Abstract
Cytosolic calcium increases were analyzed in guard cells of the Arabidopsis farnesyltransferase deletion mutant era1-2 (enhanced response to abscisic acid). At low abscisic acid (ABA) concentrations (0.1 μM), increases of guard cell cytosolic calcium and stomatal closure were activated to a greater extent in the era1-2 mutant compared with the wild type. Patch clamping of era1-2 guard cells showed enhanced ABA sensitivity of plasma membrane calcium channel currents. These data indicate that the ERA1 farnesyltransferase targets a negative regulator of ABA signaling that acts between the points of ABA perception and the activation of plasma membrane calcium influx channels. Experimental increases of cytosolic calcium showed that the activation of S-type anion currents downstream of cytosolic calcium and extracellular calcium-induced stomatal closure were unaffected in era1-2, further supporting the positioning of era1-2 upstream of cytosolic calcium in the guard cell ABA signaling cascade. Moreover, the suppression of ABA-induced calcium increases in guard cells by the dominant protein phosphatase 2C mutant abi2-1 was rescued partially in era1-2 abi2-1 double mutant guard cells, further reinforcing the notion that ERA1 functions upstream of cytosolic calcium and indicating the genetic interaction of these two mutations upstream of ABA-induced calcium increases.
INTRODUCTION
Farnesyltransferases (FTases) are heterodimeric enzymes in eukaryotes that catalyze the attachment of farnesyl lipids to the C-terminal region of target proteins (Schafer and Rine, 1992; Zhang and Casey, 1996; Nambara and McCourt, 1999). Farnesylation occurs at a conserved CaaX domain, where “C” is a Cys, “aa” are most often alipathic amino acids, and “X” is usually Cys, Met, Ser, Ala, or Gln. Farnesylated proteins play central roles in cell signaling in yeast and animal cells and include Ras, GTP binding proteins, nuclear lamin B, yeast mating factors, and protein kinases (Glomset and Farnsworth, 1994; Schmitt et al., 1996; Yalovsky et al., 1997). Attachment of a farnesyl lipid to these proteins allows targeting to a membrane surface or facilitates protein–protein interactions and therefore is essential in the construction of functional signaling complexes.
In plants, FTases have been identified at the molecular level in tomato, pea, and Arabidopsis (Yang et al., 1993; Cutler et al., 1996; Schmitt et al., 1996), and a range of farnesylated proteins have been identified through biochemical studies and the recognition of conserved CaaX domains in potential signaling proteins (Zhu et al., 1993; Nambara and McCourt, 1999; Rodríguez-Concepción et al., 1999; Yalovsky et al., 2000a, 2000b). However, in most cases, it remains unknown at which point in the various signaling cascades farnesylated signaling proteins have their effects in plants.
An Arabidopsis mutant carrying a fast neutron–induced deletion in the β-subunit of a FTase has been isolated from a screen to identify mutants with an enhanced response to abscisic acid (ABA) (Cutler et al., 1996). The era1-2 mutant allele carries a 7.5-kb deletion in the FTase β-subunit and increases the dormancy of seeds in response to ABA (Cutler, 1995; Cutler et al., 1996) but does not affect endogenous ABA levels (Ghassemian et al., 2000).
ABA reduces water loss from plants during drought stress via a signal transduction network in guard cells that leads to stomatal closure (MacRobbie, 1998; Schroeder et al., 2001b). The era1-2 FTase mutation causes ABA-hypersensitive S-type anion current activation and stomatal closure (Pei et al., 1998). Stomatal closure and anion channel activity in wild-type Arabidopsis were rendered hypersensitive to ABA by the application of the FTase inhibitor α-hydroxyfarnesylphosphonic acid, indicating that FTases may function in the guard cell ABA signal transduction pathway (Pei et al., 1998).
Enhanced ABA sensitivity in era1-2 guard cells leads to reduced rates of water loss from era1-2 plants compared with wild-type plants under drought stress (Pei et al., 1998). The era1-2 mutation deletes the only FTase β-subunit gene present in Arabidopsis and therefore affects a variety of signal transduction and developmental processes (Pei et al., 1998; Nambara and McCourt, 1999; Yalovsky et al., 2000a, 2000b; Ziegelhoffer et al., 2000). The ABA hypersensitivity of the era1-2 mutant in both germination and stomatal responses indicates that the Arabidopsis FTase targets one or more negative regulators of ABA signal transduction to a signal-transducing complex that affects ABA signaling in both Arabidopsis seeds and guard cells.
Increases in cytosolic calcium concentration ([Ca2+]cyt) in guard cells have been shown to be early events in the signaling cascade that results in ABA-induced stomatal closure in a number of plant species (McAinsh et al., 1990, 1992; Schroeder and Hagiwara, 1990; Gilroy et al., 1991; Irving et al., 1992; Grabov and Blatt, 1998; Allen et al., 1999a, 2001; Staxén et al., 1999). Recently, the kinetics of [Ca2+]cyt oscillations were shown to tightly control stomatal movements in response to stimuli such as ABA (Allen et al., 2001). Furthermore, studies in suspension culture cells have shown that ABA induction of Rab18 gene expression requires the activation of plasma membrane calcium and anion channels (Ghelis et al., 2000a, 2000b) and that phospholipase C and inositol 1,4,5-trisphosphate contribute to the ABA regulation of seed germination and KIN2 gene expression (Sanchez and Chua, 2001; Xiong et al., 2001).
These data suggest that [Ca2+]cyt increases function as a second messenger in a wide variety of ABA signal transduction cascades. Recently, signaling mutants in Arabidopsis and other species are being defined and “mapped” with respect to their effects on stimulus-induced [Ca2+]cyt signals with mutations acting either upstream or downstream of [Ca2+]cyt (Allen et al., 1999a, 2001; Baum et al., 1999; Knight et al., 1999; Wais et al., 2000; Walker et al., 2000; Hugouvieux et al., 2001; Schroeder et al., 2001a). To date, only one plant mutation has been shown to enhance the sensitivity of [Ca2+]cyt signal induction (Hugouvieux et al., 2001).
In this study, we used Arabidopsis plants expressing the calcium indicator yellow cameleon 2.1 (YC2.1) (Allen et al., 1999b; Miyawaki et al., 1999) combined with stomatal movement assays and electrophysiological measurements of guard cell S-type anion channels, potassium channels, and calcium influx channels to map the position of the era1-2 mutation with respect to [Ca2+]cyt in the guard cell ABA signaling cascade. The data presented demonstrate that the era1-2 mutation acts upstream of ABA-induced [Ca2+]cyt increases and the activation of plasma membrane Ca2+ influx channels, resulting in a ABA-hypersensitive [Ca2+]cyt increase and stomatal closure in era1-2. In addition, the era1-2 mutation can partly rescue the insensitivity of the abi2-1 mutant to ABA at the level of [Ca2+]cyt signaling and stomatal closure. These data suggest that the era1-2 mutation acts very early in the ABA signaling cascade at a point between the perception of the ABA stimulus and the activation of Ca2+ channels.
RESULTS
Hypersensitivity of ABA-Induced [Ca2+]cyt Increases in era1-2 Guard Cells
ABA elicits repetitive transients, or oscillations, in [Ca2+]cyt in stomatal guard cells maintained in low (5 mM) extracellular KCl buffers (Grabov and Blatt, 1998; Allen et al., 1999b, 2000; Staxén et al., 1999). In 75% (n = 36 from 48 cells) of wild-type (Columbia) guard cells expressing the green fluorescent protein–based calcium indicator YC2.1 (Allen et al., 1999b; Miyawaki et al., 1999), application of 10 μM ABA induced repetitive transients in [Ca2+]cyt, with an average peak-to-peak period of 7.6 ± 0.3 min and an amplitude increase of 0.42 ± 0.021 ratio units (n = 36), as shown in Figure 1A. However, in wild-type guard cells, application of only 0.1 μM ABA elicited no, or only small, [Ca2+]cyt increases (change in ratio units of 0.04 ± 0.01; n = 46) (Figure 1B).
