Abstract
Cell proliferation requires calmodulin, a protein that regulates calcium-dependent enzymes involved in signal transduction pathways in eukaryotic cells. Calmodulin-like protein (CLP) is found in certain epithelial cell types, including normal breast epithelium, and, although it closely resembles calmodulin in amino acid sequence, CLP interacts with different proteins than does calmodulin. The observation that CLP mRNA expression is dramatically reduced in transformed breast epithelial cells led to two hypotheses: (1) CLP helps to maintain the differentiated state in epithelial cells; and (2) downregulation of CLP accompanies malignant transformation of breast epithelial cells. The objective of this study was to determine if the expression of CLP in human breast cancer specimens is reduced in comparison to its expression in normal breast tissue. Eighty human breast cancer biopsy specimens were analyzed immunohistochemically for CLP expression by using a polyclonal rabbit antihuman CLP antibody. CLP expression was reduced in 79% to 88% of the invasive ductal carcinoma and lobular carcinoma specimens and in a similar fraction of the ductal carcinoma in-situ specimens, compared with normal breast specimens. None of the breast cancer specimens showed an increase in CLP expression. These findings support the hypotheses that CLP behaves as a functional tumor suppressor protein and is downregulated early in breast cancer progression.
Keywords: breast cancer, calmodulin-like protein, epithelial differentiation, immunohistochemistry, tumorigenesis
Introduction
Breast cancer is the most prevalent malignancy in women and the second leading cause of cancer mortality in women in the United States (1). Advances in molecular biology have linked genetic alterations to numerous cancers. The activation of cellular oncogenes and the inactivation of tumor suppressor genes appear to be directly involved in carcinogenesis. Whereas tumor suppressors normally regulate cell cycle progression, mutations in, or aberrant expression of, these suppressor genes allow dysregulated cellular proliferation (2). Defective tumor suppressor genes such as the p53 gene and the BRCA1 gene have been detected in some breast cancers (3). The identification of additional genes with altered expression or function in breast cancer remains an area of intense research because of its potential to yield new prognostic markers and treatment strategies (4).
As a sensor of intracellular Ca2+, calmodulin mediates many activities involved in cell proliferation. Among its many functions, calmodulin is important for normal cell cycle progression (5–7), and increased levels of calmodulin are a hallmark of rapidly proliferating cancer cells (8–10). In addition to the ubiquitous calmodulin, a number of related Ca2+-binding proteins are expressed in a cell- or tissue-restricted pattern. Among them, several members of the S100 family of Ca2+-binding proteins as well as a calmodulin-like protein (CLP) are highly expressed in epithelial cells, and their levels can vary dramatically between the normal and malignant state (11–14). The intronless CLP gene was initially characterized by Koller and Strehler in 1988 (15). Yaswen and colleagues subsequently identified CLP by subtractive hybridization of transcripts expressed in normal versus chemically immortalized human mammary epithelial cells and designated the independently cloned gene, NB-1 (11). Although the 148 amino acid sequence of CLP shares 85% identity with calmodulin, the protein-binding activity of CLP appears to differ significantly from that of calmodulin. For example, although CLP's activation of calmodulin kinase II is equivalent to that of calmodulin, its activation of phosphodiesterase and the plasma membrane Ca2+ pump is much weaker than that of calmodulin, and CLP is unable to activate calcineurin, nitric-oxide synthase, or myosin-light-chain kinase (16,17).
Evidence from studies by Yaswen and colleagues suggests that CLP may have tumor suppressor function in normal breast tissue. CLP expression is over 50-fold decreased in tumorigenically transformed human mammary epithelial cells compared with corresponding normal epithelial cells (11). Further studies with reverse transcriptase polymerase chain reaction (RT-PCR) and immunohistochemistry demonstrated CLP expression in normal breast, prostate, cervix and epidermal tissues but did not detect any CLP protein or RNA in corresponding tumor-derived cell lines. In addition, CLP expression was decreased in a small number (n=2) of primary breast carcinomas (18). Taken together, these data support the hypothesis that downregulation of CLP gene expression may accompany malignant transformation of breast epithelial cells.
