Abstract
Intercellular communication is essential for differentiation and development. In plants, plasmodesmata (PD) form cytoplasmic channels for direct communication. During plant development, programmed reduction in PD number and transport capacity creates the so-called symplasmic domains. Small fluorescent dyes and ions can diffuse among cells within a domain but not across domain boundaries. Such symplasmic isolation is thought to allow groups of cells to differentiate and develop into tissues with distinct structures and functions. Whether or how “symplasmically isolated” cells communicate with one another is poorly understood. One well-documented symplasmic domain is the sieve element–companion cell (SE-CC) complex in the phloem tissue. We report here that, when produced in the CC of transgenic tobacco, the 3a movement protein (3a MP) of Cucumber mosaic virus fused to green fluorescent protein (GFP) can traffic out of the SE-CC complex via PD. The extent of 3a MP:GFP traffic across the boundary between vascular and nonvascular tissues depends on organ type and developmental stage. Our findings provide experimental evidence that endogenous machinery exists for protein traffic between the symplasmically isolated SE-CC complex and neighboring cells. We suggest that PD-mediated traffic of selected macromolecules can be a mechanism for symplasmically isolated cells to communicate with one another.
INTRODUCTION
Plasmodesmata (PD) are cytoplasmic channels for direct communication between plant cells. In a young embryo, all cells are connected by PD, as demonstrated by structural (Schulz and Jensen, 1968; Mansfield and Briarty, 1991) and dye-coupling (McLean et al., 1997) studies. During organogenesis, cells differentiate to form specialized tissues with distinct functions. Significantly, plasmodesmal connections undergo dramatic changes in structure and function, resulting in the formation of symplasmic domains (Erwee and Goodwin, 1983; Van der Schoot and Van Bel, 1990).
Changes in symplasmic connectivity, as monitored by dye coupling, are correlated closely with specific developmental and cellular processes. Examples include symplasmic isolation of inflorescence meristems or young primordia before flowering initiation and release of this isolation later in development in Arabidopsis (Gisel et al., 1999), isolation of cotton fibers during rapid elongation and subsequent release of isolation (Ruan et al., 2001), and synchronization of mitosis between symplasmically connected cultured cells (Ehlers and Kollmann, 2000).
Symplasmic isolation may allow groups of cells to pursue distinct developmental pathways (Lucas et al., 1993; McLean et al., 1997; Ding et al., 1999; Zambryski and Crawford, 2000; Pfluger and Zambryski, 2001). On the other hand, coordinated differentiation of cells is essential for the formation of a functional plant body. How symplasmically isolated cells communicate with their neighbors is virtually unknown. One possibility is by signaling across the cell walls mediated by secreted ligands and extracellular receptors (Clark, 2001). Another possibility is by regulated symplasmic traffic of selective molecules via PD.
An important symplasmic domain is the sieve element–companion cell (SE-CC) complex in the phloem tissue. Phloem transport mediated by the SE-CC complex is essential to many plant functions, including photoassimilate partitioning, flower development, and defense signaling, and to viral movement (Bernier et al., 1993; Carrington et al., 1996; Ding, 1998; Nelson and Van Bel, 1998; Thompson and Schulz, 1999; Crawford and Zambryski, 2000; Oparka and Santa Cruz, 2000; Ruiz-Medrano et al., 2001). Entering and exiting the SE-CC complex are two control points for long-distance transport of a cargo that is originated in and/or destined for other cell types. During leaf maturation in many plant species, PD frequencies between the SE-CC complex and neighboring cells in minor veins decrease dramatically (Gamalei, 1989; Grusak et al., 1996; Turgeon et al., 2001).
The few PD connecting the SE-CC complex to neighboring cells appear to be nonfunctional for photoassimilate transport. As a result, photoassimilate derived from the mesophyll moves via PD to phloem parenchyma and is taken up subsequently by the SE-CC complex apoplastically via membrane-localized sugar transporters (Von Schaewen et al., 1990; Bush, 1993; Sauer et al., 1994; Grusak et al., 1996). Dye- and electrical-coupling experiments have demonstrated directly the symplasmic isolation of the SE-CC complex from surrounding cells in the transport phloem of stems, petioles, hypocotyls, and roots in many species (Van der Schoot and Van Bel, 1989, 1990; Van Bel and Kempers, 1991; Oparka et al., 1994; Van Bel and Van Rijen, 1994; Wright and Oparka, 1997; Knoblauch and Van Bel, 1998). Such isolation may result from the closure of PD via an energy-dependent mechanism (Wright and Oparka, 1997) and is essential to maintain the high turgor pressure in the SE-CC complex to drive long-distance transport (Oparka and Turgeon, 1999).
Symplasmic isolation of the SE-CC complex raises questions regarding how this complex communicates with neighboring cells, how various transport cargos enter and exit this complex, and why a plant keeps PD at the interface between this complex and surrounding cells if PD are not functional for transport. Keeping these PD closed constantly by consumption of energy certainly is not economical for a plant (Oparka and Turgeon, 1999). Therefore, the presence of PD at this interface, albeit at low frequency in many plant species, may serve certain functions that are indispensable for a plant.
Despite the symplasmic isolation of the SE-CC complex, plant viruses can enter and exit this complex to spread systemically (Carrington et al., 1996; Gilbertson and Lucas, 1996; Ghoshroy et al., 1997; Nelson and Van Bel, 1998). Intercellular viral movement is facilitated by the viral movement protein (MP) and other viral proteins as well, depending on the viruses. This fact suggests that these viral proteins traffic across the boundary between the SE-CC complex and the surrounding cells (Oparka and Turgeon, 1999; Oparka and Santa Cruz, 2000). However, experimental evidence for such traffic is not available.
In this study, we generated transgenic tobacco expressing the 3a MP of Cucumber mosaic virus (CMV) fused to green fluorescent protein (GFP) under the control of the CC-specific promoter of Commelina yellow mottle virus (CoYMV) (Matsuda et al., 2002). We found that, in the absence of viral replication, the fusion protein trafficked from CC to SE and further from the “symplasmically isolated” SE-CC complex into neighboring cells. This traffic is mediated specifically by 3a MP and occurs via PD. Further traffic of the fusion protein into nonvascular tissues is regulated by factors associated with organ type and developmental stage. Our data provide experimental evidence that selective proteins can traffic between symplasmically isolated cells. We present our findings and discuss their biological implications.
RESULTS
The SE-CC Complex in Tobacco Stem and Mature Leaves Is Isolated Symplasmically
To confirm that the SE-CC complex is isolated symplasmically in mature organs of tobacco, we performed dye-coupling studies using the membrane-impermeable and phloem-mobile fluorescent tracer fluorescein (334 D). The dye was loaded into the phloem stream of a photosynthetically mature (source) leaf. Confocal microscopy revealed confinement of the dye in the SE-CC complex in stems (Figure 1A) and source leaves (data not shown). However, the dye was unloaded into surrounding cells in immature, photoassimilate-sink leaves, as expected (data not shown).
