Abstract
Lad is an SH2 domain-containing adaptor protein that binds MEK kinase 2 (MEKK2), a mitogen-activated protein kinase (MAPK) kinase kinase for the extracellular signal-regulated kinase 5 (ERK5) and JNK pathways. Lad and MEKK2 are in a complex in resting cells. Antisense knockdown of Lad expression and targeted gene disruption of MEKK2 expression results in loss of epidermal growth factor (EGF) and stress stimuli-induced activation of ERK5. Activation of MEKK2 and the ERK5 pathway by EGF and stress stimuli is dependent on Src kinase activity. The Lad-binding motif is encoded within amino acids 228 to 282 in the N terminus of MEKK2, and expression of this motif blocks Lad-MEKK2 interaction, resulting in inhibition of Src-dependent activation of MEKK2 and ERK5. JNK activation by EGF is similarly inhibited by loss of Lad or MEKK2 expression and by blocking the interaction of MEKK2 and Lad. Our studies demonstrate that Src kinase activity is required for ERK5 activation in response to EGF, MEKK2 expression is required for ERK5 activation by Src, Lad and MEKK2 association is required for Src activation of ERK5, and EGF and Src stimulation of ERK5-regulated MEF2-dependent promoter activity requires a functional Lad-MEKK2 signaling complex.
Extracellular signal-regulated kinase 5 (ERK5)/big mitogen-activated kinase 1 is a member of the mitogen-activated protein kinase (MAPK) family. Efforts to define the ERK5 signaling module have led to the identification of MEK5 as a specific ERK5 kinase (61). Sequence comparisons indicate that MEK5 is most related to the ERK1/2 kinases MEK1 and MEK2 (42). However, MEK5 is not significantly phosphorylated by the MEK1/2 kinase Raf-1 or MEKK1, nor does MEK5 phosphorylate ERK1/2 or JNK (13), indicating that MEK5/ERK5 represents a distinct MAPK signaling cascade. The ERK5 pathway has been implicated in stress response and growth factor-promoted cell growth and survival (1, 20). In neurons, blocking ERK5 activation diminished retrograde survival responses initiated by neurotrophin stimulation of axon terminals (56). A recent study showed that expression of an activated form of MEK5 resulted in serial assembly of sarcomeres in cardiomyocytes and eccentric cardiac hypertrophy in transgenic mice, suggesting a role for the MEK5/ERK5 module in mediating cytokine signaling associated with cardiac hypertrophy (36). ERK5 activates the MADS box transcription factors MEF2A, -C, and -D (19, 21, 28) and the Ets-domain transcription factor Sap1a (18). One consequence of activation of the MEF2 proteins is to induce c-jun expression that is essential for cell growth and cell cycle progression (7, 19, 21, 23, 28). Additionally, ERK5 regulates muscle cell differentiation via a mechanism probably involving MEF2 proteins and myocyte-specific activators such as MyoD (12).
We and others have recently demonstrated that the two highly homologous MEK kinases, MEKK2 and -3, specifically interact with MEK5 and activate the ERK5 pathway (8, 49). Significantly, we found that MEKK2 is much more potent than MEKK3 in ERK5 activation; the reason for this is unclear but may be accounted for by a higher affinity of MEKK2 for binding to MEKK2-MEK5 (49). Our understanding of MEKK2 signaling is further advanced by the finding that MEKK2, but not MEKK3, associates with the SH2-domain adaptor protein Lad, otherwise known as RIBP (49). Lad possesses other features that potentially serve as protein-protein interaction motifs, including a zinc finger, a proline-rich region, and several tyrosine phosphorylation sites (11). Lad may be the mouse homologue of the human adaptor protein TSAd/VRAP; they exhibit 68% sequence identity and 76% similarity (43, 46, 58). Although initially thought to be restricted in T cells, Lad and TSAd are expressed in a variety of other cell types (41, 51, 58). Lad and TSAd are involved in T-cell receptor and platelet-derived growth factor receptor (PDGFR) signaling (11, 29, 41, 43), and we have shown that during T-cell activation Lad and MEKK2 colocalize at the T-cell contact site with antigen-loaded presenting cells (49). This observation suggests that Lad recruits MEKK2 to activated receptor complexes.
Adaptor proteins like Lad facilitate and promote specificity in signal transduction. The modular structure of Lad/TSAd suggests its involvement in transduction of signals from multiple receptors and protein tyrosine kinases. Indeed, Lad/TSAd interacts with the receptor tyrosine kinases PDGFR and vascular endothelial cell growth factor receptor (VEGFR) KDR, as well as several components of receptor signaling networks, including Grb2, phosphatidylinositol 3-kinase, and phospholipase Cγ (PLC-γ) (11, 41, 58). An association of Lad with the Src family kinase Lck as well as the Tec family tyrosine kinases Itk and Rlk has also been documented (11, 43). Although the implication is obvious that Lad/TSAd plays an important part in signal integration within many receptor signal transduction systems, the functional role of Lad/TSAd in these receptor-regulated responses has not been defined.
We are interested in Lad-MEKK2-regulated signal transduction. Lad and MEKK2 bind to each other and colocalize in cells, and several experimental findings indicate they may act in a common signaling pathway. Targeted gene disruptions show that both MEKK2 and Lad knockout mice are developmentally normal but exhibit defects in cell proliferation and production of certain cytokines in response to receptor engagement by antigen and specific growth factors (15, 43, 48). Chayama and colleagues have recently presented evidence that expression of tumor necrosis factor alpha, interleukin-4, and granulocyte-macrophage colony-stimulating factor in mast cells is regulated by the MEKK2/MEK5/ERK5 pathway (9). Thus, we started our present study by hypothesizing that Lad is critical in regulating activation of MEKK2 by extracellular stimuli. Herein, we show that endogenously expressed MEKK2 and Lad interact and their interaction is disrupted in response to epidermal growth factor (EGF) and oxidative or hyperosmotic stress stimuli. Lad and its interaction with MEKK2 are required for MEKK2 and ERK5 activation in response to these stimuli. Furthermore, Src family kinases are required for EGF and stress-induced activation of MEKK2 and ERK5. Our work defines the Lad-MEKK2 complex to be essential for Src-dependent activation of the ERK5 pathway.
MATERIALS AND METHODS
Antibodies, proteins, growth factors, DNA constructs, and other reagents.
The monoclonal MEKK2 antibody has been described elsewhere (K. Lobel-Rice, K. Kesavan, W. Sun, R. Lapadat, S. Webb, A. Doan, E. W. Gelfand, P. M. Henson, G. L. Johnson, and T. P. Garrington, submitted for publication). Rabbit anti-ERK5 serum and the monoclonal anti-Flag M5 antibody were purchased from Sigma-Aldrich, Inc. Polyclonal anti-JNK, polyclonal anti-ERK1/2, and monoclonal anti-phospho-ERK1/2 (E10) antibodies were commercial products of Cell Signaling Technology. The monoclonal anti-EGF receptor antibody (clone 13) was purchased from BD Biosciences. Mouse monoclonal anti-phosphotyrosine 4G10 antibody, EGF, and recombinant MKK4 were from Upstate Biotechnology. Lad antiserum was generated by injecting rabbits with full-length Lad expressed and purified from Sf9 cells. The Src kinase inhibitor PP1 was from Biomol Research Laboratories, Inc. Geneticin (G418 sulfate) and hygromycin B were obtained from Invitrogen and Calbiochem, respectively.
The constitutively active (Y527F) and dominant negative (K295R) Src mutants were created by site-directed mutagenesis. Dominant negative MEKK2, MEK5, ERK5, and MKK7 have been described elsewhere (9, 45, 49). Dominant negative MEF2C (amino acids 1 to 105) is capable of DNA binding but defective in transcriptional activation (33, 38). The MEF2-dependent reporter plasmid pGL2-MEF2-Luc was kindly provided by Saadi Khochbin (INSERM, Paris, France).
Cells, cell culture, and transfection.
The mink lung epithelial cell line CCL64 was purchased from the American Type Culture Collection. Isolation of mouse embryonic fibroblasts (MEFs) from day 14.5 embryos was as described elsewhere (Lobel-Rice et al., submitted), and cells were grown in Iscove's modified Dulbecco's medium (IMDM) with 10% fetal calf serum, 100 U of penicillin/ml, and 100 μg of streptomycin/ml. Human embryonic kidney (HEK) 293 and CCL64 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum and antibiotics. Selection for stable CCL64 and 293 cells was with 0.3 mg of hygromycin B/ml and 0.7 mg of G418/ml, respectively. Transfection was accomplished either through the use of Lipofectamine (Invitrogen) or electroporation at 280 V and 960 μF.
Yeast two-hybrid interaction mapping.