In this study, we explore the hypothesis that CLP expression is reduced in breast cancer by comparing CLP expression using immunohistochemical analysis in 80 paraffin-embedded archived human primary breast cancer specimens of varying histologic type and axillary node status to that in normal human breast tissue. The correlation between CLP downregulation and the progression of breast lesions from localized (in situ carcinoma) to invasive (node-positive tumors) was also investigated. These data show that loss of CLP expression occurs early in malignancy and may thus be a marker of the transition from the normal to an aberrant epithelial phenotype.
Materials and Methods
Generation of CLP and Characterization of CLP Antibodies
Recombinant human CLP was expressed in Escherichia coli and purified to homogeneity by phenyl-Sepharose affinity chromatography, followed by ion exchange and gel filtration chromatography as described (16,19). Polyclonal rabbit antibodies were raised against a peptide corresponding to the C-terminal residues 127 to 148 of CLP (the most divergent region between CLP and calmodulin; (15)). The peptide was synthesized in the Mayo Protein Core and attached via an extra cysteine residue to keyhole limpet hemocyanin before use as antigen. Rabbits were maintained and treated at Cocalico, Inc, Reamstown, PA. Antisera were obtained with good specificity for CLP; however, they showed considerable cross-reactivity with calmodulin (which shares 85% identity with CLP) on Western blots. For use in immunohistochemistry, one of the CLP antisera (TG7) was therefore affinity-purified by chromatography over Poros-Protein-A (PerSeptive Biosystems, Cambridge, MA), followed by adsorption of calmodulin-cross-reacting antibodies on calmodulin-agarose (Sigma, St. Louis, MO). CLP-specific antibodies in the flow through of the calmodulin-agarose column were finally isolated by CLP-Sepharose chromatography. CLP-Sepharose was prepared by coupling purified recombinant CLP to CNBr-activated Sepharose 4B (Pharmacia, Piscataway, NJ) following the manufacturers' instructions. Purified CLP antibodies showed excellent sensitivity and specificity for CLP (Figure 1, lanes 1 and 2), whereas cross-reactivity with calmodulin was strongly reduced and observed with a sensitivity at least 50-fold lower than that for CLP (Figure 1, lanes 3 and 4).
Figure 1.
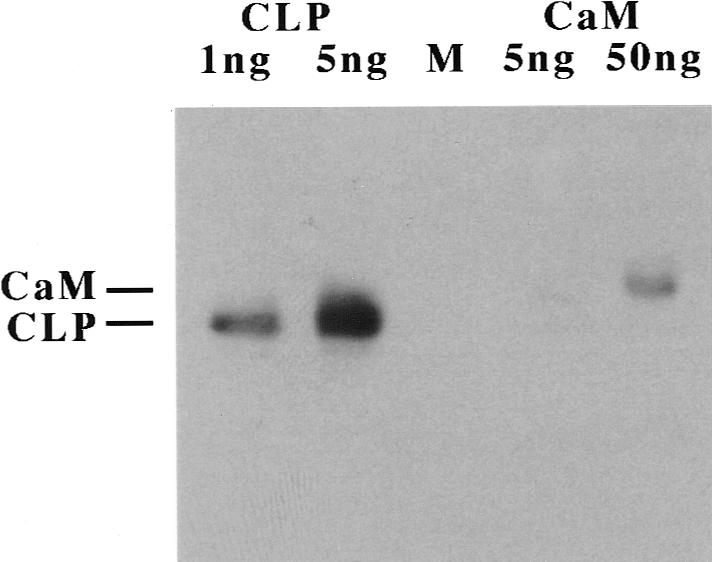
Western blot analysis to determine the specificity of CLP antibody TG7. Purified recombinant CLP (1 ng and 5 ng, left two lanes) or calmodulin (CaM) (5 ng and 50 ng, lanes labeled CaM) was added to 20 µg of carrier protein (from a total lysate of MCF-7 breast cancer cells that do not express CLP), electrophoresed on a 15% Sodium dodecyl sulfate-polyacrylamide gel, blotted onto a poly-vinylidene fluoride (PVDF) membrane, and the membrane probed with affinity-purified anti-CLP antibody TG7 (250 ng/mL). The position of CaM and CLP on the gel is indicated on the left. Note that the antibody easily detects 1 ng of CLP but shows no reaction with 5 ng of CaM and barely detects it at 50 ng. Also note the slower migration of CaM compared with CLP under the Ca2+-free conditions used (16). M, molecular mass marker lane.