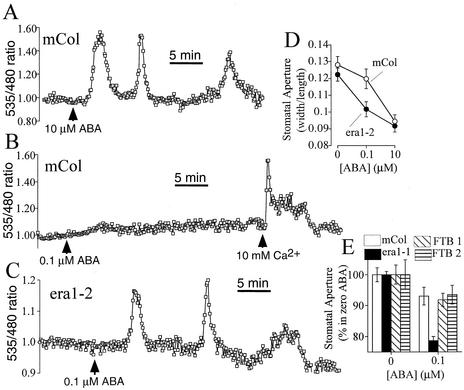
Figure 1.
ABA-Hypersensitive Induction of [Ca2+]cyt Increases and Stomatal Closure in era1-2.
(A) Repetitive [Ca2+]cyt transients induced by 10 μM ABA in wild-type Arabidopsis guard cells expressing YC2.1.
(B) ABA at 0.1 μM fails to elicit [Ca2+]cyt transients in wild type (Columbia ecotype) Arabidopsis guard cells, although application of extracellular calcium elicits [Ca2+]cyt transients.
(C) ABA at 0.1 μM elicits [Ca2+]cyt transients in era1-2 guard cells.
(D) Hypersensitive stomatal closure is induced in era1-2 stomates at 0.1 μM ABA.
(E) Hypersensitive stomatal closure is induced in era1-1 stomates at 0.1 μM ABA but not in lines FTB1 and FTB2 complemented with the tomato FTase β-subunit.
Data are means ± sem relative to n = 6 replicates comprising 120 stomates per point in (D) and relative to n = 3 replicates comprising 75 stomates per bar in (E). Fluorescence ratios were normalized relative to baseline ratios before ABA application (see Methods). mCol, Columbia.
As a positive control, 10 mM extracellular calcium ([Ca2+]ext) was applied to these cells at the end of the experiment and elicited large [Ca2+]cyt increases, indicating that they were viable to report [Ca2+]cyt changes (Figure 1B). However, when era1-2 guard cells expressing YC2.1 were treated with 0.1 μM ABA, repetitive [Ca2+]cyt transients were induced in 67% of cells (n = 29 from 43 cells; Figure 1C). Increases in [Ca2+]cyt in era1-2 guard cells induced by 0.1 μM ABA had a mean transient peak-to-peak period of 9.1 ± 0.9 min, an amplitude increase of 0.17 ± 0.033 ratio units, and mean and modal (most common) numbers of transients of 2.5 and 3, respectively (n = 29), within a recording period of up to 45 min. No significant differences in resting [Ca2+]cyt were apparent between wild-type and era1-2 guard cells (P > 0.61 for the wild type [n = 36] versus era1-2 [n = 43]).
The era1-2 mutant shows ABA-hypersensitive stomatal closure (Pei et al., 1998). Here, we analyzed ABA responses on stomatal movements using experimental conditions identical to those used in calcium imaging experiments. Hypersensitive induction of [Ca2+]cyt transients in era1-2 guard cells by ABA correlated with the hypersensitivity of ABA-induced stomatal closure, measured at 0.1 μM ABA, under identical experimental conditions (Figure 1D). Furthermore, the additional T-DNA insertion allele era1-1 (Cutler et al., 1996) also showed hypersensitive stomatal closure at 0.1 μM ABA (Figure 1E), whereas the era1-2 mutant complemented with the β-subunit of the FTase from tomato rescued the ABA hypersensitivity phenotype, with stomatal closure at 0.1 μM ABA being similar to that of the wild type (Figure 1E). Together, these data suggest that the era1 mutation renders stomata hypersensitive to ABA and affects guard cell signal transduction at a point upstream of [Ca2+]cyt increases.
Calcium Influx Mediated by Calcium-Permeable Currents Shows ABA-Hypersensitive Activation in era1-2
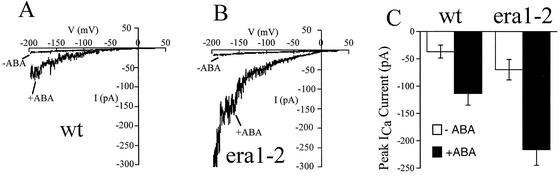
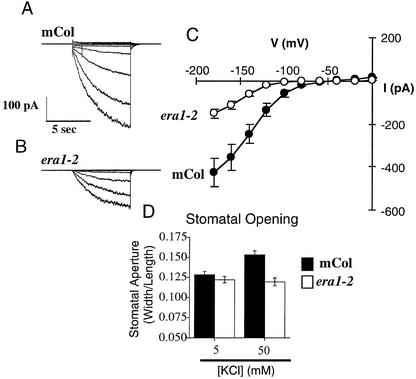
Hyperpolarization-activated calcium-permeable currents (ICa) in the guard cell plasma membrane are activated by ABA and H2O2 and mediate extracellular calcium influx (Hamilton et al., 2000; Pei et al., 2000). Using Ba2+ as a charge carrier, we measured ICa activation by low concentrations of ABA (0.1 μM) in the presence of 1 mM cytosolic NADPH in wild-type and era1-2 guard cell protoplasts, as shown in Figure 2. ICa in the presence of 0.1 μM ABA in era1-2 were significantly greater (P < 0.01) than in wild-type guard cells (Figures 2A to 2C). These data reinforce the observation that the era1 mutation affects ABA signaling in guard cells at a point upstream of ABA-induced [Ca2+]cyt signaling and show that era1-2 amplifies the ABA activation of ICa channels.
Figure 2.
ICa Channels Show Hypersensitive ABA Activation in era1-2.
(A) and (B) ICa measured before and after the addition of 0.1 μM ABA in wild-type (A) and era1-2 (B) guard cell protoplasts (n = 6 to 8 protoplasts for each).
(C) Average maximal ABA-induced ICa in wild-type and era1-2 protoplasts at −198 mV.
wt, wild type.
S-Type Anion Channel Activation by [Ca2+]cyt in era1-2 Guard Cells
To determine whether the era1-2 mutation also can affect signal transduction mechanisms downstream of [Ca2+]cyt in guard cells, we measured S-type anion channel activation by [Ca2+]cyt. S-type anion currents can be activated by both ABA (Grabov et al., 1997; Pei et al., 1997, 1998; Leonhardt et al., 1999; Li et al., 2000) and [Ca2+]cyt (Schroeder and Hagiwara, 1989; Allen et al., 1999a) and are central components of stomatal closure (Schmidt et al., 1995).
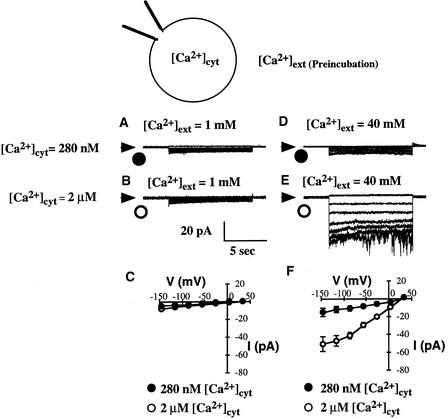
Unexpectedly, we found that [Ca2+]cyt activation of S-type anion channels in patch-clamped Arabidopsis guard cell protoplasts was dependent on the protoplasts being preincubated with high [Ca2+]ext concentrations, as illustrated in Figure 3. When protoplasts were preincubated in a solution containing 1 mM [Ca2+]ext, no significant cytosolic calcium activation of S-type anion currents was measured when [Ca2+]cyt was buffered to 280 nM (n = 9) (Figures 3A and 3C) or 2 μM (n = 26) (Figures 3B and 3C). After 1 h of preincubation with 40 mM [Ca2+]ext, only small S-type anion channel currents were recorded when [Ca2+]cyt was buffered to 280 nM (n = 13) (Figures 3D and 3F), but they were activated when [Ca2+]cyt was buffered to 2 μM (Figures 3E and 3F) (n = 8; P < 0.001 for 2 μM versus 280 nM [Ca2+]cyt at −145 mV).
