Abstract
We have previously suggested that the inhibition of RNA polymerase II-mediated transcription after exposure to UV light promotes the accumulation of p53 and the induction of apoptosis (Oncogene 13, 823–831). However, it was not clear whether p53 induction was contributing to apoptosis. Here we report that apoptosis is triggered at lower UV doses in p53-deficient Li-Fraumeni syndrome (LFS) and human papillomavirus (HPV) E6 expressing fibroblasts than in normal cells, suggesting that p53 can be protective against UV-induced apoptosis. There is no significant difference in the effect of UV-irradiation on the cell cycle distribution of normal and primary LFS fibroblasts. Importantly, the recovery of nascent mRNA synthesis in all p53-deficient fibroblasts is significantly impaired compared with control cells after exposure to relevant doses of UV light. Taken together, our results suggest that wild-type p53 can protect cells against UV-induced apoptosis by facilitating the recovery of transcription. Furthermore, we suggest that the capacity of cells to recover transcription after genotoxic damage is an important determinant of sensitivity to apoptosis.
Keywords: apoptosis, cell cycle arrest, DNA repair, p53, transcription
Introduction
The p53 tumor suppressor protein plays an important role in the protection against neoplastic transformation. The p53 gene is mutated in at least 50% of sporadic tumors (1). The inheritance of a mutant allele of p53 can cause Li-Fraumeni syndrome (LFS), which is characterized by the very early onset of a wide variety of neoplasms (2). Furthermore, the human papillomavirus (HPV) E6 protein promotes the degradation of p53, and this activity is thought to be important in the development of HPV-induced cervical cancers (1). The protection conferred by p53 against neoplastic transformation is at least in part related to the role of p53 in protection against the mutagenic effects of genotoxic agents (1). p53 is thought to exert its effect by promoting cell cycle arrest, enhancing apoptosis, and stimulating DNA repair (3). Interestingly, these functions of p53 can have opposing effects on the survival of cells after genotoxic stress (3).
We and others have previously reported a strong correlation between the sustained induction of p53 and the induction of apoptosis in primary human fibroblasts after exposure to UV light (4–6). We also found a similar relationship after exposure to cisplatin and the transcription inhibitors: 5,6-dichloro-1-b-d-ribofuranosylbenzimidazole (DRB), 1-(5-isoquinolinylsulfonyl)-3-methylpiperazine (H7), actinomycin D, and α-amanitin (7). Although there was a tight correlation between the induction of p53 and apoptosis, it was unclear whether p53 was contributing to this mechanism of cell death. Therefore, we studied the role of p53 in UV-induced apoptosis in LFS fibroblasts and primary embryonic fibroblasts expressing the HPV-E6 protein. In contrast to the prevailing dogma, we found that all p53-deficient fibroblasts had a lower threshold for the induction of apoptosis after UV-irradiation. The apparent protection against apoptosis conferred by wild-type p53 did not appear to result from the establishment of cell cycle checkpoints but correlated with an enhanced recovery of mRNA synthesis compared with p53-deficient fibroblasts. We suggest that p53 protects cells from UV-induced apoptosis by enhancing the recovery of transcription.
Materials and Methods
Cell Culture
Normal neonatal foreskin fibroblasts (NF) were provided by Drs. Mary Davis and Theodore Lawrence (University of Michigan). A second normal skin fibroblast strain (AG1522), xeroderma pigmentosum group C cells (GM671), and Cockayne syndrome group B cells (GM1629) were obtained from Coriell Repositories (Camden, NJ). Primary skin fibroblasts derived from LFS patients MDAH041 and MDAH087 (041wt/mut and 087wt/mut), and hemizygous immortalized sublines expressing only mutant p53 from these patients (041mut and 087mut) were provided by Michael Tainsky (Wayne State University). Immunoblots with a p53 monoclonal antibody (AB2, Oncogene Science Cambridge, MA) yielded the predicted p53 expression patterns for these cells (data not shown) (8). WI38 embryonic lung fibroblasts, WS1 embryonic skin fibroblasts and HPV-E6 expressing sublines (WI38-E6 and WS1-E6) were kindly provided by Geoffrey Wahl (Salk institute). All primary cells were used before passage 20. All cells were grown in minimal essential medium supplemented with 10% fetal bovine serum. Transduced cells were maintained in the presence of 200 µg/mL G418 (Sigma, St. Louis, MO).
mRNA Synthesis
The recovery of mRNA synthesis was performed as previously reported (4). Briefly, cycling cells were labeled for 2 to 3 days in [14C]thymidine. Cells were UV-irradiated with a UV source emitting at 254 nm and at various times after irradiation, cells were incubated for 30 minutes in the presence of [3H]uridine. Polyadenylated RNA was isolated from cell lysates with the Straight A's mRNA isolation system (Novagen, Madison, WI). The unbound fraction was retained and used to assess the amount of nascent nonpolyadenylated RNA. Incorporation of [3H]uridine and [14C]thymidine was quantified with a scintillation counter. [3H] counts were normalized to [14C] counts and the recovery of mRNA and nonpolyadenylated RNA were expressed as the proportion of incorporated [3H]uridine in UV-treated to mock-treated controls.
Apoptosis and Cell Cycle Distribution
Cells were prepared for flow cytometric analysis as previously described (4,9). Analysis was performed with a Coulter Epics Elite Cell Sorter (Miami, FL) and the Multicycle software package (Phoenix Flow Systems, San Diego, CA). Where indicated, zVAD-fmk (CalBiochem, Cambridge, MA) was added to the medium to a final concentration of 25 µmol/L 30 minutes before UV irradiation and was maintained in the medium after UV irradiation.