Tissue Specimens
Tissue samples were obtained from the Mayo Clinic Tissue Registry under the IRB-approved protocol ‘Request to Examine Paraffin-Embedded Specimens of Normal and Tumor Tissue from Human Breast, Prostate, Cervix, and Colon for the Immunohistological Presence of Calmodulin-Like Protein’ (IRB 692–95). Five categories of breast cancer specimens were requested from the Registry for this study: Ductal carcinoma in situ (DCIS); invasive ductal carcinoma, axillary node negative (IDC-); invasive ductal carcinoma, axillary node positive (IDC+); invasive lobular carcinoma, axillary node negative (ILC-); and invasive lobular carcinoma, axillary node positive (ILC+). The 25 most recent specimens in each of these categories were requested. Of the 125 specimens requested, 83 were available at the time of this study. Samples were excluded if no paraffin-embedded tissue blocks or representative hematoxylin-eosin (H&E)-stained slides were available. Cases were excluded if no tumor was found in the H&E slides for available blocks or if tumor-containing blocks were unavailable. The pathology reports for all the specimens were reviewed to verify correct classification. A total of 80 breast cancer specimens was finally included in this study: 9 DCIS, 16 IDC-, 19 IDC+, 24 ILC-, 12 ILC+. In addition, seven normal breast tissue specimens from patients who had undergone reduction mammoplasty were included as controls.
Immunohistochemistry
The staining technique was modified from the avidin-biotin peroxidase method of Hsu et al (20) and was performed essentially as described (21). Sections (4 µm) of paraffin-embedded tissue specimens were mounted on positively charged slides, deparaffinized, and endogenous peroxidase activity was blocked by treatment with H2O2/methanol. After heat-induced epitope retrieval in 1 mmol/L EDTA, pH 8.0, for 20 minutes, and blocking of nonspecific protein-binding sites (in 5% normal goat serum in phosphate-buffered solution (PBS)/0.05% Tween-20), the sections were immunostained by sequential incubations in affinity-purified CLP antibody (40 µg/mL in PBS, 0.05% Tween-20, 1% normal goat serum), biotinylated goat anti-rabbit IgG (1:200 dilution, DAKO Corp, Santa Barbara, CA), and peroxidase-conjugated streptavidin (1:300 dilution). The sections were developed by incubation in 3-amino-9-ethylcarbazole in the presence of H2O2, counterstained with hematoxylin, and mounted with coverslips. Negative controls were incubated with anti-CLP IgG preabsorbed with purified CLP and showed no staining. Preabsorption with calmodulin caused no reduction in staining intensity (data not shown).
Evaluation of Immunostaining
Four independent observers (M.S.R., M.A.F., T.B.C., P.C.R.) evaluated the expression patterns of CLP in the breast cancer specimens, specifically the immunohistochemical staining intensity in tumor cells compared with that in adjacent normal epithelial cells. If no normal cells were present on a slide, then the normal breast tissue slides were used as the reference. For each specimen, the CLP-staining intensity in tumor cells was scored in relation to the CLP-staining intensity in normal cells using a three-point scale: decreased (-1), unchanged (0), and increased (+1). After the four observers independently scored the cancer specimens, the scores were reviewed for consistency. A conclusive score was assigned to a specimen only if at least three of the four observers reported the identical score for that specimen. If three observers did not report the same score, the specimen's score was designated ‘inconclusive.’
