tested viral strains (2.5 to 6 times less efficiently than IgG), whereas IgA just inhibited the MN isolate.
TABLE 1.
SIAa
| Conditions and TCLA strain | EC50 (μg/ml) of antibody and class
|
||||
|---|---|---|---|---|---|
| 2G12
|
2F5
|
||||
| IgG | IgM | IgG | IgM | IgA | |
| Standard | |||||
| MN | NT | NT | 0.16 | 0.4 | 2.4 |
| RF | 0.75 | 0.21 | NT | NT | NT |
| NL4-3 | 2.4 | 0.3 | 2.4 | 21 | >50 |
| Standard plus active human complement | |||||
| RF | 0.1 | 0.1 | NT | NT | NT |
| NL4-3 | 0.3 | 0.1 | 0.2 | 10.5 | >50 |
| Standard plus inactivated human plasma | |||||
| RF | 2.9 | 0.3 | NT | NT | NT |
| NL4-3 | 1.7 | 0.6 | 2.4 | 12.5 | >50 |
Effective antibody concentrations are listed as EC50s, representing the concentrations of antibody (micrograms per milliliter) causing 100% syncytium inhibition in 50% of the replicate wells. The assay was performed with the laboratory strains MN, RF, and NL4-3 (102 to 103 50% tissue culture infective doses/ml) and AA-2 cells. Normal human serum (diluted 1:8 to 1:16) was added as a source of active human complement in some experiments. Heat-inactivated human plasma was added as a negative control for complement activation in some experiments. The table summarizes the data of two independent assays. Lack of neutralization is indicated by an EC50 of >50 μg/ml. NT, not tested.
SIA plus active human complement.
We further evaluated the contribution of human complement to the inhibitory potency of our antibodies. Active human serum—presumably the complement system—strongly enhanced the antiviral effectiveness of all 2F5 and 2G12 antibodies (Table 1). The 50% effective concentrations (EC50) of 2G12IgM, 2G12IgG, and 2F5IgG were decreased to 200 ng/ml and lower; 2F5IgM inhibited NL4-3 at 10.5 μg/ml. The EC50 of 2F5IgA was threefold reduced but was still higher than 50 μg/ml. Even inactivated human plasma, which was used as a negative control for the active complement, appeared to have an impact on syncytium inhibition and partly enhanced antibody activity (Table 1).
In vitro neutralization of primary isolates.
We determined the in vitro efficacy of the anti-HIV antibodies against infection of human PBMCs with a panel of primary isolates. HIV-1 strain P7/366 or P3/366 was chosen for relative insensitivity to 2F5IgG or 2G12IgG neutralization. The results are represented as effective antibody concentrations, resulting in a 50, 90, and 99% reduction of virus in the PBMC culture (Table 2).
TABLE 2.
Neutralization values for antibodies 2F5 and 2G12 against primary HIV-1 isolatesa
| Isolate | % EC | Result (μg/ml) for antibody:
|
||||
|---|---|---|---|---|---|---|
| 2G12IgG | 2G12IgM | 2F5IgG | 2F5IgM | 2F5IgA | ||
| P7/366 dual tropic | 50 | >50 | 1.1 | >50 | 2.3 | 36.5 |
| 90 | >50 | >25 | >50 | >40 | >40 | |
| 99 | >50 | >25 | >50 | >40 | >40 | |
| S2/04 M-tropic | 50 | 22.8 | 3.6 | 1.7 | 3.2 | >40 |
| 90 | >50 | >25 | 18.6 | 33.7 | >40 | |
| 99 | >50 | >25 | >50 | >40 | >40 | |
| P3/366 M-tropic | 50 | >50 | 0.7 | 35.2 | 8.3 | >40 |
| 90 | >50 | 16.9 | >50 | >40 | >40 | |
| 99 | >50 | >25 | >50 | >40 | >40 | |
| P2/71 M-tropic | 50 | >50 | 2.9 | 5.6 | 0.1 | 4.0 |
| 90 | >50 | 7.1 | 15.4 | 10.6 | 10.0 | |
| 99 | >50 | 23.3 | 25.2 | 19.2 | 19.1 | |
| S2/03 | 50 | NT | NT | 2.9 | >50 | 22.7 |
| 90 | NT | NT | 9.5 | >50 | >40 | |
| 99 | NT | NT | 21.2 | >50 | >40 | |
| 92/BR/30 M-tropic B clade | 50 | 5.0 | 0.5 | 15.1 | >50 | NT |
| 90 | >50 | 1.5 | 41.6 | >50 | NT | |
| 99 | >50 | 5.9 | >50 | >50 | NT | |
| 92/UG/29 T-tropic A clade | 50 | 46.7 | 1.62 | 16.1 | >50 | NT |
| 90 | >50 | 7.86 | 24.6 | >50 | NT | |
| 99 | >50 | >20 | >50 | >50 | NT | |
| 92/UG/37 M-tropic A-clade | 50 | 0.2 | 0.7 | 3.9 | >50 | >40 |
| 90 | 49.7 | 4.7 | 9.6 | >50 | >40 | |
| 99 | >50 | >20 | 22.9 | >50 | >40 | |
The inhibition of virus production was assessed by measuring p24 concentrations in HIV-infected PBMC cultures. The neutralization titers of the antibodies were calculated. The EC50s, EC90s, and EC99s represent the concentrations of antibody (micrograms/milliliter) causing a 50, 90, and 99% reduction of HIV-infected PBMC. No neutralization is indicated by an EC of >50, >40, or >25 μg/ml, each representing the maximal antibody concentration used in the assay. NT, not tested.