Results
Wild-Type p53 is Protective Against Apoptosis Induced by Moderate Doses of UV Light
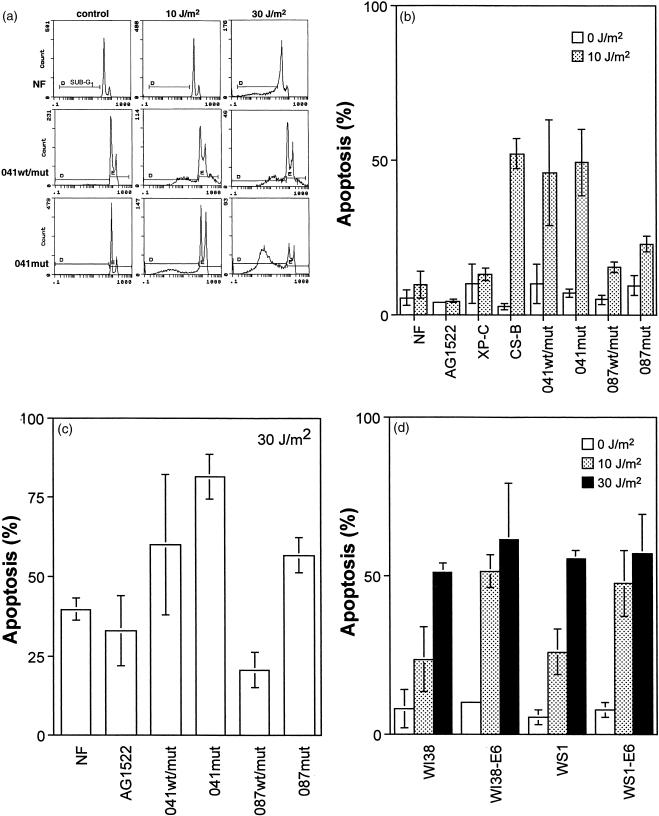
p53 has been reported to be important for the induction of apoptosis after exposure to UV light (10,11). Recently, the induction of apoptosis in normal fibroblasts was found to be highly dose dependent and correlated strongly with a robust induction of p53 (4–6). However, it was unclear whether this dose-dependent threshold for the induction of apoptosis was related to wild-type p53 function. Consistent with the previous reports (4–6), we report here that normal fibroblast and XP-C strains did not undergo apoptosis after exposure to 10 J/m2 UV light, but these strains were sensitive to the induction of apoptosis at 30 J/m2 (Figure 1A–C). However, a significant increase in the sub-G1 population of cells was induced even after exposure to doses as low as 10 J/m2 UV light in all four LFS fibroblast strains (Figure 1A and B). In fact the induction of apoptosis in 041wt/mut and 041mut cells was similar to the extent of apoptosis observed in Cockayne syndrome group B (CS-B) cells (Figure 1B), which are known to be transcription-coupled repair (TCR)-deficient (12) and hypersensitive to UV-induced apoptosis (4,5). Furthermore, HPV-E6 expression in embryonic fibroblasts sensitized cells to UV-induced apoptosis indicating that p53 was protective against apoptosis at these doses of UV light (Figure 1D). After exposure to 30 J/m2, significant apoptosis was induced in all fibroblast strains tested (Figure 1A, C, D). Clearly, the effect of p53 disruption on the sensitivity of fibroblasts to UV-induced apoptosis was highly dose-dependent. Whereas p53 disruption sensitized all cells to apoptosis at moderate doses of UV light, this was not evident in all cells after exposure to 30 J/m2 (Figure 1C and D). We conclude that p53 is protective against the induction of apoptosis after exposure to moderate but not high doses of UV light.
Figure 1.
p53-deficient fibroblasts are hypersensitive to apoptosis induced by moderate doses of UV light. (A) Irradiated (10 or 30 J/m2) and unirradiated fibroblasts were collected 72 hr after treatment, fixed in ethanol, and stained with propidium iodide. The proportion of cells with a sub-G1 DNA content was determined by flow cytometry for normal, XP-C, CS-B, and LFS cells. Representative experiments for normal, 041wt/mut and 041mut fibroblasts are presented. (B) UV doses of 10 J/m2 induced apoptosis in CS-B and all LFS fibroblasts but not in normal (NF or AG1522) or XP-C fibroblasts (P>.20). After exposure to 10 J/m2 UV light, there was a significant increase in the sub-G1 population in 041wt/mut (P<.05), 041mut (P<.005), 087wt/mut cells (P<.05) and 087mut (P<.02) compared with the normal fibroblast strains. (C) Apoptosis was induced in all fibroblasts after exposure to 30 J/m2. (D) The induction of apoptosis after exposure to 10 J/m2 UV light was significantly elevated in WI38-E6 (P<.05) and WS1-E6 (P<.02) cells compared with their respective parental strains. Values in (B), (C) and (D) represent the mean (±standard error) from a minimum of three independent experiments. P values were determined by using 1-tailed Student t tests.
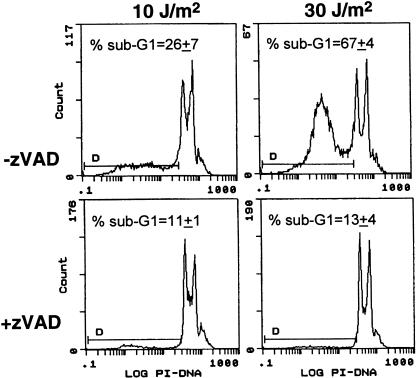
The immortalized LFS strains used in this study were thought to be resistant to UV-induced apoptosis compared with heterozygous parental strains (11). Therefore, to ensure that the increase we observed in the sub-G1 population of cells represented apoptosis, experiments were performed in which UV-irradiated cells were incubated in the presence of the caspase inhibitor zVAD-fmk. Caspases are proteases important for the execution of apoptosis, and inhibition of caspase activity can prevent the generation of several characteristics associated with apoptosis (13). The UV-induced generation of a sub-G1 population of cells was efficiently blocked by incubation of UV-irradiated cells in the presence of the caspase inhibitor zVAD-fmk (Figure 2). These results indicate that the sub-G1 population of cells detected in our study arose in the course of caspase-mediated apoptosis.
Figure 2.
