Abstract
In plants, the role of mitogen-activated protein kinase (MAPK) in reactive oxygen species (ROS)–based signal transduction processes is elusive. Despite the fact that ROS can induce MAPK activation, no direct genetic evidence has linked ROS-induced MAPK activation with the hypersensitive response, a form of programmed cell death. In tobacco, the major ROS-induced MAPK is salicylate-induced protein kinase (SIPK). We found through gain-of-function and loss-of-function approaches that both overexpression and RNA interference–based suppression of SIPK render the plant sensitive to ROS stress. Transgenic lines overexpressing a nonphosphorylatable version of SIPK were not ROS sensitive. Analysis of the MAPK activation profiles in ROS-stressed transgenic and wild-type plants revealed a striking interplay between SIPK and another MAPK (wound-induced protein kinase [WIPK]) in the different kinotypes. During continuous ozone exposure, abnormally prolonged activation of SIPK was seen in the SIPK-overexpression genotype, without WIPK activation, whereas strong and stable activation of WIPK was observed in the SIPK-suppressed lines. Thus, one role of activated SIPK in tobacco cells upon ROS stimulation appears to be control of the inactivation of WIPK.
INTRODUCTION
Mitogen-activated protein kinase (MAPK) modules form a key part of the eukaryotic signal transduction network that links environmental inputs to a wide range of modifications of cellular functions, ranging from cell division to cell death. In plants, MAPK signaling has been implicated in defense against pathogens and herbivores, in cellular responses to auxin, abscisic acid, and other phytohormones, in cell cycle control, in the induction of programmed cell death, and in responses to abiotic stresses such as UV light and ozone (Zhang and Klessig, 1997; Kovtun et al., 1998; Romeis et al., 1999; Heimovaara-Dijkstra et al., 2000; Samuel et al., 2000; Nishihama et al., 2001; Yang et al., 2001; Miles et al., 2002).
A variety of stress responses have been found to involve the rapid activation of a specific subset of plant MAPKs, notably Arabidopsis MPK6 (Ichimura et al., 2000; Kovtun et al., 2000; Nühse et al., 2000; Yuasa et al., 2001) and its orthologs in other species, such as salicylic acid–induced protein kinase (SIPK) in tobacco (Zhang and Klessig, 1998a, 1998b; Romeis et al., 1999; Mikolajczyk et al., 2000; Samuel et al., 2000; Zhang et al., 2000) and salt stress-induced MAPK (SIMK) in alfalfa (Cardinale et al., 2000). Because many biotic and abiotic stressors (virus infection, treatment with microbial elicitors, wounding, and osmotic stress) elicit a very rapid oxidative burst in plant cells, the apparent convergence of disparate stress signals on this particular MAPK node may be related to the sensitive response of MPK6/SIPK to redox perturbation.
Exposure to ozone immediately creates an oxidizing environment in plant tissues and triggers an array of cellular responses, including the accumulation of antioxidants, elicitation of pathogenesis-related proteins, deposition of phenols, induction of ethylene synthesis, suppression of primary metabolic activities such as photosynthesis, and eventually cell death (Darrall, 1989; Schraudner et al., 1992; Conklin and Last, 1995; Sharma and Davis, 1997; Tuomainen et al., 1997). Ozone enters the plant mesophyll through the stomata and diffuses through inner air spaces. In the cell wall and plasmalemma, it is converted spontaneously to reactive oxygen species (ROS) by contact with either water or membrane components (Sharma and Davis, 1997). The ozone-induced cell death process is influenced by the interaction of multiple signaling molecules, including salicylic acid, jasmonic acid, and ethylene (Orvar and Ellis, 1997; Overmyer et al., 2000; Rao et al., 2000).
One of the earliest responses elicited by ozone and other ROS generators in plants is the activation of specific MAPKs (Samuel et al., 2000; Desikan et al., 2001). The primary ROS-activated tobacco MAPK has been identified as the 46-kD SIPK; a second MAPK, the 44-kD wound-induced protein kinase (WIPK), usually responds more weakly (Kumar and Klessig, 2000; Samuel et al., 2000).
The rapid activation of these MAPKs suggests that their action on downstream targets could be important for the modulation of the cellular response to increased oxidative damage, but direct evidence for that role is lacking in plants. No intracellular substrates have been identified for either SIPK or WIPK, nor have loss-of-function genotypes been assessed for their ability to control redox stress. Stable overexpression or suppression of SIPK or WIPK in transgenic tobacco apparently did not result in the alteration of its activity (Yang et al., 2001). By contrast, transient overexpression of SIPK or its upstream activator, NtMEK2, in an active form has been shown to lead to the activation of either SIPK or both SIPK and WIPK, with associated induction of defense genes and hypersensitive response (HR)–like cell death (Yang et al., 2001; Zhang and Liu, 2001). This finding suggests that SIPK may play a role as a positive regulator in the cell death pathway.
The previously reported inability to produce SIPK-suppressed lines, and the lack of phenotype or alteration of SIPK activity reported for overexpression lines (Yang et al., 2001), have suggested that the normal functioning of this kinase may be essential for cell survival. However, we report here, using RNA interference (RNAi) technology, the recovery and analysis of transgenic tobacco plants in which SIPK is either overexpressed ectopically or largely eliminated. These plants display distinctive ozone response phenotypes that confirm the importance of SIPK activation for the effective control of ROS damage and also reveal an unexpected interplay between the activities of SIPK and WIPK.
RESULTS
Infiltration of fully grown tobacco leaves with a suspension of Agrobacterium tumefaciens cells carrying a SIPK-FLAG overexpression construct resulted in the accumulation of the epitope-tagged SIPK protein in the infiltrated tissue within 48 h. In unstressed cells, endogenous SIPK was not phosphory-lated at the TXY motif found in the activation loop of the kinase, as indicated by the absence of any signal in the control lane of a protein gel blot (Figure 1C) prepared using an anti-pMAPK antibody that specifically recognized the doubly phosphorylated protein. In the infiltrated tissue, however, at least a portion of the pool of SIPK became activated by 48 h after infiltration, with even greater activation observed by 72 h. In the same period, the infiltrated zones showed signs of tissue collapse, and by 96 h, these zones became completely necrotic (Figure 1A).
Figure 1.
Post-Transcriptional Gene Silencing-Induced Suppression of Cell Death Triggered by Transient Overexpression of SIPK.
Coinfiltration with Agrobacterium containing the SIPK-FLAG construct along with the SIPK-RI construct inhibited the cell death process induced by transient overexpression of SIPK-FLAG alone (A). Protein samples extracted at different times after infiltration of the constructs were immunoblotted with either anti-FLAG antibody (B) or phospho-MAPK-specific antibody (anti-pERK) (C). −, +, ++, and +++ indicate the extent of visible lesions appearing in the infiltrated zones. EV, agrobacterium carrying empty vector.
When leaves were coinfiltrated with Agrobacterium carrying the SIPK-FLAG overexpression construct plus an RNAi construct that targeted SIPK, both expression and activation of SIPK-FLAG were suppressed completely (Figures 1B and 1C). The cell death induced by the overexpression of SIPK-FLAG in the infiltrated zones also was eliminated (Figure 1A).