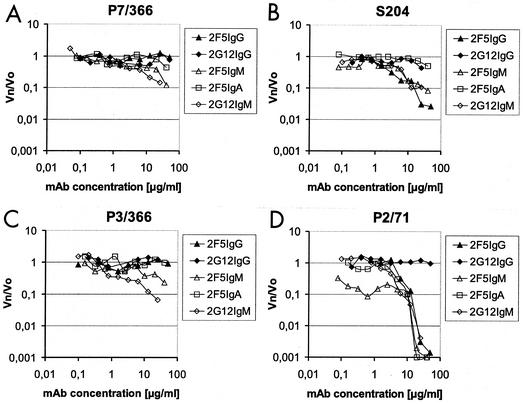
In our experiments, 2G12 was clearly more potent as the IgM isotype than as the IgG isotype. 2G12IgM was able to neutralize every virus that we tested and achieved a 50% inhibition of isolates P7/366 and S2/04, a 90% reduction of isolates UG92/37, UG92/29, and P3/366, and even a 99% reduction of isolates BR92/30 and P2/71. 2G12IgM attained neutralization at an EC90 between 1.5 and 20 μg/ml, which correlates to concentrations achievable in vivo for monoclonal antibodies. The correlation between neutralization and antibody concentration is illustrated in Fig. 3. The graphs suggest that 2G12IgM, which was not used at concentrations higher than 25 μg/ml, may achieve at higher concentrations a 2 log reduction of virus for isolate P3/366 (Fig. 3C).
FIG. 3.
Neutralization curves for 2F5 and 2G12 antibodies against primary isolates. The dose-dependent inhibition of primary isolates was assessed by measuring p24 concentrations in HIV-infected PBMC cultures. The antibody dilutions (in micrograms/milliliter) are plotted against the p24 levels relative to p24 in the blank (without addition of antibody) (Vn/V0). The horizontal lines on the y axes indicate the points of 50, 90, and 99% virus reduction. The primary isolates used were P7/366 (A), S2/04 (B), P3/366 (C), and P2/71 (D). MAb, monoclonal antibody.
The 2F5IgG isotype was the most potent of the 2F5 isotypes (Table 2), although the viral strains P7/366, P3/366, and P2/71 were more sensitive to 2F5IgM and were neutralized at an EC50 of 0.1 to 3.2 μg/ml. The efficiency of 2F5IgG has strongly been associated with the expression of the ELDKWA epitope on gp41 (15, 38). Nevertheless, there were significant variations in the activity of 2F5 antibodies against different HIV-1 isolates, but no explicit correlation between the isolate sensitivity to 2F5IgG and to the polymeric 2F5 antibodies could be found.
Transwell assay.
We used a transwell assay to assess the ability of 2F5 and 2G12 antibodies to inhibit HIV-1 infection at mucosal sites. A tight polarized epithelial layer was grown on permeable supports, generating an apical and a basal compartment. Infectious virus was applied from the apical side by using HIV-plus-PBMC cultures while antibody was applied from the basal side.