Incubation of UV-irradiated LFS fibroblasts with the caspase inhibitor zVAD-fmk blocks the generation of a sub-G1 population of cells. 041mut cells were incubated with the caspase inhibitor zVAD-fmk (25 µmol/L) during the post-UV-incubation period (48 hr). Values inset represent the mean sub-G1 population (±standard error) from two independent experiments.
Ultraviolet Light Did Not Induce G1 Arrest in Primary Human Fibroblasts
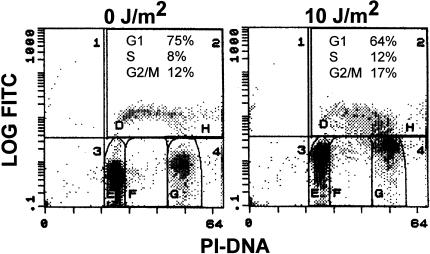
G1 arrest mediated by p21WAF1/CIP1 has been suggested to be protective against UV-induced apoptosis (5,14). To determine whether the protection conferred by wild-type p53 against UV-induced apoptosis in normal fibroblasts was related to p53-mediated cell cycle arrest, the cell cycle distribution of UV-irradiated cells was assessed by flow cytometry of propidium iodide-stained cells. Surprisingly, we find no evidence for a G1 arrest in any of the fibroblast strains examined after exposure to 10 J/m2 UV light (Table 1). In fact, there was a decrease in the proportion of cells in G1 and an increase in the proportion of cells in S phase in normal and LFS strains, 24 hours after UV irradiation (Table 1). Importantly, the increase in the proportion of cells in S phase and the decrease in the proportion of cells in G1 phase was similar in normal and heterozygous LFS cells (Table 1). The absence of a UV-induced G1 arrest in normal fibroblasts may be considered to be somewhat surprising; therefore the effect of UV irradiation on the cell cycle distribution of normal fibroblasts was assessed by two-parameter flow cytometry. Quantitatively similar results were obtained using this more sensitive method. (Compare Figure 3 and Table 1). Therefore, the protective effect of p53 against UV-induced apoptosis in normal fibroblasts after exposure to 10 J/m2 UV light appears to involve a mechanism other than the activation of a G1 checkpoint.
Table 1.
Cell Cycle Distribution of UV-Irradiated Normal and Li-Fraumeni Syndrome Cells.
| Cells | G1 | S | G2/M | ||||||
| C* | UV | Ratio | C | UV | Ratio | C | UV | Ratio | |
| NF | 68±2† | 59±5 | 0.87 | 18±1 | 21±4 | 1.17 | 14±1 | 20±3 | 1.43 |
| 041wt/mut | 43±0 | 36±1 | 0.84 | 33±2 | 41±4 | 1.24 | 23±2 | 24±3 | 1.04 |
| 087wt/mut | 59±5 | 52±1 | 0.88 | 28‖6 | 27±1 | 1.29 | 20±4 | 21±2 | 1.05 |
| 041mut | 55±7 | 25±2 | 0.45 | 21±0 | 56±5 | 2.00 | 17±3 | 19±4 | 1.12 |
| 087mut | 43 | 28 | 0.65 | 24 | 22 | 0.92 | 33 | 50 | 1.52 |
C, unirradiated control; UV, treated with 10 J/m2; ratio=% UV treated to % control.
Percentage of cells in the indicated phase of the cell cycle (± standard error).
Figure 3.
G1 arrest is not induced in normal fibroblasts exposed to 10 J/m2 UV light. UV-treated (10 J/m2) and unirradiated normal fibroblasts were assessed by two-parameter flow cytometry as described in Materials and Methods. The distribution of cells in various phases of the cell cycle is inset in each panel.
p53 Disruption Delays the Recovery of mRNA Synthesis After UV Irradiation
TCR is a specialization of nucleotide excision repair which is thought to remove transcription-blocking UV lesions to permit the rapid recovery of mRNA synthesis (12). We have previously found that it is TCR and not GGR that is protective against UV-induced apoptosis (4,5). Recently, we reported that a series of transcription inhibitors all induced apoptosis and p53 at doses that significantly inhibited mRNA synthesis without inducing detectable DNA strand breaks (7). Based on these results, we have hypothesized that stalled RNA polymerase II may signal both p53 accumulation and the induction of apoptosis (4,5,7). Although the induction of p53 and apoptosis occurred at similar doses, it was not clear whether p53 was required for apoptosis when transcription was impaired. Because the LFS and HPV-E6 expressing cells were found to be hypersensitive to UV-induced apoptosis (see Figure 1), we sought to determine if these cells were impaired in their capacity to recover mRNA synthesis.
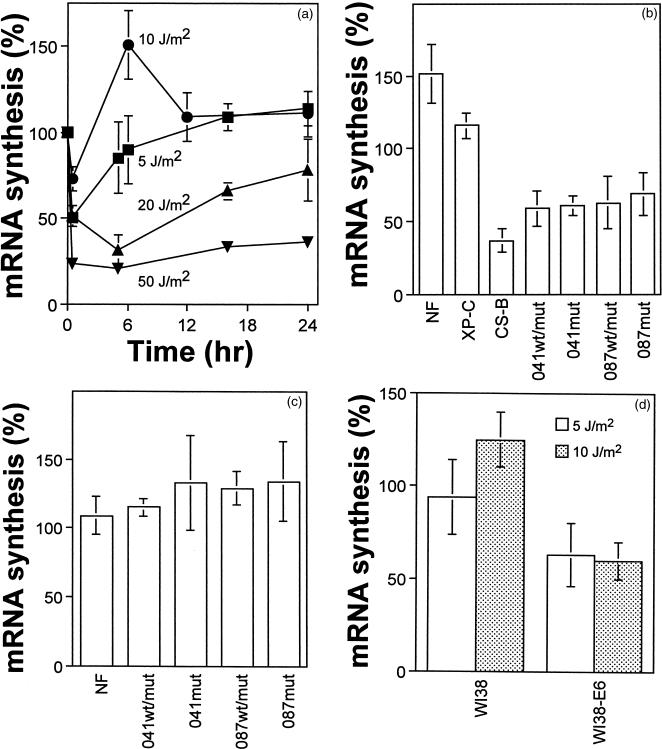
The time course for the recovery of nascent mRNA synthesis was assessed in normal fibroblasts after exposure to several doses of UV light. Nascent mRNA synthesis recovered within 6 hours after either 5 or 10 J/m2, but at higher UV doses there was a striking delay in the recovery of nascent mRNA synthesis (Figure 4A). The recovery of mRNA synthesis at 6 hours after UV irradiation reflected the TCR capacity of cells because TCR-proficient XP-C cells recovered mRNA synthesis within this time, but TCR-deficient CS-B cells did not (Figure 4B). Interestingly, not only did mRNA synthesis recover fully in normal fibroblasts within 6 hours, it exceeded that in unirradiated control cells suggesting that UV-irradiated cells compensate for the initial inhibition of transcription by increasing the rate of initiation of transcription, as recently suggested (15). Because the normal and p53-deficient cells differed in their sensitivity to apoptosis after exposure to 10 J/m2, the recovery of nascent mRNA synthesis was assessed in p53-deficient fibroblasts after this dose.