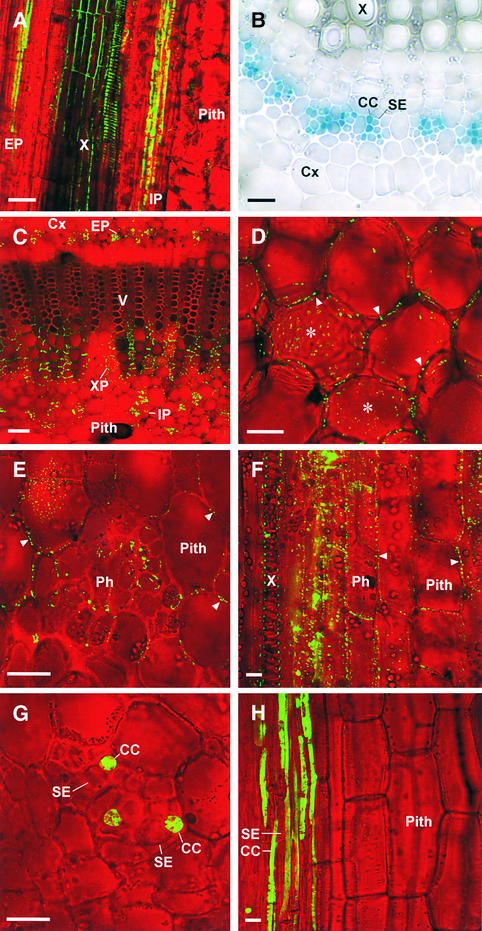
Figure 1.
Traffic of 3a MP:GFP out of the Symplasmically Isolated SE-CC Complex in Stems of Transgenic Tobacco.
Bright-field images were taken in grayscale and pseudocolored in red.
(A) Longitudinal section of the mature stem of a nontransgenic tobacco plant. Transported fluorescein is confined to the SE-CC complex of the external phloem (EP) and internal phloem (IP). The autofluorescence of xylem (X) cell walls appears green by confocal microscopy.
(B) Transverse view of the internal phloem bundles of a vein of CoYMV:GUS transgenic tobacco. GUS activity is confined in CCs. Cx, cortex.
(C) to (F) Confocal images of stem sections from CoYMV:3a MP:GFP transgenic tobacco. The presence of 3a MP:GFP as punctate dots in cell walls is indicated by arrowheads.
(C) Low-magnification transverse view. 3a MP:GFP produced in CCs of the internal and external phloem traffics into neighboring cells such as xylem parenchyma (XP). V, vessel.
(D) 3a MP:GFP is present in cell walls of pith in the center of the stem. Asterisks indicate tangential section of cell walls with punctate fluorescent dots.
(E) High-magnification view of the phloem (Ph) and surrounding tissues.
(F) Longitudinal view. 3a MP:GFP is present in cell walls of phloem (Ph) as well as pith cells.
(G) and (H) Longitudinal (G) and transverse (H) views of stem sections from CoYMV:dimeric GFP transgenic tobacco. Dimeric GFP is confined to CCs.
Bars in (A) to (D) = 50 μm; bars in (E) to (H) = 20 μm.
3a. MP:GFP Trafficked out of the Symplasmically Isolated SE-CC Complex in Transgenic Tobacco
To determine whether a protein could traffic from the symplasmically isolated SE-CC complex into surrounding cells, we used the CoYMV promoter to drive the expression of the 3a MP:GFP fusion in CCs of transgenic tobacco. This promoter is active in CCs of all organs of different stages, as shown using β-glucuronidase (GUS) as a reporter (Matsuda et al., 2002) (Figure 1B). We obtained three lines of transgenic plants that expressed 3a MP:GFP under the control of the CoYMV promoter. The presence of 3a MP:GFP fluorescence as punctate dots in the cell walls allowed us to trace the movement of the fusion protein (Itaya et al., 1997, 1998).
In all transgenic lines, confocal microscopy detected 3a MP:GFP in the phloem as well as in the xylem parenchyma regardless of organ type and developmental stage examined (Figures 1C and 2). The traffic of the protein within the vascular tissue became more extensive as a given organ matured, as indicated by the presence of the protein in more cells (Figures 2A and 2B). The protein also was detected in nonvascular cells, such as pith in the stem (Figures 1D and 1F). However, the presence of 3a MP:GFP in nonvascular tissues appeared to be influenced by organ type and plant development, as will be discussed below.
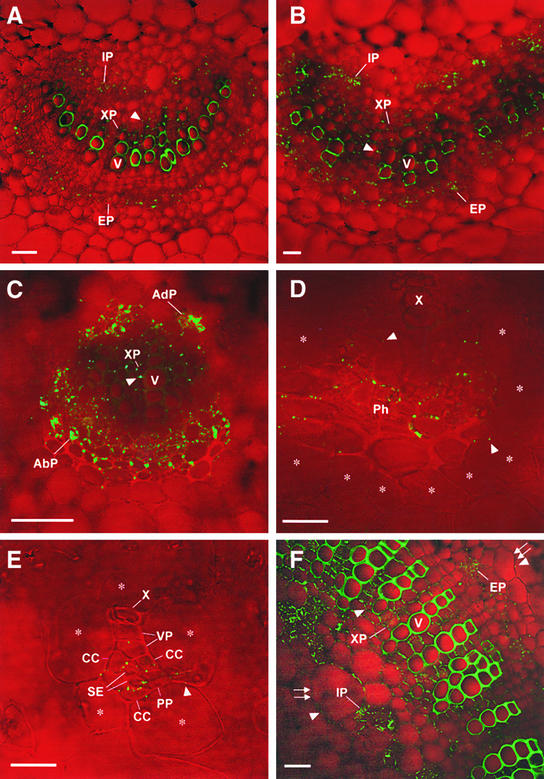
Figure 2.
Traffic of 3a MP:GFP out of the Symplasmically Isolated SE-CC Complex in Leaves and Petioles of Transgenic Tobacco.
The results described above strongly suggested that 3a MP:GFP traffics out of the SE-CC complex. However, it was possible that 3a MP:GFP was translated outside of CCs if the CoYMV promoter had leaky activity and/or if 3a MP:GFP mRNAs trafficked out of CCs. We performed the following series of experiments to resolve this issue.
Dimeric GFP Was Confined in CCs
We generated transgenic tobacco expressing dimeric GFP (GFP:GFP fusion; 54 kD) under the control of the CoYMV promoter. Confocal microscopic analysis showed that in the transgenic plant, the dimeric GFP stayed in CCs in mature stems (Figures 1G and 1H). Data indicate that CoYMV promoter activity is in fact CC specific and suggest strongly that the presence of 3a MP:GFP (57 kD) in cells other than CCs was attributable to traffic. These data also suggest that the traffic of 3a MP:GFP out of CCs is a regulated process mediated by 3a MP and does not occur by diffusion.
3a MP:GFP mRNA Was Localized in CCs
To determine whether 3a MP:GFP mRNA would be present outside of CCs, we performed in situ hybridization using antisense 3a MP or antisense GFP RNA probes. As shown in Figures 3A and 3B, 3a MP:GFP mRNA was localized exclusively in CCs of CoYMV:3a MP:GFP transgenic tobacco. In situ hybridization of nontransgenic tobacco did not produce any hybridization signals (Figures 3C and 3D). Data confirmed the CC-specific expression of the 3a MP:GFP fusion gene and suggested that 3a MP:GFP did not potentiate the traffic of its mRNA. Therefore, the presence of 3a MP:GFP outside of CCs must be caused by the traffic of the protein. The inability of the 3a MP:GFP fusion protein to traffic its mRNA is consistent with the observation that 3a MP:GFP does not potentiate the cell-to-cell movement of viral RNA (Canto et al., 1997).
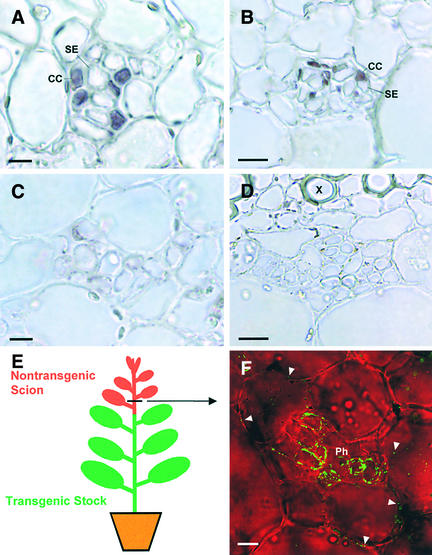
Figure 3.