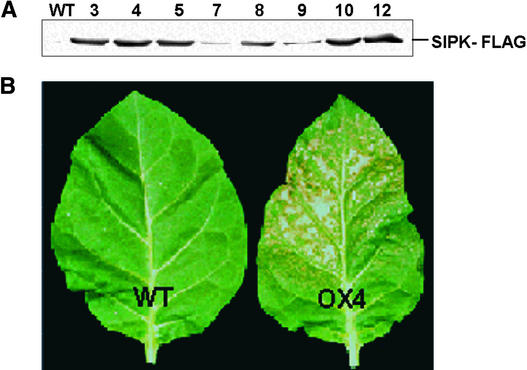
The cell death associated with the spontaneous activation of SIPK in overexpression (OX) transgenic cells suggested that it might be difficult to recover stably transformed lines using this construct, but cocultivation of tobacco leaf discs with the appropriate Agrobacterium culture and selection on kanamycin yielded a number of transgenic lines that were found to ectopically express a range of levels of SIPK-FLAG (Figure 2A). No spontaneous activation of SIPK was detected in these lines, all of which displayed normal growth and development phenotypes.
Figure 2.
Transgenic Tobacco Plants Overexpressing SIPK-FLAG Show Increased Ozone Sensitivity.
(A) Proteins (40 μg) extracted from leaves of the different OX lines ectopically expressing SIPK-FLAG were immunoblotted using anti-FLAG antibody.
(B) Transgenic tobacco line OX4 and wild-type tobacco (Xanthi-nc) plants (n = 25) were exposed to ozone (500 ppb) for 8 h. The treated leaves were photographed 24 h after exposure.
WT, wild type.
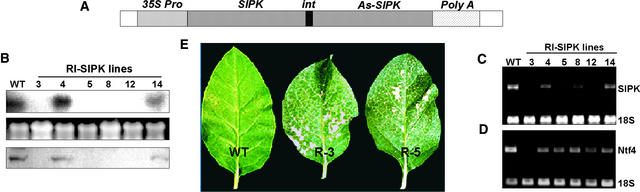
Transformation of tobacco leaf discs with the SIPK-RI construct also yielded stable transgenic lines, although with a sharply reduced frequency. In the recovered RI lines, silencing of endogenous SIPK expression was observed to varying degrees, ranging from partial reduction in both SIPK mRNA and protein to elimination of both products (Figures 3B and 3C). The specificity of this silencing was shown by the continued expression in most of the recovered RI lines of the closely related NTF4 MAPK gene, whose cDNA sequence is 89% identical to that of SIPK (Figure 3D). The RI lines again showed largely normal growth and development phenotypes, although the most severely suppressed lines showed some modest tendency to dwarfing (data not shown).
Figure 3.
SIPK-Suppressed Lines Also Are Sensitive to Ozone.
(A) RNAi construct under the control of the 35S promoter of Cauliflower mosaic virus.
(B) SIPK is suppressed in four of the six PCR-positive lines. RNA gel blot analysis was performed using total RNA (15 μg) extracted from wild-type and SIPK-RI lines and probed with the radiolabeled C-terminal fragment of the SIPK ORF. Autoradiography revealed essentially no SIPK mRNA in four of the six PCR-positive lines (top). Ethidium bromide staining of the gel showed equal loading of RNA (middle). Immunoblot analysis of protein samples from the same lines indicated the absence of detectable amounts of SIPK protein in all four SIPK-suppressed lines (bottom).
(C) Similar results were observed when reverse transcriptase–mediated PCR was conducted using SIPK-specific primers.
(D) NTF4 gene expression in the SIPK-suppressed lines was analyzed by reverse transcriptase–mediated PCR using gene-specific primers.
(E) SIPK-suppressed lines R3 and R5 display ozone-sensitive phenotypes. Plants (n = 15) of SIPK-suppressed tobacco transgenic lines R3 and R5, together with wild-type plants, were exposed to ozone (500 ppb) for 8 h per day for 2 days. The treated leaves were photographed 24 h after the end of 2 days of exposure.
WT, wild type.
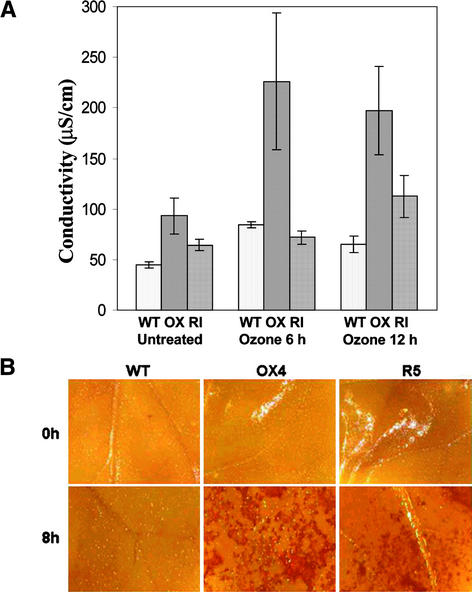
Plants of both the OX and RI lines showed no signs of spontaneous cell death under normal growth conditions. However, exposure of mature OX or RI leaves to levels of ozone that caused no visible injury to wild-type plants (500 parts per billion [ppb]) resulted in the rapid appearance of small necrotic lesions on leaves of both the transgenic genotypes (Figures 2B and 3E). The kinetics of this oxidative stress damage were quite different. Lesions consistently appeared on the leaves of OX plants as early as 4 to 6 h, but visually similar lesions only appeared on RI leaves ∼24 h later. In plants challenged with lower ozone concentrations (250 ppb), an analogous pattern was observed except that the necrotic responses were delayed until 48 h (OX) and 72 h (RI) (data not shown). When leaf discs prepared from the wild-type, OX, and RI genotypes were assayed for the loss of membrane integrity and associated ion leakage resulting from ozone exposure (500 ppb), differential timing of the damage response also was observed (Figure 4).
Figure 4.
Quantitation of Ozone-Induced Cell Death and Hydrogen Peroxide Accumulation in SIPK Kinotypes.
(A) Ion leakage from leaf discs (five each) of the third and fourth leaves of wild-type, OX4, and RI5 lines was assessed as an indicator of the loss of membrane integrity 6 and 12 h after the initiation of ozone exposure (500 ppb). The data presented are means and standard deviations from three independent experiments.
(B) 3,3′-Diaminobenzidine staining to detect hydrogen peroxide accumulation in ozone-treated leaves of SIPK kinotypes.
WT, wild type.
To assess in situ the relative levels of hydrogen peroxide accumulation induced by ozone exposure, control and ozone-treated leaf halves were infiltrated with 3,3′-diaminobenzidine solution. The staining patterns revealed no detectable levels of hydrogen peroxide in untreated leaves of any of the genotypes or in leaves of wild-type plants after 8 h of ozone exposure. However, strong 3,3′-diaminobenzidine staining was observed in both the OX and RI lines after ozone treatment (Figure 4B).