Figure 4.
The recovery of mRNA synthesis after UV-irradiation is impaired in p53-deficient fibroblasts. (A) The time course for the recovery of mRNA synthesis in normal fibroblasts was determined after exposure to 5 (squares), 10 (circles), 20 (triangles), and 50 J/m2 (inverted triangles) of UV light. The synthesis of nascent mRNA was reduced (P<.0025) in all LFS strains compared with normal fibroblasts at 6 (B) but not 12 hr (C) after exposure to 10 J/m2 of UV light. As observed in normal cells, TCR-proficient XP-C fibroblasts recovered mRNA synthesis within 6 hr but CS-B cells did not. As observed in LFS fibroblasts, there was a significant decrease in the synthesis of nascent mRNA in WI38-E6 cells compared with parental cells 6 hr after exposure to 10 (shaded; P<.005) but not 5 J/m2 (hollow; 0.25>P>0.10). Each point represents the mean (± standard error) from between 3 and 12 determinations.
The initial decrease in mRNA synthesis was similar in all fibroblasts (data not shown). In striking contrast to the enhanced recovery of mRNA synthesis observed in normal fibroblasts after exposure to 10 J/m2, LFS fibroblasts did not recover mRNA synthesis within 6 hours (Figure 4B). However, mRNA synthesis recovered fully in these cells within 12 hours (Figure 4C). Similarly, HPV-E6 expressing cells did not recover mRNA synthesis within 6 hours after exposure to 10 J/m2 (Figure 4D). Interestingly, the recovery of nonpolyadenylated RNA was not consistently reduced in LFS fibroblasts compared with normal cells (data not shown). These results support a role for p53 in a process required for efficient recovery of RNA polymerase II-mediated transcription after UV irradiation.
Discussion
Wild-Type p53 is Protective Against UV-Induced Apoptosis at Moderate UV Doses
The ability of p53 to contribute to the induction of apoptosis is well documented (1). We have previously suggested that persistent transcription-blocking UV lesions trigger the induction of p53 and apoptosis (4,5). Furthermore, we have reported a strong correlation between the inhibition of transcription and the induction of p53 and apoptosis after treatment of cells with a series of transcriptional inhibitors (7). Although p53 induction strongly correlated with the induction of apoptosis in our previous studies, it was not clear whether p53 induction was required for apoptosis. Here we show that both primary and spontaneously immortalized LFS fibroblasts were sensitive to the induction of apoptosis after exposure to 10 J/m2 of UV light. The generation of a sub-G1 population of cells in LFS cells could be inhibited by incubation of UV-irradiated cells in the presence of the caspase inhibitor zVAD-fmk (see Figure 2), indicating that the sub-G1 population of cells resulted from caspase-mediated apoptotic events. In contrast, two different normal fibroblast strains, one xeroderma pigmentosum group C and one ataxia telangiectasia fibroblast strain (data not shown) did not undergo apoptosis after this dose of UV light. We also found that HPV-E6 expressing embryonic fibroblasts were hypersensitive to apoptosis compared with their parental control strains. We conclude that the induction of apoptosis in response to UV-irradiation is not dependent on the induction of wild-type p53 in primary human fibroblasts.
p53 acts as a transcriptional activator of genes harboring a p53 recognition sequence. Target genes include the gene encoding the cyclin dependent kinase inhibitor p21WAF1/CIP1, which is required for p53-mediated G1 arrest after exposure of cells to ionizing radiation (16). We did not find any evidence of a G1 checkpoint in normal diploid fibroblasts after exposure to 10 J/m2 UV light. Also, there was no significant difference between the cell cycle distributions of UV-treated normal and heterozygous LFS cells, even though these latter strains were hypersensitive to UV-induced apoptosis. In our previous studies, we have found that this dose of UV light did not strongly induce p53 or p21WAF1/CIP1 in normal fibroblasts (4,5). Furthermore, we did not observe a strong G1 arrest after this dose in wild-type p53 expressing colorectal carcinoma cells, whereas ionizing radiation induced a pronounced G1 arrest (9). We suggest that UV light is not a good inducer of G1 arrest because p53 is only induced at UV doses that significantly affect transcription, and thus transactivation of p53-responsive genes such as p21WAF1/CIP1 is not efficient (5,17). We conclude that the protection conferred by p53 against UV-induced apoptosis is independent of a p53-mediated G1 arrest.
It is becoming well established that p53 contributes to the repair of UV-induced DNA damage (3,6,11,17–25). Therefore, one might expect that hypersensitivity to UV-induced apoptosis may stem from the previously reported DNA repair defects in these strains. However, 041wt/mut and 087wt/mut have near normal DNA repair capacity (11), but these cells are hypersensitive to UV-induced apoptosis (see Figure 1B). Furthermore, the 041mut, 087mut and HPV-E6 expressing strains have been reported to be GGR deficient but TCR proficient (11,18,19). Importantly, this DNA repair phenotype is very similar to that in XP-C cells (11,18,19), yet XP-C cells are as resistant as normal fibroblasts to apoptosis after exposure to 10 J/m2 UV light (Figure 1B) (4,5). Therefore, it appears that the DNA repair deficiencies reported in these p53-deficient fibroblasts can be dissociated from the hypersensitivity to UV-induced apoptosis reported here.