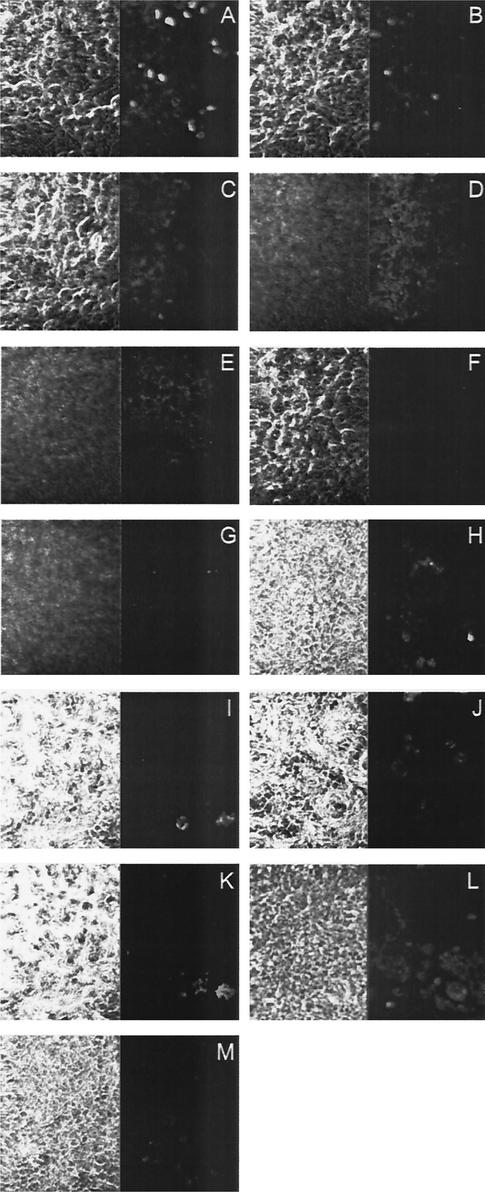
The specific role of polymeric antibodies in virus neutralization at mucosal surfaces has been reported to correlate with the ability to be actively secreted across the epithelial layer via the polymeric immunoglobulin receptor (25, 42). Therefore, we first confirmed the transcellular transport of 2F5IgA and IgM antibodies, but not human polyclonal sIgA, along the basal-to-apical axis of the epithelial monolayer (Fig. 4). This finding suggested mucosal functionality of 2F5IgA, 2F5IgM, and 2G12IgM, a feature that has been reported to be associated with the integration of J-chain into the polymeric antibodies (8). The IgG antibodies were also found to bind and cross the epithelial layer (Fig. 4M). The mechanism of IgG transport probably depends on receptors other than the polymeric immunoglobulin receptor, like the FcRn receptor, which has been reported to be expressed on human epithelial cells (24).
FIG. 4.
Immunofluorescence analysis for transcytosis of 2F5 and 2G12 antibodies. A polarized monolayer of Me-180 cells grown on transwells was incubated with 2F5IgA, human sIgA, 2G12IgM, 2F5IgM, or 2F5IgG from the basal side. After 2 h, the cells were fixed and stained for the human κ light chain. The membrane cell layer was scanned from the basal to the apical side. Pictures A (basal) to E (apical) represent spaced confocal pictures of 2F5IgA-loaded cells. Pictures F and G represent pictures of the basal and the apical side of a cell layer loaded with human sIgA. Pictures H (basal) and I (intra-cellular) show cells loaded with 2G12IgM, pictures J (basal) and K (intracellular) show cells loaded with 2F5IgM, and picture L shows the basal side of cells loaded with 2F5IgG. Picture M shows the negative control (basal) without the addition of antibodies. The left side shows the micrograph, the right side shows the fluorescent signal.
Transepithelial neutralization.
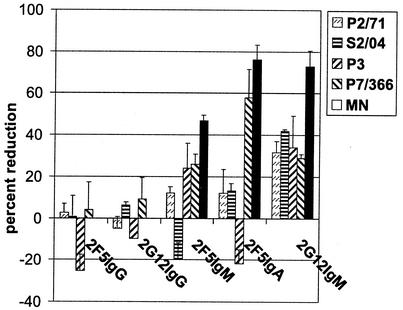
We analyzed the ability of 2F5 and 2G12 antibodies to neutralize viral transepithelial penetration of HIV-1 in vitro. The inhibitory activity of the antibodies is presented as the percent reduction of transcytosed virus in Fig. 5. All polymeric anti-HIV antibodies proved to inhibit the viral transcytosis of all primary HIV isolates while neither 2F5IgG nor 2G12IgG was able to interfere with HIV entry. 2F5IgA and 2G12IgM exhibited the highest potency, and the degree of transepithelial neutralization ranged between 24 and 76%. Our results from the transwell assay did not appear to correlate with the results from the standard neutralization assay (Table 2). Additionally, we observed in some cases low levels of inhibition and enhancement for antibodies of all isotypes, which may be caused by variations in protein content, antibody concentration and composition, cellular growth, amount of PBMCs, or their state of infection between singular assays. Although the duplicate wells showed only little variability, we assumed that any observed effect lower than ±20% may be a result of these variations. The presence of antibodies may of course also result in enhanced transport of HIV via Fc receptors.
FIG. 5.