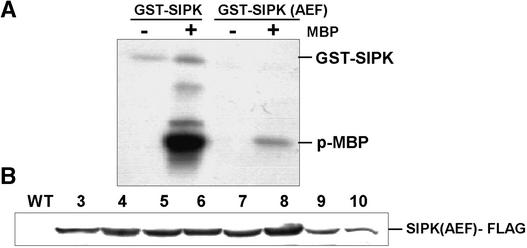
The observation that overexpression of SIPK-FLAG in infiltrated leaves was accompanied by the spontaneous activation of MAPK and by cell death raised the question of whether activation of the ectopically expressed protein was necessary for the induction of cell death. Therefore, site-directed mutagenesis was used to create a version of SIPK-FLAG in which the TEY motif found in the activation loop of SIPK had been converted to an AEF sequence. This modification yielded a kinase that retained a low level of basal activity when the recombinant protein was assayed in vitro against myelin basic protein (Figure 5A), but it could not be activated further through dual phosphorylation of the activation loop by upstream MAPK kinases. Unlike the SIPK-FLAG construct, when transiently expressed in tobacco leaves, the SIPK(AEF)-FLAG construct failed to cause cell death in the infiltrated zone (data not shown).
Figure 5.
Activity and Expression of Mutagenized SIPK.
(A) The recombinant SIPK activation loop mutant is less active than wild-type SIPK. Myelin basic protein (MBP)-phosphorylating activities of SIPK and SIPK(AEF) were measured by incubating recombinant proteins (5 μg) with 5 μg of MBP, as described in Methods. The phospho-MBP product was visualized through autoradiography after SDS-PAGE fractionation.
(B) SIPK(AEF) transgenic lines show high expression of the transgene product. Proteins (40 μg) extracted from the different lines overexpressing SIPK(AEF)-FLAG were fractionated by SDS-PAGE and immunoblotted using anti-FLAG antibody.
WT, wild type.
Stably transformed tobacco plants expressing high levels of SIPK(AEF)-FLAG also were recovered readily after Agrobacterium cocultivation, and these plants displayed no visibly altered phenotype. Despite accumulating similar levels of the epitope-tagged kinase (Figure 5B), the ozone sensitivity of these SIPK(AEF) transgenic lines did not differ from that of wild-type plants (data not shown). This finding indicates that the heightened ozone sensitivity observed in SIPK-OX transgenic lines requires not only that the ectopically expressed kinase be expressed at high levels within the plant cell but that it have the capacity to become activated.
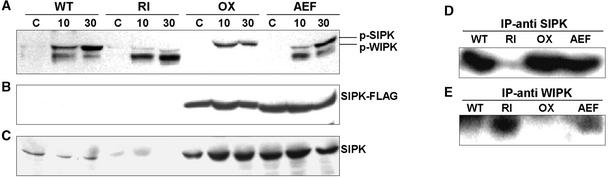
The activation status of both SIPK and WIPK in tobacco tissue extracts can be assessed either on protein gel blots using a phosphospecific antibody or by immunoprecipitation with antibodies that discriminate between SIPK and WIPK, followed by in gel or in vitro kinase activity assays. When the various transgenic and wild-type tobacco lines were monitored during a 30-min period of ozone exposure, striking differences in the pattern of kinase activation were observed among these genotypes (Figure 6).
Figure 6.
Differential Ozone-Induced Activation of SIPK and WIPK in SIPK Kinotypes.
(A) Crude protein extracts prepared from ozone-exposed tissues from T1 lines of the different SIPK kinotypes (RI5, OX4, and AEF8) and the wild type were resolved on a 10% polyacrylamide gel, blotted, and probed with an anti-phospho-ERK antibody to recognize phospho-MAPK forms.
(B) The same blot was probed subsequently using an anti-FLAG antibody to detect ectopic expression of the transgene product in the different kinotypes.
(C) A replicate protein gel blot was analyzed using a SIPK-specific antibody, revealing high expression of SIPK forms in the overexpressor lines and its absence in the SIPK-suppressed lines.
(D) and (E) Protein samples prepared from ozone-exposed (30 min) tissues from the different kinotypes were immunoprecipitated with either SIPK-specific (D) or WIPK-specific (E) antibodies. The immunoprecipitates were subjected to an in gel kinase assay, as described in Methods.
WT, wild type.
As reported previously (Samuel et al., 2000), ozone treatment led to the rapid activation of SIPK in leaves of wild-type plants. This was accompanied by a much weaker activation of the smaller kinase, WIPK (Figure 6A). In the OX4 genotype, ozone exposure also led to SIPK activation, but the level of activation appeared to be depressed relative to the wild-type response, despite the presence of far greater amounts of ectopically expressed SIPK in the OX cells (Figures 6B and 6C). No activation of WIPK was detected in the OX tissue samples.
The SIPK(AEF) genotype presented a kinase activation profile that was very similar to that of the wild type. This indicates that flooding the cell with a nonactivatable version of SIPK (a potential dominant-negative form) does not interfere with the ability of the upstream MAPK cascade elements to transmit oxidant-induced signals to their cognate MAPKs.
Exposure of the RI genotype to ozone, on the other hand, yielded a very different MAPK activation profile. Very weak or no SIPK activation was detected, as would be predicted for a genotype in which SIPK expression has been suppressed by post-transcriptional gene silencing (Figures 6A and 6C). Instead, ozone exposure produced strong and specific activation of WIPK. The identity of these highly activated kinases in ozone-treated leaves of each genotype was confirmed through immunoprecipitation of the 30-min ozone-treated protein extracts with either SIPK- or WIPK-specific antibodies, followed by in gel kinase assays (Figures 6D and 6E).
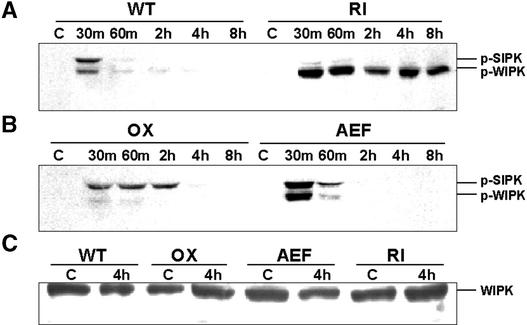
Aside from the unexpected massive activation of WIPK, the stability of that activation also was strikingly different in this genetic background. Normally, when oxidants trigger a rapid activation of SIPK, it is a transient response. The activation is effectively lost within 1 h, even under conditions of continuous oxidant stimulus, as seen in Figure 7A (wild-type lane). However, in the RI genotype, WIPK was not only activated rapidly but the pool of this MAPK remained continuously active for up to 8 h after the initiation of the response (Figure 7A, RI lane). Although normally there is far less WIPK than SIPK present in tobacco leaves (Zhang and Klessig, 1998b), the high activation signal observed in the RI tissue extracts did not appear to reflect increased levels of WIPK protein in this genotype compared with wild-type plants, as assessed by protein gel blot analysis (Figure 7C).
Figure 7.
Loss of SIPK Has Differential Effects on the Expression of Ozone-Induced Genes and Leads to the Hyperactivation of WIPK.