The Relationship Between Clonogenic Survival and Apoptosis
The assessment of cell death with short-term apoptosis or viability assays does not always correlate well with clonogenic survival assays (6,26). For example, a UV dose of 10 J/m2 results in a decrease in clonogenic survival of at least 60%, whereas this dose does not lead to the induction of apoptosis in normal fibroblasts (Table 2) (4–6). The lack of correlation between clonogenic survival and the sensitivity to UV-induced apoptosis is even more pronounced in XP-C cells. A dose of 10 J/m2 reduces clonogenic survival of XP-C fibroblasts by more than 99% (Table 2) but these cells are as resistant to UV-induced apoptosis as normal fibroblasts (4,5). In contrast, the percent decrease in clonogenic survival observed in the immortalized LFS cells correlates well with the induction of apoptosis (Table 2). Therefore, the induction of apoptosis is probably the primary mechanism contributing to the loss of clonogenic survival in these immortalized LFS fibroblasts, whereas clonogenic survival in normal and heterozygous LFS cells is greatly affected by one or more additional processes.
Table 2.
Relationship Between Clonogenic Survival and the Induction of Apoptosis After 10 J/m2 UV light.
| Cells | Decrease in CFA (%)* | Apoptosis (%)† |
| Normal | 61±8 | 8±3‡ |
| XP-C | >99 | 13±2 |
| 041wt/mut | 72±23 | 46±17 |
| 087wt/mut | 70±16 | 16±2 |
| 041mut | 35±4 | 49±11 |
| 087mut | 21±1 | 23±3 |
Values for the percent decrease (± standard error) in colony forming ability (43). Similar results were reported elsewhere (11,22).
Values for percent apoptosis in XP-C cells were determined from a single experiment but confirm our previous reports (4,5).
Mean determined by using two different normal fibroblast strains.
p53, TCR, and the Recovery of mRNA Synthesis
UV-induced cyclobutane pyrimidine dimer (CPD) are removed more rapidly from the transcribed strand of class II but not class I or class III genes. It is thought that the recovery of RNA synthesis is limited primarily by the repair of transcription-blocking UV lesions (12). The LFS- and HPV-E6 expressing fibroblast strains used in this study are reportedly TCR proficient (11,18,19). Based on this DNA repair phenotype, one would predict that mRNA synthesis would recover efficiently in LFS- and HPV-E6 expressing fibroblasts after UV irradiation. In distinct contrast to this prediction, p53-deficient fibroblasts did not efficiently recover transcription. Whereas normal fibroblasts recovered transcription fully within 6 hours, there was no recovery of nascent mRNA synthesis within this time in LFS cells. These results support two previous studies in which subtle defects in the recovery of total RNA synthesis were reported in other primary LFS strains (6,20). However, specifically examining the recovery of the polyadenylated fraction of RNA, we show that wild-type p53 plays a very pronounced role in the recovery of transcription of class II genes. We have similarly found a role for p53 in the recovery of transcription in colon cancer cells stably expressing a temperature-sensitive allele of p53. At the permissive temperature, these cells recover mRNA synthesis significantly more efficiently than the same cells maintained at the restrictive temperature indicating that wild-type but not mutant p53 can stimulate the recovery of transcription (manuscript in preparation). At this point, it is not entirely clear whether the role of p53 in the recovery of mRNA synthesis is to enhance some aspect of the DNA repair process or to stimulate the recovery of transcription through another mechanism.
Although a role for p53 in TCR has been suggested (6,20–23,27), the strand-specific removal of UV lesions is apparently normal in the LFS- and HPV-E6 expressing strains examined here (Table 3) (11,18,19). However, one must consider that endonuclease-sensitive site assays such as those that used to assess TCR in these strains (11,18,19), only assess preincision steps in the repair of UV lesions. A potential role for wild-type p53 in a postincision process required to repair transcription-blocking UV lesions cannot be excluded.
Table 3.
Nucleotide Excision Repair, the Recovery of mRNA Synthesis and the Induction of Apoptosis.
| Cells | TCR* | GGR* | mRNA† | Apoptosis‡ |
| Normal | + | + | + | - |
| XP-C | + | - | + | - |
| CS-B | - | + | - | + |
| XP-A | - | - | - | + |
| 041wt/mut | + | + | - | + |
| 087wt/mut | + | - | - | + |
| 041mut | + | + | - | + |
| 087mut | + | - | - | + |
The transcription-coupled repair (TCR) and global genome repair (GGR) phenotypes of these fibroblasts have been reported (11,12,18).
The ability of cells to recover mRNA synthesis after UV-irradiation. Data for XP-C, CS-B and XP-A cells have been reported (4).
The sensitivity of cells to apoptosis induced by 10 J/m2 UV light. Data for XP-C, CS-B and XP-A cells have been reported previously (4,5).
An alternative mechanism by which p53 could enhance the recovery of mRNA synthesis after UV-irradiation is by stimulating the transcriptional bypass of UV-induced lesions in the transcribed strand of active genes (Figure 4). Previous studies have suggested that RNA polymerase II may be able to perform translesion RNA synthesis in both the human metallothionein gene and the rodent dihydrofolate reductase gene after exposure to 10 J/m2 of UV light (15,28). Interestingly, nascent mRNA synthesis recovered in normal fibroblasts within 6 hours after exposure to 10 J/m2 yet one would expect less than 70% of CPD to be removed from the transcribed strand of active genes within this time (11,18,29,30). Therefore, our results are consistent with the existence of a mechanism for translesion RNA synthesis. So, although the defect in the recovery of mRNA synthesis in p53-deficient cells is consistent with a defect in TCR, the role of p53 in the recovery of transcription may be at least partially dissociated from the excision of transcription-blocking UV lesions and could involve translesion RNA synthesis.