Figure 3.
S-Type Anion Channel Activation by [Ca2+]cyt in Arabidopsis Guard Cell Protoplasts Requires Preincubation at High [Ca2+]ext Calcium Concentrations.
(A) S-type anion channel currents in a wild-type Arabidopsis guard cell protoplast preincubated in 1 mM [Ca2+]ext with [Ca2+]cyt buffered to 280 nM via the patch pipette solution.
(B) S-type anion channel currents in a guard cell protoplast preincubated in 1 mM [Ca2+]ext with [Ca2+]cyt buffered to 2 μM.
(C) Current-voltage relationships for S-type anion currents recorded from guard cell protoplasts preincubated in 1 mM [Ca2+]ext with [Ca2+]cyt buffered at 280 nM (closed symbols; n = 9) or 2 μM (open symbols; n = 26) as in (A) and (B).
(D) S-type anion channel currents in a guard cell protoplast preincubated in 40 mM [Ca2+]ext with [Ca2+]cyt buffered to 280 nM.
(E) S-type anion channel currents in a guard cell protoplast preincubated in 40 mM [Ca2+]ext with [Ca2+]cyt buffered to 2 μM.
(F) Current-voltage relationships for S-type anion currents recorded from guard cell protoplasts preincubated in 40 mM [Ca2+]ext with [Ca2+]cyt buffered at 280 nM (closed symbols; n =13) or 2 μM (open symbols; n = 8) as in (D) and (E).
Consistent with these findings, [Ca2+]cyt activation of anion currents measured previously in Arabidopsis guard cell protoplasts followed gigaohm seal formation in a bath solution containing 40 mM CaCl2 (Allen et al., 1999a). In the experiments represented in Figures 3A to 3F, protoplasts were sealed and recorded in bath solution containing 1 mM CaCl2 after preincubation in either 1 or 40 mM [Ca2+]ext, indicating that the continued presence of high [Ca2+]ext per se is not essential for [Ca2+]cyt activation of anion currents, although a previous exposure to high [Ca2+]ext is required.
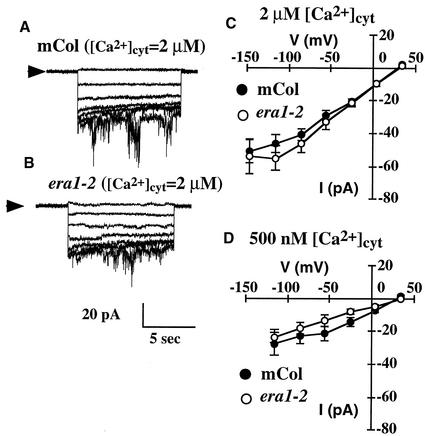
Calcium activation of S-type anion currents was compared in wild-type and era1-2 guard cell protoplasts at 1 mM [Ca2+]ext after preincubation in 40 mM [Ca2+]ext, as shown in Figure 4. S-type anion currents were similar in the wild type and era1-2 at [Ca2+]cyt levels of 500 nM (P > 0.79) or 2 μM (P > 0.58) (Figure 4). Because the activation of anion currents occurs downstream of [Ca2+]cyt (Schroeder and Hagiwara, 1989; Allen et al., 1999a), similar [Ca2+]cyt activation of S-type currents is consistent with the positioning of the era1-2 mutation upstream of [Ca2+]cyt signaling in ABA-induced stomatal closure (Figures 1 and 2).
Figure 4.
S-Type Anion Channel Activation by [Ca2+]cyt Is Similar in Wild-Type and era1-2 Guard Cell Protoplasts.
(A) S-type anion channel currents in a wild-type guard cell protoplast with [Ca2+]cyt buffered to 2 μM.
(B) S-type anion channel currents in an era1-2 guard cell protoplast with [Ca2+]cyt buffered to 2 μM.
(C) Current-voltage relationships for S-type anion currents recorded from wild-type (closed symbols; n = 8) or era1-2 (open symbols; n = 8) guard cell protoplasts with [Ca2+]cyt buffered at 2 μM as in (A) and (B).
(D) Current-voltage relationships for S-type anion currents recorded from wild-type (closed symbols; n = 8) or era1-2 (open symbols; n = 7) guard cell protoplasts with [Ca2+]cyt buffered to 500 nM.
In all experiments, protoplasts were preincubated in 40 mM [Ca2+]ext and then perfused with 1 mM [Ca2+]ext before patch clamping (see Results and Figure 2). mCol, Columbia.
The era1-2 Mutation Reduces Inwardly Rectifying K+ Current Channel Activity in Guard Cells
To determine if the era1-2 mutation affects other ion channels involved in stomatal movements, inwardly rectifying K+ (K+in) currents were measured in guard cell protoplasts. As shown in Figure 5, K+in currents were reduced significantly (2.8-fold lower at −180 mV; P < 0.0001; n = 26) in era1-2 guard cells compared with the wild type. K+in channels provide an important route for K+ uptake during stomatal opening (Schroeder et al., 1987; Thiel et al., 1992; Kwak et al., 2001). To determine whether lower K+in currents affected stomatal movements in era1-2, stomatal opening was measured in low and high extracellular K+ (Figure 5D). In low K+ (5 mM KCl and 50 μM CaCl2), stomatal opening was similar in the wild type and era1-2 (Figure 5D, left bars; P > 0.46; see also Figure 1D).
Figure 5.
K+in Channel Current and Extracellular K+-Dependent Stomatal Opening Are Reduced in era1-2.
(A) Representative K+in currents in a wild-type guard cell protoplast.
(B) Representative K+in currents in an era1-2 guard cell protoplast.
(C) Current-voltage relationships for K+in currents recorded from wild-type (closed symbols; n = 13) or era1-2 (open symbols; n = 13) guard cell protoplasts.
(D) Stomatal apertures measured after a 2.5-h opening period in the light in the wild type and era1-2. Apertures were measured in 5 mM KCl and 50 μM CaCl2, pH 6.15, opening buffer (left bars) or in 50 mM KCl and 50 μM CaCl2, pH 6.15, opening buffer (right bars). Data are means ± sem relative to n = 3 replicates comprising 120 stomates per bar. mCol, Columbia.
However, when extracellular K+ was increased (50 mM KCl and 50 μM CaCl2), wild-type stomata opened farther than those in era1-2 (Figure 5D, right bars). Similarly, at 20 mM KCl, stomatal apertures were smaller in era1-2 compared with the wild type in the absence of ABA (Pei et al., 1998). These data suggest that the era1-2 mutation can act as a limiting factor on K+in channel activity in guard cells, reducing stomatal opening.
Epistatic Interaction between abi2-1and era1-2 in [Ca2+]cyt Signaling in Guard Cells
The Arabidopsis mutants abi1-1 and abi2-1 are dominant mutations in homologous protein phosphatases of type 2C, which render stomatal closure insensitive to ABA (Koornneef et al., 1984). Using fura-2 dye–loaded Arabidopsis guard cells, it was demonstrated that abi1-1 and abi2-1 both act upstream of ABA-induced [Ca2+]cyt increases in guard cells, suppressing ABA-induced [Ca2+]cyt transients (Allen et al., 1999a). Furthermore, experimental induction of [Ca2+]cyt increases in abi1-1 and abi2-1 resulted in the recovery of S-type anion channel activation and stomatal closing, indicating that processes downstream of [Ca2+]cyt were unaffected in guard cells by these protein phosphatase type 2C mutants (Allen et al., 1999a).