Transwell assay of in vitro transepithelial neutralization. An epithelial monolayer was preincubated with the antibodies from the basal side before HIV-infected PBMCs were added from the apical side. After 3 h of coincubation, p24 levels on both sides of the transwell were determined by ELISA. The reduction is presented as the percentage of p24 found in the basal medium when incubated with the antibodies compared to p24 levels in the basal medium of the isotype control, representing uninhibited transcytosis of the virus.
DISCUSSION
We have switched the classes of human monoclonal anti-HIV-1 antibodies 2F5 and 2G12 as IgM and IgA, respectively, to introduce new biological properties, such as avidity, mucosal activity, or complement activation. The polymeric isotypes display different binding characteristics than the monomers, and they probably bind with high avidity, provided that the epitope is present at a high density and is well exposed.
2F5IgG and 2G12IgG are highly potent neutralizing antibodies against TCLA strain and primary HIV-1 isolates. The isotype switch from IgG to IgA or IgM had a clear impact on the ability to interact with viral infection (Tables 1 and 2). For 2G12 antibodies, we found a direct correlation between valence and its antiviral potency. The 2G12IgM was the only antibody able to neutralize every primary HIV-1 isolate tested. It mostly provided protection at lower effective concentrations (up to 28-fold) than 2G12IgG and achieved up to 99% reduction of HIV-1 primary strain infection. 2G12IgG is directed against a conserved discontinuous motif located around the base of the V3 and V4 loops on the outer domain of gp120. The epitope recognition is dependent on mannose elements from a number of N-linked glycan chains (44, 46). 2G12IgG has been proposed to sterically impair the interaction between the oligomeric envelope complex and the coreceptor after CD4 binding (52), due to the proximity of its epitope to the receptor binding site (44). The large and bulky pentamer of 2G12IgM is likely to inhibit viral attachment even more effectively, which may explain the high level of protection at low concentrations that we found in our experiments.
In contrast to 2G12, the class switch did not clearly improve the neutralizing activity of 2F5. Neutralization by 2F5IgG is probably dependent on the presence of a linear amino acid sequence ELDKWA (amino acids 662 to 667) at the C-terminal gp41 ectodomain (36, 35, 38). 2F5IgG is assumed to neutralize virus during the fusion process, possibly at the transition from the prehairpin intermediate to the trimeric hairpin structure. In the functional HIV-1 trimer of gp120/gp41, the accessibility of the peptide for a dimeric or pentameric 2F5 antibody is questionable, which may be a physical restriction to its neutralization potency. Additionally the impact of both antibody flexibility (13) and antibody valency (12) on viral neutralization of different isolates has been described. This may also have an impact on 2F5 neutralization, since its epitope may not be well exposed, unlike the 2G12 epitope.
Direct lysis of HIV by the complement system in the presence of specific antibodies has been described as an efficient mechanism of neutralization (47). In our experiments, 2F5IgG, 2G12IgG, and 2G12IgM together with active human serum inhibited HIV-1 at concentrations as low as 200 ng/ml (Table 1). It was demonstrated before that 2G12IgG-dependent activation of complement leads to enhanced neutralization of HIV-1 particles in vitro (51) and vigorous complement activation in patients (48). Human serum conditions may facilitate virus neutralization by 2G12-like antibodies.
Complement proteins like complement factor H (CFH) have been found to be protective for HIV, preventing its effective destruction (29). It has been shown that one CFH binding site overlaps with the 2F5 epitope ELDKWA on gp41 (49). In this context, 2F5IgG binding to ELDKWA could abolish the protective effect of CFH, resulting in efficient viral lysis (50). Surprisingly the contribution of active human complement to neutralization was relatively stronger for 2F5IgG than for the polymeric isotypes (Table 1). On the other hand, 2F5IgG isotype proved to recognize its epitope more effectively than 2F5IgM, which may of course give 2F5IgG a greater ability to displace CFH from the common binding site.
HIV is commonly transmitted through mucosal surfaces as a result of either sexual contact or oral exposure, for example, during breastfeeding. It has been demonstrated before that HIV-1-specific mucosal IgA (and IgM) can interfere with viral infection at mucosal sites (3, 7, 21, 42). Our results demonstrate that 2F5IgA, 2F5IgM, and 2G12IgM, but not 2F5IgG or 2G12IgG, were able to neutralize transepithelial HIV-1 entry in vitro, blocking transmission involving cell-to-cell spread of HIV-1 across an epithelial layer (Fig. 5). Remarkably, 2F5IgA, which displayed only poor activity in the standard neutralization assay, blocked up to 76% of viral transport in the mucosal assay. Similar results have been found by Bomsel et al. (7). In their experiments, polyclonal anti-envelope dimeric IgA neutralized up to 60%, and an IgM reactive with a peptide overlapping with the 2F5 epitope neutralized up to 80% of viral transcytosis, and 2F5IgG did not interfere with HIV-1 entry.