The best characterized activity of p53 is its sequence-specific DNA binding and transactivation function. However, it seems counterintuitive that the transcription of p53-regulated genes would be required to recover transcription. Interestingly, the levels of p53 regulated proteins in unstressed cells can be affected by wild-type p53 (31,32). Therefore, it is possible that the regulation of p53 responsive genes before UV exposure is important for the recovery of transcription (17). Alternatively, wild-type p53 could directly participate in this recovery process (17). These mechanisms are currently being investigated.
We have found a very strong correlation between resistance to UV-induced apoptosis and the ability of cells to recover mRNA synthesis (Table 3) (4,5). The defect in the recovery of mRNA synthesis in these p53-deficient fibroblasts was only observed at early times after UV irradiation, yet apoptosis was only detected at later times. Similarly, direct activation of death receptors in the tumor necrosis factor family by UV light has been invoked in UV-induced apoptosis (33–35). Activation of death receptors and the recruitment of adapter molecules occur within 30 minutes of UV irradiation. At this time, it is unclear what rate-limiting events delay the onset of apoptosis, but one could speculate that ongoing transcription of genes encoding inhibitors of these apoptotic pathways could be required to survive the activation of death receptors. At moderate doses of UV light, the rapid recovery of mRNA synthesis in normal fibroblasts may be sufficient to overcome activation of death pathways, whereas the delay in recovery of transcription in LFS- and HPV-E6 expressing cells leads to a commitment to apoptosis, which is only apparent at later times. Alternatively, stalled RNA polymerase II could trigger additional signaling events that are more sustained in cells that recover mRNA synthesis slowly. In either scenario, cells that have lost this biological function would be preferentially eliminated by apoptosis.
Does Hypersensitivity to UV-Induced Apoptosis in p53-Deficient Cells Contribute to the Latency Observed in the Development of Nonmelanoma Skin Cancers?
It is well established that p53 mutations are common in UV-induced nonmelanoma skin cancers, and these mutations are thought to arise at an early stage in the development of these cancers (36,37). It has been hypothesized that mutations in the p53 gene can act at two levels in skin carcinogenesis. First, p53-mutated cells are expected to be hypermutable, and second, it has been suggested that these cells are more resistant to UV-induced apoptosis (37). It has been estimated that as many as 4% of keratinocytes in sun-exposed skin express mutant p53 (38), but few, if any, of these cells will become neoplastic and only after a long latency period (37,39). Although patients with LFS show an early onset of a wide spectrum of internal tumors, there is no report of nonmelanoma skin cancer in LFS. Therefore, p53 disruption appears to overcome the latency associated with the development of many internal cancers but not of nonmelanoma skin cancers.
In this study, we show that p53 is not required for UV-induced apoptosis in human skin fibroblasts, but rather, p53 is protective against apoptosis induced by moderate doses of UV light in these cells. It is tempting to speculate that mutant p53-expressing keratinocytes could be preferentially eliminated by apoptosis after sunlight exposure. Thus, a critical rate-limiting step in the development of these tumors would be to overcome UV-induced apoptosis. Interestingly, almost all basal cell carcinomas express high levels of the antiapoptotic protein Bcl-2 (40). A related antiapoptotic protein, Bcl-XL, may contribute to the development of squamous cell carcinomas (41). Therefore, the Bcl-2 family of proteins is likely to play an important role in overcoming the induction of apoptosis in nonmelanoma skin cancers, whereas mutations in p53 are unlikely to suffice (42).
It has been suggested that decreased apoptosis in mutant p53-expressing cells would result in the preferential expansion of mutant p53-expressing clones in sun-exposed skin (37,38). The results presented here suggest that loss of wild-type p53 function enhances UV-induced apoptosis, which could result in the preferential loss of p53 mutant cells in sun-exposed skin. We have recently found that UV light promotes S phase entry in a manner antagonized by wild-type p53 (9). Therefore, clonal selection of p53 mutations in sun-exposed skin may be explained by a difference in growth rate and not death rate between wild type and mutant p53-expressing cells.
In summary, we find that p53 is not required for UV-induced apoptosis, but rather p53 protects cells from apoptosis after exposure to moderate doses of UV light. The protection conferred by p53 does not appear to result from the induction of cell cycle arrest but correlates with an enhanced recovery of mRNA synthesis after exposure to UV light. Based on these and other results from our laboratory (4,5,7), we suggest that the capacity of cells to support transcription after exposure to genotoxic agents is an important determinant of the sensitivity of cells to apoptosis.
Acknowledgements
We would like to thank Drs. Mary Davis, Theodore Lawrence, Michael Tainsky, Geoffrey Wahl, and Al Rehemtulla for providing cell lines and reagents used in this study. We would also like to thank the technical staff of the Flow Cytometric Core at the University of Michigan Medical Center. This work was supported by a grant from the University of Michigan Comprehensive Cancer Center's Institutional Grant from the American Cancer Society.