Further Evidence for 3a MP:GFP Traffic out of the SE-CC Complex.
(A) and (B) CoYMV:3a:GFP transgenic tobacco. 3a MP:GFP mRNA is localized in CCs but not in any other cells by in situ hybridization using antisense 3a MP RNA probes.
(A) External phloem of a petiole.
(B) Internal phloem of a stem.
(C) and (D) Nontransgenic tobacco. In situ hybridization using antisense 3a MP RNA probes produced no hybridization signals.
(C) External phloem of a petiole.
(D) Internal phloem of a stem. X, xylem.
(E) Diagram of a graft union consisting of a CoYMV:3a MP:GFP transgenic stock and a nontransgenic scion.
(F) Transverse view of the internal phloem of a nontransgenic scion stem. Bright-field images were taken in grayscale and pseudocolored in red. 3a MP:GFP is present in pith cells (arrowheads) as well as in the phloem (Ph).
Bars in (A) and (C) = 20 μm; bars in (B), (D), and (F) = 10 μm.
3a. MP:GFP Trafficked from a Transgenic Rootstock to a Nontransgenic Scion
To further verify the ability of 3a MP:GFP to traffic out of the symplasmically isolated SE-CC complex, we generated graft unions between CoYMV:3a MP:GFP transgenic stocks and nontransgenic scions (Figure 3E). Two weeks after grafting, we examined transverse and longitudinal sections of the scion stems for the presence of 3a MP:GFP. As shown in Figure 3F, the fusion protein was detected in the phloem of scion stems up to 6 cm above the grafting interface. This finding indicates that 3a MP:GFP produced in CCs entered SEs in the stocks and then translocated into nontransgenic scions through SEs. Importantly, the fusion protein was detected in phloem as well as pith cells of the scion (Figure 3F). Because there was no CoYMV promoter activity in the scion, this result provided the most compelling evidence that 3a MP:GFP can traffic from the symplasmically isolated SE-CC complex into neighboring cells.
Traffic of 3a MP:GFP into Nonvascular Cells Was Regulated by Organ Type and Plant Developmental Stage
3a MP:GFP trafficked from the SE-CC complex to other parenchymatous vascular cells (i.e., phloem parenchyma and xylem parenchyma) regardless of vein type, organ type, or developmental stage examined, as described above (Figures 1C to 1F and 2). However, further traffic of 3a MP:GFP beyond the vascular tissue depended on the type and developmental stage of an organ (Figure 4). In all classes of veins in both sink and source leaves (see Figure 4 for vein classification) and in young petioles and stems (which are associated with sink leaves), the traffic of 3a MP:GFP into nonvascular tissues was limited if not restricted. In these organs, the fusion protein trafficked a distance of zero to two cells (average was less than one cell) away from the vascular bundle (Table 1).
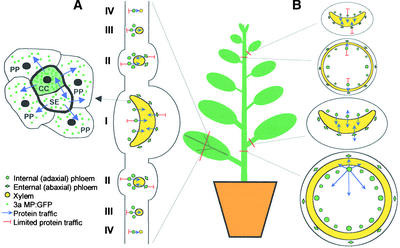
Figure 4.
Regulation of 3a MP:GFP Traffic by Organ Type and Developmental Stage.
(A) Idealized transverse view of a tobacco leaf. A phloem bundle is enlarged at left, highlighting 3a MP:GFP traffic from CC into SE and phloem parenchyma (PP). Traffic of the fusion protein from phloem to xylem parenchyma occurs in all veins. However, traffic into nonvascular tissues is limited in all veins. Roman numerals denote classes of veins (Ding et al., 1988). Class I veins include the midrib and proximal ends of secondary branches. Class II consists mostly of secondary branches. Class III veins include the tertiary branches and terminal ends of secondary branches. Class IV and V veins (minor veins) are subsequent branches from class III veins.
(B) Idealized transverse views of tobacco petioles and stems. The traffic of the fusion protein from phloem to xylem parenchyma is not influenced by the developmental stage of these organs. Traffic of 3a MP:GFP into nonvascular tissues is limited in young petioles and stems (top two details) but is extensive in mature petioles and stems (bottom two details).
Table 1.
Quantification of the Regulation of 3a MP:GFP Traffic by Organ Type and Developmental Stage
| Distance of 3a MP:GFP Traffic
|
|||
|---|---|---|---|
| Organ | Developmental Stage | Within Vascular Tissue | Outside Vascular Tissue |
| Leaf | Sink and source | 6.0a (100–200 μm)b | 1.0 (20 μm) |
| Petiole | Young | 7.0 (100 μm) | 0.5 (10 μm) |
| Stem | Young | 7.0 (100 μm) | 0.5 (10 μm) |
| Petiole | Mature | 10.0 (200 μm) | 3.5 (100 μm) |
| Stem | Mature | 10.0 (200 μm) | 50.0 (5000 μm) |
Traffic distance outside of vascular tissue was determined as the farthest point from the phloem at which 3a MP:GFP could be detected. The center point between internal (adaxial) phloem and external (abaxial) phloem is defined as the traffic distance within the vascular tissue. For each organ and developmental stage, data presented are average measurements on sections from three independent lines of transgenic plants.
Number of cells.
Physical distance.
These nonvascular cells included cortex in class I (Figures 2A and 2B) and class II (Figure 2C) veins and bundle sheath cells in class III (Figure 2D) and minor (Figure 2E) veins. Interestingly, this limitation of 3a MP:GFP trafficking into nonvascular tissues was released in petioles (Figures 2F and 4) and stems (Figures 1C to 1F and 4) as they matured. In petioles of mature source leaves, the fusion protein trafficked an average distance of 3.5 cells from the vascular bundle (Table 1). In mature stems, 3a MP:GFP trafficked into all pith cells (Figure 1D). It should be noted that the center of pith is ∼50 cells away from the vascular bundles (Table 1).
3a. MP:GFP Was Localized to PD between CC and Neighboring Cells
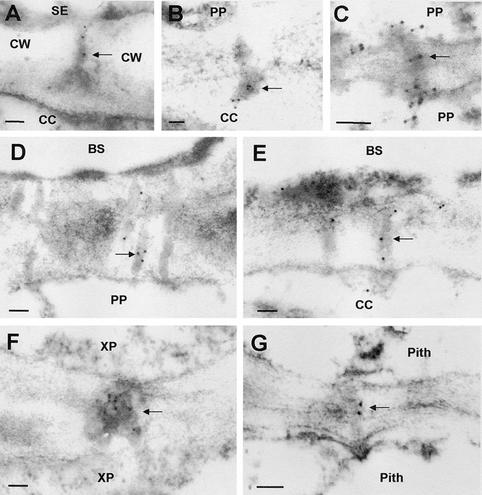
3a MP:GFP accumulated as punctate dots in the cell walls (Figures 1C to 1F and 2), suggesting targeting of the fusion protein to PD. To determine whether 3a MP:GFP was in fact localized to PD, especially to those at the boundary between the SE-CC complex and its neighbors, we performed immunolabeling experiments. Using a polyclonal antibody against 3a MP and a gold-conjugated secondary antibody (Itaya et al., 1998), we localized the fusion protein to PD between various cells: SE and CC, CC and phloem parenchyma cells, CC and bundle sheath, xylem parenchyma cells, and pith cells (Figure 5). The data suggest strongly that the fusion protein trafficked symplasmically through PD from CCs into other cells.
Figure 5.
Immunogold Electron Microscopic Localization of 3a MP:GFP to PD in CoYMV:3a MP:GFP Transgenic Tobacco.