Mapping of the Lad/RIBP-binding sites of MEKK2 was based on the previous study that the N-terminal fragment of MEKK2 associates with Lad. Serial truncations of this MEKK2 region were cloned in frame to the LexA DNA-binding protein in the yeast vector pBTM116 (4). The pBTM116-derived plasmids were then cotransformed with a plasmid expressing a Gal4 activation domain fusion of Lad (pACT2-Lad; see reference 49) into the yeast reporter strain L40 (17). Strength of interaction was estimated by the abilities of transformant Saccharomyces cerevisiae cells to grow on minimal plates lacking histidine but supplemented with 3 mM 3-aminotriazole (3-AT) (Sigma). Quantitation of two-hybrid interaction was carried out as previously described (50).
Yeast three-hybrid analysis.
The three-hybrid vector pBridge was purchased from Clontech (53). Full-length MEKK2 was expressed constitutively from the ADH1 promoter in pBridge as a Gal4 DNA-binding domain fusion protein, and a Lad-binding MEKK2 fragment (either amino acids [aa] 228 to 282 or aa 241 to 282) was conditionally expressed under the methionine-repressible promoter PMET25. Lad, fused C terminal to the Gal4 activation domain, was cloned in a second plasmid (pACT2). After cotransformation, yeast cells were grown on histidine plates. Colonies of roughly equal size were picked and suspended in 500 μl of H2O. Two microliters of the suspensions and their tenfold serial dilutions were each spotted on three types of minimal plates: with histidine, or without histidine but supplemented with 20 mM 3-AT plus 2 mM or no methionine (see Fig. 5A). Yeast cells were incubated at 30°C for 2 days and photographed.
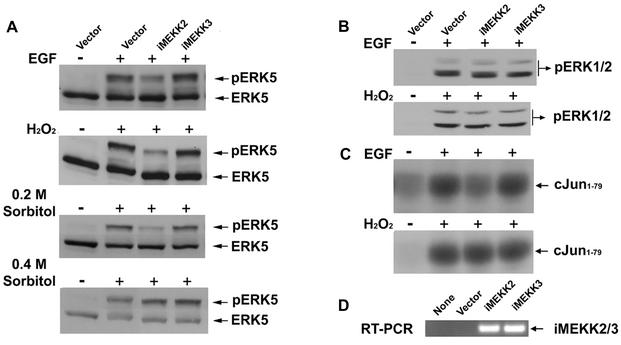
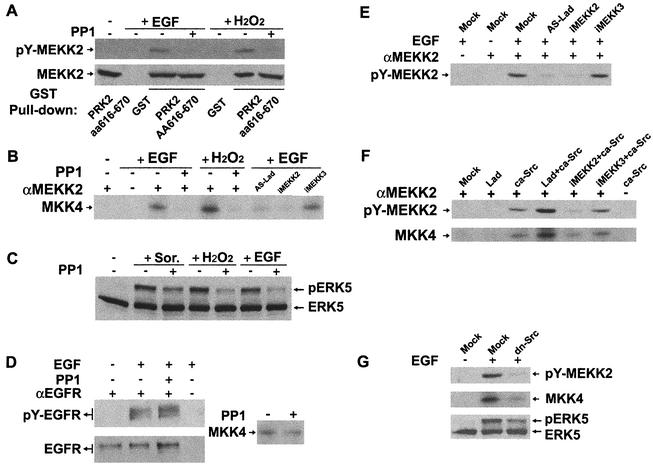
FIG. 5.
Requirement of Lad-MEKK2 association for specific MAPK signaling. (A) Lad-MEKK2 interaction is required for ERK5 activation by EGF, H2O2, and low (0.2 M) but not high (0.4 M) concentrations of sorbitol. Shown are results of the analysis of ERK5 activation in HEK293 cells stably expressing iMEKK2 or iMEKK3. (B) The Lad-MEKK2 complex is not required for ERK1/2 activation. Blotting cell lysates with anti-ERK1/2 indicated that endogenous ERK1/2 were expressed similarly across each cell line (data not shown). (C) Lad-MEKK2 interaction plays a role in EGF- but not H2O2-elicited JNK activation. (D) Semiquantitative RT-PCR analysis of iMEKK2 and iMEKK3 RNA expression in HEK293 cells.
Coimmunoprecipitation and in vitro binding analysis.
293 or CCL64 cells were washed with ice-cold phosphate-buffered saline buffer and lysed in a lysis buffer consisting of 50 mM Tris-Cl (pH 7.5), 100 mM NaCl, 50 mM NaF, 5 mM Na4P2O7, 1 mM Na3VO4, 0.5% Triton X-100, 1 mM EDTA, 1 mM EGTA, 50 μM leupeptin, 10 μg of pepstatin A/ml, 1 mM phenylmethylsulfonyl fluoride, and 2 μg of aprotinin/ml. After brief clearance by high-speed centrifugation, cell lysates were immunoprecipitated at 4°C for 2 to 3 h, followed by a 2-h incubation with prewashed protein G beads. The beads were washed three times with lysis buffer, and bead-bound proteins of interest were examined by Western blotting.
For the in vitro binding study, the MEKK2 fragment spanning residues 228 to 282 and the corresponding region from MEKK3 (aa 239 to 293) were produced in bacteria as glutathione S-transferase (GST) fusions. The fusion proteins, prebound to glutathione-Sepharose 4B beads (Amersham Pharmacia Biotech), were incubated with Lad-transfected CCL64 cell lysates for 3 h. After extensive washing, the samples were Western blotted with M5 to detect the presence of Lad.
RT-PCR.
Reverse transcriptase PCR (RT-PCR) was performed on mRNA prepared from cultured cells by using Trizol (Life Technologies, Inc.). Reverse transcription and PCRs were carried out using rTth DNA polymerase following the manufacturer's instructions (Perkin-Elmer Life Sciences). The amplifying primers were complementary to the vector (pcDNA3.1) sequences flanking the multiple cloning site 5′-TAATACGACTCACTATAGGG-3′ and 5′-TAGAAGGCACAGTCGAGG-3′. RT-PCR of glyceraldehyde-3-phosphate dehydrogenase mRNA served as an internal standard.
Analysis of MEKK2 and MAPK activities.
CCL64 or HEK293 and derivative cell lines expressing different constructs were serum starved for 3 to 24 h in DMEM (or overnight in IMDM for MEFs) with 0.5% fetal bovine serum before treatment. Stimulation with EGF, H2O2 (Sigma), and sorbitol (Sigma) was for 20 min at 37°C, at concentrations of 2.5 ng/ml, 250 nM, and 0.2 or 0.4 M, respectively. Treatment with the Src kinase inhibitor PP1 was at 10 μM for 30 min before stimulation. Analysis of ERK5 activation was based on a gel-shift protocol according to the established observation that phosphorylated and activated ERK5 has a significantly reduced mobility on sodium dodecyl sulfate (SDS)-polyacrylamide gels (19, 20, 49). This was done by running cell lysates on 7% gels at 4°C and Western blotting with anti-ERK5 antiserum. ERK1/2 activation was determined by blotting with the phospho-ERK1/2-specific antibody E10. An in vitro kinase assay for JNK was done using GST-cJun1-79 as described previously (50). To assay MEKK2 kinase activity, endogenous MEKK2 was immunoprecipitated from cell lysates and, after extensive wash in high-stringency lysis buffer (50), was used in an immunocomplex kinase reaction using purified recombinant MKK4 as a specific substrate.
Luciferase reporter gene assay.
CCL64 cells were transfected with the MEF2-dependent reporter plasmid pGL-MEF2-Luc in combination with pRL-tk and grown overnight. Cells were then placed in fresh serum-free medium with or without EGF for 8 h. Aliquots of cell lysates were assayed for firefly and Renilla luciferase activities according to the instructions provided in the Dual-Luciferase reporter assay system (Promega).
RESULTS
MEKK2 and Lad are in a complex.
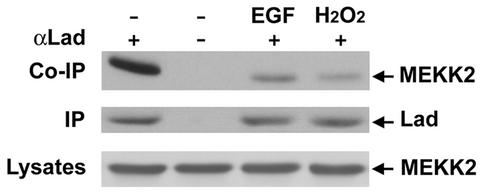
The adaptor protein Lad was identified as a MEKK2-binding protein in a yeast two-hybrid screen using the N terminus of MEKK2 (49). To demonstrate endogenous Lad-MEKK2 interactions, CCL64 cell lysates were immunoprecipitated with Lad antiserum and Western blotted with a monoclonal antibody against MEKK2. Results shown in Fig. 1 demonstrate that endogenous Lad and MEKK2 are in a complex in serum-starved cells. Treatment of cells with EGF or H2O2, stimuli that activate MEKK2 and ERK5 (see below), caused a significant reduction in the MEKK2 that coimmunoprecipitated with Lad. The total MEKK2 in lysates and immunoprecipitated Lad was similar for basal and stimulated cells. Thus, the Lad-MEKK2 interaction is stable in resting cells and declines in response to EGF and oxidative stress. This is in agreement with our previous transient expression studies showing phosphorylation of Lad by MEKK2-induced dissociation of the Lad-MEKK2 complex (49).
FIG. 1.