Abbreviations
- CPD
cyclobutane pyrimidine dimer
- CS-B
Cockayne syndrome complementation group B
- HPV
human papillomavirus
- GGR
Global genome repair
- LFS
Li-Fraumeni syndrome
- TCR
transcription-coupled repair
- XP-C
xeroderma pigmentosum complementation group C
References
- 1.Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 2.Malkin D. p53 and the Li-Fraumeni syndrome. Biochim Biophys Acta. 1994;1198:197–213. doi: 10.1016/0304-419x(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 3.Smith ML, Fornace AJ. p53-mediated protective responses to UV irradiation. Proc Natl Acad Sci USA. 1997;94:12255–12257. doi: 10.1073/pnas.94.23.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ljungman M, Zhang F. Blockage of RNA polymerase as a possible trigger for UV light-induced apoptosis. Oncogene. 1996;13:823–831. [PubMed] [Google Scholar]
- 5.McKay BC, Ljungman RM, Rainbow AJ. Persistent DNA damage induced by ultraviolet light inhibits p21waf1 and bax expression: Implications for DNA repair, UV sensitivity and the induction of apoptosis. Oncogene. 1998;17:545–555. doi: 10.1038/sj.onc.1201963. [DOI] [PubMed] [Google Scholar]
- 6.Barley RDC, Enns L, Paterson MC, Mirzayans R. Aberrant p21WAF1-dependent growth arrest as the possible mechanism of abnormal resistance to ultraviolet light cytotoxicity in Li-Fraumeni syndrome fibroblast strains heterozygous for TP53 mutations. Oncogene. 1998;17:533–543. doi: 10.1038/sj.onc.1202271. [DOI] [PubMed] [Google Scholar]
- 7.Ljungman M, Zhang F, Chen F, Rainbow A, McKay B. Inhibition of RNA polymerase II as a trigger for the p53 response. Oncogene. 1999;18:583–592. doi: 10.1038/sj.onc.1202356. [DOI] [PubMed] [Google Scholar]
- 8.Loignon M, Fetni R, Gordon AJE, Drobetsky EA. A p53-independent pathway for induction of p21(waf1cip1) and concomitant G(1) arrest in UV-irradiated human skin fibroblasts. Cancer Res. 1997;57:3390–3394. [PubMed] [Google Scholar]
- 9.Chang D, Chen F, Zhang F, McKay BC, Ljungman M. Dose-dependent effects of DNA-damaging agents on p53-mediated cell cycle arrest. Cell Growth Differ. 1999;10:155–162. [PubMed] [Google Scholar]
- 10.Ziegler A, Jonason AS, Leffell DJ, Simon JA, Sharma HW, Kimmelman J, Remington L, Jacks T, Brash DE. Sunburn and p53 in the onset of skin cancer. Nature. 1994;372:773–776. doi: 10.1038/372773a0. [DOI] [PubMed] [Google Scholar]
- 11.Ford JM, Hanawalt PC. Li-Fraumeni syndrome fibroblasts homozygous for p53 mutations are deficient in global DNA repair but exhibit normal transcription-coupled repair and enhanced UV resistance. Proc Natl Acad Sci USA. 1995;92:8876–8880. doi: 10.1073/pnas.92.19.8876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanawalt PC. Transcription-coupled repair and human disease. Science. 1994;266:1957–1958. doi: 10.1126/science.7801121. [DOI] [PubMed] [Google Scholar]
- 13.Nunez G, Benedict MA, Hu Y, Inohara N. Caspases: the proteases of the apoptotic pathway. Oncogene. 1998;17:3237–3245. doi: 10.1038/sj.onc.1202581. [DOI] [PubMed] [Google Scholar]
- 14.Bissonnette N, Hunting DJ. p21-induced cell cycle arrest in G1 protects cells from apoptosis induced by UV-irradiation or RNA polymerase II blockage. Oncogene. 1998;16:3461–3469. doi: 10.1038/sj.onc.1201899. [DOI] [PubMed] [Google Scholar]
- 15.Ljungman M. Recovery of RNA synthesis from the DHFR gene following UV-irradiation precedes the removal of pyrimidine dimers from the transcribed strand. Carcinogenesis. 1999;20:395–399. doi: 10.1093/carcin/20.3.395. [DOI] [PubMed] [Google Scholar]
- 16.Dulic V, Kaufmann WK, Wilson SJ, Tisty TD, Lees E, Harper JW, Elledge SJ, Reed SI. p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell. 1994;76:1013–1023. doi: 10.1016/0092-8674(94)90379-4. [DOI] [PubMed] [Google Scholar]
- 17.McKay BC, Ljungman M, Rainbow AJ. Potential roles for p53 in nucleotide excision repair. Carcinogenesis. 1999 doi: 10.1093/carcin/20.8.1389. (in press) [DOI] [PubMed] [Google Scholar]
- 18.Ford JM, Hanawalt PC. Expression of wild-type p53 is required for efficient global genomic nucleotide excision repair in UV-irradiated human fibroblasts. J Biol Chem. 1997;272:28073–28080. doi: 10.1074/jbc.272.44.28073. [DOI] [PubMed] [Google Scholar]
- 19.Ford JM, Baron EL, Hanawalt PC. Human fibroblasts expressing the human papillomavirus E6 gene are deficient in global genomic nucleotide excision repair and sensitive to ultraviolet irradiation. Cancer Res. 1998;58:599–603. [PubMed] [Google Scholar]
- 20.Mirzayans R, Enns L, Dietrich K, Barley RDC, Paterson MC. Faulty DNA polymerase delta/epsilon-mediated excision repair in response to gamma radiation or ultraviolet light in p53-deficient fibroblast strains from affected members of a cancer-prone family with Li-Fraumeni syndrome. Carcinogenesis. 1996;17:691–698. doi: 10.1093/carcin/17.4.691. [DOI] [PubMed] [Google Scholar]
- 21.McKay BC, Rainbow AJ. Heat-shock enhanced reactivation of a UV-damaged reporter gene in human cells involves the transcription coupled DNA repair pathway. Mutat Res. 1996;363:125–135. doi: 10.1016/0921-8777(96)00013-4. [DOI] [PubMed] [Google Scholar]
- 22.McKay BC, Francis MA, Rainbow AJ. Wild-type p53 is required for heat shock and ultraviolet light enhanced repair of a UV-damaged reporter gene. Carcinogenesis. 1997;18:245–249. doi: 10.1093/carcin/18.2.245. [DOI] [PubMed] [Google Scholar]