Images are from leaf ([A] to [F]) and stem (G) samples. Arrows indicate PD. BS, bundle sheath; CW, cell wall; PP, phloem parenchyma; XP, xylem parenchyma. Bars = 100 nm.
DISCUSSION
Endogenous Mechanisms Exist for Selective Protein Traffic out of the Symplasmically Isolated SE-CC Complex
We have presented evidence from several lines of studies that CMV 3a MP mediates the traffic of a fusion protein out of the SE-CC complex via PD in transgenic tobacco. First, dimeric GFP transgenically produced in CCs remained in such cells. Second, in situ hybridization localized 3a MP:GFP mRNA only in CCs. Third, in the scion that imported the 3a MP:GFP from the transgenic rootstock, the fusion protein trafficked out of the SE-CC complex. Fourth, immunoelectron microscopy localized 3a MP:GFP directly in PD between CCs and neighboring cells.
The traffic of 3a MP:GFP out of the SE-CC complex is in sharp contrast to the restriction of small molecules such as fluorescein in this complex (Figure 1A). The traffic of 3a MP:GFP also contradicts the restriction of some well-characterized plant proteins within the SE-CC complex. These include RTM1 and RTM2 (Chisholm et al., 2001), the phloem proteins PP1 and PP2 (Dannenhoffer et al., 1997; Golecki et al., 1999), the Suc transporter SUT1 (Kühn et al., 1997), thioredoxin h (Ishiwatari et al., 1998), and CmPP16 (Xoconostle-Cázares et al., 1999).
Interestingly, some of these phloem proteins that are limited naturally to the SE-CC complex can traffic between cells in nonphloem tissues such as the mesophyll after microinjection (Balachandran et al., 1997; Ishiwatari et al., 1998; Lucas, 1999). Therefore, it appears that PD that connect the SE-CC complex to surrounding cells can prevent the diffusion and/or traffic of small molecules and some proteins and yet allow the traffic of selective proteins out of the complex. How PD prevent the diffusion of small molecules and yet allow the traffic of proteins is a significant issue to be resolved in future studies. Because GUS and dimeric GFP remain in the CC, the traffic of 3a MP:GFP out of the SE-CC complex must be mediated by a motif(s) that resides in 3a MP.
Our data suggest that protein traffic can be a mechanism for symplasmically isolated cells to communicate with one another. This protein-based communication may play a significant role in the coordination of developmental processes. For example, in the case of signaling during flower development in Arabidopsis, it has been postulated that flowering factors have already been transported into the apical meristems before symplasmic isolation or that such factors are produced locally, based on the assumption that symplasmic isolation terminates all transport through the PD (Gisel et al., 1999). In light of the results presented here, it will be interesting to determine whether specific signaling molecules can be transported symplasmically via an active mechanism into the “isolated” apical meristem.
Molecular Recognition Is a Mechanism for Selective Protein Traffic from CC to SE
The transport of some proteins from CC to SE is essential to maintain the longevity of enucleate SE (Raven, 1991) and perhaps also to regulate developmental and physiological processes (Mezitt and Lucas, 1996). However, the mechanisms of traffic between these two cell types are not well understood. Because of the large size exclusion limit of PD between CC and SE, as shown by the movement of 10-kD dextran (Kempers and Van Bel, 1997) and 27-kD GFP (Imlau et al., 1999; Oparka et al., 1999), it has been speculated that proteins diffuse from CC to SE largely by default (Santa Cruz, 1999; Oparka and Santa Cruz, 2000; Zambryski and Crawford, 2000). To prevent unwanted loss of certain molecules from CC, these molecules may be anchored to cytoplasmic structures (Imlau et al., 1999; Oparka et al., 1999; Crawford and Zambryski, 2000; Oparka and Santa Cruz, 2000). In addition, lost molecules may be reclaimed by CC (Turgeon, 2000).
Larger proteins are suggested to be trafficked selectively from CC to SE (Fisher and Cash-Clark, 2000; Oparka and Santa Cruz, 2000; Wu et al., 2002), with mechanisms of selectivity that are unknown. In this study, we showed that dimeric GFP was confined in CC, but 3a MP:GFP trafficked from CC to SE. Because GFP diffuses out of CC (Imlau et al., 1999; Oparka et al., 1999), the confinement of dimeric GFP in CC is unlikely to be the result of active retention in the cytoplasm; rather, it is caused by the absence of a traffic motif(s) in GFP. On the other hand, 3a MP must have a motif(s) to mediate the traffic of the 3a MP:GFP fusion from CC to SE.
Therefore, our data provide experimental evidence for the selective traffic of a protein from CC to SE, presumably mediated by molecular recognition. Such selective protein traffic may account for the presence of proteins of 60 to 200 kD in the phloem exudate of many plant species (Balachandran et al., 1997; Hayashi et al., 2000). In some cases, a protein may be anchored to membranes and cleavage of the protein may precede active traffic from CC to SE (Xoconostle-Cázares et al., 2000).
Developmental Factors Control the Traffic of a Protein from Vascular Tissue into Neighboring Tissues
3a MP:GFP traffics from CCs into vascular cells regardless of organ type and developmental stage examined. In particular, the fusion protein can be detected in the xylem parenchyma as far as 200 μm from the CCs in all organs examined (Figures 1 and 2, Table 1). In contrast, traffic of the fusion protein into nonvascular tissues, such as bundle sheath and cortex, is limited in leaves, young stems, and petioles (Figure 4, Table 1). The rare presence of 3a MP:GFP in nonvascular tissues is unlikely to be the result of the rapid turnover of 3a MP:GFP or the inability of the protein to target to PD, because 3a MP:GFP is localized to PD in nonvascular tissues in 35S:3a MP:GFP transgenic tobacco (Itaya et al., 1998). Therefore, we interpret our data as an indication of the polar traffic of 3a MP:GFP mediated by specific molecular interactions. Interestingly, the transcription factor DEFICIENS also traffics unidirectionally in the floral apex of snapdragon (Perbal et al., 1996).
The restriction of 3a MP:GFP traffic into nonvascular cells is released in mature stems and in petioles of mature leaves. Most notably, the fusion protein traffics into all pith cells over a distance of 5000 μm in mature stems. Therefore, the traffic of a protein out of the vascular tissue is controlled by factors associated with specific cellular boundaries and developmental stages of the organ. The developmental regulation of 3a MP:GFP traffic also was observed in tobacco leaf epidermis (Itaya et al., 1998). Specifically, 3a MP:GFP targeted to and trafficked through PD in the epidermis of source leaves but not in the epidermis of sink leaves.
Similar developmental regulation was reported for PD targeting of Tobacco mosaic virus (TMV) MP:GFP in tobacco (Roberts et al., 2001). However, Crawford and Zambryski (2001) reported that the traffic of TMV MP:GFP was not correlated to leaf developmental stage. The reason for the inconsistent reports regarding TMV MP:GFP is unclear. The maize transcription factor KN1, when produced ectopically as a GFP fusion protein in the vascular tissue of Arabidopsis, also can traffic into surrounding cells (Kim et al., 2002). The specific vascular cell type in which the fusion protein was produced is unknown. Whether the traffic is regulated developmentally remains to be investigated.
Selective Protein Traffic out of Vascular Tissue May Be Important for Plant Function
Although numerous proteins have been identified in the phloem sap, their biological functions remain speculative (Mezitt and Lucas, 1996; Crawford and Zambryski, 1999; Thompson and Schulz, 1999; Oparka and Santa Cruz, 2000). One important question is whether some proteins traffic out of the SE-CC complex to perform certain functions. The traffic of 3a MP:GFP out of the SE-CC complex suggests the existence of mechanisms for the traffic of endogenous proteins. Identification of such plant proteins should provide new opportunities to study the regulation of plant developmental and physiological processes.