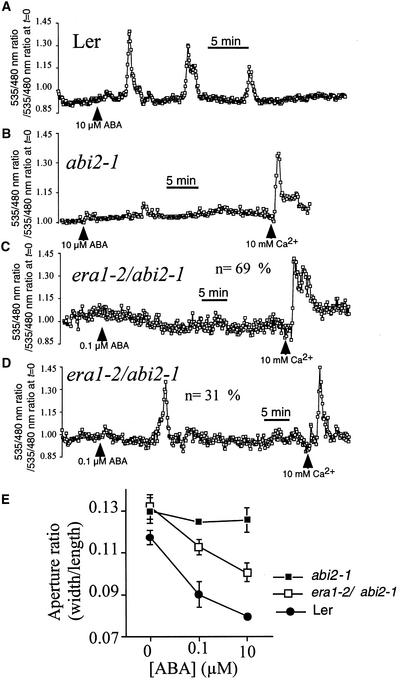
A recent study showed that the abi2-1 mutation disrupts calcium signaling downstream of ABA perception but upstream of ICa activation (Murata et al., 2001), analogous to the positioning of era1-2 found here. Therefore, we analyzed interactions of the abi2-1 and era1-2 mutations and their effects on guard cell signaling. As shown in Figure 6, we confirmed the abi2-1 impairment of ABA-induced [Ca2+]cyt increases using abi2-1–expressing YC2.1. ABA at 10 μM induced repetitive [Ca2+]cyt transients in wild-type (Landsberg erecta) guard cells (Figure 6A) (n = 36 of 42 cells) but failed to elicit significant changes in [Ca2+]cyt in all abi2-1 guard cells tested (n = 26) (Figure 6B). Positive controls using the addition of 10 mM [Ca2+]ext induced [Ca2+]cyt transients in abi2-1 (Figure 6B).
Figure 6.
The era1-2 Mutation Partly Restores ABA-Induced [Ca2+]cyt Increases and Stomatal Closure in the era1-2 abi2-1 Double Mutant.
(A) Repetitive [Ca2+]cyt transients induced by 10 μM ABA in wild-type (Landsberg erecta) Arabidopsis guard cells expressing YC2.1.
(B) [Ca2+]cyt transients are not induced by 10 μM ABA in abi2-1 guard cells expressing YC2.1, although application of extracellular calcium elicits [Ca2+]cyt transients.
(C) ABA at 0.1 μM fails to elicit [Ca2+]cyt transients in the majority (69%; n = 29 from 42 cells) of era1-2 abi2-1 guard cells, although application of extracellular calcium elicits [Ca2+]cyt transients.
(D) ABA at 0.1 μM elicits single [Ca2+]cyt transients in a fraction (31%; n = 13 from 42 cells) of era1-2 abi2-1 guard cells.
(E) ABA-induced stomatal closure is impaired in the abi2-1 mutant but is partly restored in the era1-2 abi2-1 double mutant at 0.1 μM ABA using the same bathing solutions that were used for calcium imaging studies. Data are means ± sem relative to three replicates comprising 120 stomates per point.
Ler, Landsberg erecta.
To determine whether the hypersensitivity of ABA-induced [Ca2+]cyt signals in era1-2 (Figure 1C) or the insensitivity of ABA-induced [Ca2+]cyt increases in abi2-1 (Figure 6B) was epistatic in relation to ABA-induced [Ca2+]cyt signals, we measured [Ca2+]cyt and stomatal closure in the era1-2 abi2-1 double mutant in response to low levels of ABA (0.1 μM). (Note that Landsberg erecta/Columbia cross-controls did not show any significant variation from either ecotype in ABA sensitivity in seed germination assays [data not shown].) In the majority of double mutant guard cells (69%; n = 29 from 42 cells tested), there was no induction of [Ca2+]cyt transients after the application of 0.1 μM ABA (Figure 6C), although subsequent application of 10 mM [Ca2+]ext as a control was able to induce [Ca2+]cyt transients.
However, in some era1-2 abi2-1 guard cells (31%; n = 13 from 42 cells tested), 0.1 μM ABA induced a transient increase in [Ca2+]cyt (Figure 6D). In these responsive guard cells of the double mutant, a single [Ca2+]cyt transient was induced in all cells that responded to 0.1 μM ABA (n = 13). Therefore, [Ca2+]cyt signaling in guard cells of the double mutant showed a phenotype that was intermediate between those of the two mutant phenotypes (cf. Figures 1C and 6B).
The partial recovery of the abi2-1 [Ca2+]cyt signaling phenotype is reflected in ABA-induced stomatal closure in the double mutant. Under the same experimental conditions used for [Ca2+]cyt imaging here, ABA-induced closure in era1-2 abi2-1 was significantly greater than in abi2-1 (Figure 6E) but less than in the wild type or in era1-2 alone (Figures 1D and 6E) (see Pei et al. [1998] for other experimental conditions). These data support the notion that both era1-2 and abi2-1 mutations act upstream of [Ca2+]cyt signaling in guard cells but that neither has a clear epistatic effect.
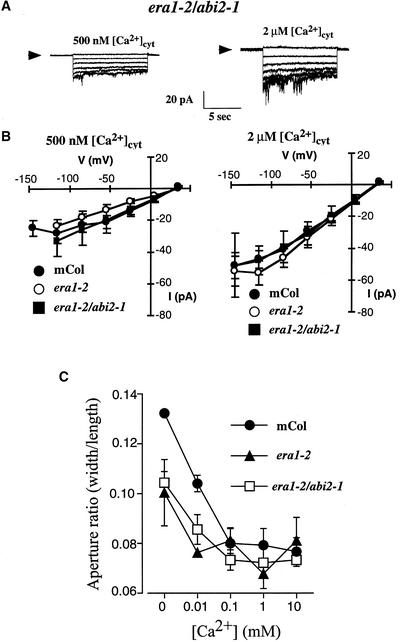
To test this finding further, [Ca2+]cyt activation of S-type anion currents and [Ca2+]ext-induced stomatal closure were measured in the era1-2 abi2-1 double mutant, as illustrated in Figure 7. S-type anion currents were activated (after a 40 mM [Ca2+]ext preincubation, as in Figures 3 and 4) by [Ca2+]cyt at 500 nM and 2 μM (Figure 7A), and activation was not significantly different from that in the wild-type or era1-2 guard cell protoplasts (Figure 7B) (n = 13 era1-2 abi2-1 guard cells; P > 0.58).
Figure 7.
S-Type Anion Channel Activation by [Ca2+]cyt- and [Ca2+]ext-Induced Stomatal Closure Is Not Impaired in the era1-2 abi2-1 Double Mutant.
(A) S-type anion channel currents in era1-2 abi2-1 double mutant guard cell protoplasts preincubated in 40 mM [Ca2+]ext with [Ca2+]cyt buffered to 500 nM (left) and 2 μM (right).
(B) Current-voltage relationships for S-type anion currents recorded from wild-type (closed circles), era1-2 (open circles), or era1-2 abi2-1 double mutant (closed squares) guard cell protoplasts preincubated in 40 mM [Ca2+]ext with [Ca2+]cyt buffered at 2 μM (right) or 500 nM (left) as in (A). The wild-type and era1-2 data are as presented in Figures 4C and 4D and are shown for direct comparison with era1-2 abi2-1 data.
(C) [Ca2+]ext-induced stomatal closure in the wild type (closed circles), era1-2 (closed triangles), and era1-2 abi2-1 double mutant (open squares). Note that stomata were opened in buffer containing 5 mM KCl and no CaCl2, pH 6.15, and that in the absence of [Ca2+]ext, era1-2 and era1-2 abi2-1 double mutant stomates failed to open to the same aperture as the wild type. Data are means ± sem relative to three replicates comprising 120 stomates per point.
mCol, Columbia.
The addition of [Ca2+]ext to preopened stomates can cause [Ca2+]cyt increases and oscillations (McAinsh et al., 1995; Allen et al., 1999a, 2000) that cause stomatal closure. Stomatal closure induced by [Ca2+]ext showed no dramatic differences between the wild type, era1-2, and the era1-2 abi2-1 double mutant (Figure 7C), although interestingly, at 0.1 mM [Ca2+]ext, stomatal apertures in the era1-2 mutant were consistently larger than at 0.01 or 1 mM (Figure 7C). The signaling mechanism underlying this phenotype is not known, but this finding suggests additional points of action of the FTase.