Interaction of Lad with MEKK2. CCL64 cells were serum starved overnight and then stimulated with vehicle, 2.5 ng of EGF/ml, or 250 nM H2O2 for 20 min. Cell lysates were immunoprecipitated with Lad antiserum or preimmune antiserum (as negative control). Western blotting with anti-MEKK2 monoclonal antibody indicated MEKK2 was coimmunoprecipitated with Lad in resting cells but was disrupted upon cell activation (top panel). Blotting the immunoprecipitates with anti-Lad (middle panel) or cell lysates with anti-MEKK2 (bottom panel) showed that Lad and MEKK2 expression was unaffected by treatment with either stimulus.
MEKK2 is activated by EGF and stress stimuli.
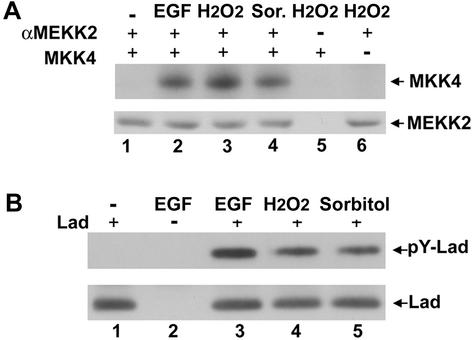
MEKK2 is a MEK kinase for the ERK5 and JNK pathways (6, 49), which are known to respond to a wide spectrum of stimulants, including growth factors and stress agents such as H2O2 and sorbitol. We investigated if MEKK2 was activated in response to these stimuli. The activity of endogenous MEKK2 immunoprecipitated from CCL64 mink lung epithelial cells treated with EGF, H2O2, or sorbitol was analyzed in an in vitro immune complex kinase assay (Fig. 2A). Since bacterially expressed MEK5 was not soluble, we used recombinant MKK4 as a MEKK2 substrate. The activity of MEKK2 was significantly stimulated by each of the stimuli tested. Phosphorylation of MKK4 was minimal for MEKK2 immune complexes from H2O2-treated cells incubated with a control antibody (lane 5) and was nonexistent without the MKK4 substrate (lane 6), demonstrating specific phosphorylation of MKK4 by MEKK2.
FIG. 2.
MEKK2 is activated and Lad tyrosine phosphorylated in response to EGF and stress stimuli. (A) MEKK2 is activated by EGF (2.5 ng/ml), H2O2 (250 nM), and sorbitol (0.2 M). Stimulated CCL64 cells were lysed and immunoprecipitated with a monoclonal anti-MEKK2 antibody. MEKK2 activity was analyzed in an in vitro kinase assay using purified recombinant MKK4 as a substrate. Western blot analysis confirmed equal immunoprecipitation of MEKK2 (lower panel). (B) Lad is tyrosine phosphorylated in cells exposed to EGF, H2O2, and sorbitol. Following stimulation with EGF, H2O2, or sorbitol, cells stably expressing Flag-Lad were lysed and Lad immunoprecipitated using an anti-Flag antibody. Phosphorylation of Lad was detected by Western blotting using the anti-phosphotyrosine antibody 4G10 (upper panel). Lad was immunoprecipitated similarly from cell lysates of different cellular stimulations (lower panel). No band was observed in cells transfected with vector alone (lane 2).
We next examined the tyrosine phosphorylation of Lad by EGF, H2O2, and sorbitol. CCL64 cells stably expressing Flag-Lad were treated with or without EGF, H2O2, or sorbitol, and Flag-Lad was immunoprecipitated followed by Western blotting with an antiphosphotyrosine antibody (Fig. 2B). Lad was tyrosine phosphorylated in cells treated with each of the stimuli (lanes 3 to 5), but not in control cells without stimulation (lane 1) or mock-transfected cells (lane 2). These results show that Lad is tyrosine phosphorylated in response to EGF and oxidative and hyperosmotic stresses that also stimulate MEKK2 kinase activity. Our results are consistent with those of Choi et al. (11), who showed Lad was phosphorylated on tyrosines by Lck during T-cell activation.
Lad and MEKK2 are required for ERK5 activation.
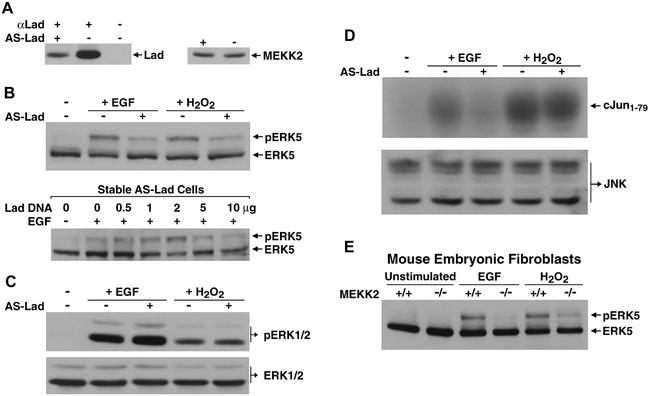
Our findings suggested that Lad might be responsible for coupling extracellular stimulation to MEKK2 activation, leading to ERK5 and JNK activation. This possibility was investigated using an antisense (AS) RNA interference approach. Stable transfection with an AS-Lad construct substantially decreased the protein level of endogenous Lad, while the expression of endogenous MEKK2 was unaffected (Fig. 3A), demonstrating the specificity of AS-Lad inhibition. Endogenous ERK5 activities were analyzed based on the established protocol that activated ERK5 migrates during SDS-polyacrylamide gel electrophoresis at a reduced mobility relative to nonphosphorylated, inactive ERK5 (2, 19, 49). As shown in Fig. 3B, AS-Lad considerably diminished ERK5 responses to stimulation by EGF or H2O2. To show that the AS-Lad interference was a direct consequence of Lad knockdown, the ERK5 response could be reconstituted by transfection of Lad. Thus, the AS-Lad effect is directly a result of knockdown of Lad protein expression and is reversed by transfection of a Lad cDNA and increased expression of Lad protein. At the highest levels of add-back Lad expression, ERK5 activation was inhibited, probably due to excess Lad relative to MEKK2. Correlating with the observed AS-Lad inhibition of ERK5 activation, we found that EGF-dependent activation of MEKK2 was also inhibited by AS-Lad (see data in Fig. 6B). In additional experiments, the knockdown of MEKK3 had no effect on ERK5 activation by EGF or stress stimuli (data not shown), further demonstrating the specificity of Lad and MEKK2 in the ERK5 response. As shown in Fig. 3C, no suppression of ERK1/2 activity was detected in cells expressing AS-Lad, demonstrating a specific requirement for Lad in ERK5 signaling. The dependence of ERK5 activity on MEKK2 is more pronounced in EGF-stimulated fibroblasts than under the condition of oxidative stress (Fig. 3E). Lad has somewhat of a stimulus-specific role in JNK signaling; there was a significant inhibition by AS-Lad of EGF-stimulated JNK activity but not for oxidative stress-activated JNK (Fig. 3D). This is consistent with several different pathways activated by oxidative stress leading to JNK activation. For example, MEKK1 has been shown to contribute to JNK activation in response to oxidative stress (32). There is also a requirement of MEKK2 for ERK5 activation by both EGF and oxidative stress (Fig. 3E). The targeted disruption of MEKK2 expression (15) results in the loss of ERK5 activation by EGF and a significant but partial inhibition of ERK5 activation in response to oxidative stress. The MEKK2 knockout and AS-Lad give similar inhibition of ERK5 activation, consistent with MEKK2 and Lad being in a signaling complex required for EGF and H2O2 activation of MEKK2 and ERK5.
FIG. 3.
Requirement of Lad and MEKK2 in specific MAPK signaling. (A) AS-Lad specifically inhibited endogenous Lad expression. CCL64 cells were stably transfected with a DNA construct encoding AS-Lad (from −51 to +345 relative to the ATG start site) or control mock vector. Left panel: Cell lysates were incubated with preimmune or Lad antiserum, and immunoprecipitates were Western blotted with anti-Lad. Right panel: Cell lysates were blotted with anti-MEKK2. (B) Lad is required for ERK5 activation by EGF and H2O2. ERK5 activation was analyzed in a gel-shift assay based on the previous finding that activated ERK5 has a characteristic reduced mobility on SDS-polyacrylamide gels (upper panel). AS-Lad inhibition of EGF-stimulated ERK5 activation can be reversed by transient expression of Lad (lower panel). Increasing amounts of Lad were transiently expressed for 6 h in AS-Lad cells, which were subsequently treated with EGF after 3 h of serum starvation. ERK5 activities were determined by Western blotting. (C) Lad is dispensable for ERK1/2 signaling. EGF- or H2O2-treated AS-Lad or mock-transfected cells were analyzed for ERK1/2 responses by Western blotting using a phospho-ERK1/2-specific antibody (upper panel). Immunoblotting cell lysates with anti-ERK1/2 showed no difference in endogenous ERK1/2 expression (lower panel). (D) Lad contributes to EGF- but not H2O2-induced JNK activation. Endogenous JNK was pulled down with GST-cJun1-79 and used in an in vitro kinase assay (upper panel). Blotting with an anti-JNK antibody showed that AS-Lad does not affect JNK expression (lower panel). (E) MEKK2 is required for EGF and H2O2 activation of ERK5. MEKK2-knockout MEFs or wild-type MEFs were stimulated as indicated. Analysis of ERK5 activities was as described in the legend for panel B.