(A) and (B) Extended ozone exposure reveals strong and prolonged activation of WIPK in the RI line. A temporal profile of the phosphorylation status of SIPK and WIPK was generated through anti-pERK immunoblotting of crude proteins extracted from tissues of either wild-type and RI lines (A) or OX and AEF lines (B) exposed to ozone for different times (0 to 8 h).
(C) Alteration of SIPK does not lead to changes in the amount of WIPK. Protein extracts from untreated and 4-h ozone-treated tissues from different kinotypes were immunoblotted with anti-WIPK antibody.
WT, wild type.
Interestingly, kinase activation by ozone in the OX genotype also was prolonged abnormally, relative to that seen in ozone-treated wild-type plants, but in this case, the active kinase was SIPK rather than WIPK (Figure 7B). In addition, unlike the hyperactivated WIPK pool, the extended activation of SIPK in the OX line was more transient and disappeared within 4 h. This is approximately the time at which visible lesions began appearing on ozone-treated OX leaves.
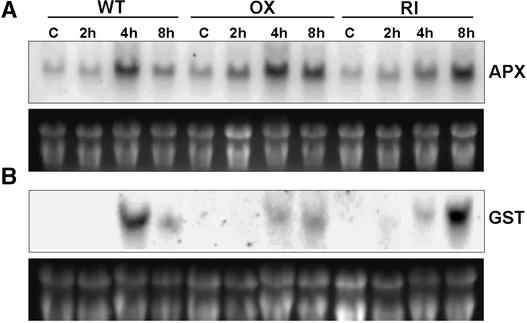
Examination of the temporal response of the two genes (GST [glutathione S-transferase] and cAPX [cytosolic ascorbate peroxidase]) whose expression was induced strongly by ozone treatment revealed that the loss of SIPK signaling in the RI genotype resulted in a delayed response in the expression of both genes. In the OX line, the prolonged activation of SIPK signaling resulted in the suppression of GST induction, whereas APX gene expression was unaffected (Figures 8A and 8B).
Figure 8.
Alteration of SIPK Signaling Affects the Expression of GST and cAPX.
RNA gel blot analysis of the accumulation of cAPX (A) and GST mRNA (B) in wild-type, SIPK-overexpressing, and SIPK-suppressed transgenic tobacco. Plants were exposed to ambient air (C) or 500 ppb of ozone for 2, 4, and 8 h, and total RNA was harvested from the third and fourth leaves.
WT, wild type.
DISCUSSION
Plant cells must deal constantly with ROS from a range of sources, including photooxidation, mitochondrial electron transport, flavin oxidase by-products, and environmental insults such as UV light, ozone, and ionizing radiation. Against this background, ROS pulses (“oxidative bursts”) also can occur within cells, usually as very early responses to localized challenges to cellular integrity such as wounding and pathogen assault. These pulses may serve in multiple functions, including activation of redox protection mechanisms, modulation of intracellular signal transduction pathways, and transmission of systemic signals to neighboring cells.
A severe oxidative challenge that overwhelms local protective measures ultimately will lead to cell death. The archetype for this outcome is the HR response induced during incompatible host–pathogen interactions. Similar lesions are induced by exposure to increased levels of ozone or UV light. The exact process by which cellular integrity fails is unclear, but the notion that HR represents a form of genetically programmed cell death is supported by the identification of numerous mutants affected in the process of lesion formation (Richberg et al., 1998).
The correlation of ROS pulses with the cell death process has been described extensively. Treatments such as chilling, wounding, pathogen infection, UV irradiation, and ozone exposure rapidly induce ROS accumulation in plant cells, followed later by lesion development. However, despite these correlative observations, a functional link between ROS accumulation and local lesion formation has yet to be defined.
It is striking that so many stresses that elicit ROS accumulation in plant cells consistently appear to activate MAPK modules as one of their earliest effects (Seo et al., 1995; Zhang and Klessig, 1998b; Allan et al., 2001; Desikan et al., 2001; Orozco-Cardenas et al., 2001). The MAPK observed most consistently to be activated by both applied stresses and ROS is SIPK in tobacco (Samuel et al., 2000; Miles et al., 2002) or its apparent orthologs in other species, such as MPK6 in Arabidopsis (Kovtun et al., 2000; Yuasa et al., 2001) and SIMK in alfalfa (Cardinale et al., 2000). This pattern suggests that SIPK activation might play an important role in determining the response and ultimate fate of the stressed cells.
Links between ROS-associated cell death and MAPK signaling have been reported for a number of nonplant systems. Hydrogen peroxide–induced cell death in cultured mammalian oligodendrocyte cells is inhibited by PD98059, a specific inhibitor of MEK, the upstream kinase of the ERK1/2 MAPK (Bhat and Zhang, 1999), whereas delayed and prolonged activation of p44 and p42 MAPKs is critical for genistein-induced programmed cell death in rat primary cortical neurons (Linford et al., 2001). Similarly, delayed and persistent activation of ERK1/2 is associated with glutamate-induced oxidative cytotoxicity in neuronal cell lines (Stanciu et al., 2000).
There also is evidence that ROS-activated MAPKs may play analogous roles in plant cells. Cell death induced in Arabidopsis cell suspension cultures by treatment with a bacterial elicitor (harpin) is inhibited when the cells are treated with the MEK inhibitor PD98059 (Desikan et al., 1999), whereas pretreatment of tobacco cells with staurosporine, a general protein kinase inhibitor, suppresses the cell death normally induced by exposure to fungal elicitors (Suzuki et al., 1999).
Genetic manipulation experiments also have implicated MAPK activation in the cell death process. In Arabidopsis plants overexpressing constitutively active forms of the MAPK kinases AtMEK4 and AtMEK5 under the control of an inducible promoter, HR-like lesions appeared after induction with dexamethasone, and lesion formation was preceded by the activation of endogenous MAPKs and the accumulation of hydrogen peroxide (Ren et al., 2002). Transient overexpression of a constitutively active form of a MAPK kinase (NtMEK2) in tobacco also led to the sustained activation of MAPKs, identified as SIPK and WIPK, and to the death of the infiltrated tissue (Yang et al., 2001). Transient overexpression of SIPK itself was shown subsequently to result in the formation of HR-like lesions, but only in young leaves (Zhang and Liu, 2001).
We have confirmed that ectopic SIPK overexpression leads to the appearance of high levels of the activated kinase in Agrobacterium-infiltrated tobacco tissue and to rapid cell death (Figure 1). On the other hand, when stably transformed tobacco plants were produced that overexpressed epitope-tagged SIPK (Figure 2), they displayed no visible phenotype. When exposed to ozone, however, the transgenic SIPK-OX plants proved to be much more sensitive than the nontransgenic parental line, indicating that ROS-induced cell death was controlled less effectively in the overexpression genotype.
Although this pattern is consistent with the results of NtMEK2 or SIPK-OX transient expression, its physiological relevance remains uncertain, because we know little about the effects of the accumulation of nonphysiological levels of active signal components on cellular function. To unambiguously identify a functional relationship between ROS activation of SIPK and ROS-induced cell death, we turned to the creation of defined loss-of-function mutants.