Together, the [Ca2+]ext-induced stomatal closure in the wild type, era1-2, and the era1-2 abi2-1 double mutant (Figure 7C) and the calcium activation of anion currents (Figures 7A and 7B) reinforce the finding that the era1-2 and abi2-1 mutations both affect guard cell signaling upstream of [Ca2+]cyt.
DISCUSSION
The role of cytosolic calcium in controlling plant cell signal transduction is becoming more fully understood with the application of new molecular genetic and imaging techniques (Sanders et al., 1999; Rudd and Franklin-Tong, 2001). In particular, the ability to map the positions of various mutants in relation to the calcium signal is revealing interactions and relationships in defined signaling cascades (Allen and Schroeder, 2001). However, the identities of many signaling components remain to be identified, and their interactions with calcium-based signaling pathways remain unknown.
To date, five Arabidopsis mutants have been shown to affect guard cell calcium signaling. These mutants fall into three categories: the mutants abi1-1 and abi2-1 impair ABA-induced calcium oscillations (Allen et al., 1999a); the mutant abh1 enhances the sensitivity of ABA-induced calcium signals (Hugouvieux et al., 2001); and the mutations det3 and gca2 affect the kinetics and dynamics of stimulus-induced calcium signals (Allen et al., 2000, 2001). In these mutants, the changes in the guard cell calcium signaling phenotypes correlate well with equivalent changes in stomatal movement and ion channel regulation phenotypes, underscoring the importance of cytosolic calcium signaling in the control of guard cell turgor.
In this study, we investigated the effect of the FTase deletion mutant era1-2 on calcium signaling in guard cells. This mutation renders stomatal closure hypersensitive to the hormonal stimulus ABA (Pei et al., 1998) (Figure 1D). Here, we show that cytosolic calcium increases could be induced in this mutant at low ABA concentrations that were ineffective in the wild type (Figure 1).
Recent studies have suggested that calcium influx into guard cells across the plasma membrane plays a critical role in ABA-induced stomatal closure (Hamilton et al., 2000; MacRobbie, 2000; Pei et al., 2000). In particular, at high ABA concentrations (1 to 10 μM) in Commelina communis, guard cell Ca2+ influx predominated, whereas at low ABA concentrations (0.1 μM), internal calcium release was more prevalent (MacRobbie, 2000). These data correlate with only a small activation of ICa in wild-type Arabidopsis guard cells at 0.1 μM ABA (Figure 2A) and an increase in this activity in the era1-2 mutant (Figure 2B).
Hyperpolarization-activated calcium channels that mediate Ca2+ influx have been identified in tomato suspension cells (Gelli and Blumwald, 1997), in Arabidopsis root cells (Kiegle et al., 2000; Véry and Davies, 2000), and in Arabidopsis and Vicia guard cells (Hamilton et al., 2000; Pei et al., 2000). The ICa channels in guard cells are activated by reactive oxygen species (ROS) (Pei et al., 2000), and ABA can generate ROS in both Arabidopsis (Pei et al., 2000) and Vicia (Zhang et al., 2001) guard cells and in maize embryos (Guan et al., 2000). In era1-2 guard cells, activation of the ICa was hypersensitive to ABA, indicating that era1-2 modulates signaling between ABA perception and the activation of ROS-sensitive ICa (Figure 2).
Our data do not exclude additional effects of ERA1 in modulating intracellular calcium release mechanisms (reviewed in Schroeder et al., 2001a). The era1-2 mutant is hypersensitive to ABA, suggesting that the FTase deleted in this mutant acts to target a negative regulator of ABA signaling to the plasma membrane or to an ABA signaling complex in guard cells. Modulation of the calcium channel or a closely related protein is possible. The positioning of a negative regulator between ABA perception and ICa activation might act to prevent spurious ICa activation in response to low levels of ABA.
The activation of S-type anion currents by an increase of cytosolic calcium has been shown to be a component of guard cell turgor reduction (Schroeder and Hagiwara, 1989; Grabov and Blatt, 1998; Allen et al., 1999a; Leonhardt et al., 1999). To further test the positioning of era1-2 in relation to cytosolic calcium in guard cells, we measured the activation of S-type anion channels in guard cells. S-type anion currents activated by calcium were identical in wild-type and era1-2 guard cells, reinforcing the finding that era1-2 acts upstream of cytosolic calcium increases and that mechanisms downstream of [Ca2+]cyt remain largely unaffected by era1-2.
A previous report has shown that S-type anion current activation in era1-2 guard cell protoplasts was hypersensitive to ABA (Pei et al., 1998) when protoplasts were preincubated in ABA and subsequently patch clamped in the presence of ABA and cytosolic calcium was buffered at the intermediate level of 280 nM, as described by Pei et al. (1997). In the present study, ABA was applied to guard cells 15 to 30 min after whole-cell configurations had been achieved to measure rapid events in ABA-induced calcium signaling. With these shorter ABA treatments, ICa were activated to a greater extent in the era1-2 mutant (Figure 2), showing that ERA1 acts upstream of calcium influx.
These data suggest two possible explanations for the ABA-hypersensitive anion channel phenotypes observed previously (Pei et al., 1998). Cytosolic calcium increases occur in protoplasts preexposed to ABA and result in the ABA-hypersensitive activation of anion channels, even though the calcium is buffered subsequently to 280 nM during the patch clamp experiments (Pei et al., 1997), and/or anion channel activation may occur via a calcium-independent pathway that has been proposed (Allan et al., 1994; Li et al., 2000), and this pathway also may be hypersensitive to ABA in era1-2 guard cells. To unequivocally characterize parallel Ca2+-independent pathways, Arabidopsis mutants or experimental conditions that show no ABA-induced [Ca2+]cyt increases but that continue to show stomatal closure would be required.
Interestingly, a new component of the regulation of S-type anion currents by calcium was revealed in this study (Figure 3). Cytosolic calcium activated S-type currents most effectively when guard cells were preincubated in high external calcium. High extracellular calcium concentrations are known to cause [Ca2+]cyt oscillations in guard cells (McAinsh et al., 1995; Allen et al., 2000), and [Ca2+]cyt oscillations program long-term stomatal closure (Allen et al., 2001). The external calcium enhancement of S-type anion current activation by [Ca2+]cyt may be linked to the programming of stomatal responses. Importantly, the requirement for [Ca2+]ext incubation found here provides a new and powerful approach for dissecting mechanisms and mutations that affect the [Ca2+]cyt sensitivity and regulation of S-type anion channels.
Another new effect of the era1-2 mutant on guard cell K+in channel activity also was characterized here (Figure 5). K+in currents were significantly lower in era1-2 compared with wild-type guard cells. This was not observed to have an effect on stomatal aperture under the imposed conditions of low extracellular K+ and Ca2+ concentrations (Figure 1D), but it led to reduced apertures relative to those in the wild type in era1-2 at higher extracellular K+ (Figure 5) (Pei et al., 1998) or at low extracellular K+ in the absence of extracellular calcium (Figure 7C).
The idea that ERA1 acts upstream of [Ca2+]cyt was further strengthened by analyzing era1-2 and abi2-1 interactions. The hypersensitivity of ABA-induced cytosolic calcium increases in era1-2 guard cells could partly rescue the impairment of ABA-induced calcium increases in the abi2-1 protein phosphatase type 2C mutant (Figure 6). These data correlate with the intermediate ABA-induced stomatal closing phenotype in the era1 abi2 double mutant (Pei et al., 1998) (Figure 6E). Double mutant analyses presented here show that both of these mutants act upstream of calcium in guard cells. The intermediate phenotype of the double mutant may be explained in part by the genetic dominance of abi2-1. This double mutant analysis suggests that the signaling pathway between ABA perception and calcium increases is complex and contains many distinct signaling elements.