Systemic administration of 2F5IgG and 2G12IgG was able to confer protection against oral SHIV challenge in neonatal macaques (22). Since saliva samples of these infants did not contain neutralizing antibodies, IgG-dependent protection was considered to occur in the subepithelial compartment after the virus crossed the mucosal barrier. We now observed transepithelial protection by polymeric 2F5IgA/M and 2G12IgM but not the IgGs in the transwell assay. This suggests a cross-epithelial neutralization mechanism for polymeric 2F5 and 2G12 antibodies distinct from the antiviral activity of IgG observed in the macaque model. The inhibition possibly includes intracellular neutralization and/or excretory IgA neutralization, features that are attributed to polymeric mucosal antibodies (34). The higher mucosal efficacy of polymeric antibodies may also be explained by increased steric inhibition of attachment to the epithelium. While this may be true for the 2G12IgM, it is unlikely to apply to 2F5IgA or 2F5IgM, since the standard neutralization assays did not show enhanced efficacy of polymeric 2F5 antibodies compared to the monomeric IgG. The sites in HIV-1 envelope that are critical for cell-associated viral attachment to epithelial cells differ from the sites involved in CD4+ cell infection and probably include the ELDKWA motif on gp41 (1). 2F5 and its epitope ELDKWA may hence play a specific role in the neutralizing response to HIV-1 entry at mucosal surfaces.
We conclude that 2F5- and 2G12-like antibodies of all isotypes are highly promising agents against relevant steps of natural HIV-1 transmission. This supports the assumption that mucosal neutralizing antibodies are critical for both passive or active vaccine designs.
Acknowledgments
We thank Kristen Khanna and Richard Markham for generous assistance with the transwell assays and Reingard Grabherr and Friedemann Hesse for critical reading of the manuscript.
This work was supported by Polymun Scientific Immunbiologische Forschung GmbH.
REFERENCES
- 1.Alfsen, A., P. Iniguez, E. Bouguyon, and M. Bomsel. 2001. Secretory IgA specific for a conserved epitope on gp41 envelope glycoprotein inhibits epithelial transcytosis of HIV-1. J. Immunol. 166:6257-6265. [DOI] [PubMed] [Google Scholar]
- 2.Baba, T. W., V. Liska, R. Hofmann-Lehmann, J. Vlasak, W. Xu, S. Ayehunie, L. A. Cavacini, M. R. Posner, H. Katinger, G. Stiegler, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, Y. Lu, J. E. Wright, T. C. Chou, and R. M. Ruprecht. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200-206. [DOI] [PubMed] [Google Scholar]
- 3.Battle-Miller, K., C. A. Eby, A. L. Landay, M. H. Cohen, B. E. Sha, and L. L. Baum. 2002. Antibody-dependent cell-mediated cytotoxicity in cervical lavage fluids of human immunodeficiency virus type 1-infected women. J. Infect. Dis. 185:439-447. [DOI] [PubMed] [Google Scholar]
- 4.Berdoz, J., C. T. Blanc, M. Reinhardt, J. P. Kraehenbuhl, and B. Corthesy. 1999. In vitro comparison of the antigen-binding and stability properties of the various molecular forms of IgA antibodies assembled and produced in CHO cells. Proc. Natl. Acad. Sci. USA 96:3029-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bomsel, M. 1997. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat. Med. 3:42-47. [DOI] [PubMed] [Google Scholar]
- 6.Bomsel, M., K. Prydz, R. G. Parton, J. Gruenberg, and K. Simons. 1989. Endocytosis in filter-grown Madin-Darby canine kidney cells. J. Cell Biol. 109:3243-3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bomsel, M., M. Heyman, H. Hocini, S. Lagaye, L. Belec, C. Dupont, and C. Desgranges. 1998. Intracellular neutralization of HIV transcytosis across tight epithelial barriers by anti-HIV envelope protein dIgA or IgM. Immunity 9:277-287. [DOI] [PubMed] [Google Scholar]
- 8.Brandtzaeg, P., and H. Prydz. 1984. Direct evidence for an integrated function of J chain and secretory component in epithelial transport of immunoglobulins. Nature 311:71-73. [DOI] [PubMed] [Google Scholar]