The modification of SIPK function in transgenic tobacco plants using either conventional gene-silencing methods (cosuppression and antisense-mediated suppression) or overexpression of dominant-negative forms proved ineffective (Yang et al., 2001) (data not shown). However, expression of an intron-containing “hairpin RNA” (Smith et al., 2000) designed to target a unique tract within the SIPK coding sequence yielded a number of transgenic plants in which SIPK expression was suppressed severely and specifically through post-transcriptional gene silencing. Loss of SIPK had no obvious phenotypic consequences for plants grown under normal greenhouse conditions.
Given the sensitivity of SIPK-OX lines to ozone, it might have been predicted that the absence of this kinase would have no effects, or perhaps even positive effects, on the ozone sensitivity of the SIPK-RI lines. Instead, after ozone treatments that induced no visible damage on wild-type plants, the SIPK-RI lines developed numerous lesions on their middle leaves within 24 h. Thus, the inability of the suppressed genotype to generate and activate SIPK compromises the cell's ability to manage ROS stress and to control cell death, although apparently on a different time scale from that observed in SIPK-OX plants.
Which facet of ROS-stress management has been compromised in SIPK-OX and SIPK-RI plants is not clear. No constitutive hydrogen peroxide accumulation was detected in any of the genotypes, suggesting that their heightened ozone sensitivity is not the consequence of a preexisting accumulation of ROS. Instead, it appears that alteration of the normal ozone-induced MAPK activation process, through either unregulated overexpression or suppression, creates an inability to cope with increased redox stress. Examination of the transcriptional activity of two genes whose mRNAs accumulate rapidly after ozone exposure showed that the response of both genes was affected differently (Figure 8).
Expression of cAPX, which encodes a major ROS-scavenging enzyme, was induced less effectively by ozone in RI plants, whereas it was unaffected in the OX line. Antisense suppression of cAPX was shown previously to create hypersensitivity to both ozone (Orvar and Ellis, 1997) and pathogens (Mittler et al., 1999) in transgenic tobacco plants. On the other hand, ozone-induced expression of GST, a general cellular protectant, was suppressed strongly in the OX line, but its expression was delayed markedly in the RI line. In Arabidopsis, both hydrogen peroxide and ozone induce GST expression (Clayton et al., 1999; Grant et al., 2000), and this expression has been demonstrated to require the activity of an unidentified 48-kD MAPK and calcium ion influx. Calcium channel activity also is essential for the ROS activation of SIPK in tobacco (Samuel et al., 2000).
The delayed response of the antioxidant genes in the RI line could result in increased early accumulation of ROS (Figure 4B), which could lead to a necrotic cell death process. In the OX line, although the cAPX gene response to ozone appeared to be normal, the antioxidant response clearly was unable to contain the increasing ROS levels associated with extended SIPK activation (Figure 4B). MAPK activation has been linked previously to increased ROS accumulation in Arabidopsis (Ren et al., 2002). A broader comparison of transcript profiles should generate useful insights into other connections between the transmission of redox signals by SIPK and the ability of the cell to avoid oxidative cell death.
Another aspect of the link between SIPK activation and cell death is revealed in the pattern of MAPK activation in ROS-stressed plants. The activation of SIPK by ozone occurred within 10 min in SIPK-OX plants but was not reversed for 4 h, by which time cell death already was becoming visible. This outcome is similar to the association of the prolonged activation of mammalian ERK with the induction of programmed cell death in neurons (Stanciu et al., 2000). Considered together with the results of the transient expression experiments, this finding demonstrates that the unregulated continuous activity of SIPK within plant cells profoundly affects normal homeostatic mechanisms.
The absence of SIPK in the SIPK-RI genotype also led to premature cell death under redox stress conditions, but in this case, the hyperactivated species observed was WIPK rather than SIPK. There have been other indications that WIPK plays a central role in plant stress signaling. This gene was identified originally on the basis of its rapid and transient induction upon wounding of tobacco leaves (Seo et al., 1995), and the gene product was shown later to be activated transiently by wounding (Seo et al., 1999) and by various other stresses (Romeis et al., 1999; Zhang et al., 2000). WIPK activation usually is accompanied by the activation of SIPK, but SIPK and WIPK do not always respond in unison; some oxidative stresses appear to activate SIPK preferentially and leave WIPK unaffected (Kumar and Klessig, 2000; Samuel et al., 2000).
WIPK activity, either alone or together with SIPK, has been suggested to be involved in the induction of cell death in cultured tobacco cells by specific fungal elicitor treatments (Zhang et al., 2000). Pretreatment of the elicited cells with staurosporine and K252A (protein kinase inhibitors) completely suppressed both WIPK activation and cell death. However, transient overexpression of WIPK did not result in its activation and failed to induce cell death in infiltrated tobacco leaves, unlike overexpression of SIPK (Zhang and Liu, 2001). In another study, the stable overexpression of WIPK in transgenic tobacco was accompanied by the constitutive expression of protease inhibitor II and the accumulation of methyl jasmonate (Seo et al., 1999), but the oxidative stress sensitivity of the WIPK-OX lines was not reported.
How SIPK elimination leads to the prolonged hyperactivation of WIPK is unknown, but various possibilities suggest themselves. If NtMEK2 is the sole upstream MAPK kinase responsible for the activation of both SIPK and WIPK, these two MAPKs may normally compete for binding to NtMEK2. However, basal levels of SIPK in unstimulated tobacco cells are much higher (10-fold) than those of WIPK (Zhang and Klessig, 1998b). In the absence of competition from SIPK, activation of WIPK by NtMEK2 activation in SIPK-RI cells may be much more efficient than usual. This scenario also might explain why WIPK remains largely inactivated in ozone-treated SIPK-OX tissues in which an excess of SIPK is present. However, although this model accounts for WIPK hyperactivation, it does not necessarily explain why that activation is prolonged abnormally.
Alternatively, one of the normal roles of activated SIPK may be the direct or indirect regulation of WIPK activity. Both dual-specificity phosphoprotein phosphatases (MKP) and Ser/Thr phosphatases have been implicated in inactivating MAPK pathways in mammalian and plant models (Brondello et al., 1997; Meskiene et al., 1998; Ulm et al., 2001; Westermarck et al., 2001). If SIPK activity is required for the induction or activation of a protein phosphatase that normally acts upon phospho-WIPK, the absence of SIPK from oxidant-stressed SIPK-RI cells would create a situation in which WIPK could be activated by its cognate MAPK kinase but could not be inactivated subsequently.
In this regard, it is interesting that Arabidopsis plants in which a dual-specificity phosphatase (AtMKP-1) has been mutated by T-DNA insertional mutagenesis display increased activation of an unidentified ∼49-kD MAPK and are more susceptible to ROS-generating stresses (e.g., UV light) (Ulm et al., 2001). On the other hand, MP2C, an alfalfa Ser/Thr phosphatase belonging to the PP2C class, has been shown to be a negative regulator of the MAPK pathway involving stress-activated MAPK, an apparent ortholog of WIPK (Meskiene et al., 1998). Both classes of protein phosphatase could be involved in cross-regulation mechanisms. Resolution of this question, and of the relative importance of the loss of SIPK activity versus the enhancement of WIPK activity in controlling oxidant-induced cell death, will require the development and analysis of other relevant single and multiple loss-of-function genotypes. These studies are now under way.