FIG. 6.
MEKK2 and ERK5 activation requires Lad expression and is regulated by Src. (A) MEKK2 is tyrosine phosphorylated by Src kinases. CCL64 cells pretreated with the Src kinase inhibitor PP1 (10 μM; 30 min) or vehicle (dimethyl sulfoxide [DMSO]) were stimulated with EGF or H2O2. Endogenous MEKK2 was pulled down by bead-bound GST-PRK2aa616-670 and blotted with 4G10 (upper panel). Blotting an aliquot of the beads with anti-MEKK2 showed that MEKK2 was pulled down equally under each condition (lower panel). (B) MEKK2 activation is regulated by Src kinases and Lad. PP1-pretreated (or DMSO-pretreated) wild-type cells or cells stably expressing AS-Lad, iMEKK2, or iMEKK3 were stimulated with EGF or H2O2. MEKK2 was immunoprecipitated from cell lysates, and kinase activity towards MKK4 was determined in an in vitro immune complex kinase assay. Equal MEKK2 immunoprecipitation was achieved as confirmed by Western blotting, as described in the legend for panel A. RT-PCR analysis indicated iMEKK2 and iMEKK3 RNAs were similarly expressed in CCL64 cells, similar to results with HEK293 cells. (C) Src kinases regulate ERK5 signaling in response to EGF and stress stimuli. Western blot analysis of ERK5 inhibition by PP1 was done as described in the legend for Fig. 3B. (D) PP1 at 10 μM does not interfere with EGFR phosphorylation or significantly decrease MEKK2 activity in vitro. Cells were treated with PP1 (or DMSO) and then with or without EGF as described in the legend for panel A (left panel). Cell lysates were incubated with a monoclonal anti-EGFR antibody. The immunoprecipitates were blotted with either 4G10 or anti-EGFR. Immunoprecipitated MEKK2 from H2O2-treated cells was used for an in vitro kinase assay in the presence or absence of PP1 (right panel). (E) EGF-stimulated MEKK2 tyrosine phosphorylation requires interaction with Lad. Lysates of the indicated stable cell lines were immunoprecipitated with anti-MEKK2 and blotted with 4G10. (F) Lad and Src play a synergistic role in MEKK2 tyrosine phosphorylation and activation. Cells were transfected with the indicated expression constructs. Immunoprecipitated MEKK2 was analyzed either for tyrosine phosphorylation (upper panel) or kinase activity on MKK4 (lower panel). (G) EGF signaling to the MEKK2→ERK5 pathway was interfered with by dominant negative Src. Assays for MEKK2 tyrosine phosphorylation and activation of MEKK2 and ERK5 were as described above.
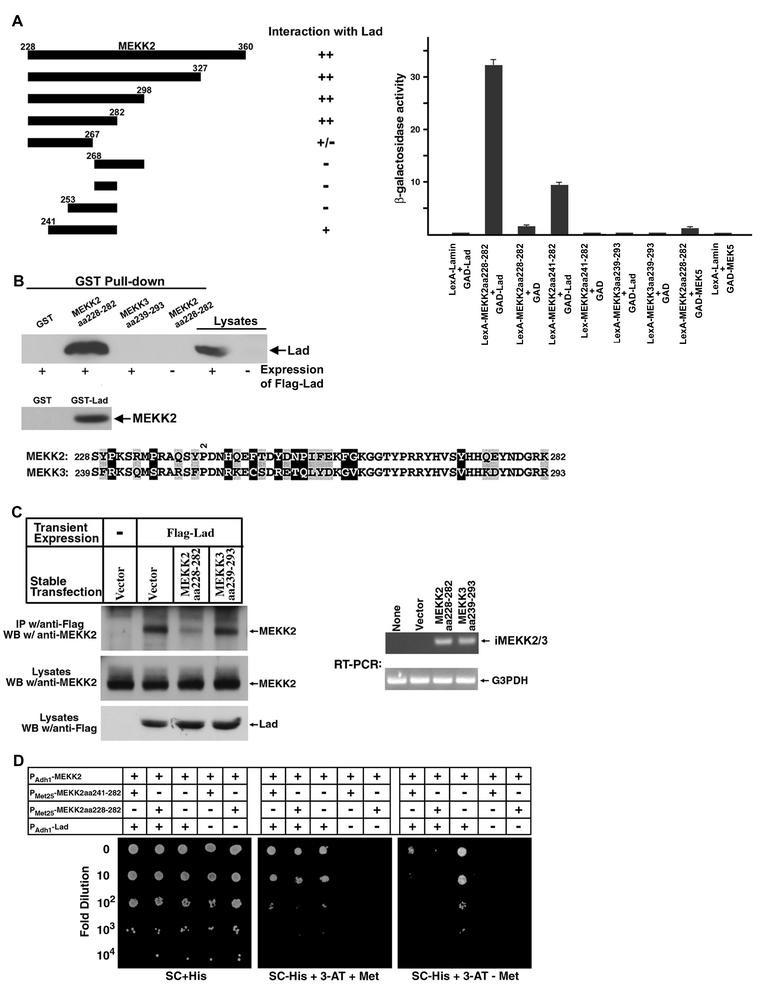
Mapping the interaction between MEKK2 and Lad.
The region of MEKK2 from aa 228 to 360 (MEKK2aa228-360) was originally used as bait in a yeast two-hybrid screen that identified Lad as a MEKK2-binding protein (49). Starting with this region of MEKK2, we examined a series of truncated MEKK2 fragments for their interactions with Lad by the criterion of their ability to support growth of the yeast reporter strain L40 on minimal plates lacking histidine (Fig. 4A, upper panel). Gradual deletion of MEKK2aa228-360 from the C terminus showed that amino acids after residue 282 were dispensable for binding Lad. However, further removal of the C-terminal 15 residues of MEKK2aa228-282 resulted in a nearly complete loss of interaction. Truncation of the MEKK2aa228-282 fragment from the N terminus revealed that MEKK2aa241-282 could still interact with Lad with significant, although reduced, affinity. In support of these results, quantitative analysis of the lacZ reporter gene showed that MEKK2aa228-282-Lad interaction-driven β-galactosidase activity was fourfold higher than that of yeast L40 cells transformed with MEKK2aa241-282 and Lad. The region of MEKK3 that is homologous to MEKK2aa228-282, from residues 239 to 293, did not show any appreciable interaction with Lad in the yeast two-hybrid analysis, although MEKK2 and MEKK3 are 82% conserved (58% identical) between these regions. The lacZ reporter assay also revealed that the Lad-binding site of MEKK2 failed to interact with MEK5. Confirming the two-hybrid data, the bacterially expressed and purified GST fusion of MEKK2aa228-282 could readily pull down Lad from CCL64 cell lysates, whereas GST-MEKK3aa239-293 clearly failed to do so (Fig. 4B, upper panel). Importantly, a GST-Lad fusion protein was able to pull down MEKK2 from cell lysates (Fig. 4B). However, neither N- nor C-terminal fragments nor the SH2 domain of Lad bound MEKK2 (data not shown), suggesting that proper folding of only the full-length Lad protein gave binding activity for MEKK2. The findings define a specific Lad-binding motif within aa 228 to 282 of MEKK2.
FIG.4.