Overall, the data presented in this study demonstrate how a combination of genetics and a multifaceted cell biological approach can be a powerful tool for dissecting signal transduction pathways and placing mutations at specific points in defined signaling networks. For the era1-2 mutant, this analysis has placed the action of this mutant in guard cells at a point downstream of ABA perception and upstream of plasma membrane calcium influx channel activation.
METHODS
Plant Growth
Arabidopsis thaliana plants were grown in soil (Redi-Earth Peat-Lite Mix; Scotts, Marysville, OH) in a controlled-environment growth chamber (Conviron model E15; Controlled Environments, Asheville, NC) under a 16-h-light/8-h-dark cycle at a photon fluence rate of 75 μmol·m−2·s−1 and a temperature of 20°C. Pots were watered every 2 to 3 days with deionized water. The wild-type background was Columbia for the era1-2 mutant and Landsberg erecta for abi2-1. The era1-2 mutant line used in this study had been backcrossed previously into the wild type (Columbia) three times (Cutler, 1995). All mutants and wild types were transformed with yellow cameleon 2.1 (YC2.1) by direct Agrobacterium tumefaciens transformation using floral dip (Allen et al., 1999b). At least three independent YC2.1-transformed lines were used for each wild type and mutant, except for the era1-2 abi2-1 double mutant, for which only two lines were available.
Calcium Imaging
Calcium imaging was performed as described previously using Arabidopsis expressing the green fluorescent protein–based calcium indicator YC2.1 (Allen et al., 1999b, 2001). To open stomata, abaxial epidermal strips were incubated for 2.5 h in the light (125 μmol·m−2·s−1) in 5 mM KCl, 50 μM CaCl2, and 10 mM Mes-Tris, pH 6.15, before cytosolic calcium concentration ([Ca2+]cyt) measurements. In all ecotypes and mutants analyzed, ∼30% of all guard cells exhibited spontaneous oscillations in [Ca2+]cyt. These cells were not included in the analysis; only cells exhibiting a stable resting [Ca2+]cyt level were used to analyze stimulus-induced [Ca2+]cyt increases. (Note that because measurements of [Ca2+]cyt in this study were collected from two different microscopes that used charge-coupled device cameras with different 480-nm sensitivities, the 535:480-nm ratios were normalized by dividing all measured ratios in any given recording by the initial, steady state ratio from that recording that represents prestimulus [Ca2+]cyt). The yellow fluorescent protein of YC2.1 bleaches at a faster rate than the cyan fluorescent protein, leading to a slow decline in baseline 535:480-nm ratios. Baseline decline was eliminated by a linear correction factor that was calculated from the rate of decrease in baseline ratio before the application of a stimulus.
Aperture Measurements
Stomatal apertures were measured using whole leaves as described previously (Pei et al., 1997; Allen et al., 2000). To open stomates, leaves were floated for 2.5 h (at light intensity of 125 μmol·m−2·s−1 and 20°C) on buffer containing 5 mM KCl, 50 μM CaCl2, and 10 mM Mes-Tris, pH 6.15, for abscisic acid (ABA)–induced stomatal closure experiments, or 5 mM KCl and 10 mM Mes-Tris, pH 6.15, for [Ca2+]ext-induced closure experiments. After 2.5 h, ABA or CaCl2 was added as indicated, and incubation was continued for another 3 h. Then, leaves were blended briefly (15 s) in the same opening buffer in a Waring blender, and the epidermal fragments were collected on a 30-μm (pore size) nylon mesh. Fragments were washed gently onto a microscope slide with 500 μL of opening buffer and covered with a cover slip, and aperture size was measured (pore width/length) by focusing on the focal plane of guard cells in epidermal strips, as described previously (Allen et al., 1999a).
Electrophysiology
Arabidopsis guard cell protoplasts were prepared from rosette leaves of 4- to 6-week-old plants, and patch clamp electrophysiology was performed in the whole cell mode as described previously (Pei et al., 1997; Allen et al., 1999a).
To measure calcium influx currents (ICa), the pipette solution contained 10 mM BaCl2, 0.1 mM DTT, 4 mM EGTA, and 10 mM Hepes-Tris, pH 7.1, and the bath solution contained 100 mM BaCl2, 0.1 mM DTT, and 10 mM Mes-Tris, pH 5.6. NAD(P)H (1 mM) was added to the pipette for the measurement of ABA-activated ICa activity. The addition of 5 mM NAD(P)H to the pipette (Pei et al., 2000; Murata et al., 2001) caused unstable, bursting activity of background ICa in the absence of ABA in the present study, particularly in the era1-2 mutant (data not shown). The voltage was ramped from +30 mV to −196 mV and back to +30 mV in 3 s. Sixteen ramps were applied at 40-s intervals before ABA addition and after the activated currents had stabilized. ABA was added 15 to 30 min after whole cell configurations had been achieved.
To measure S-type anion currents, the pipette solution contained 150 mM CsCl, 2 mM MgCl2, 6.7 mM EGTA, 5 mM MgATP, 10 mM Hepes-Tris, pH 7.1, and a concentration of CaCl2 calculated to give the desired free Ca2+ concentration. Free calcium concentrations were calculated with the program CALCIUM (Foehr et al., 1993). The bath solution contained 30 mM CsCl, 2 mM MgCl2, 1 mM CaCl2, and 10 mM Mes-Tris, pH 5.6. The liquid junction potential in this solution was 0.45 mV. Preincubation in 1 or 40 mM [Ca2+]ext was for 30 to 60 min in either standard bath solution (1 mM CaCl2) or bath solution supplemented with 39 mM CaCl2. (Note that gigaohm seal formation and current recording were performed in the 1 mM CaCl2 standard bath solution.) Steady state currents were sampled during the last 3 s of voltage pulses.
The standard voltage protocol stepped the voltage from a holding potential of +30 mV to −145 mV for 40 s. Subsequent voltage steps were reduced by 30 mV per pulse. The interpulse period was 12 s. No leak subtraction was made. All recordings were made 7 to 10 min after access to the whole cell configuration. There was no preincubation of protoplasts with ABA in these experiments (in contrast to the procedure reported by Pei et al. [1997][1998]).
To measure inwardly rectifying K+ currents, the pipette solution contained 30 mM KCl, 70 mM K-glutamate, 2 mM MgCl2 6.7 mM EGTA, 3.35 mM CaCl2, 5 mM Mg-ATP, and 10 mM Hepes-Tris, pH 7.1. The bath solution contained 30 mM KCl, 1 mM CaCl2, 2 mM MgCl2, and 10 mM Mes-Tris, pH 5.6. From a holding potential of 0 mV, the voltage was stepped to −180 mV in −20-mV increments for 5 s each. Steady state current was sampled in the last 100 ms of these voltage steps.
Acknowledgments
We dedicate this work to the memory of our friend Gethyn Allen and his pioneering research in plant Ca2+ signaling. We thank Roger Tsien for the use of a CALCIUM imaging setup for some experiments, Peter McCourt (University of Toronto) for providing the era1-2 and era1-1 mutants, Shaul Yalovsky (Tel Aviv University) for providing the complemented era1-2 lines, and Justin Borevitz (Salk Institute) for Landsberg erecta/Columbia crosses. This research was supported by grants from the National Institutes of Health (Grant GM60396-1P42ES10337), the U.S. Department of Energy (Grant 94ER201807), the National Science Foundation (Grant MCB 0077791), the Torry Mesa Research Institute Syngenta, and the U.S.–Israel Binational Agricultural Research and Development Fund to J.I.S.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.010448.