A recent study provides elegant evidence that the well-regulated traffic of a transcription factor from the vascular tissue into neighboring cells can control cell differentiation. The Arabidopsis protein SHORT-ROOT is produced in the stele and traffics into one layer of adjacent cells to confer positional information for endodermis formation in the root (Nakajima et al., 2001). Perhaps the traffic of specific proteins is responsible for the regulation of photosynthetic cell development by vascular tissue (Langdale et al., 1988) and for the control of carbon partitioning (Lucas et al., 1996).
Traffic of an MP into and out of the Symplasmically Isolated SE-CC Complex May Be Key for Viral Systemic Infection
Despite the symplasmic isolation of the SE-CC complex in mature stems and leaves, some viruses, such as TMV (Cheng et al., 2000) and CMV (Dufour et al., 1989; Andrianifahanana et al., 1997; Guerini and Murphy, 1999), can establish systemic infection in susceptible plant species by entering and exiting this complex. The ability of a viral MP or other viral proteins to traffic between the symplasmically isolated SE-CC complex and surrounding cells has been suggested to be an important factor for viral systemic movement (Oparka and Turgeon, 1999). Our results support this hypothesis.
Blackman et al. (1998) demonstrated that 3a MP enters SE from CC through PD during CMV infection, suggesting an important role of the MP in the viral entry into SE. How the virus exits the SE-CC complex remains unknown. The traffic of 3a MP:GFP between SE and CC and then out of the SE-CC complex into surrounding tissues suggests that 3a MP also is involved in viral exit out of the SE-CC complex. Some viruses are restricted to the phloem (Sanger et al., 1994; Wang et al., 1996; Ghoshroy et al., 1998), whereas others cannot enter the phloem (Ding et al., 1998; Wang et al., 1998). It will be revealing to test MPs from these viruses for their ability to traffic through PD at the boundary between the SE-CC complex and neighboring cells.
The limited traffic of 3a MP:GFP out of the vascular tissue in leaves or young tissues raises the question of how CMV moves beyond the vascular tissue during systemic infection. It is known that other CMV proteins, such as coat protein (Suzuki et al., 1991; Canto et al., 1997; Kaplan et al., 1998; Schmitz and Rao, 1998; Nagano et al., 2001), replicase (Gal-On et al., 1994), and 2b protein (Ding et al., 1995), are involved in CMV movement. In particular, 2b protein is required for systemic movement in tobacco and many other species (Ding et al., 1995). A future challenge is to elucidate how these proteins function in concert with 3a MP to facilitate CMV systemic movement.
Conclusion
In summary, we have obtained experimental evidence that a viral MP, in the absence of viral replication, can traffic out of the SE-CC complex that is isolated symplasmically for the transport of small molecules. Therefore, deduced transport functions of PD based on dye coupling (Lucas et al., 1993; Pfluger and Zambryski, 2001) and PD frequencies (Gamalei, 1989; Botha and Van Bel, 1992) need to be reevaluated using biologically relevant molecules. Our study also reveals regulatory points for protein traffic at intercellular (between CC and SE) and tissue (between vascular and nonvascular tissues) levels. These regulations are influenced by organ type and developmental stage. We suggest that regulated macromolecular traffic is one of the means of communication between symplasmic domains to coordinate plant development, plant physiology, and interactions with pathogens.
METHODS
Plant Growth
Tobacco (Nicotiana tabacum) plants were grown at 23 ± 3°C under 40-W cool-white fluorescent lights kept on a daily 16-h-light/8-h-dark cycle in growth chambers.
Dye-Coupling Experiment
Fluorescein (334 D; Sigma, St. Louis, MO) was loaded into the phloem of fully expanded tobacco leaves. Fluorescein at a concentration of 1% in 0.1 M potassium-sodium phosphate buffer, pH 5.3, was applied to the gently abraded surface of a fully expanded leaf, which was then covered with plastic wrap. After 3 to 12 h, free-hand sections of the upper stems were examined for the presence of fluorescein using a PCM-2000 confocal laser scanning microscope equipped with argon and green HeNe lasers (Nikon, Tokyo, Japan).
Binary Vector Construction
To construct the dimeric green fluorescent protein (GFP) gene, the monomeric GFP gene without a stop codon was amplified by PCR from pEGFP-1 (Clontech, Palo Alto, CA) using primers designed to incorporate the NcoI restriction site at the 5′ end and the SacI site at the 3′ end. The amplified fragment was cloned at the NcoI and SacI sites of vector pRTL2 (Carrington and Freed, 1990; Restrepo et al., 1990). Similarly, another monomeric GFP gene with a stop codon was amplified by PCR to incorporate the SacI site at the 5′ end and the BamHI site at the 3′ end and was cloned at the SacI and BamHI sites downstream of the first GFP gene.
The fusion DNA fragments Tobacco etch virus leader sequence:3a MP:GFP (Itaya et al., 1997) and the dimeric GFP in plasmid pRTL2 were excised by double digestion with EcoRI and BamHI or NcoI and BamHI, respectively. The isolated DNA fragments were cloned by conventional blunt-end ligation at the SmaI site of binary vector pGPTV-Kan containing the promoter of Commelina yellow mottle virus (CoYMV) (from Neil Olszewski, University of Minnesota, St. Paul). Each plasmid was used to transform Agrobacterium tumefaciens (LBA4404), as described previously (Itaya et al., 1998).
Tobacco Transformation
A standard Agrobacterium-mediated leaf disc transformation method (Horsch et al., 1985) was used to generate transgenic tobacco plants. Expression of the 3a movement protein (3a MP):GFP or dimeric GFP in the phloem was confirmed by examining the plants with a Nikon E600 fluorescence microscope with a filter set consisting of a blue excitation filter (420 to 490 nm), a dichroic mirror (510 nm), and a green barrier filter (520 to 560 nm). Plants of subsequent generations were subjected to detailed analysis. Free-hand sections of various organs were examined for 3a MP:GFP or dimeric GFP localization using the PCM-2000 confocal microscope.
Grafting Experiments
Nontransgenic tobacco plants were grafted onto rootstocks (with six or seven source leaves) of CoYMV:3a MP:GFP transgenic tobacco plants. Two weeks after grafting, sections from nontransgenic scions were examined for the presence and localization of 3a MP:GFP using the PCM-2000 confocal microscope.
In Situ Hybridization
3a MP and GFP genes were cloned at the EcoRI and BamHI sites in the pGEM-4Z cloning vector (Promega, Madison, WI). Plasmids linearized with EcoRI were used as templates for in vitro transcription using digoxigenin-UTP and T7 polymerase to produce digoxigenin-labeled antisense RNA probe, as described by Zhu et al. (2001). To obtain cryosections for in situ hybridization, 2 × 2-mm segments of transgenic leaves and stems were fixed in a mixture of 3.7% paraformaldehyde, 0.1% glutaraldehyde, 0.2% picric acid, 50 mM potassium phosphate, and 5 mM EGTA for 2 to 3 h. The fixed samples were infiltrated sequentially with embedding mixture (two parts 20% Suc and one part optimal cutting temperature compound [Ted Pella Inc., Redding, CA]):potassium phosphate:EGTA buffer at room temperature for 1 h.
Afterward, the samples were infiltrated with pure embedding mixture for 2 h at room temperature, embedded, and frozen at −20°C. The frozen samples were sectioned to 12 μm thickness using a Microm HM500 cryostat (Microm International, Walldorf, Germany). Sections were collected onto microscope slides coated with 1% gelatin and 0.1% chromium alum. The slides with sections were incubated at 42°C for at least 3 h and then stored at 4°C. In situ hybridization was performed as described by Zhu et al. (2001).