METHODS
Plant Material and Treatment
Tobacco (Nicotiana tabacum) plants of all genotypes were grown for 6 weeks in soil under controlled environmental conditions (25/20°C, 16-h-light/8-h-dark cycle) and then exposed to ozone (500 parts per billion) and harvested as described previously (Orvar and Ellis, 1997).
Recombinant Protein Production
The open reading frame (ORF) of salicylate-induced protein kinase (SIPK) was amplified by reverse transcriptase–mediated (RT) PCR using gene-specific primers and RNA isolated from untreated leaves of tobacco cv Xanthi-nc. The amplicon was cloned in frame into the expression vector pGEX 4T-3. Mutations in the activation loop of SIPK were introduced using a PCR-mediated approach, taking advantage of the unique NheI restriction site close to the activation loop. The mutational primers were designed so that the mutant form would code for AEF instead of TEY at amino acid positions Thr-218 and Tyr-220.
The SIPK(AEF) gene construct then was cloned into pGEX 4T-3. The recombinant glutathione S-transferase (GST) fusion proteins were expressed in Escherichia coli BL21 cells by induction with 0.1 mM isopropylthio-β-galactoside for 4 h at 25°C, followed by purification according to the manufacturer's protocol (Amersham Pharmacia). The different constructs were sequenced to confirm the changes and the absence of mismatches.
Intron-Spliced Hairpin Loop RNA–SIPK Construct
The double-stranded RNA interference construct was tailored through a PCR-mediated approach using the N-terminal sequence of the SIPK ORF. A minimal intron based on the splice junctions and flanking regions of the fourth intron of AtMPK6 (the Arabidopsis ortholog of SIPK) was incorporated into the sense-strand primer. The sense strand then was amplified using a primer combination that generated an EcoRI cleavage site and intron-XbaI sequence on the opposite ends of the product, whereas the antisense strand was amplified using a primer combination that added BamHI and XbaI sites on the opposite ends of the product. These two products were directionally cloned into EcoRI-BamHI–processed Bin19/pRT101 through a triple ligation, which placed the RNA interference construct under the control of the 35S promoter of Cauliflower mosaic virus (Figure 3A).
Binary Vector Construction and Plant Transformation
The different SIPK overexpression constructs were tagged with a C-terminal FLAG epitope through a PCR-mediated approach, followed by ligation into the plant expression vector Bin19/pRT101, which contains an nptII-selectable marker. All of the constructs were sequenced to confirm the presence of appropriate changes. The recombinant binary vector was used to transform competent Agrobacterium tumefaciens (EHA105) cells by a freeze-thaw transformation procedure.
Agrobacterium-mediated transformation of tobacco (cv Xanthi-nc) was performed using a leaf disc cocultivation procedure. Transformants were selected on half-strength Murashige and Skoog (1962) culture medium containing 50 mg/L kanamycin. Surviving plantlets were screened by PCR using 35S forward and gene-specific reverse primer combinations. Positive transformants then were screened by protein gel blot analysis (see below) using an anti-FLAG antibody for the SIPK overexpression lines and anti-SIPK antibodies to assess the RI suppression lines.
The confirmed transgenic lines were transferred to soil and grown to maturity, and seeds were collected. The T1 seeds were germinated on half-strength Murashige and Skoog (1962) medium with 50 mg/L kanamycin, and antibiotic-resistant plants were transferred to soil and grown under controlled conditions.
Transient Transformation Using Agrobacterium Infiltration
Four- to 6-week-old wild-type tobacco plants (cv Xanthi-nc) were used for infiltration experiments as described previously (Yang et al., 2001). This involved leaf infiltration with a mixed culture (OD of 0.4 at 600 nm) of Agrobacterium EHA105 containing the SIPK-FLAG overexpression construct plus an equal population of Agrobacterium containing either the empty vector or the SIPK-RI construct. At the indicated times (Figure 1), the infiltrated area was cut from the leaf, frozen in liquid nitrogen, and stored at −80°C until further analysis.
RNA Gel Blot and RT-PCR Analysis
Total RNA (15 μg) was resolved on 1% agarose-formaldehyde gels, blotted, and probed as described previously (Orvar and Ellis, 1997). The 600-bp C-terminal fragment of the SIPK ORF and PCR-amplified fragments of the cytosolic ascorbate peroxidase (cAPX) and GST were used as probes. Gene-specific primers were used to amplify the ORFs of cAPX (forward, 5′-AGAACAATTGCTATGGGTAAGTG-3′; reverse, 5′-GCAAGCTTAAGCTTCAGCAAAT-3′) and GST (forward, 5′-ATGGCGATCAAAGTCCATGGTA-3′; reverse, 5′-TTTTTGCAG-CTTCTCCAATCCC-3′) using cDNA as the template.
The cDNA was synthesized from total RNA extracted from control and ozone-exposed tissues of the different genotypes/treatments using a first-strand cDNA synthesis kit (Invitrogen, Carlsbad, CA). RT-PCR was performed using gene-specific primers designed to target either SIPK (25 cycles) or NTF4 (30 cycles). The number of cycles was adjusted so that the amplification was within the linear range. As an internal control, 18S ribosomal cDNA was amplified using a 1:4 ratio of 18S-specific primers to competitor's DNA fragments provided by Ambion (Austin, TX).
Protein Extraction and Protein Gel Blot Analysis
Total protein extracts were prepared (40 to 80 μg) and used for protein gel blot analysis as described previously (Samuel et al., 2000). A primary antibody dilution of 1:1000 was used for anti-pERK (New England Biolabs, Beverly, MA), and a dilution of 1:5000 was used for anti-SIPK, anti–wound-induced protein kinase (WIPK) (Seo et al., 1999; Y. Ohashi, personal communication), and anti-FLAG (Sigma) antibodies.
Immune Complex Kinase Assay
Immunoprecipitations were performed as described previously (Samuel et al., 2000) using 250 μg of extracted protein together with 5 μg of either anti-SIPK or anti-WIPK antibodies. The immunoprecipitates were analyzed in an in gel kinase assay as described previously using myelin basic protein as the substrate (Zhang and Klessig, 1997).
In Vitro Kinase Assays
GST fusion proteins (5 μg) of the wild-type and mutant SIPK(AEF) were incubated with 5 μg of myelin basic protein and 10 μCi of γ-32P– labeled ATP (>5000 Ci/mmol) (Amersham Pharmacia) in a 20-μL reaction mixture (20 mM Hepes, pH 7.5, 5 mM MgCl2, 1 mM EGTA, 5 mM β-mercaptoethanol, 2 mM Na3VO4, and 20 mM β-glycerophosphate) at 30°C for 30 min. The reaction was stopped with 6 × SDS loading buffer, and the samples were resolved on a 15% polyacrylamide gel, blotted onto a nylon membrane, and visualized by autoradiography.