- 9.Buchacher, A., R. Predl, K. Strutzenberger, W. Steinfellner, A. Trkola, M. Purtscher, G. Gruber, C. Tauer, F. Steindl, A. Jungbauer, et al. 1994. Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res. Hum. Retrovir. 10:359-369. [DOI] [PubMed] [Google Scholar]
- 10.Carayannopoulos, L., E. E. Max, and J. D. Capra. 1994. Recombinant human IgA expressed in insect cells. Proc. Natl. Acad. Sci. USA 91:8348-8352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castilla, J., I. Sola, and L. Enjuanes. 1997. Interference of coronavirus infection by expression of immunoglobulin G (IgG) or IgA virus-neutralizing antibodies. J. Virol. 71:5251-5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cavacini, L. A., C. L. Emes, J. Power, M. Duval, and M. R. Posner. 1994. Effect of antibody valency on interaction with cell-surface expressed HIV-1 and viral neutralization. J. Immunol. 152:2538-2545. [PubMed] [Google Scholar]
- 13.Cavacini, L. A., C. L. Emes, J. Power, F. D. Desharnais, M. Duval, D. Montefiori, and M. R. Posner. 1995. Influence of heavy chain constant regions on antigen binding and HIV-1 neutralization by a human monoclonal antibody. J. Immunol. 155:3638-3644. [PubMed] [Google Scholar]
- 14.Chang, A. C., J. H. Nunberg, R. J. Kaufman, H. A. Erlich, R. T. Schimke, and S. N. Cohen. 1978. Phenotypic expression in E. coli of a DNA sequence coding for mouse dihydrofolate reductase. Nature 275:617-624. [DOI] [PubMed] [Google Scholar]
- 15.Conley, A. J., J. A. Kessler II, L. J. Boots, J. S. Tung, B. A. Arnold, P. M. Keller, A. R. Shaw, and E. A. Emini. 1994. Neutralization of divergent human immunodeficiency virus type 1 variants and primary isolates by IAM-41-2F5, an anti-gp41 human monoclonal antibody. Proc. Natl. Acad. Sci. USA 91:3348-3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis, A. C., K. H. Roux, and M. J. Shulman. 1988. On the structure of polymeric IgM. Eur. J. Immunol. 18:1001-1008. [DOI] [PubMed] [Google Scholar]
- 17.Davis, A. C., C. Collins, and M. J. Shulman. 1989. Differential glycosylation of polymeric and monomeric IgM. Mol. Immunol. 26:147-152. [DOI] [PubMed] [Google Scholar]
- 18.Flanagan, J. G., and T. H. Rabbitts. 1982. Arrangement of human immunoglobulin heavy chain constant region genes implies evolutionary duplication of a segment containing gamma, epsilon and alpha genes. Nature 300:709-713. [DOI] [PubMed] [Google Scholar]
- 19.Harindranath, N., G. Donadel, G. Sigounas, and A. L. Notkins. 1993. Comparison of complete nucleotide sequence of the human IgM heavy chain constant region of polyreactive and monoreactive antibodies. Mol. Immunol. 30:111-112. [DOI] [PubMed] [Google Scholar]
- 20.Hirt, R. P., O. Poulain-Godefroy, J. Billotte, J. P. Kraehenbuhl, and N. Fasel. 1992. Highly inducible synthesis of heterologous proteins in epithelial cells carrying a glucocorticoid-responsive vector. Gene 111:199-206. [DOI] [PubMed] [Google Scholar]
- 21.Hocini, H., L. Belec, S. Iscaki, B. Garin, J. Pillot, P. Becquart, and M. Bomsel. 1997. High-level ability of secretory IgA to block HIV type 1 transcytosis: contrasting secretory IgA and IgG responses to glycoprotein 160. AIDS Res. Hum. Retrovir. 13:1179-1185. [DOI] [PubMed] [Google Scholar]
- 22.Hofmann-Lehmann, R., J. Vlasak, R. A. Rasmussen, B. A. Smith, T. W. Baba, V. Liska, F. Ferrantelli, D. C. Montefiori, H. M. McClure, D. C. Anderson, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, H. Katinger, G. Stiegler, L. A. Cavacini, M. R. Posner, T. C. Chou, J. Andersen, and R. M. Ruprecht. 2001. Postnatal passive immunization of neonatal macaques with a triple combination of human monoclonal antibodies against oral simian-human immunodeficiency virus challenge. J. Virol. 75:7470-7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horton, R. M., Z. L. Cai, S. N. Ho, and L. R. Pease. 1990. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. BioTechniques 8:528-535. [PubMed] [Google Scholar]