Mapping of the MEKK2-binding motif for Lad. (A) Quantitation of two-hybrid interaction. Serial truncations of the MEKK2aa228-360 fragment were fused to the bacterial DNA-binding protein LexA in vector pBTM116 and tested for their interactions with Lad in a yeast two-hybrid prototrophy assay. The strength of interactions was determined by growth of transformant yeast cells on synthetic complete (SC)-His plates supplemented with 3 mM 3-AT. Liquid cultures of yeast transformant cells were assayed for β-galactosidase activities, and the results are represented as the mean ± standard deviation. The alignment of the Lad-interacting MEKK2 sequence with the corresponding region from MEKK3 is shown. Nonconserved residues are highlighted in black, and conserved but nonidentical amino acids are shaded in gray. (B) Confirmation of yeast two-hybrid results by in vitro binding analysis. Bead-bound and purified GST fusion proteins were incubated with CCL64 cell lysates that did or did not express Flag-Lad as indicated (upper panel). Binding with Lad was examined by Western blotting with M5 antibody. Cell lysates were also loaded in parallel as controls. A GST fusion of full-length Lad was incubated with CCL64 cell lysate, and pull down of endogenous MEKK2 was analyzed by Western blotting (lower panel). (C) Expression of MEKK2aa228-282 in HEK293 cells blocked MEKK2 binding to Lad. HEK293 cells stably transfected with MEKK2aa228-282, MEKK3aa239-293, or an empty vector were transiently transfected with or without Flag-Lad. After limited (6 h) expression, cell lysates were subjected to immunoprecipitation with anti-Flag M5 antibody. Western blotting was then employed to detect the amounts of endogenous MEKK2 associated with Lad (top panel). Western blotting of cell lysates showed that the levels of endogenous MEKK2 or transiently expressed Lad were similar (middle and bottom panels, respectively). Semiquantitative RT-PCR analysis of MEKK2aa228-282 and MEKK3aa239-293 demonstrated similar RNA expression in stably expressing 293 cells. The primers used were complementary to the vector (pcDNA3.1) sequences flanking the multiple cloning site. Amplification of the housekeeping gene for glyceraldehyde-3-phosphate dehydrogenase (G3PDH) indicated equal mRNA recovery and RT-PCR efficiency. (D) Three-hybrid analysis demonstrates that MEKK2 from aa 228 to 282 but not the related sequence in MEKK3 can specifically disrupt Lad-MEKK2 interaction. MEKK2aa228-282 or -aa241-282 was cloned under the methionine-repressible MET25 promoter in the yeast three-hybrid vector pBridge, and full-length MEKK2 was expressed as a Gal4 DNA-binding domain fusion protein from the constitutive ADH1 promoter in the same vector. Either of the pBridge-derived plasmids was cotransformed with GAD-Lad into the yeast reporter strain CG1945. After growth on SC-His plates, similarly sized transformant colonies were randomly picked and spotted in 10-fold serial dilutions on SC-His plates (as controls), or on SC-His plus 20 mM 3-AT plates supplemented with or without methionine. Photography was taken after cells were incubated at 30°C for 2 days. The figure represents similar results from six independent experiments.
The finding that MEKK2aa228-282 binds Lad suggested that it could be used to selectively disrupt Lad interactions with MEKK2. To test this prediction, cells stably expressing MEKK2aa228-282, or the corresponding MEKK3 sequence (aa 239 to 293) that does not bind Lad, were transiently transfected with Flag-Lad. After limited expression (6 h posttransfection), immunoprecipitation with the anti-Flag M5 antibody was performed to assess the amounts of Lad-associated endogenous MEKK2. Western blotting of cell lysates with an anti-MEKK2 or anti-Flag antibody showed that the expression levels of MEKK2 and Lad were constant across each cell line (Fig. 4C, middle and bottom panels, respectively). Examination by RT-PCR also found that MEKK2aa228-282 and MEKK3aa239-293 were expressed at similar RNA levels, predicting similar protein expression (Fig. 4C). Lanes 1 and 2 (Fig. 4C, top panel) showed that Lad bound endogenous MEKK2. The association was significantly disrupted by MEKK2aa228-282 (lane 3) but was unaffected by expression of MEKK3aa239-293 (lane 4).
We then took advantage of the yeast three-hybrid system to confirm that expression of MEKK2aa228-282 or MEKK2aa241-282 in cells could block MEKK2 association with Lad. In the three-hybrid system, MEKK2 and Lad were constitutively expressed, and the MEKK2 fragment aa 228 to 282 or aa 241 to 282 was under the transcriptional control of the methionine-repressible promoter Met25 (PMET25) (53). After transformation, individual yeast colonies were spotted on three sets of minimal plates in 10-fold serial dilutions, as shown in Fig. 4D. In the presence of histidine where pressure on selection for MEKK2-Lad interaction was not applied (left panel), all the transformant yeast cells grew at about the same density, showing that a roughly equal number of cells were spotted. In plates depleted of histidine (but with methionine so that transcription from PMET25 was suppressed) (middle panel), cells without expression of Lad could not grow, even without dilution (Fig. 4D, columns 3 and 4), again demonstrating the requirement for Lad-MEKK2 interaction. Further omission of methionine from the yeast plates induced expression of the MEKK2 fragments aa 241 to 282 and aa 228 to 282 from PMET25 (right panel). Under these conditions, growth of cells expressing either of the cloned Lad-binding fragments was severely impaired (columns 1 and 2) compared to cells not bearing the MEKK2 fragments (column 3), which grew equally well in the presence or absence of methionine. The results also showed that aa 228 to 282 had a greater ability than aa 241 to 282 to inhibit Lad-MEKK2 interaction and consequently cell growth, correlating nicely with the yeast two-hybrid binding-site mapping study. We therefore designated MEKK2aa228-282 as iMEKK2, for inhibitory fragment of MEKK2 and, for convenience, MEKK3aa239-293 is hereafter referred to as iMEKK3.
Lad-MEKK2 interaction is required for ERK5 activation in response to growth factor ligation and extracellular stresses.
Results from the above experiments indicated that Lad likely coupled MEKK2 to activation by upstream regulators. The mapping of the Lad-MEKK2 interaction provided an excellent tool to test this question, as we showed that iMEKK2 specifically disrupted this interaction. Expression of iMEKK2 exhibited a strong inhibitory effect on the activation of ERK5 (Fig. 5A) and MEKK2 (Fig. 6B); in contrast, iMEKK3, which was expressed at a similar RNA level (Fig. 5D) but did not interact with Lad, had no effect on the activation of either kinase. Thus, the results demonstrated that ERK5 activation induced by growth factors and oxidative or hyperosmotic stresses required Lad interaction with MEKK2. Interestingly, at a high concentration (0.4 versus 0.2 M) of sorbitol, the effect of iMEKK2 on ERK5 became insignificant (Fig. 5A, bottom panel), indicating that additional signaling pathways at high hyperosmolarity bypass the requirement for Lad-MEKK2 interaction. The results also show that the ERK5 response pathway is functional to an appropriate stimulus.
In line with the results that signaling from the cell surface to the ERK1/2 cascade is largely independent of Lad (Fig. 3C), uncoupling MEKK2 from Lad by using iMEKK2 showed no inhibition of ERK1/2 activation to stimulation by EGF or H2O2 (Fig. 5B). Expression of iMEKK2 partially inhibited EGF but not H2O2 activation of JNK, consistent with the finding when using AS-Lad (Fig. 3D), that Lad-MEKK2 interaction has a more prominent role in EGF than in H2O2 activation of JNK (Fig. 5C). The combined results with MEKK2−/− MEFs, iMEKK2, and AS-Lad indicate that Lad-MEKK2 interaction is critical for ERK5 activation and is involved in EGF receptor regulation of JNK, but it is not required for ERK1/2 regulation.
The Lad/MEKK2→ERK5 pathway is regulated by Src kinases.
We next investigated if MEKK2, like Lad, was tyrosine phosphorylated in response to EGF or H2O2. Endogenous MEKK2 was isolated from CCL64 cell lysates with a purified MEKK2-binding fragment of PRK2 (residues 616 to 670) expressed as a GST fusion (50). Western blotting with an anti-MEKK2 monoclonal antibody confirmed equal pull-downs by GST-PRK2aa616-670 (Fig. 6A, lower panel). Blotting with the antiphosphotyrosine antibody 4G10 showed that MEKK2 was tyrosine phosphorylated by treatment with EGF or H2O2 (Fig. 6A, upper panel). The 4G10-reactive bands were MEKK2, as they were not precipitated from EGF- or H2O2-treated cells incubated with GST alone. Importantly, pretreatment of cells with PP1, a Src kinase inhibitor (25), inhibited MEKK2 tyrosine phosphorylation (Fig. 6A). At this concentration of PP1 (10 μM), phosphorylation of the EGF receptor (EGFR) was unaffected (Fig. 6D, left panel), indicating that Src kinases are likely involved in MEKK2 tyrosine phosphorylation. Correlating with the requirement for Src kinases for MEKK2 tyrosine phosphorylation, MEKK2 kinase activity in cells treated with EGF or H2O2 was strongly inhibited by pretreatment of cells with PP1 (Fig. 6B). However, PP1 did not inhibit the in vitro kinase activity of MEKK2 isolated from stimulated CCL64 cells (Fig. 6D, right panel). PP1 treatment of cells also inhibited ERK5 activation (Fig. 6C), consistent with the loss of MEKK2 activation in PP1-treated cells. Supporting a critical role for Src kinases in MEKK2/MEK5/ERK5 signaling, a dominant negative form of Src (dn-Src) substantially reduced EGF stimulation of MEKK2 tyrosine phosphorylation and activity as well as ERK5 activation (Fig. 6G). In addition, transient expression of a constitutively active Src mutant resulted in a significant stimulation of both MEKK2 tyrosine phosphorylation and catalytic activity, which were impeded by iMEKK2 (but not iMEKK3) but were synergized with coexpression of Lad (Fig. 6F). These results established a requirement for Lad and its interaction with MEKK2 in MEKK2 tyrosine phosphorylation in response to EGF. Tyrosine phosphorylation of Lad by the Src kinase Lck has been shown to induce Lad binding to the Lck SH2 domain, recruiting it to the T-cell receptor signaling complex (11).