Ion-Leakage Assay
Five leaf discs (9 mm) were cut from each of the third and fourth leaves of ozone-exposed and untreated plants of the wild type, OX4, and RI5 lines. The 10 leaf discs were incubated in 5 mL of deionized water at 25°C on a gyratory shaker at 110 rpm for 4 h, and the conductivity of the solution was measured as described previously (Mittler et al., 1999).
In Situ Staining for Hydrogen Peroxide
Hydrogen peroxide was visualized in situ by 3,3′-diaminobenzidine staining performed essentially according to Torres et al. (2002). Leaf halves were collected after 8 h of ozone exposure (500 parts per billion) and vacuum infiltrated with the 3,3′-diaminobenzidine (1 mg/mL) solution. Infiltrated leaves were placed under high humidity until brown precipitation was observed (5 to 6 h) and then fixed with a solution of ethanol:lactic acid:glycerol (3:1:1, v/v) for 2 days, followed by further clearing in methanol. Unless indicated otherwise, all experiments were repeated with consistent results.
Upon request, all novel materials described in this article will be made available in a timely manner for noncommercial research purposes. No restrictions or conditions will be placed on the use of any materials described in this article that would limit their use for noncommercial research purposes.
Acknowledgments
We thank Y. Ohashi for providing the anti-WIPK and anti-SIPK antibodies and H. Hall for assistance with the 3,3′-diaminobenzidine staining experiments. Funding for this research was provided by the Natural Sciences and Engineering Research Council of Canada.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.002337.
References
- Allan, A.C., Lapidot, M., Culver, J.N., and Fluhr, R. (2001). An early tobacco mosaic virus-induced oxidative burst in tobacco indicates extracellular perception of the virus coat protein. Plant Physiol. 126, 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat, N.R., and Zhang, P. (1999). Hydrogen peroxide activation of multiple mitogen-activated protein kinases in an oligodendrocyte cell line: Role of extracellular signal-regulated kinase in hydrogen peroxide-induced cell death. J. Neurochem. 72, 112–119. [DOI] [PubMed] [Google Scholar]
- Brondello, J.M., Brunet, A., Pouyssegur, J., and McKenzie, F.R. (1997). The dual specificity mitogen-activated protein kinase phosphatase-1 and -2 are induced by the p42/p44 MAPK cascade. J. Biol. Chem. 272, 1368–1376. [DOI] [PubMed] [Google Scholar]
- Cardinale, F., Jonak, C., Ligterink, W., Niehaus, K., Boller, T., and Hirt, H. (2000). Differential activation of four specific MAPK pathways by distinct elicitors. J. Biol. Chem. 275, 36734–36740. [DOI] [PubMed] [Google Scholar]
- Clayton, H., Knight, M.R., Knight, H., McAinsh, M.R., and Hetherington, A.M. (1999). Dissection of the ozone-induced calcium signature. Plant J. 17, 575–579. [DOI] [PubMed] [Google Scholar]
- Conklin, P.L., and Last, R.L. (1995). Differential accumulation of antioxidant mRNAs in Arabidopsis thaliana exposed to ozone. Plant Physiol. 109, 203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrall, N.M. (1989). The effect of air pollutants on physiological processes in plants. Plant Cell Environ. 12, 1–30. [Google Scholar]
- Desikan, R., Clarke, A., Atherfold, P., Hancock, J.T., and Neill, S.J. (1999). Harpin induces mitogen-activated protein kinase activity during defence responses in Arabidopsis thaliana suspension cultures. Planta 210, 97–103. [DOI] [PubMed] [Google Scholar]
- Desikan, R., Hancock, J.T., Ichimura, K., Shinozaki, K., and Neill, S.J. (2001). Harpin induces activation of the Arabidopsis mitogen-activated protein kinases AtMPK4 and AtMPK6. Plant Physiol. 126, 1579–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant, J.J., Yun, B.W., and Loake, G.J. (2000). Oxidative burst and cognate redox signalling reported by luciferase imaging: Identification of a signal network that functions independently of ethylene, SA and Me-JA but is dependent on MAPKK activity. Plant J. 24, 569–582. [DOI] [PubMed] [Google Scholar]
- Heimovaara-Dijkstra, S., Testerink, C., and Wang, M. (2000). Mitogen-activated protein kinase and abscisic acid signal transduction. Results Probl. Cell Differ. 27, 131–144. [DOI] [PubMed] [Google Scholar]
- Ichimura, K., Mizoguchi, T., Yoshida, R., Yuasa, T., and Shinozaki, K. (2000). Various abiotic stresses rapidly activate Arabidopsis MAP kinases ATMPK4 and ATMPK6. Plant J. 24, 655–665. [DOI] [PubMed] [Google Scholar]
- Kovtun, Y., Chiu, W.-L., Tena, G., and Sheen, J. (2000). Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc. Natl. Acad. Sci. USA 97, 2940–2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovtun, Y., Chiu, W.L., Zeng, W., and Sheen, J. (1998). Suppression of auxin signal transduction by a MAPK cascade in higher plants. Nature 395, 716–720. [DOI] [PubMed] [Google Scholar]
- Kumar, D., and Klessig, D.F. (2000). Differential induction of tobacco MAP kinases by the defense signals nitric oxide, salicylic acid, ethylene, and jasmonic acid. Mol. Plant-Microbe Interact. 13, 347–351. [DOI] [PubMed] [Google Scholar]
- Linford, N.J., Yang, Y., Cook, D.G., and Dorsa, D.M. (2001). Neuronal apoptosis resulting from high doses of the isoflavone genistein: Role for calcium and p42/44 mitogen-activated protein kinase. J. Pharmacol. Exp. Ther. 299, 67–75. [PubMed] [Google Scholar]
- Meskiene, I., Bogre, L., Glase, W., Balog, J., Brandstotter, M., Zwerger, K., Ammerer, G., and Hirt, H. (1998). MP2C, a plant protein phosphatase 2C, functions as a negative regulator of mitogen-activated protein kinase pathways in yeast and plants. Proc. Natl. Acad. Sci. USA 95, 1938–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikolajczyk, M., Awotunde, O.S., Muszynska, G., Klessig, D.F., and Dobrowolska, G. (2000). Osmotic stress induces rapid activation of a salicylic acid-induced protein kinase and a homolog of protein kinase ASK1 in tobacco cells. Plant Cell 12, 165–178. [PMC free article] [PubMed] [Google Scholar]
- Miles, G.P., Samuel, M.A., and Ellis, B.E. (2002). Suramin inhibits oxidant-induced MAPK signalling in plants. Plant Cell Environ. 25, 521–527. [Google Scholar]
- Mittler, R., Herr, E.H., Orvar, B.L., van Camp, W., Willekens, H., Inze, D., and Ellis, B.E. (1999). Transgenic tobacco plants with reduced capability to detoxify reactive oxygen intermediates are hyperresponsive to pathogen infection. Proc. Natl. Acad. Sci. USA 96, 14165–14170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15, 473–497. [Google Scholar]