PCR Analysis of CLP Gene Status in Tumor Samples
Genomic DNA samples from microdissected breast tumor tissue and peripheral blood from six patients were a kind gift from Dr. J.S. Kovach (City of Hope National Medical Center, Duarte, CA). One microliter (corresponding to 400–800 ng DNA) of each sample was subjected to PCR in a 50-µL reaction with primers CLP-1051 (5′-CAC CCA CGC CGC GGC CGC TGG CAT GGC C-3′) and CLP-1536r (5′-GGT GGG CGC CGG CCT CAT TTG GAC AC-3′) by using a touchdown cycling profile (35 cycles) with a 94°C denaturation (30 seconds), an annealing temperature beginning at 64°C, decreasing 1°C per cycle for 8 cycles, and remaining at 56°C for the remainder of the profile (30 seconds), and an extension temperature of 72°C (1 minute). The resulting products were analyzed by electrophoresis in a 2% agarose gel and directly sequenced with the primer CLP-1534r (5′-TGG GCG AAG CTT TCA CTT GGA CAC CAG CAC ACG GAC AAA CTC CTC G-3′) in the Mayo molecular biology core facility.
Results
Staining for CLP Immunoreactivity in Normal Tissue and Breast Tumors
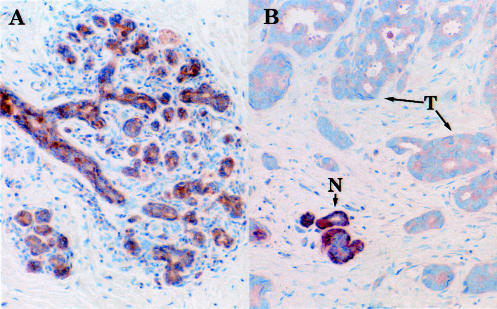
By using a polyclonal rabbit antihuman CLP antibody, expression of CLP was readily detected in epithelial cells lining the ducts of normal mammary glands (Figure 2A) as well as in histologically normal breast tissue adjacent to the tumors (Figure 2B). This finding is in agreement with the observation that CLP is specifically expressed in normal epithelial cells of a variety of organs including the breast (18). No CLP staining was observed in the myoepithelial or stromal cells or in the endothelial cells of blood vessels. This result independently confirms the specificity of our CLP antibody by demonstrating the absence of significant cross-reactivity with calmodulin (which is abundantly expressed in all cell types). CLP staining occurred primarily in the cytoplasm of the normal epithelial cells, but a small proportion of the cells (less than 5%) demonstrated nuclear staining in addition to cytoplasmic staining. In normal ducts, CLP staining was particularly intense in the basal portion of the epithelial cell cytoplasm.
Figure 2.
Immunohistochemical staining of normal breast tissue and breast tumors for CLP. Sections of normal and tumor tissue were stained with affinity-purified CLP antibody TG7 and counterstained with a light hematoxylin as described in Materials and Methods. (A) Normal breast tissue. (B) Invasive ductal carcinoma (node negative). Note the uniform and specific staining for CLP in the epithelial cells lining the ducts of a normal mammary gland (A) and the absence of staining in the surrounding myoepithelial and stromal cells. Staining for CLP is also evident in epithelial cells of normal-appearing tissue ‘islands’ (N) in the tumor specimen (B) but is weak or absent in the surrounding tumor cells (T).
In contrast with the normal epithelial cells, cells in the majority of tumors of all histological types showed a decrease in CLP immunoreactivity. No increase in CLP staining was observed in any of the tumor specimens. The decrease in overall CLP staining was observed equally in DCIS and in IDC-, IDC+, ILC-, ILC+. A representative example is shown in Figure 2B; the tumor cells in this ductal carcinoma are entirely devoid of CLP staining. The distinction of malignant and normal cells with respect to CLP staining was particularly striking in cases in which an island of normal-looking tissue was found interspersed with the tumor tissue (N versus T in Figure 2B). Nuclear CLP staining was rarely seen in the tumor cells.