The Src/Lad/MEKK2/MEK5/ERK5 cascade transmits EGF stimulation to MEF2-dependent gene expression.
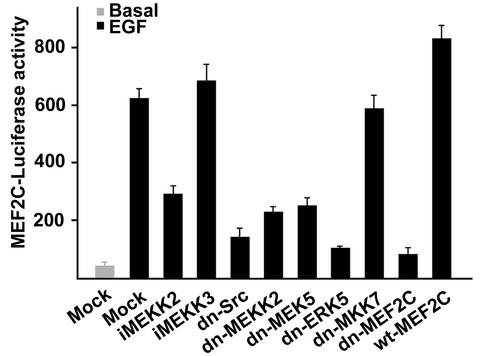
A well-established ERK5 target are the MEF2 transcription factors (19), which are essential for differentiation of muscle lineages as well as expression of many other non-muscle-specific genes, including the proto-oncogene c-jun (5, 16, 34). Expression from a MEF2-dependent luciferase reporter gene was augmented approximately 12-fold by EGF treatment of cells (Fig. 7). This response was inhibited by dominant negative mutants of Src, MEKK2, MEK5, and ERK5 but unaffected by dn-MKK7, showing that the JNK pathway was not involved. Further substantiating the specificity of the reporter gene assay was the fact that EGF-stimulated luciferase activity was essentially eliminated by dn-MEF2C but was moderately enhanced by expression of wild-type MEF2C. Expression of iMEKK2 but not iMEKK3 also inhibited EGF activation of the reporter gene. The partial inhibition with iMEKK2 in blocking MEF2-driven transcription compared to ERK5 activation was probably a result of a weak p38 MAPK activation in response to EGF that is able to regulate MEF2 activation (16, 28, 59, 60).
FIG. 7.
EGF-induced gene transcription from a MEF2-dependent promoter is regulated by Src kinases, Lad, and the MEKK2/MEK5/ERK5/MEF2C cascade, but it is independent of the JNK pathway. Transcriptional activation was shown as relative firefly or Renilla luciferase light units (mean ± standard deviation of triplicate determinations).
DISCUSSION
The present study for the first time demonstrates (i) Src kinase activity is required for ERK5 activation in response to EGF, (ii) MEKK2 expression is required for ERK5 activation by Src, (iii) Lad and MEKK2 association is required for Src activation of ERK5, and (iv) EGF and Src stimulation of MEF2-dependent promoter activity requires a functional Lad-MEKK2 signaling complex. Three independent experimental approaches define the function of MEKK2 in EGF and Src kinase regulation of ERK5 activation. Two approaches involved inhibition of protein expression by using MEKK2 gene knockout and Lad AS RNA interference. Loss of MEKK2 expression and inhibition of Lad by AS RNA interference abolished or substantially reduced ERK5 activation not only in response to growth factors but also oxidative stress and hyperosmolarity. The third independent approach involved uncoupling Lad-MEKK2 association by expression of a Lad-binding MEKK2 fragment. Expression of the iMEKK2 peptide strongly diminished MEKK2 and ERK5 activation and also resulted in a significant inhibition of EGF-induced JNK activity. These effects are specific, as iMEKK2 disruption of MEKK2 binding to Lad has little or no effect on ERK1/2 or H2O2 activation of JNK. It is not surprising that JNK is activated by pathways independent of Lad-MEKK2 association, given that the many proteins and lipids that are oxidized in cells in response to H2O2 could activate other MEKKs in the JNK pathway. Cumulatively, these three independent approaches defined a pathway of Src/Lad/MEKK2/MEK5/ERK5 for the regulation of MEF2-activated gene expression.
Lad encodes several well-defined protein interaction modules (11). The SH2 domain of Lad associates with the kinase insert domains of the PDGF and VEGF receptors upon receptor activation and tyrosine phosphorylation (41, 58), and Lad subsequently undergoes tyrosine phosphorylation, which provides binding sites for interaction with the SH2 or phosphotyrosine-binding domains of other proteins such as Src kinases and Grb2 (3, 11, 41). Lad can also bind the SH3 domain-containing enzymes phosphatidylinositol 3-kinase and PLC-γ, probably via interaction with the proline-rich region of the adaptor proteins (58). Thus, there are potentially many proteins through which the Lad-MEKK2 complex can interact within receptor signaling networks for the regulation of ERK5. MEKK2 may also regulate the activity of other proteins in this complex (49). Of note, MEKK2 can also bind PRK2, a Nck-binding PKC-related kinase that is an effector for the small GTPases Rho and Rac (50). The multiple interactions that are possible suggest a signaling module of which Lad-MEKK2 is a part for the integrated regulation of ERK5 and JNK.
In addition to growth factors, Lad-MEKK2 interaction is also required for ERK5 responses to stress stimuli such as hyperosmolarity and oxidants. In the budding yeast S. cerevisiae, osmotic imbalances in the environment are detected by two sensor systems, the histidine kinase receptor Sln1 and the four-pass transmembrane receptor Sho1p (27, 39). Much less is known about osmotic signaling in mammalian cells, but there is evidence that the osmotic stress responses utilize specific growth factor and cytokine receptors (40, 44). Similarly, oxidative stress responses are known to involve the activation of Src kinases and the EGFR (10, 14, 24, 31, 52, 55). Thus, stress activation of ERK5 may involve pathways regulated by the EGFR and Src kinases through Lad regulation of MEKK2.
Lad and MEKK2 are clearly tyrosine phosphorylated by a Src-dependent mechanism. MEKK2 has also been shown to be tyrosine phosphorylated in T cells stimulated with anti-CD3 antibody (48). Similarly, Lad is phosphorylated by Lck in T cells (11, 43). Src kinases also phosphorylate additional proteins that are involved in binding Lad, including receptor tyrosine kinases (22, 26, 47), PLC-γ (35, 37, 54, 57), and Shc (30). Our studies have not yet defined how Src activates MEKK2. Our preliminary studies have found that MEKK2 is phosphorylated on one and possibly two threonines in the activation loop sequence 504ASKRLQTICLSGTGMKSVTGTPYWM528 that is between kinase subdomains VII and VIII (unpublished studies). We currently have no evidence that the single tyrosine, Y526, within the kinase subdomain VIII sequence 524TPYWMSPE531 is phosphorylated. There are no other tyrosines within the activation loop of the MEKK2 kinase domain. Our prediction is that Src-dependent tyrosine phosphorylation of Lad and MEKK2 is involved in formation of a protein complex that regulates MEKK2 activation.
Finally, the use of the iMEKK2 peptide strongly corroborates the antisense Lad and MEKK2 knockout experiments that define the Lad/MEKK2/MEK5/ERK5 signaling pathway regulated by Src. Based on the following considerations, the iMEKK2 effect on MAPK signaling is a specific consequence resulting from disruption of the Lad-MEKK2 interaction: (i) no inhibitory effect was caused by iMEKK3, which is 82% conserved with iMEKK2 but incapable of binding Lad. (ii) iMEKK2 does not bind MEK5 or ERK5 and hence does not directly interfere with the MEKK2/MEK5/ERK5 module. (iii) iMEKK2 inhibition of ERK5 could be reversed by moderate expression of Lad or a high concentration of sorbitol, showing the intactness of the MEKK2/MEK5/ERK5 module and the requirement of Lad for its signaling. (iv) The effect of iMEKK2 was stimulus and pathway specific. It did not hamper ERK1/2 activation and was required for EGF but not H2O2 activation of JNK. (v) iMEKK2 suppressed MEF2-dependent gene expression independent of JNK, consistent with Lad regulation of ERK5 through MEKK2. (vi) Two-hybrid and three-hybrid analyses defined the specificity of iMEKK2 disruption of Lad-MEKK2 interactions. The use of a selective inhibitor of protein-protein interactions is a powerful tool to define specific functions of two proteins that interact but have additional domains and protein interactions. Using such an approach combined with gene knockouts and antisense analysis defined the requirement of an interaction between Lad and MEKK2 when the expression of neither protein was altered. We propose that such an approach should be used in combination with knockdown experiments such as RNA interference, antisense, and knockouts to define the specificity of signal transduction pathways.
Acknowledgments
This research was partially supported by NIH grants DK37871, CA18587, and AI42246.
REFERENCES
- 1.Abe, J.-I., M. Kusuhara., R. J. Ulevitch, B. C. Berk, and J. D. Lee. 1996. Big mitogen-activated protein kinase 1 (BMK1) is a redox-sensitive kinase. J. Biol. Chem. 271:16586-16590. [DOI] [PubMed] [Google Scholar]
- 2.Abe, J.-I., M. Takahashi, M. Ishida, J. D. Lee, and B. C. Berk. 1997. c-Src is required for oxidative stress-mediated activation of big mitogen-activated protein kinase 1 (BMK1). J. Biol. Chem. 272:20389-20394. [DOI] [PubMed] [Google Scholar]
- 3.Anafi, M., F. Kiefer, G. D. Gish, G. Mbamalu, N. N. Iscove, and T. Pawson. 1997. SH2/SH3 adaptor proteins can link tyrosine kinases to a Ste20-related protein kinase, HPK1. J. Biol. Chem. 272:27804-27811. [DOI] [PubMed] [Google Scholar]
- 4.Bartel, P. L., C. Chien, R. Sternglanz, and S. Fields. 1993. Using the two-hybrid system to detect protein-protein interactions, p. 153-179. In D. A. Hartley (ed.), Cellular interactions in development: a practical approach. IRL Press, Oxford, United Kingdom.