Immunocytochemistry and Electron Microscopy
Tissue sections were prepared and probed with a rabbit-derived polyclonal antibody (IgG) against 3a MP from Cucumber mosaic virus (CMV) and then with a goat-derived and 10-nm gold–conjugated anti-rabbit IgG antibody (Sigma) as described previously (Itaya et al., 1998). All sections were examined with a CM-12 transmission electron microscope (Philips, Eindhoven, The Netherlands) operated at 80 kV.
Upon request, all novel materials described in this article will be made available in a timely manner for noncommercial research purposes. No restrictions or conditions will be placed on the use of any materials described in this article that would limit their use for noncommercial research purposes.
Acknowledgments
We express our gratitude to Neil Olszewski at the University of Minnesota for generously providing the CoYMV promoter and to Chikara Masuta at Hokkaido University for CMV 3a MP antibody. We thank Robert Turgeon for helpful discussions and Yan Xun for technical assistance. This study was supported by grants from the U.S. Department of Agriculture National Research Initiative Competitive Grants Program (1998-35304-6509, 1998-35304-10251, and 2001-35304-09928) and from the Samuel Roberts Noble Foundation.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.003954.
References
- Andrianifahanana, M., Lovins, K., Dute, R., Sikora, E., and Murphy, J.F. (1997). Pathway for phloem-dependent movement of pepper mottle potyvirus in the stem of Capsicum annuum. Phytopathology 87, 892–898. [DOI] [PubMed] [Google Scholar]
- Balachandran, S., Xiang, Y., Schobert, C., Thompson, G.A., and Lucas, W.J. (1997). Phloem sap proteins from Cucurbita maxima and Ricinus communis have the capacity to traffic cell to cell through plasmodesmata. Proc. Natl. Acad. Sci. USA 94, 14150–14155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier, G., Havelange, A., Houssa, C., Petitjean, A., and Lejeune, P. (1993). Physiological signals that induce flowering. Plant Cell 5, 1147–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman, L.M., Boevink, P., Cruz, S.S., Palukaitis, P., and Oparka, K.J. (1998). The movement protein of cucumber mosaic virus traffics into sieve elements in minor veins of Nicotiana clevelandii. Plant Cell 10, 525–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botha, C.E.J., and Van Bel, A.J.E. (1992). Quantification of symplastic continuity as visualised by plasmodesmograms: Diagnostic value of phloem-loading pathways. Planta 187, 359–366. [DOI] [PubMed] [Google Scholar]
- Bush, D.R. (1993). Proton-coupled sugar and amino acid transporters in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 44, 513–542. [Google Scholar]
- Canto, T., Prior, D.A., Hellwald, K.H., Oparka, K.J., and Palukaitis, P. (1997). Characterization of cucumber mosaic virus. IV. Movement protein and coat protein are both essential for cell-to-cell movement of cucumber mosaic virus. Virology 237, 237–248. [DOI] [PubMed] [Google Scholar]
- Carrington, J.C., and Freed, D.D. (1990). Cap-independent enhancement of translation by a plant potyvirus 5′ nontranslated region. J. Virol. 64, 1590–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington, J.C., Kasschau, K.D., Mahajan, S.K., and Schaad, M.C. (1996). Cell-to-cell and long distance transport of viruses in plants. Plant Cell 8, 1669–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, N.H., Su, C.L., Carter, S.A., and Nelson, R.S. (2000). Vascular invasion routes and systemic accumulation patterns of tobacco mosaic virus in Nicotiana benthamiana. Plant J. 23, 349–362. [DOI] [PubMed] [Google Scholar]
- Chisholm, S.T., Parra, M.A., Anderberg, R.J., and Carrington, J.C. (2001). Arabidopsis RTM1 and RTM2 genes function in phloem to restrict long-distance movement of tobacco etch virus. Plant Physiol. 127, 1667–1675. [PMC free article] [PubMed] [Google Scholar]
- Clark, S.E. (2001). Cell signalling at the shoot meristem. Nat. Rev. Mol. Cell Biol. 2, 276–284. [DOI] [PubMed] [Google Scholar]
- Crawford, K.M., and Zambryski, P.C. (1999). Plasmodesmata signaling: Many roles, sophisticated statutes. Curr. Opin. Plant Biol. 2, 382–387. [DOI] [PubMed] [Google Scholar]
- Crawford, K.M., and Zambryski, P.C. (2000). Subcellular localization determines the availability of non-targeted proteins to plasmodesmatal transport. Curr. Biol. 10, 1032–1040. [DOI] [PubMed] [Google Scholar]
- Crawford, K.M., and Zambryski, P.C. (2001). Non-targeted and targeted protein movement through plasmodesmata in leaves in different developmental and physiological states. Plant Physiol. 125, 1802–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenhoffer, J.M., Schulz, A., Skaggs, M.I., Bostwick, D.E., and Thompson, G.A. (1997). Expression of the phloem lectin is developmentally linked to vascular differentiation in cucurbits. Planta 201, 405–414. [Google Scholar]
- Ding, B. (1998). Intercellular protein trafficking through plasmodesmata. Plant Mol. Biol. 38, 279–310. [PubMed] [Google Scholar]
- Ding, B., Itaya, A., and Woo, Y. (1999). Plasmodesmata and cell-to-cell communication in plants. Int. Rev. Cytol. 190, 251–316. [Google Scholar]
- Ding, B., Parthasarathy, M.V., Niklas, K., and Turgeon, R. (1988). A morphometric analysis of the phloem-unloading pathway in developing tobacco leaves. Planta 176, 307–318. [DOI] [PubMed] [Google Scholar]
- Ding, S.W., Li, W.X., and Symons, R.H. (1995). A novel naturally occurring hybrid gene encoded by a plant RNA virus facilitates long distance virus movement. EMBO J. 14, 5762–5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, X.S., Carter, S.A., Deom, C.M., and Nelson, R.S. (1998). Tobamovirus and potyvirus accumulation in minor veins of inoculated leaves from representatives of the Solanaceae and Fabaceae. Plant Physiol. 116, 125–136. [PMC free article] [Google Scholar]
- Dufour, O., Palloix, A., Selassie, K.G., Pochard, E., and Marchoux, G. (1989). The distribution of cucumber mosaic virus in resistant and susceptible plants of pepper. Can. J. Bot. 67, 655–660. [Google Scholar]
- Ehlers, K., and Kollmann, R. (2000). Synchronization of mitotic activity in protoplast-derived Solanum nigrum L. microcalluses is correlated with plasmodesmal connectivity. Planta 210, 269–278. [DOI] [PubMed] [Google Scholar]
- Erwee, M.G., and Goodwin, P.B. (1983). Characterisation of the Egeria densa Planch. leaf symplast. Planta 158, 320–328. [DOI] [PubMed] [Google Scholar]
- Fisher, D.B., and Cash-Clark, C.E. (2000). Sieve tube unloading and post-phloem transport of fluorescent tracers and proteins injected into sieve tubes via severed aphid stylets. Plant Physiol. 123, 125–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal-On, A., Kaplan, I., Roossinck, M.J., and Palukaitis, P. (1994). The kinetics of infection of zucchini squash by cucumber mosaic virus indicate a function for RNA 1 in virus movement. Virology 205, 280–289. [DOI] [PubMed] [Google Scholar]
- Gamalei, Y. (1989). Structure and function of leaf minor veins in trees and herbs. Trees 3, 96–110. [Google Scholar]
- Ghoshroy, S., Freedman, K., Lartey, R., and Citovsky, V. (1998). Inhibition of plant viral systemic infection by non-toxic concentrations of cadmium. Plant J. 13, 591–602. [DOI] [PubMed] [Google Scholar]
- Ghoshroy, S., Lartey, R., Sheng, J., and Citovsky, V. (1997). Transport of proteins and nucleic acids through plasmodesmata. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48, 27–50. [DOI] [PubMed] [Google Scholar]
- Gilbertson, R.L., and Lucas, W.J. (1996). How do viruses traffic on the ‘vascular highway’? Trends Plant Sci. 1, 260–268. [Google Scholar]
- Gisel, A., Barella, S., Hempel, F.D., and Zambryski, P.C. (1999). Temporal and spatial regulation of symplastic trafficking during development in Arabidopsis thaliana apices. Development 126, 1879–1889. [DOI] [PubMed] [Google Scholar]
- Golecki, B., Schulz, A., and Thompson, G.A. (1999). Translocation of structural P proteins in the phloem. Plant Cell 11, 127–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grusak, M.A., Beebe, D.U., and Turgeon, R. (1996). Phloem loading. In Photoassimilate Distribution in Plants and Crops: Source-Sink Relationships, E. Zamski and A.A. Schaffer, eds (New York: Marcel Dekker), pp. 209–227.