Downregulation of CLP Expression in Breast Tumors
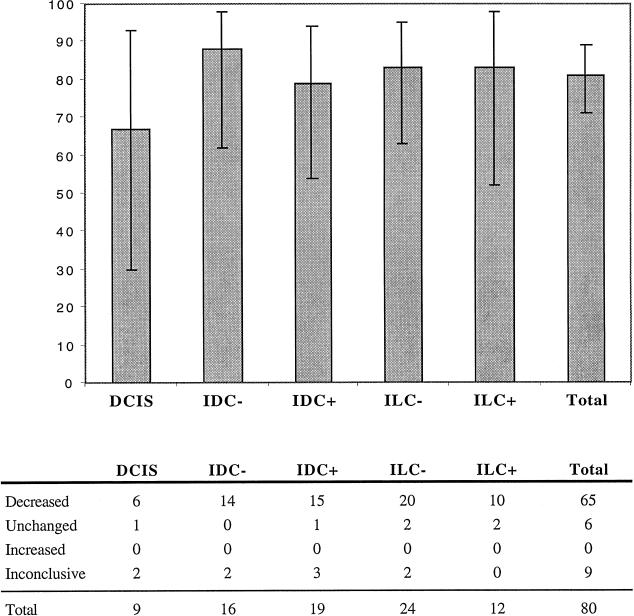
To obtain information on the prevalence of CLP downregulation in different breast tumors, an immunohistochemical analysis of CLP expression was performed on 87 archival specimens. The control group consisted of seven normal human breast specimens. The experimental group consisted of 80 archival human breast cancer specimens divided into subgroups based on the histological type and axillary nodal status at the time of surgical resection of the cancer. Sections of each specimen were stained for CLP and evaluated by four independent observers as described in Materials and Methods. The results are summarized in Figure 3. A decrease in CLP staining was obvious in a majority of all tumor types when compared to adjacent normal tissue or the average staining intensity of normal control tissue. Reduction or complete loss of CLP staining was found in 67% of DCIS (n=9), 88% of IDC- (n=16), 79% of IDC+ (n=19), 83% of ILC- (n=24), and 83% of ILC+ (n=12) tumors. In a minority of cases (ranging from 0%–17% in the different tumor types), no change in staining was found in the tumors compared to normal tissue. Although the fraction of samples with reduced CLP staining appears to be somewhat lower in DCIS than in the other tumors, this is not significant (Fisher's exact test) and can be explained by the relatively large number (22%) of ‘inconclusive’ samples in this rather small cohort (Figure 3). Specimens listed as inconclusive generally were those where two of the observers reported a decrease in CLP staining and the other two observers reported no change. Indeed, this was the case for the two ‘inconclusive’ DCIS specimens and for 7 of the 9 inconclusive samples listed in Figure 3.
Figure 3.
Results of CLP immunostaining in breast tumors compared with normal breast epithelium. Top, percent of samples showing a decrease in CLP staining relative to adjacent normal tissue; 95% confidence intervals are indicated. Bottom, summary of the results. Decreased, unchanged, and increased indicate agreement among at least three of four observers that CLP staining was decreased, unchanged, or increased, respectively, relative to adjacent normal tissue. Inconclusive indicates disagreement on the scoring by at least two of the four observers.
PCR Analysis of CLP Gene Status in Tumors
Six tumors and peripheral blood from the same patients were analyzed for the presence of an intact CLP gene. PCR analysis of DNA from both tumor and peripheral blood yielded 480 bp products (corresponding to the CLP gene) in all samples (data not shown). Direct sequencing of these PCR products showed no differences between these sequences and the published CLP sequence (GenBank accession number X13461). Together with data from Yaswen et al (18), these results show that the downregulation of CLP expression in breast cancer occurs at the transcriptional level and is not due to the loss of the CLP gene or to deleterious mutations in its coding sequence.
Discussion
Calmodulin-like protein represents the closest relative of calmodulin in humans, yet its highly epithelial-specific expression, overlapping but distinct biochemical properties (16,17), and apparently different role in cell growth and differentiation suggest that it is not an isoform of calmodulin. This is also supported by the inability of CLP to rescue the lethal calmodulin knockout phenotype in yeast (22). Moreover, the apparent incompatibility of CLP expression with a transformed cell phenotype is opposite to the properties of calmodulin, which is required for cell cycle progression and upregulated in many transformed cells (5–9,23).