- 5.Black, B. L., and E. N. Olson. 1998. Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins. Annu. Rev. Cell Dev. Biol. 14:167-196. [DOI] [PubMed] [Google Scholar]
- 6.Blank, J. L., P. Gerwins, E. M. Elliott, S. Sather, and G. L. Johnson. 1996. Molecular cloning of mitogen-activated protein/ERK kinase kinases (MEKK) 2 and 3. Regulation of sequential phosphorylation pathways involving mitogen-activated protein kinase and c-Jun kinase. J. Biol. Chem. 271:5361-5368. [DOI] [PubMed] [Google Scholar]
- 7.Carter, R., S. C. Cosenza, A. Pena, K. Lipson, D. R. Soprano, and K. J. Soprano. 1991. A potential role for c-Jun in cell cycle progression through late G1 and S. Oncogene 6:229-235. [PubMed] [Google Scholar]
- 8.Chao, T.-H., M. Hayashi, R. I. Tapping, Y. Kato, and J. D. Lee. 1999. MEKK3 directly regulates MEK5 activity as part of the big mitogen-activated protein kinase 1 (BMK1) signaling pathway. J. Biol. Chem. 274:36305-36308. [DOI] [PubMed] [Google Scholar]
- 9.Chayama, K., P. J. Papst, T. P. Garrington, J. C. Pratt, T. Ishizuka, S. Webb, S. Ganiatsas, L. I. Zon, W. Sun, G. L. Johnson, and E. W. Gelfand. 2001. Role of MEKK2-MEK5 in the regulation of TNF-α gene expression and MEKK2-MKK7 in the activation of c-Jun N-terminal kinase in mast cells. Proc. Natl. Acad. Sci. USA 98:4599-4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, K., J. A. Vita, B. C. Berk, and J. F. Keaney. 2001. c-Jun N-terminal kinase activation by hydrogen peroxide in endothelial cells involves Src-dependent epidermal growth factor receptor transactivation. J. Biol. Chem. 276:16045-16050. [DOI] [PubMed] [Google Scholar]
- 11.Choi, Y. B., C. K. Kim, and Y. Yun. 1999. Lad, an adaptor protein interacting with the SH2 domain of p56lck, is required for T cell activation. J. Immunol. 163:5242-5249. [PubMed] [Google Scholar]
- 12.Dinev, D., B. W. M. Jordan, B. Neufeld, J. D. Lee, D. Lindemann, U. R. Rapp, and S. Ludwig. 2001. Extracellular signal regulated kinase 5 (ERK5) is required for the differentiation of muscle cells. EMBO Rep. 2:829-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.English, J. M., C. A. Vanderbilt, S. Xu, S. Marcus, and M. H. Cobb. 1995. Isolation of MEK5 and differential expression of alternatively spliced forms. J. Biol. Chem. 270:28897-28902. [DOI] [PubMed] [Google Scholar]
- 14.Gamou, S., and N. Shimizu. 1995. Hydrogen peroxide preferentially enhances the tyrosine phosphorylation of epidermal growth factor receptor. FEBS Lett. 357:161-164. [DOI] [PubMed] [Google Scholar]
- 15.Garrington, T. P., T. Ishizuka, P. J. Papst, K. Chayama, S. Webb, T. Yujiri, W. Sun, S. Sather, D. M. Russell, S. B. Gibson, G. Keller, E. W. Gelfand, and G. L. Johnson. 2000. MEKK2 gene disruption causes loss of cytokine production in response to IgE and c-Kit ligand stimulation of ES cell-derived mast cells. EMBO J. 19:5387-5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han, J., Y. Jiang, Z. Li, V. V. Kravchenko, and R. T. Ulevitch. 1997. Activation of the transcription factor MEF2C by the MAP kinase p38 in inflammation. Nature 386:296-299. [DOI] [PubMed] [Google Scholar]
- 17.Hollenberg, S. M., R. Sternglanz, P. F. Cheng, and H. Weintraub. 1995. Identification of a new family of tissue-specific basic helix-loop-helix proteins with a two-hybrid system. Mol. Cell. Biol. 15:3813-3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamakura, S., T. Moriguchi, and E. Nishida. 1999. Activation of the protein kinase ERK5/BMK1 by receptor tyrosine kinases. Identification and characterization of a signaling pathway to the nucleus. J. Biol. Chem. 274:26563-26571. [DOI] [PubMed] [Google Scholar]
- 19.Kato, Y., V. V. Kravchenko, R. I. Tapping, J. Han, R. J. Ulevitch, and J. D. Lee. 1997. BMK1/ERK5 regulates serum-induced early gene expression through transcription factor MEF2C. EMBO J. 16:7054-7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kato, Y., R. I. Tapping, S. Huang, M. H. Watson, R. J. Ulevitch, and J. D. Lee. 1998. Bmk1/Erk5 is required for cell proliferation induced by epidermal growth factor. Nature 395:713-716. [DOI] [PubMed] [Google Scholar]
- 21.Kato, Y., M. Zhao, A. Morikawa, T. Sugiyama, D. Chakravortty, N. Koide, T. Yoshida, R. I. Tapping, Y. Yang, T. Yokochi, and J. D. Lee. 2000. Big mitogen-activated kinase regulates multiple members of the MEF2 protein family. J. Biol. Chem. 275:18534-18540. [DOI] [PubMed] [Google Scholar]
- 22.Kitagawa, D., S. Tanemura, S. Ohata, N. Shimizu, J. Seo, G. Nishitai, T. Watanabe, K. Nakagawa, H. Kishimoto, T. Wada, T. Tezuka, T. Yamamoto, H. Nishina, and T. Katada. 2002. Activation of extracellular signal-regulated kinase by ultraviolet is mediated through Src-dependent epidermal growth factor receptor phosphorylation. J. Biol. Chem. 277:366-371. [DOI] [PubMed] [Google Scholar]
- 23.Kovary, K., and R. Bravo. 1991. The Jun and Fos protein families are both required for cell cycle progression in fibroblasts. Mol. Cell. Biol. 11:4466-4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leserer, M., A. Gschwind, and A. Ullrich. 2000. Epidermal growth factor receptor signal transduction. IUBMB Life 49:405-409. [DOI] [PubMed] [Google Scholar]
- 25.Liu, Y., A. Bishop, L. Witucki, B. Kraybill, E. Shimizu, J. Tsien, J. Ubersax, J. Blethrow, D. O. Morgan, and K. M. Shokat. 1999. Structural basis for selective inhibition of Src family kinases by PP1. Chem. Biol. 6:671-678. [DOI] [PubMed] [Google Scholar]
- 26.Luttrell, L. M., G. J. Della Rocca, T. van Biesen, D. K. Luttrell, and R. J. Lefkowitz. 1997. Gβγ subunits mediate Src-dependent phosphorylation of the epidermal growth factor receptor. J. Biol. Chem. 272:4637-4644. [DOI] [PubMed] [Google Scholar]
- 27.Maeda, T., M. Takekawa, and H. Saito. 1995. Activation of yeast PBS2 MAPKK by MAPKKKs or by binding of an SH3-containing osmosensor. Science 269:554-558. [DOI] [PubMed] [Google Scholar]
- 28.Marinissen, M. J., M. Chiariello, M. Pallante, and J. S. Gutkind. 1999. A network of mitogen-activated protein kinases link G protein-coupled receptors to the c-jun promoter. A role for c-Jun NH2 terminal kinase, p38s, and extracellular signal-regulated kinase 5. Mol. Cell. Biol. 19:4289-4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marti, F., N. H. Post, E. Chan, and P. D. King. 2001. A transcription function for the T cell-specific adaptor (TSAd) protein in T cells: critical role of the TSAd Src homology 2 domain. J. Exp. Med. 193:1425-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGlade, J., A. Cheng, G. Pelicci, P. G. Pelicci, and T. Pawson. 1992. Shc proteins are phosphorylated by regulated by the v-Src and v-Fps protein tyrosine kinases. Proc. Natl. Acad. Sci. USA 89:8869-8873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meves, A., S. N. Stock, A. Beyerle, M. R. Pittelkow, and D. Peus. 2001. H2O2 mediates oxidative stress-induced epidermal growth factor receptor phosphorylation. Toxicol. Lett. 122:205-214. [DOI] [PubMed] [Google Scholar]