- 23.McKay BC, Winrow C, Rainbow AJ. Capacity of UV-irradiated human fibroblasts to support adenovirus DNA synthesis correlates with transcription-coupled repair and is reduced in SV40-transformed cells and cells expressing mutant p53. Photochem Photobiol. 1997;66:659–664. doi: 10.1111/j.1751-1097.1997.tb03203.x. [DOI] [PubMed] [Google Scholar]
- 24.Eller MS, Maeda T, Magnoni C, Atwal D, Gilchrest BA. Enhancement of DNA repair in human skin cells by thymidine dinucleotides: Evidence for a p53-mediated mammalian SOS response. Proc Natl Acad Sci USA. 1997;94:12627–12632. doi: 10.1073/pnas.94.23.12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith ML, Chen IT, Zhan QM, Oconnor PM, Fornace AJ. Involvement of the p53 tumor suppressor in repair of UV-type DNA damage. Oncogene. 1995;10:1053–1059. [PubMed] [Google Scholar]
- 26.Brown JM, Wouters BG. Apoptosis, p53, and tumor cell sensitivity to anticancer agents. Cancer Res. 1999;59:1391–1399. [PubMed] [Google Scholar]
- 27.Wang XW, Yeh H, Schaeffer L, Roy R, Moncollin V, Egly JM, Wang Z, Friedberg EC, Evans MK, Taffe BG, Bohr VA, Weeda G, Hoeijmakers JHJ, Forrester K, Harris CC. p53 modulation of TFIIH-associated nucleotide excision repair activity. Nat Genet. 1995;10:188–195. doi: 10.1038/ng0695-188. [DOI] [PubMed] [Google Scholar]
- 28.Leadon SA, Snowden MM. Differential repair of DNA damage in the human metallothionein gene family. Mol Cell Biol. 1988;8:5331–5338. doi: 10.1128/mcb.8.12.5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mellon I, Spivak G, Hanawalt PC. Selective removal of transcription-blocking DNA damage from the transcribed strand of the mammalian DHFR gene. Cell. 1987;51:241–249. doi: 10.1016/0092-8674(87)90151-6. [DOI] [PubMed] [Google Scholar]
- 30.van Hoffen A, Venema J, Meschini R, Vanzeeland AA, Mullenders LHF. Transcription-coupled repair removes both cyclobutane pyrimidine dimers and 6-4 photoproducts with equal efficiency and in a sequential way from transcribed DNA in xeroderma pigmentosum group C fibroblasts. EMBO J. 1995;14:360–367. doi: 10.1002/j.1460-2075.1995.tb07010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang H-Y, Zhao K, Pizzolato JF, Fonarev M, Langer JC, Manfredi JJ. Constitutive expression of the cyclin-dependent kinase inhibitor p21 is transcriptionally regulated by the tumor suppressor protein p53. J Biol Chem. 1998;273:29156–29163. doi: 10.1074/jbc.273.44.29156. [DOI] [PubMed] [Google Scholar]
- 32.Hwang BJ, Ford JM, Hanawalt PC, Chu G. Expression of the p48 xeroderma pigmentosum gene is p53-dependent and is involved in global genomic repair. Proc Natl Acad Sci USA. 1999;96:424–428. doi: 10.1073/pnas.96.2.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rehemtulla A, Hamilton CA, Chinnaiyan AM, Dixit VM. Ultraviolet radiation-induced apoptosis is mediated by activation of CD-95 (Fas/APO-1) J Biol Chem. 1997;272:25783–25786. doi: 10.1074/jbc.272.41.25783. [DOI] [PubMed] [Google Scholar]
- 34.Aragane Y, Kulms D, Metze D, Wilkes G, Pöppelmann B, Luger T, Schwarz T. Ultraviolet light induces apoptosis via direct activation of CD95 (fas/APO-1) independently of its ligand CD95L. J Cell Biol. 1998;140:171–182. doi: 10.1083/jcb.140.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheikh MS, Antinore MJ, Huang Y, Fornace AJ. Ultraviolet-irradiation-induced apoptosis is mediated via ligand independent activation of tumor necrosis factor receptor 1. Oncogene. 1998;17:2555–2563. doi: 10.1038/sj.onc.1202292. [DOI] [PubMed] [Google Scholar]
- 36.Daya-Grosjean L, Dumaz N, Sarasin A. The specificity of p53 mutation spectra in sunlight induced human cancers. J Photochem Photobiol B. 1995;26:115–124. doi: 10.1016/1011-1344(95)07130-t. [DOI] [PubMed] [Google Scholar]
- 37.Brash DE. Sunlight and the onset of skin cancer. Trends Genet. 1997;13:410–414. doi: 10.1016/s0168-9525(97)01246-8. [DOI] [PubMed] [Google Scholar]
- 38.Jonason AS, Kuala S, Price GJ, Restifo RJ, Spinelli HM, Persing JA, Leffell OJ, Tarone RE, Brash DE. Frequent clones of p53-mutated keratinocytes in normal human skin. Proc Natl Acad Sci USA. 1996;93:14025–14029. doi: 10.1073/pnas.93.24.14025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kraemer KH. Sunlight and skin cancer: Another link revealed. Proc Natl Acad Sci USA. 1997;94:11–14. doi: 10.1073/pnas.94.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morales-Ducret CR, van de Rijn M, LeBrun DP, Smoller BR. bcl-2 expression in primary malignancies of the skin. Arch Dermatol. 1995;131:909–912. [PubMed] [Google Scholar]
- 41.Penna JC, Rudin CM, Thompson CB. A Bcl-xL transgene promotes malignant conversion of chemically initiated skin papillomas. Cancer Res. 1997;58:2111–2116. [PubMed] [Google Scholar]
- 42.Wikonkal NM, Berg RJ, van Haselen CW, Horkay I, Remenik E, Begany A, Hunyadi J, van Vloten WA, de Gruijl FR. bcl-2 vs. p53 protein expression and apoptotic rate in human non melanoma skin cancers. Arch Dermatol. 1997;133:599–602. [PubMed] [Google Scholar]
- 43.McKay BC. The Relationship Between The Repair Of Ultraviolet Light Induced DNA Damage and the p53 Tumour Suppressor [PhD Thesis] Hamilton, Canada: Department of Biology, McMaster University; 1997. [Google Scholar]