Downregulation of CLP expression has previously been noted in several carcinomas including lesions of breast, prostate, and cervix (18), but only small numbers of primary tumor samples have been studied so far. The data presented here on the expression of CLP in 80 breast cancer specimens of various histological types and axillary node status demonstrate for the first time that a reduction or loss of CLP expression is a frequent finding in breast cancers of all types. The decrease in CLP expression, as determined by immunohistochemical staining of archival paraffin-embedded specimens, is evident in localized DCIS and equally striking in the more advanced ILC+ and IDC+. None of the tumor specimens showed an increase in CLP staining, underlining the strong correlation between a downregulation (and not merely dysregulation) of CLP and the malignant cell phenotype. In some cases where histologically normal tissue is tightly interspersed with tumorigenic tissue, the difference in CLP reactivity is particularly striking: ‘Islands’ of CLP-positive epithelial cells lining a morphologically normal duct are found immediately adjacent to CLP-negative tumor cells. The downregulation of CLP expression appears to be specific for malignant cells because CLP staining in a small number of benign breast hyperplasia samples is unaffected (M.S.R., unpublished observation).
Previous studies with models for tumorigenesis, combined with the present data on primary breast tumors show that CLP downregulation likely occurs as a result of decreased CLP gene transcription. The existence of some residual CLP staining in tumor samples, combined with our ability to amplify the CLP gene from six out of six tumor samples, suggests that the CLP gene remains intact during the transition from the normal to the tumorigenic phenotype. Because our PCR primers flank only the coding portion of the gene, the possibility of mutations/deletions in the promoter and/or the untranslated regions of the CLP gene cannot be excluded. Regardless, however, our data strongly suggest that CLP downregulation during breast cancer development occurs at the transcriptional level. This is further supported by data published by Yaswen et al (18), who demonstrated that in immortalized cells and established breast cancer cell lines, the decrease in CLP protein levels is always accompanied by a decrease in CLP mRNA levels. Although the mechanism of the transcriptional downregulation of CLP expression is unknown, aberrant cytosine methylation of the gene's regulatory regions is an attractive possibility. Hypermethylation of selective cytosine residues in CpG dinucleotides has been shown to be a frequent epigenetic lesion leading to the silencing of several tumor suppressor genes such as the p16 cyclin-dependent kinase inhibitor or the Van Hippel Lindau gene (reviewed in (24)). Site-specific CpG hypermethylation has also recently been shown to be responsible for the tumor-specific repression of the S100A2 gene (25). Like CLP, S100A2 is a member of the EF-hand family of Ca2+ binding proteins, and its expression in normal breast epithelial cells and downregulation in tumor cells are reminiscent of the expression pattern of CLP.
Whereas it is now clear that CLP downregulation is a common event in breast tumorigenesis, the role of CLP in normal cells is still unknown. It is unlikely, however, that CLP is simply a calmodulin isoform. Experiments with gel overlay assays indicate that CLP interacts with a number of cellular proteins distinct from those recognized by calmodulin, and a recent two-hybrid search identified an unconventional myosin as a potential CLP-specific interacting protein (M.S.R. and E.E.S., unpublished observation). Participation in regulating a molecular motor-driven process in the nucleus (nuclear CLP staining is observed in a small fraction of normal cells) or the cytoplasm of epithelial cells would be an attractive possibility for CLP's function. Whatever its role may be, the fact that CLP expression is strongly reduced early in the transition of an epithelial cell toward a malignant phenotype suggests that the expression of CLP can serve as a marker for the nonmalignant state.
Because loss of CLP expression appears to occur early in tumorigenesis and is observed in all breast tumors, determination of the CLP expression status is unlikely to provide prognostic information concerning disease progression. Rather, the untimely loss of CLP expression appears to be linked with a failure of epithelial cells to undergo normal terminal differentiation. Unraveling the processes that are affected by the lack of CLP may, however, allow the development of intervention strategies to reverse the transition to malignancy, even at later stages in tumorigenesis.