References
- Allan, A.C., Fricker, M.D., Ward, J.L., Beale, M.H., and Trewavas, A.J. (1994). Two transduction pathways mediate rapid effects of abscisic acid in Commelina guard cells. Plant Cell 6, 1319–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, G.J., Chu, S.P., Harrington, C.L., Schumacher, K., Hoffmann, T., Tang, Y.Y., Grill, E., and Schroeder, J.I. (2001). A defined range of guard cell calcium oscillation parameters encodes stomatal movements. Nature 411, 1053–1057. [DOI] [PubMed] [Google Scholar]
- Allen, G.J., Chu, S.P., Schumacher, K., Shimazaki, C.T., Vafeados, D., Kemper, A., Hawke, S.D., Tallman, G., Tsien, R.Y., Harper, J.F., Chory, J., and Schroeder, J.I. (2000). Alteration of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science 289, 2338–2342. [DOI] [PubMed] [Google Scholar]
- Allen, G.J., Kuchitsu, K., Chu, S.P., Murata, Y., and Schroeder, J.I. (1999. a). Arabidopsis abi1-1 and abi2-1 phosphatase mutations reduce abscisic acid-induced cytosolic calcium rises in guard cells. Plant Cell 11, 1785–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, G.J., Kwak, J.M., Chu, S.P., Llopis, J., Tsien, R.Y., Harper, J.F., and Schroeder, J.I. (1999. b). Cameleon calcium indicator reports cytoplasmic calcium dynamics in Arabidopsis guard cells. Plant J. 19, 735–747. [DOI] [PubMed] [Google Scholar]
- Allen, G.J., and Schroeder, J.I. (2001). Combining genetics and cell biology to crack the code of plant cell calcium signaling. Science's STKE. (http://stke.sciencemag.org/cgi/content/full/OC_ sigtrans;2001/102/re13). [DOI] [PubMed]
- Baum, G., Long, J.C., Jenkins, G.I., and Trewavas, A.J. (1999). Stimulation of the blue light phototropic receptor NPH1 causes a transient increase in cytosolic Ca2+. Proc. Natl. Acad. Sci. USA 96, 13554–13559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler, S. (1995). Isolation and Characterization of an Arabidopsis Mutant Supersensitive to Abscisic Acid. Master's thesis (Toronto, Canada: University of Toronto).
- Cutler, S., Ghassemian, M., Bonetta, D., Cooney, S., and McCourt, P. (1996). A protein farnesyl transferase involved in abscisic acid signal transduction in Arabidopsis. Science 273, 1239–1241. [DOI] [PubMed] [Google Scholar]
- Foehr, K.J., Worchol, W., and Gratzel, M. (1993). Calculation and control of free divalent cations in solutions used for membrane fusion studies. Methods Enzymol. 221, 149–157. [DOI] [PubMed] [Google Scholar]
- Gelli, A., and Blumwald, E. (1997). Hyperpolarization-activated Ca2+-permeable channels in the plasma membrane of tomato cells. J. Membr. Biol. 155, 35–45. [DOI] [PubMed] [Google Scholar]
- Ghassemian, M., Nambara, E., Cutler, S., Kawaide, H., Kamiya, Y., and McCourt, P. (2000). Regulation of abscisic acid signaling by the ethylene response pathway in Arabidopsis. Plant Cell 12, 1117–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghelis, T., Dellis, O., Jeannette, E., Bardat, F., Cornel, D., Miginiac, E., Rona, J.P., and Sotta, B. (2000. a). Abscisic acid specific expression of RAB18 involves activation of anion channels in Arabidopsis thaliana suspension cells. FEBS Lett. 474, 43–47. [DOI] [PubMed] [Google Scholar]
- Ghelis, T., Dellis, O., Jeannette, E., Bardat, F., Miginiac, E., and Sotta, B. (2000. b). Abscisic acid plasmalemma perception triggers a calcium influx essential for RAB18 gene expression in Arabidopsis thaliana suspension cells. FEBS Lett. 483, 67–70. [DOI] [PubMed] [Google Scholar]
- Gilroy, S., Fricker, M.D., Read, N.D., and Trewavas, A.J. (1991). Role of calcium in signal transduction of Commelina guard cells. Plant Cell 3, 333–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glomset, J.A., and Farnsworth, C.C. (1994). Role of protein modification reactions in programming interactions between ras-related GTPases and cell membranes. Annu. Rev. Cell Biol. 10, 181–205. [DOI] [PubMed] [Google Scholar]
- Grabov, A., and Blatt, M.R. (1998). Membrane voltage initiates Ca2+ waves and potentiates Ca2+ increases with abscisic acid in stomatal guard cells. Proc. Natl. Acad. Sci. USA 95, 4778–4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabov, A., Leung, J., Giraudat, J., and Blatt, M.R. (1997). Alteration of anion channel kinetics in wild-type and abi1-1 transgenic Nicotiana benthamiana guard cells by abscisic acid. Plant J. 12, 203–213. [DOI] [PubMed] [Google Scholar]
- Guan, L.Q.M., Zhao, J., and Scandalios, J.G. (2000). Cis-elements and trans-factors that regulate expression of the maize Cat1 antioxidant gene in response to ABA and osmotic stress: H2O2 is the likely intermediary signaling molecule for the response. Plant J. 22, 87–95. [DOI] [PubMed] [Google Scholar]
- Hamilton, D.W.A., Hills, A., Köhler, B., and Blatt, M.R. (2000). Ca2+ channels at the plasma membrane of stomatal guard cells are activated by hyperpolarization and abscisic acid. Proc. Natl. Acad. Sci. USA 97, 4967–4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugouvieux, V., Kwak, J.M., and Schroeder, J.I. (2001). An mRNA cap binding protein, ABH1, modulates early abscisic acid signal transduction in Arabidopsis. Cell 106, 477–487. [DOI] [PubMed] [Google Scholar]
- Irving, H.R., Gehring, C.A., and Parish, R.W. (1992). Changes in cytosolic pH and calcium of guard cells precede stomatal movements. Proc. Natl. Acad. Sci. USA 89, 1790–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiegle, E., Gilliham, M., Haseloff, J., and Tester, M. (2000). Hyperpolarisation-activated calcium currents found only in cells from the elongation zone of Arabidopsis thaliana roots. Plant J. 21, 225–229. [DOI] [PubMed] [Google Scholar]
- Knight, H., Veale, E.L., Warren, G.J., and Knight, M.R. (1999). The sfr6 mutation in Arabidopsis suppresses low-temperature induction of genes dependent on the CRT/DRE sequence motif. Plant Cell 11, 875–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef, M., Reuling, G., and Karssen, C.M. (1984). The isolation and characterization of abscisic acid-insensitive mutants of Arabidopsis thaliana. Physiol. Plant. 61, 377–383. [Google Scholar]
- Kwak, J.M., Murata, Y., Baizabal-Aguirre, V.M., Merrill, J., Wang, M., Kemper, A., Hawke, S.D., Tallman, G., and Schroeder, J.I. (2001). Dominant negative guard cell K+ channel mutants reduce inward-rectifying K+ currents and light-induced stomatal opening in Arabidopsis. Plant Physiol. 127, 473–485. [PMC free article] [PubMed] [Google Scholar]
- Leonhardt, N., Vavasseur, A., and Forestier, C. (1999). ATP binding cassette modulators control abscisic acid-regulated slow anion channels in guard cells. Plant Cell 11, 1141–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J., Wang, X.-Q., Watson, M.B., and Assmann, S.M. (2000). Regulation of abscisic acid-induced stomatal closure and anion channels by guard cell AAPK kinase. Science 287, 300–303. [DOI] [PubMed] [Google Scholar]
- MacRobbie, E.A.C. (1998). Signal transduction and ion channels in guard cells. Philos. Trans. R. Soc. Lond. 1374, 1475–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRobbie, E.A.C. (2000). ABA activates multiple Ca2+ fluxes in stomatal guard cells, triggering vacuolar K+(Rb+) release. Proc. Natl. Acad. Sci. USA 97, 12361–12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAinsh, M.R., Brownlee, C., and Hetherington, A.M. (1990). Abscisic acid-induced elevation of guard cell cytosolic Ca2+ precedes stomatal closure. Nature 343, 186–188. [Google Scholar]