- Nishihama, R., Ishikawa, M., Araki, S., Soyano, T., Asada, T., and Machida, Y. (2001). The NPK1 mitogen-activated protein kinase kinase kinase is a regulator of cell-plate formation in plant cytokinesis. Genes Dev. 15, 352–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nühse, T.S., Peck, S.C., Hirt, H., and Boller, T. (2000). Microbial elicitors induce activation and dual phosphorylation of the Arabidopsis thaliana MAPK 6. J. Biol. Chem. 17, 7521–7526. [DOI] [PubMed] [Google Scholar]
- Orozco-Cardenas, M., Narvaez-Vasquez, J., and Ryan, C. (2001). Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. Plant Cell 13, 179–191. [PMC free article] [PubMed] [Google Scholar]
- Orvar, B.L., and Ellis, B.E. (1997). Transgenic tobacco plants expressing antisense RNA for cytosolic ascorbate peroxidase show increased susceptibility to ozone injury. Plant J. 11, 1297–1305. [Google Scholar]
- Overmyer, K., Tuominen, H., Kettunen, R., Betz, C., Langebartels, C., Sandermann, H., Jr., and Kangasjärvi, J. (2000). Ozone-sensitive Arabidopsis rcd1 mutant reveals opposite roles for ethylene and jasmonate signaling pathways in regulating superoxide-dependent cell death. Plant Cell 12, 1849–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao, M.V., Lee, H., Creelman, R.A., Mullet, J.E., and Davis, K.R. (2000). Jasmonic acid signaling modulates ozone-induced hypersensitive cell death. Plant Cell 12, 1633–1646.11006337 [Google Scholar]
- Ren, D., Yang, H., and Zhang, S. (2002). Cell death mediated by MAPK is associated with hydrogen peroxide production in Arabidopsis. J. Biol. Chem. 277, 559–565. [DOI] [PubMed] [Google Scholar]
- Richberg, M.H., Aviv, D.H., and Dangl, J.L. (1998). Dead cells do tell tales. Curr. Opin. Plant Biol. 1, 480–485. [DOI] [PubMed] [Google Scholar]
- Romeis, T., Piedras, P., Zhang, S., Klessig, D.F., Hirt, H., and Jones, J. (1999). Rapid Avr9- and Cf-9-dependent activation of MAP kinases in tobacco cell cultures and leaves: Convergence of resistance gene, elicitor, wound, and salicylate responses. Plant Cell 11, 273–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel, M.A., Miles, G.P., and Ellis, B.E. (2000). Ozone treatment rapidly activates MAP kinase signalling in plants. Plant J. 22, 367–376. [DOI] [PubMed] [Google Scholar]
- Schraudner, M., Ernst, D., Langebartels, C., and Sandermann, H. (1992). Biochemical responses to ozone. III. Activation of the defense-related proteins β-1,3-glucanase and chitinase in tobacco leaves. Plant Physiol. 99, 1321–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo, S., Okamoto, M., Seto, H., Ishizuka, K., Sano, H., and Ohashi, Y. (1995). Tobacco MAP kinase: A possible mediator in wound signal transduction pathways. Science 270, 1988–1992. [DOI] [PubMed] [Google Scholar]
- Seo, S., Sano, H., and Ohashi, Y. (1999). Jamonate-based wound signal transduction requires activation of WIPK, a tobacco mitogen-activated protein kinase. Plant Cell 11, 289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, Y.K., and Davis, K.R. (1997). The effects of ozone on antioxidant responses in plants. Free Radical Biol. Med. 23, 480–488. [DOI] [PubMed] [Google Scholar]
- Smith, N.A., Singh, S.P., Wang, M.B., Stoutjesdijk, P.A., Green, A.G., and Waterhouse, P.M. (2000). Total silencing by intron-spliced hairpin RNAs. Nature 407, 319–320. [DOI] [PubMed] [Google Scholar]
- Stanciu, M., Wang, Y., Kentor, R., Burke, N., Watkins, S., Kress, G., Reynolds, I., Klann, E., Angiolieri, M.R., Johnson, J.W., and DeFranco, D.B. (2000). Persistent activation of ERK contributes to glutamate-induced oxidative toxicity in a neuronal cell line and primary cortical neuron cultures. J. Biol. Chem. 275, 12200–12206. [DOI] [PubMed] [Google Scholar]
- Suzuki, K., Yano, A., and Shinshi, H. (1999). Slow and prolonged activation of the p47 protein kinase during hypersensitive cell death in a culture of tobacco cells. Plant Physiol. 119, 1465–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres, M.A., Dangl, J.L., and Jones, J.D.G. (2002). Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc. Natl. Acad. Sci. USA 99, 517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomainen, J., Betz, C., Kangasjärvi, J., Ernst, D., Yin, Z., Langebartels, C., and Sandermann, H. (1997). Ozone induction of ethylene emission in tomato plants: Regulation by differential transcript accumulation for the biosynthetic enzymes. Plant J. 12, 1151–1162. [Google Scholar]
- Ulm, R., Revenkova, E., di Sansebastiano, G.P., Bechtold, N., and Paszkowski, J. (2001). Mitogen-activated protein kinase phosphatase is required for genotoxic stress relief in Arabidopsis. Genes Dev. 15, 699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermarck, J., Li, S.P., Kallunki, T., Han, J., and Kahari, V.M. (2001). p38 mitogen-activated protein kinase-dependent activation of protein phosphatases 1 and 2A inhibits MEK1 and MEK2 activity and collagenase 1 (MMP-1) gene expression. Mol. Cell. Biol. 21, 2373–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, K.Y., Liu, Y., and Zhang, S. (2001). Activation of a mitogen-activated protein kinase pathway is involved in disease resistance in tobacco. Proc. Natl. Acad. Sci. USA 98, 741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuasa, T., Ichimura, K., Mizoguchi, T., and Shinozaki, K. (2001). Oxidative stress activates ATMPK6, an Arabidopsis homologue of MAP kinase. Plant Cell Physiol. 42, 1012–1016. [DOI] [PubMed] [Google Scholar]
- Zhang, S., and Klessig, D.F. (1997). Salicylic acid activates a 48-kD MAP kinase in tobacco. Plant Cell 9, 809–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S., and Klessig, D.F. (1998. a). Resistance gene N-mediated de novo synthesis and activation of a tobacco mitogen-activated protein kinase by tobacco mosaic virus infection. Proc. Natl. Acad. Sci. USA 95, 7433–7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S., and Klessig, D.F. (1998. b). The tobacco wounding-activated mitogen-activated protein kinase is encoded by SIPK. Proc. Natl. Acad. Sci. USA 95, 7225–7230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S., and Liu, Y. (2001). Activation of salicylic acid-induced protein kinase, a mitogen-activated protein kinase, induces multiple defense responses in tobacco. Plant Cell 13, 1877–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S., Liu, Y., and Klessig, D.F. (2000). Multiple levels of tobacco WIPK activation during the induction of cell death by fungal elicitins. Plant J. 23, 339–347. [DOI] [PubMed] [Google Scholar]