Acknowledgements
We thank Tammy Greenwood (Mayo Foundation, Rochester, MN) for help with the characterization of antibodies. Dr. John S. Kovach (City of Hope National Medical Center, Duarte, CA) for supplying tumor and normal DNA, Steve Ziesmer (Mayo Immunohistochemistry Lab, Rochester, MN) for expert help with immunohistochemistry, and Dr. Vera Suman (Mayo Cancer Center, Rochester, MN) for assistance with data analysis. Supported by grants from the Breast Cancer Research Foundation (BCR #1-01), the National Cancer Institute (P20 CA 65800-01), and the Fraternal Order of Eagles' Cancer Research Fund (Eagles #157). M.S.R. was supported by predoctoral training grants from the U.S. Army Medical Research and Materiel Command (DAMD17-94-J-4116) and from the National Cancer Institute (T32CA75926).
Abbreviations
- CLP
calmodulin-like protein
- DCIS
ductal carcinoma in situ
- IDC-
invasive ductal carcinoma—node negative
- IDC+
invasive ductal carcinoma—node positive
- ILC-
invasive lobular carcinoma—node negative
- ILC+
invasive lobular carcinoma—node positive
- H&E
hematoxylin-eosin
References
- 1.Boring CC, Squires TS, Tong T, Montgomery S. Cancer statistics. CA Cancer J Clin. 1994;44:7–26. doi: 10.3322/canjclin.44.1.7. [DOI] [PubMed] [Google Scholar]
- 2.Walker RA, Varley JM. The molecular pathology of human breast cancer. Cancer Surv. 1993;16:31–57. [PubMed] [Google Scholar]
- 3.Wolman SR, Heppner GH, Wolman E. New directions in breast cancer research. FASEB J. 1997;11:535–543. doi: 10.1096/fasebj.11.7.9212077. [DOI] [PubMed] [Google Scholar]
- 4.de la Chapelle A, Peltomäki P. The genetics of hereditary common cancers. Curr Op Genetics & Development. 1998;8:298–303. doi: 10.1016/s0959-437x(98)80085-3. [DOI] [PubMed] [Google Scholar]
- 5.Rasmussen CD, Means AR. Calmodulin is involved in regulation of cell proliferation. EMBO J. 1987;6:3961–3968. doi: 10.1002/j.1460-2075.1987.tb02738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rasmussen CD, Means AR. Calmodulin is required for cell-cycle progression during G1 and mitosis. EMBO J. 1989;8:73–82. doi: 10.1002/j.1460-2075.1989.tb03350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu KP, Means AR. Regulation of the cell cycle by calcium and calmodulin. Endocrine Rev. 1993;14:40–58. doi: 10.1210/edrv-14-1-40. [DOI] [PubMed] [Google Scholar]
- 8.Takemoto D, Jilka K. Increased content of calmodulin in human leukemic cells. Leukemia Res. 1983;7:97–100. doi: 10.1016/0145-2126(83)90062-0. [DOI] [PubMed] [Google Scholar]
- 9.Wei J-W, Hickie RA. Increased content of calmodulin in Morris hepatoma 5123 tc (h) Biochim Biophys Res Commun. 1981;256:1562–1568. doi: 10.1016/0006-291x(81)90697-5. [DOI] [PubMed] [Google Scholar]
- 10.Takuwa N, Zhou W, Takuwa Y. Calcium, calmodulin and cell cycle progression. Cell Signalling. 1995;7:93–104. doi: 10.1016/0898-6568(94)00074-l. [DOI] [PubMed] [Google Scholar]
- 11.Yaswen P, Smoll A, Peehl DM, Trask DK, Sager R, Stampfer MR. Down-regulation of a calmodulin-related gene during transformation of human mammary epithelial cells. Proc Natl Acad. Sci USA. 1990;87:7360–7364. doi: 10.1073/pnas.87.19.7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee SW, Tomasetto C, Swisshelm K, Keyomarsi K, Sager R. Down-regulation of a member of the S100 gene family in mammary carcinoma cells and reexpression by azadeoxycytidine treatment. Proc Natl Acad Sci USA. 1992;89:2504–2508. doi: 10.1073/pnas.89.6.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schafer BW, Heizmann CW. The S100 family of EF-hand calcium-binding proteins: functions and pathology. TIBS. 1996;21:134–140. doi: 10.1016/s0968-0004(96)80167-8. [DOI] [PubMed] [Google Scholar]