- McAinsh, M.R., Brownlee, C., and Hetherington, A.M. (1992). Visualizing changes in cytosolic-free Ca2+ during the response of stomatal guard cells to abscisic acid. Plant Cell 4, 1113–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAinsh, M.R., Webb, A.A.R., Taylor, J.E., and Hetherington, A.M. (1995). Stimulus-induced oscillations in guard cell cytosolic free calcium. Plant Cell 7, 1207–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki, A., Griesbeck, O., Heim, R., and Tsien, R.Y. (1999). Dynamic and quantitative Ca2+ measurements using improved cameleons. Proc. Natl. Acad. Sci. USA 96, 2135–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata, Y., Pei, Z.-M., Mori, I.C., and Schroeder, J.I. (2001). Abscisic acid activation of plasma membrane Ca2+ channels in guard cells requires cytosolic NAD(P)H and is differentially disrupted upstream and downstream of reactive oxygen species production in the abi1-1 and abi2-1 protein phosphatase 2C mutants. Plant Cell 13, 2513–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambara, E., and McCourt, P. (1999). Protein farnesylation in plants: A greasy tale. Curr. Opin. Plant Biol. 2, 388–392. [DOI] [PubMed] [Google Scholar]
- Pei, Z.-M., Ghassemian, M., Kwak, C.M., McCourt, P., and Schroeder, J.I. (1998). Role of farnesyltransferase in ABA regulation of guard cell anion channels and plant water loss. Science 282, 287–290. [DOI] [PubMed] [Google Scholar]
- Pei, Z.-M., Kuchitsu, K., Ward, J.M., Schwarz, M., and Schroeder, J.I. (1997). Differential abscisic acid regulation of guard cell slow anion channels in Arabidopsis wild-type and abi1 and abi2 mutants. Plant Cell 9, 409–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei, Z.-M., Murata, Y., Benning, G., Thomine, S., Klüsener, B., Allen, G.J., Grill, E., and Schroeder, J.I. (2000). Calcium channels activated by hydrogen peroxide mediate abscisic acid signaling in guard cells. Nature 406, 731–734. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Concepción, M., Yalovsky, S., Zik, M., Fromm, H., and Gruissem, W. (1999). The prenylation status of a novel plant calmodulin directs plasma membrane or nuclear localization of the protein. EMBO J. 18, 1996–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd, J.J., and Franklin-Tong, V.E. (2001). Unravelling response-specificity in Ca2+ signalling pathways in plant cells. New Phytol. 151, 7–33. [DOI] [PubMed] [Google Scholar]
- Sanchez, J.-P., and Chua, N.-H. (2001). Arabidopsis PLC1 is required for secondary responses to abscisic acid signals. Plant Cell 13, 1143–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders, D., Brownlee, C., and Harper, J.F. (1999). Communicating with calcium. Plant Cell 11, 691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer, W.R., and Rine, J. (1992). Protein prenylation: Genes, enzymes, targets, and functions. Annu. Rev. Biochem. 26, 209–237. [DOI] [PubMed] [Google Scholar]
- Schmidt, C., Schelle, I., Liao, Y.J., and Schroeder, J.I. (1995). Strong regulation of slow anion channels and abscisic acid signaling in guard cells by phosphorylation and dephosphorylation events. Proc. Natl. Acad. Sci. USA 92, 9535–9539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt, D., Callan, K., and Gruissem, W. (1996). Molecular and biochemical characterization of tomato farnesyl-protein transferase. Plant Physiol. 112, 767–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder, J.I., Allen, G.J., Hugouvieux, V., Kwak, J.M., and Waner, D. (2001. a). Guard cell signal transduction. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52, 627–658. [DOI] [PubMed] [Google Scholar]
- Schroeder, J.I., and Hagiwara, S. (1989). Cytoplasmic calcium regulates ion channels in the plasma membrane of Vicia faba guard cells. Nature 338, 427–430. [Google Scholar]
- Schroeder, J.I., and Hagiwara, S. (1990). Repetitive increases in cytosolic Ca2+ of guard cells by abscisic acid activation of non-selective Ca2+ permeable channels. Proc. Natl. Acad. Sci. USA 87, 9305–9309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder, J.I., Kwak, J.M., and Allen, G.J. (2001. b). Guard cell abscisic acid signalling and engineering drought hardiness in plants. Nature 410, 327–330. [DOI] [PubMed] [Google Scholar]
- Schroeder, J.I., Raschke, K., and Neher, E. (1987). Voltage dependence of K+ channels in guard cell protoplasts. Proc. Natl. Acad. Sci. USA 84, 4108–4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staxén, I., Pical, C., Montgomery, L.T., Gray, J.E., Hetherington, A.M., and McAinsh, M.R. (1999). Abscisic acid induces oscillations in guard-cell cytosolic free calcium that involve phosphoinositide-specific phospholipase C. Proc. Natl. Acad. Sci. USA 96, 1779–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel, G., MacRobbie, E.A.C., and Blatt, M.R. (1992). Membrane transport in stomatal guard cells: The importance of voltage control. J. Membr. Biol. 126, 1–18. [DOI] [PubMed] [Google Scholar]
- Véry, A.A., and Davies, J.M. (2000). Hyperpolarization-activated calcium channels at the tip of Arabidopsis root hairs. Proc. Natl. Acad. Sci. USA 97, 9801–9806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wais, R.J., Galera, C., Oldroyd, G., Catoria, R., Penmetsa, R.V., Cook, D., Gough, C., Dénarie, J., and Long, S.R. (2000). Genetic analysis of calcium spiking responses in nodulation mutants of Medicago truncatula. Proc. Natl. Acad. Sci. USA 97, 13407–13412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, S.A., Viprey, V., and Downie, J.A. (2000). Dissection of nodulation signaling using pea mutants defective for calcium spiking induced by Nod factors and chitin oligomers. Proc. Natl. Acad. Sci. USA 97, 13413–13418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, L., Lee, B.-h., Ishitani, M., Lee, H., Zhang, C., and Zhu, J.-K. (2001). FIERY1 encoding an inositol polyphosphate 1-phosphatase is a negative regulator of abscisic acid and stress signaling in Arabidopsis. Genes Dev. 15, 1971–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalovsky, S., Kulukian, A., Rodríguez-Concepción, M., Young, C.A., and Gruissem, W. (2000. a). Functional requirement of plant farnesyltransferase during development in Arabidopsis. Plant Cell 12, 1267–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalovsky, S., Rodríguez-Concepción, M., Bracha, K., Toledo-Ortiz, G., and Gruissem, W. (2000. b). Prenylation of the floral transcription factor APETALA1 modulates its function. Plant Cell 12, 1257–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalovsky, S., Trueblood, C.E., Callan, K.L., Narita, J.O., Jenkins, S.M., Rine, J., and Gruissem, W. (1997). Plant farnesyltransferase can restore yeast Ras signaling and mating. Mol. Cell. Biol. 17, 1986–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Z.B., Cramer, C.L., and Watson, J.C. (1993). Protein farnesyltransferase in plants: Molecular cloning and expression of a homolog of the β-subunit from the garden pea. Plant Physiol. 101, 667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, F.L., and Casey, P.J. (1996). Protein prenylation: Molecular mechanisms and functional consequences. Annu. Rev. Biochem. 65, 241–269. [DOI] [PubMed] [Google Scholar]
- Zhang, X., Zhang, L., Dong, F., Gao, J., Galbraith, D.W., and Song, C.-P. (2001). Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol. 126, 1438–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, J., Bressan, R.A., and Hasegawa, P.M. (1993). Isoprenylation of the plant molecular chaperone ANJ1 facilitates membrane association and function at high temperature. Proc. Natl. Acad. Sci. USA 90, 8557–8561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegelhoffer, E.C., Medrano, L.J., and Meyerowitz, E.M. (2000). Cloning of the Arabidopsis WIGGUM gene identifies a role for farnesylation in meristem development. Proc. Natl. Acad. Sci. USA 97, 7633–7638. [DOI] [PMC free article] [PubMed] [Google Scholar]