- 24.Israel, E. J., S. Taylor, Z. Wu, E. Mizoguchi, R. S. Blumberg, A. Bhan, and N. E. Simister. 1997. Expression of the neonatal Fc receptor, FcRn, on human intestinal epithelial cells. Immunology 92:69-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaetzel, C. S., J. K. Robinson, K. R. Chintalacharuvu, J. P. Vaerman, and M. E. Lamm. 1991. The polymeric immunoglobulin receptor (secretory component) mediates transport of immune complexes across epithelial cells: a local defense function for IgA. Proc. Natl. Acad. Sci. USA 88:8796-8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kunert, R., F. Ruker, and H. Katinger. 1998. Molecular characterization of five neutralizing anti-HIV type 1 antibodies: identification of nonconventional D segments in the human monoclonal antibodies 2G12 and 2F5. AIDS Res. Hum. Retrovir. 14:1115-1128. [DOI] [PubMed] [Google Scholar]
- 27.Li, A., H. Katinger, M. R. Posner, L. Cavacini, S. Zolla-Pazner, M. K. Gorny, J. Sodroski, T. C. Chou, T. W. Baba, and R. M. Ruprecht. 1998. Synergistic neutralization of simian-human immunodeficiency virus SHIV-vpu+ by triple and quadruple combinations of human monoclonal antibodies and high-titer anti-human immunodeficiency virus type 1 immunoglobulins. J. Virol 72:3235-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li, A., T. W. Baba, J. Sodroski, S. Zolla-Pazner, M. K. Gorny, J. Robinson, M. R. Posner, H. Katinger, C. F. Barbas III, D. R. Burton, T. C. Chou, and R. M. Ruprecht. 1997. Synergistic neutralization of a chimeric SIV/HIV type 1 virus with combinations of human anti-HIV type 1 envelope monoclonal antibodies or hyperimmune globulins. AIDS Res. Hum. Retrovir. 13:647-656. [DOI] [PubMed] [Google Scholar]
- 29.Marschang, P., J. Sodroski, R. Wurzner, and M. P. Dierich. 1995. Decay-accelerating factor (CD55) protects human immunodeficiency virus type 1 from inactivation by human complement. Eur. J. Immunol. 25:285-290. [DOI] [PubMed] [Google Scholar]
- 30.Mascola, J. R., M. K. Louder, T. C. VanCott, C. V. Sapan, J. S. Lambert, L. R. Muenz, B. Bunow, D. L. Birx, and M. L. Robb. 1997. Potent and synergistic neutralization of human immunodeficiency virus (HIV) type 1 primary isolates by hyperimmune anti-HIV immunoglobulin combined with monoclonal antibodies 2F5 and 2G12. J. Virol. 71:7198-7206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mascola, J. R., G. Stiegler, T. C. VanCott, H. Katinger, C. B. Carpenter, C. E. Hanson, H. Beary, D. Hayes, S. S. Frankel, D. L. Birx, and M. G. Lewis. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6:207-210. [DOI] [PubMed] [Google Scholar]
- 32.Mascola, J. R., M. G. Lewis, G. Stiegler, D. Harris, T. C. VanCott, D. Hayes, M. K. Louder, C. R. Brown, C. V. Sapan, S. S. Frankel, Y. Lu, M. L. Robb, H. Katinger, and D. L. Birx. 1999. Protection of Macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J. Virol. 73:4009-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Max, E. E., and S. J. Korsmeyer. 1985. Human J chain gene. Structure and expression in B lymphoid cells. J. Exp. Med. 161:832-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mazanec, M. B., J. G. Nedrud, C. S. Kaetzel, and M. E. Lamm. 1993. A three-tiered view of the role of IgA in mucosal defense. Immunol. Today 14:430-435. [DOI] [PubMed] [Google Scholar]
- 35.Muster, T., F. Steindl, M. Purtscher, A. Trkola, A. Klima, G. Himmler, F. Ruker, and H. Katinger. 1993. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J. Virol. 67:6642-6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muster, T., R. Guinea, A. Trkola, M. Purtscher, A. Klima, F. Steindl, P. Palese, and H. Katinger. 1994. Cross-neutralizing activity against divergent human immunodeficiency virus type 1 isolates induced by the gp41 sequence ELDKWAS. J. Virol. 68:4031-4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parren, P. W., J. P. Moore, D. R. Burton, and Q. J. Sattentau. 1999. The neutralizing antibody response to HIV-1: viral evasion and escape from humoral immunity. AIDS 13:S137-S162. [PubMed] [Google Scholar]
- 38.Purtscher, M., A. Trkola, A. Grassauer, P. M. Schulz, A. Klima, S. Dopper, G. Gruber, A. Buchacher, T. Muster, and H. Katinger. 1996. Restricted antigenic variability of the epitope recognized by the neutralizing gp41 antibody 2F5. AIDS 10:587-593. [DOI] [PubMed] [Google Scholar]