- 14.Rogers MS, Strehler EE. Calmodulin-like proteins. In: Celio M, editor. Guidebook to Calcium-Binding Proteins. Oxford, UK: Oxford University Press; 1996. pp. 41–43. [Google Scholar]
- 15.Koller M, Strehler EE. Characterization of an intronless human calmodulin-like pseudogene. FEBS Lett. 1988;239:121–128. doi: 10.1016/0014-5793(88)80558-1. [DOI] [PubMed] [Google Scholar]
- 16.Rhyner JA, Koller M, Durussel-Gerber I, Cox JA, Strehler EE. Characterization of the human calmodulin-like protein expressed in Escherichia coli. Biochemistry. 1992;31:12826–12832. doi: 10.1021/bi00166a017. [DOI] [PubMed] [Google Scholar]
- 17.Edman CF, George SE, Means AR, Schulman H, Yaswen P. Selective activation and inhibition of calmodulin-dependent enzymes by a calmodulin-like protein found in human epithelial cells. Eur J Biochem. 1994;226:725–730. doi: 10.1111/j.1432-1033.1994.tb20101.x. [DOI] [PubMed] [Google Scholar]
- 18.Yaswen P, Smoll A, Hosoda J, Parry G, Stampfer MR. Protein product of a human intronless calmodulin-like gene shows tissue-specific expression and reduced abundance in transformed cells. Cell Growth Differentiation. 1992;3:335–345. [PubMed] [Google Scholar]
- 19.Qian H, Rogers MS, Schleucher J, Edlund U, Strehler EE, Sethson I. Sequential assignment of 1H, 15N, 13C resonances and secondary structure of human calmodulin-like protein determined by NMR spectroscopy. Protein Sci. 1998;7:2421–2430. doi: 10.1002/pro.5560071120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu S-M, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- 21.Hartmann LC, Ingle JN, Wold LE, Farr G, Jr, Grill JP, Su JQ, Maihle NJ, Krook JE, Witzig TE, Roche PC. Prognostic value of c-erbB2 overexpression in axillary lymph node positive breast cancer. Results from a randomized adjuvant treatment protocol. Cancer. 1994;74:2956–2963. doi: 10.1002/1097-0142(19941201)74:11<2956::aid-cncr2820741111>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 22.Harris E, Yaswen P, Thorner J. Gain-of-function mutations in a human calmodulin-like protein identify residues critical for calmodulin action in yeast. Mol Gen Genet. 1995;247:137–147. doi: 10.1007/BF00705643. [DOI] [PubMed] [Google Scholar]
- 23.Hickie RA, Graham MJ, Buckmeier JA, Meyskens FL., Jr Comparison of calmodulin gene expression in human neonatal melanocytes and metastatic melanoma cell lines. J Invest Dermatol. 1992;99:764–773. doi: 10.1111/1523-1747.ep12614725. [DOI] [PubMed] [Google Scholar]
- 24.Baylin SB, Herman JG, Graff JR, Vertino PM, Issa JP. Alterations in DNA methylation: a fundamental aspect of neoplasia. Adv Cancer Res. 1998;72:141–196. [PubMed] [Google Scholar]
- 25.Wicki R, Franz C, Scholl FA, Heizmann CW, Schafer BW. Repression of the candidate tumor suppressor gene S100A2 in breast cancer is mediated by site-specific hypermethylation. Cell Calcium. 1997;22:243–254. doi: 10.1016/s0143-4160(97)90063-4. [DOI] [PubMed] [Google Scholar]