- 32.Minamino, T., T. Yujiri, P. J. Papst, E. D. Chan, G. L. Johnson, and N. Terada. 1999. MEKK1 suppresses oxidative stress-induced apoptosis of embryonic stem cell-derived cardiac myocytes. Proc. Natl. Acad. Sci. USA 96:15127-15132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Molkentin, J. D., B. L. Black, J. F. Martin, and E. N. Olson. 1996. Mutational analysis of the DNA binding, dimerization, and transcriptional activation domains of MEF2C. Mol. Cell. Biol. 16:2627-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morin, S., F. Charron, L. Robitaille, and M. Nemer. 2000. GATA-dependent recruitment of MEF2 proteins to target promoters. EMBO J. 19:2046-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakanishi, O., F. Shibasaki, M. Hidaka, Y. Homma, and T. Takenawa. 1993. Phospholipase C-γ1 associates with viral and cellular Src kinases. J. Biol. Chem. 268:10754-10759. [PubMed] [Google Scholar]
- 36.Nicol, R. L., N. Frey, G. Pearson, M. Cobb, J. Richardson, and E. N. Olson. 2001. Activated MEK5 induces serial assembly of sarcomeres and eccentric cardiac hypertrophy. EMBO J. 20:2757-2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohmori, T., Y. Yatomi, Y. Wu, M. Osada, K. Satoh, and Y. Ozaki. 2001. Wheat germ agglutinin-induced platelet activation via platelet endothelial cell adhesion molecule-1: involvement of rapid phospholipase Cγ-2 activation by Src family kinases. Biochemistry 40:12992-13001. [DOI] [PubMed] [Google Scholar]
- 38.Okamoto, S., D. Krainc, K. Sherman, and S. A. Lipton. 2000. Antiapoptotic role of the p38 mitogen-activated protein kinase-myocyte enhancer factor 2 transcription factor pathway during neuronal differentiation. Proc. Natl. Acad. Sci. USA 97:7561-7566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ota, I. M., and A. Varshavsky. 1993. A yeast protein similar to bacterial two-component regulators. Science 262:566-569. [DOI] [PubMed] [Google Scholar]
- 40.Ouwens, D. M., D. S. Gomes de Mesquita, J. Dekker, and J. A. Maassen. 2001. Hyperosmotic stress activates the insulin receptor in CHO cells. Biochim. Biophys. Acta 1540:97-106. [DOI] [PubMed] [Google Scholar]
- 41.Park, D., Y. B. Choi, M.-K. Han, U.-H. Kim, J. Shin, and Y. Yun. 2001. Adaptor protein Lad relays PDGF signal to Grb2 in lung cells: a tissue-specific PDGF signal transduction. Biochem. Biophys. Res. Commun. 284:275-281. [DOI] [PubMed] [Google Scholar]
- 42.Pearson, G., F. Robinson, T. B. Bibbson, B.-E. Xu, M. Karandikar, K. Berman, and M. H. Cobb. 2001. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr. Rev. 22:153-183. [DOI] [PubMed] [Google Scholar]
- 43.Rajagopal, K., C. L. Sommers, D. C. Decker, E. O. Mitchell, U. Korthauer, A. I. Sperling, C. A. Kozak, P. E. Love, and J. A. Bluestone. 1999. RIBP, a novel Rlk/Txk- and Itk-binding adaptor protein that regulates T cell activation. J. Exp. Med. 190:1657-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosette, C., and M. Karin. 1996. Ultraviolet light and osmotic stress: activation of the JNK cascade through multiple growth factor and cytokine receptors. Science 274:1194-1197. [DOI] [PubMed] [Google Scholar]
- 45.Schaefer, B. C., M. F. Ware, P. Marrack, G. R. Fanger, J. W. Kappler, G. L. Johnson, and C. R. F. Monks. 1999. Live cell fluorescence imaging of T cell MEKK2: redistribution and activation in response to antigen stimulation of the T cell receptor. Immunity 11:411-421. [DOI] [PubMed] [Google Scholar]
- 46.Spurkland, A., J. E. Brinchmann, G. Markussen, F. Pedeutour, E. Munthe, T. Lea, F. Vartdal, and H.-C. Aasheim. 1998. Molecular cloning of a T cell-specific adaptor protein (TASd) containing a Src homology (SH) 2 domain and putative SH3 and phosphotyrosine binding sites. J. Biol. Chem. 273:4539-4546. [DOI] [PubMed] [Google Scholar]
- 47.Stover, D. R., M. Becker, J. Liebetanz, and N. B. Lydon. 1995. Src phosphorylation of the epidermal growth factor receptor at novel sites mediates receptor interaction with Src and P85 alpha. J. Biol. Chem. 270:15591-15597. [DOI] [PubMed] [Google Scholar]
- 48.Su, B., J. Cheng, J. Yang, and Z. Guo. 2001. MEKK2 is required for T-cell receptor signals in JNK activation and interleukin-2 gene expression. J. Biol. Chem. 276:14784-14790. [DOI] [PubMed] [Google Scholar]
- 49.Sun, W., K. Kesavan, B. C. Schaefer, T. P. Garrington, M. Ware, N. L. Johnson, E. W. Gelfand, and G. L. Johnson. 2001. MEKK2 associates with the adapter protein Lad/RIBP and regulates the MEK5-BMK1/ERK5 pathway. J. Biol. Chem. 276:5093-5100. [DOI] [PubMed] [Google Scholar]
- 50.Sun, W., S. Vincent, J. Settleman, and G. L. Johnson. 2000. MEK kinase 2 binds and activates protein kinase C-related kinase 2: bifurcation of kinase regulatory pathways at the level of a MAPK kinase kinase. J. Biol. Chem. 275:24421-24428. [DOI] [PubMed] [Google Scholar]
- 51.Sundvold, V., K. M. Torgersen, N. H. Post, F. Marti, P. D. King, J. A. Rottingen, A. Spurkland, and T. Lea. 2000. T cell-specific adapter protein inhibits T cell activation by modulating Lck activity. J. Immunol. 165:2927-2931. [DOI] [PubMed] [Google Scholar]
- 52.Takeyama, K., K. Dabbagh, J. J. Shim, T. Dao-Pick, I. F. Ueki, and J. A. Nadel. 2000. Oxidative stress causes mucin synthesis via transactivation of epidermal growth factor receptor: roles of neutrophils. J. Immunol. 164:1546-1552. [DOI] [PubMed] [Google Scholar]
- 53.Tirode, F., C. Malaguti, F. Romero, R. Attar, J. Camonis, and J. M. Egly. 1997. A conditionally expressed third partner stabilizes or prevents the formation of a transcriptional activator in a three-hybrid system. J. Biol. Chem. 272:22995-22999. [DOI] [PubMed] [Google Scholar]
- 54.Veri, M. C., K. E. DeBell, M. C. Seminario, A. DiBaldassarre, I. Reischl, R. Rawat, L. Graham, C. Noviello, B. L. Rellahan, S. Miscia, R. L. Wange, and E. Bonvini. 2001. Membrane raft-dependent regulation of phospholipase Cγ-1 activation in T lymphocytes. Mol. Cell. Biol. 21:6939-6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang, X., K. D. McCullough, T. F. Franke, and N. J. Holbrook. 2000. Epidermal growth factor receptor-dependent Akt activation by oxidative stress enhances cell survival. J. Biol. Chem. 275:14624-14631. [DOI] [PubMed] [Google Scholar]
- 56.Watson, F. L., H. M. Heerssen, A. Bhattacharyya, L. Klesse, M. Z. Lin, and R. A. Segal. 2001. Neurotrophins use the Erk5 pathway to mediate a retrograde survival response. Nat. Neurosci. 4:981-988. [DOI] [PubMed] [Google Scholar]
- 57.Weiss, A., G. Koretzky, R. C. Schatzman, and T. Kadlecek. 1991. Functional activation of the T-cell antigen receptor induces tyrosine phosphorylation of phospholipases C-gamma 1. Proc. Natl. Acad. Sci. USA 88:5484-5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu, L. W., L. D. Mayo, J. D. Dunbar, K. M. Kessler, O. N. Ozes, R. S. Warren, and D. B. Donner. 2000. VRAP is an adaptor protein that binds KDR, a receptor for vascular endothelial cell growth factor. J. Biol. Chem. 275:6059-6062. [DOI] [PubMed] [Google Scholar]
- 59.Yang, S.-H., A. Galanis, and A. D. Sharrocks. 1999. Targeting of p38 mitogen-activated protein kinases to MEF2 transcription factors. Mol. Cell. Biol. 19:4028-4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao, M., L. New, V. V. Kravchenko, Y. Kato, H. Gram, F. di Padova, E. N. Olson, R. J. Ulevitch, and J. Han. 1999. Regulation of the MEF2 family of transcription factors by p38. Mol. Cell. Biol. 19:21-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou, G., Z. Q. Bao, and J. E. Dixon. 1995. Components of a new human protein kinase signal transduction pathway. J. Biol. Chem. 270:12665-12669. [DOI] [PubMed] [Google Scholar]