- 39.Purtscher, M., A. Trkola, G. Gruber, A. Buchacher, R. Predl, F. Steindl, C. Tauer, R. Berger, N. Barrett, A. Jungbauer, et al. 1994. A broadly neutralizing human monoclonal antibody against gp41 of human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 10:1651-1658. [DOI] [PubMed] [Google Scholar]
- 40.Randall, T. D., L. B. King, and R. B. Corley. 1990. The biological effects of IgM hexamer formation. Eur. J. Immunol. 20:1971-1979. [DOI] [PubMed] [Google Scholar]
- 41.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 42.Renegar, K. B., and P. A. Small, Jr. 1991. Passive transfer of local immunity to influenza virus infection by IgA antibody. J. Immunol. 146:1972-1978. [PubMed] [Google Scholar]
- 43.Renegar, K. B., G. D. Jackson, and J. Mestecky. 1998. In vitro comparison of the biologic activities of monoclonal monomeric IgA, polymeric IgA, and secretory IgA. J. Immunol. 160:1219-1223. [PubMed] [Google Scholar]
- 44.Sanders, R. W., M. Venturi, L. Schiffner, R. Kalyanaraman, H. Katinger, K. O. Lloyd, P. D. Kwong, and J. P. Moore. 2002. The mannose-dependent epitope for neutralizing antibody 2G12 on human immunodeficiency virus type 1 glycoprotein gp120. J. Virol. 76:7293-7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sattentau, Q. J., and J. P. Moore. 1995. Human immunodeficiency virus type 1 neutralization is determined by epitope exposure on the gp120 oligomer. J. Exp. Med. 182:185-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scanlan, C. N., R. Pantophlet, M. R. Wormald, E. Ollmann Saphire, R. Stanfield, I. A. Wilson, H. Katinger, R. A. Dwek, P. M. Rudd, and D. R. Burton. 2002. The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of alpha1→2 mannose residues on the outer face of gp120. J. Virol. 76:7306-7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spear, G. T., B. L. Sullivan, A. L. Landay, and T. F. Lint. 1990. Neutralization of human immunodeficiency virus type 1 by complement occurs by viral lysis. J. Virol. 64:5869-5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stiegler, G., C. Armbruster, B. Vcelar, H. Stoiber, R. Kunert, N. L. Michael, L. L. Jagodzinski, C. Ammann, W. Jager, J. Jacobson, N. Vetter, and H. Katinger. 2002. Antiviral activity of the neutralizing antibodies 2F5 and 2G12 in asymptomatic HIV-1-infected humans: a phase I evaluation. AIDS 16:2019-2025. [DOI] [PubMed] [Google Scholar]
- 49.Stoiber, H., C. Ebenbichler, R. Schneider, J. Janatova, and M. P. Dierich. 1995. Interaction of several complement proteins with gp120 and gp41, the two envelope glycoproteins of HIV-1. AIDS 9:19-26. [DOI] [PubMed] [Google Scholar]
- 50.Stoiber, H., C. Pinter, A. G. Siccardi, A. Clivio, and M. P. Dierich. 1996. Efficient destruction of human immunodeficiency virus in human serum by inhibiting the protective action of complement factor H and decay accelerating factor (DAF, CD55). J. Exp. Med. 183:307-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trkola, A., M. Purtscher, T. Muster, C. Ballaun, A. Buchacher, N. Sullivan, K. Srinivasan, J. Sodroski, J. P. Moore, and H. Katinger. 1996. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J. Virol. 70:1100-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wyatt, R., P. D. Kwong, E. Desjardins, R. W. Sweet, J. Robinson, W. A. Hendrickson, and J. G. Sodroski. 1998. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature 393:705-711. [DOI] [PubMed] [Google Scholar]
- 53.Xu, W., B. A. Smith-Franklin, P. L. Li, C. Wood, J. He, Q. Du, G. J. Bhat, C. Kankasa, H. Katinger, L. A. Cavacini, M. R. Posner, D. R. Burton, T. C. Chou, and R. M. Ruprecht. 2001. Potent neutralization of primary human immunodeficiency virus clade C isolates with a synergistic combination of human monoclonal antibodies raised against clade B. J. Hum. Virol. 4:55-61. [PubMed] [Google Scholar]
- 54.Yoo, E. M., M. J. Coloma, K. R. Trinh, T. Q. Nguyen, L. U. Vuong, S. L. Morrison, and K. R. Chintalacharuvu. 1999. Structural requirements for polymeric immunoglobulin assembly and association with J chain. J. Biol. Chem. 274:33771-33777. [DOI] [PubMed] [Google Scholar]