- Guerini, M.N., and Murphy, J.F. (1999). Resistance of Capsicum annuum ‘Avelar’ to pepper mottle potyvirus and alleviation of this resistance by co-infection with cucumber mosaic cucumovirus are associated with virus movement. J. Gen. Virol. 80, 2785–2792. [DOI] [PubMed] [Google Scholar]
- Hayashi, H., Fukuda, A., Suzui, N., and Fujimaki, S. (2000). Proteins in the sieve element-companion cell complexes: Their detection, localization and possible functions. Aust. J. Plant Physiol. 27, 489–496. [Google Scholar]
- Horsch, R.B., Fry, J., Hoffmann, N.L., Wallroth, M., Eichholtz, D., Rogers, S.G., and Fraley, R.T. (1985). A simple and general method for transferring genes into plants. Science 227, 1229–1231. [DOI] [PubMed] [Google Scholar]
- Imlau, A., Truernit, E., and Sauer, N. (1999). Cell-to-cell and long-distance trafficking of the green fluorescent protein in the phloem and symplastic unloading of the protein into sink tissues. Plant Cell 11, 309–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiwatari, Y., Fujiwara, T., McFarland, K.C., Nemoto, K., Hayashi, H., Chino, M., and Lucas, W.J. (1998). Rice phloem thioredoxin h has the capacity to mediate its own cell-to-cell transport through plasmodesmata. Planta 205, 12–22. [DOI] [PubMed] [Google Scholar]
- Itaya, A., Hickman, H., Bao, Y., Nelson, R., and Ding, B. (1997). Cell-to-cell trafficking of cucumber mosaic virus movement protein:green fluorescent protein fusion produced by biolistic bombardment in tobacco. Plant J. 12, 1223–1230. [Google Scholar]
- Itaya, A., Woo, Y.M., Masuta, C., Bao, Y., Nelson, R.S., and Ding, B. (1998). Developmental regulation of intercellular protein trafficking through plasmodesmata in tobacco leaf epidermis. Plant Physiol. 118, 373–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan, I.B., Zhang, L., and Palukaitis, P. (1998). Characterization of cucumber mosaic virus. V. Cell-to-cell movement requires capsid protein but not virions. Virology 246, 221–231. [DOI] [PubMed] [Google Scholar]
- Kempers, R., and Van Bel, A.J.E. (1997). Symplasmic connections between sieve element and companion cell in the stem phloem of Vicia faba L. have a molecular exclusion limit of at least 10 kDa. Planta 201, 195–201. [Google Scholar]
- Kim, J.Y., Yuan, Z., Cilia, M., Khalfan-Jagani, Z., and Jackson, D. (2002). Intercellular trafficking of a KNOTTED1 green fluorescent protein fusion in the leaf and shoot meristem of Arabidopsis. Proc. Natl. Acad. Sci. USA 99, 4103–4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblauch, M., and Van Bel, A.J.E. (1998). Sieve tube in action. Plant Cell 10, 35–50. [Google Scholar]
- Kühn, C., Franceschi, V.R., Schulz, A., Lemoine, R., and Frommer, W.B. (1997). Macromolecular trafficking indicated by localization and turnover of sucrose transporters in enucleate sieve elements. Science 275, 1298–1300. [DOI] [PubMed] [Google Scholar]
- Langdale, J.A., Zetlitch, I., Miller, E., and Nelson, T. (1988). Cell position and light influence C4 versus C3 patterns of photosynthetic gene expression in maize. EMBO J. 7, 3643–3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas, W.J. (1999). Plasmodesmata and the cell-to-cell transport of proteins and nucleoprotein complexes. J. Exp. Bot. 50, 979–987. [Google Scholar]
- Lucas, W.J., Balachandran, S., Park, J., and Wolf, S. (1996). Plasmodesmal companion cell-mesophyll communication in the control over carbon metabolism and phloem transport: Insights gained from viral movement proteins. J. Exp. Bot. 47, 1119–1128. [DOI] [PubMed] [Google Scholar]
- Lucas, W.J., Ding, B., and Van der Schoot, C. (1993). Plasmodesmata and the supracellular nature of plants. New Phytol. 125, 435–476. [DOI] [PubMed] [Google Scholar]
- Mansfield, S.G., and Briarty, L.G. (1991). Early embryogenesis in Arabidopsis thaliana. II. The developing embryo. Can. J. Bot. 69, 461–476. [Google Scholar]
- Matsuda, Y., Liang, G., Zhu, Y., Ma, F., Nelson, R.S., and Ding, B. (2002). The Commelina yellow mottle virus promoter drives companion cell-specific gene expression in multiple organs of transgenic tobacco. Protoplasma 10.1007/s00709-002-0027-6. [DOI] [PubMed]
- McLean, B.G., Hempel, F.D., and Zambryski, P.C. (1997). Plant intercellular communication via plasmodesmata. Plant Cell 9, 1043–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezitt, L.A., and Lucas, W.J. (1996). Plasmodesmal cell-to-cell transport of proteins and nucleic acids. Plant Mol. Biol. 32, 251–273. [DOI] [PubMed] [Google Scholar]
- Nagano, H., Mise, K., Furusawa, I., and Okuno, T. (2001). Conversion in the requirement of coat protein in cell-to-cell movement mediated by the cucumber mosaic virus movement protein. J. Virol. 75, 8045–8053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima, K., Sena, G., Nawy, T., and Benfey, P.N. (2001). Intercellular movement of the putative transcription factor SHR in root patterning. Nature 413, 307–311. [DOI] [PubMed] [Google Scholar]
- Nelson, R.S., and Van Bel, A.J.E. (1998). The mystery of virus trafficking into, through and out of vascular tissue. Prog. Bot. 59, 476–533. [Google Scholar]
- Oparka, K.J., Duckett, C.M., Prior, D.A.M., and Fisher, D.B. (1994). Real-time imaging of phloem unloading in the root tip of Arabidopsis. Plant J. 6, 759–766. [Google Scholar]
- Oparka, K.J., Roberts, A.G., Boevink, P., Santa Cruz, S., Roberts, I., Pradel, K.S., Imlau, A., Kotlizky, G., Sauer, N., and Epel, B. (1999). Simple, but not branched, plasmodesmata allow the nonspecific trafficking of proteins in developing tobacco leaves. Cell 97, 743–754. [DOI] [PubMed] [Google Scholar]
- Oparka, K.J., and Santa Cruz, S. (2000). The great escape: Phloem transport and unloading of macromolecules. Annu. Rev. Plant Physiol. Plant Mol. Biol. 51, 323–347. [DOI] [PubMed] [Google Scholar]
- Oparka, K.J., and Turgeon, R. (1999). Sieve elements and companion cells: Traffic control centers of the phloem. Plant Cell 11, 739–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbal, M.C., Haughn, G., Saedler, H., and Schwarz-Sommer, Z. (1996). Non-cell-autonomous function of the Antirrhinum floral homeotic proteins DEFICIENS and GLOBOSA is exerted by their polar cell-to-cell trafficking. Development 122, 3433–3441. [DOI] [PubMed] [Google Scholar]
- Pfluger, J., and Zambryski, P.C. (2001). Cell growth: The power of symplasmic isolation. Curr. Biol. 11, 436–439. [DOI] [PubMed] [Google Scholar]
- Raven, J.A. (1991). Long-term functioning of enucleate sieve elements: Possible mechanisms of damage avoidance and damage repair. Plant Cell Environ. 14, 139–146. [Google Scholar]
- Restrepo, M.A., Freed, D.D., and Carrington, J.C. (1990). Nuclear transport of plant potyviral proteins. Plant Cell 2, 987–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, I.M., Boevink, P., Roberts, A.G., Sauer, N., Reichel, C., and Oparka, K.J. (2001). Dynamic changes in the frequency and architecture of plasmodesmata during the sink-source transition in tobacco leaves. Protoplasma 218, 31–44. [DOI] [PubMed] [Google Scholar]
- Ruan, Y.L., Llewellyn, D.J., and Furbank, R.T. (2001). The control of single-celled cotton fiber elongation by developmentally reversible gating of plasmodesmata and coordinated expression of sucrose and K+ transporters and expansin. Plant Cell 13, 47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Medrano, R., Xoconostle-Cázares, B., and Lucas, W.J. (2001). The phloem as a conduit for inter-organ communication. Curr. Opin. Plant Biol. 4, 202–209. [DOI] [PubMed] [Google Scholar]
- Sanger, M., Passmore, B., Falk, B.W., Bruening, G., Ding, B., and Lucas, W.J. (1994). Symptom severity of beet western yellow virus strain ST9 is conferred by the ST9-associated RNA and is not associated with virus release from the phloem. Virology 200, 48–55. [DOI] [PubMed] [Google Scholar]
- Santa Cruz, S. (1999). Phloem transport of viruses and macromolecules: What goes in must come out. Trends Microbiol. 7, 237–241. [DOI] [PubMed] [Google Scholar]
- Sauer, N., Baier, K., Gahrtz, M., Stadler, R., Stolz, J., and Truernit, E. (1994). Sugar transport across the plasma membrane of higher plants. Plant Mol. Biol. 26, 1671–1679. [DOI] [PubMed] [Google Scholar]
- Schmitz, I., and Rao, A.L. (1998). Deletions in the conserved amino-terminal basic arm of cucumber mosaic virus coat protein disrupt virion assembly but do not abolish infectivity and cell-to-cell movement. Virology 248, 323–331. [DOI] [PubMed] [Google Scholar]
- Schulz, R., and Jensen, W.A. (1968). Capsella embryogenesis: The egg, zygote and young embryo. Am. J. Bot. 55, 807–819. [Google Scholar]
- Suzuki, M., Kuwata, S., Kataoka, J., Masuta, C., Nitta, N., and Takanami, Y. (1991). Functional analysis of deletion mutants of cucumber mosaic virus RNA3 using an in vitro transcription system. Virology 183, 106–113. [DOI] [PubMed] [Google Scholar]
- Thompson, G.A., and Schulz, A. (1999). Macromolecular trafficking in the phloem. Trends Plant Sci. 4, 354–360. [DOI] [PubMed] [Google Scholar]
- Turgeon, R. (2000). Plasmodesmata and solute exchange in the phloem. Aust. J. Plant Physiol. 27, 521–529. [Google Scholar]
- Turgeon, R., Medville, R., and Nixon, K.C. (2001). The evolution of minor vein phloem and phloem loading. Am. J. Bot. 88, 1331–1339. [PubMed] [Google Scholar]
- Van Bel, A.J.E., and Kempers, R. (1991). Symplasmic isolation of the sieve element-companion cell complex in the phloem of Ricinus communis and Salix alba stems. Planta 183, 69–76. [DOI] [PubMed] [Google Scholar]
- Van Bel, A.J.E., and Van Rijen, H.V.M. (1994). Microelectrode-recorded development of the symplasmic autonomy of the sieve element/companion cell complex in the stem phloem of Lupinus luteus L. Planta 192, 165–175. [Google Scholar]
- Van der Schoot, C., and Van Bel, A.J.E. (1989). Glass microelectrode measurements of sieve tube membrane potentials in internode discs and petiole strips of tomato (Solanum lycopersicum L.). Protoplasma 149, 144–154. [Google Scholar]
- Van der Schoot, C., and Van Bel, A.J.E. (1990). Mapping membrane potential differences and dye-coupling in internodal tissues of tomato (Solanum lycopersicum L.). Planta 182, 9–21. [DOI] [PubMed] [Google Scholar]
- Von Schaewen, A., Stitt, M., Schmidt, R., Sonnewald, U., and Willmitzer, L. (1990). Expression of a yeast-derived invertase in the cell wall of tobacco and Arabidopsis plants leads to accumulation of carbohydrate and inhibition of photosynthesis and strongly influences growth and phenotype of transgenic tobacco plants. EMBO J. 9, 3033–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H.L., Gilbertson, R.L., and Lucas, W.J. (1996). Spatial and temporal distribution of bean dwarf mosaic geminivirus in Phaseolus vulgaris and Nicotiana benthamiana. Phytopathology 86, 1204–1214. [Google Scholar]
- Wang, H.L., Wang, Y., Giesman-Cookmeyer, D., Lommel, S.A., and Lucas, W.J. (1998). Mutations in viral movement protein alter systemic infection and identify an intercellular barrier to entry into the phloem long-distance transport system. Virology 245, 75–89. [DOI] [PubMed] [Google Scholar]
- Wright, K.M., and Oparka, K.J. (1997). Metabolic inhibitors induce symplastic movement of solutes from the transport phloem of Arabidopsis roots. J. Exp. Bot. 48, 1807–1814. [Google Scholar]
- Wu, X., Weigel, D., and Wigge, P.A. (2002). Signaling in plants by intercellular RNA and protein movement. Genes Dev. 16, 151–158. [DOI] [PubMed] [Google Scholar]
- Xoconostle-Cázares, B., Ruiz-Medrano, R., and Lucas, W.J. (2000). Proteolytic processing of CmPP36, a protein from the cytochrome b(5) reductase family, is required for entry into the phloem translocation pathway. Plant J. 24, 735–747. [DOI] [PubMed] [Google Scholar]
- Xoconostle-Cázares, B., Xiang, Y., Ruiz-Medrano, R., Wang, H.L., Monzer, J., Yoo, B.C., McFarland, K.C., Franceschi, V.R., and Lucas, W.J. (1999). Plant paralog to viral movement protein that potentiates transport of mRNA into the phloem. Science 283, 94–98. [DOI] [PubMed] [Google Scholar]
- Zambryski, P., and Crawford, K. (2000). Plasmodesmata: Gatekeepers for cell-to-cell transport of developmental signals in plants. Annu. Rev. Cell Dev. Biol. 16, 393–421. [DOI] [PubMed] [Google Scholar]
- Zhu, Y., Green, L., Woo, Y.-M., Owens, R., and Ding, B. (2001). Cellular basis of potato spindle tuber viroid systemic movement. Virology 279, 69–77. [DOI] [PubMed] [Google Scholar]