Abstract
Proper control of cell cycle progression is critical for the constant self-renewal, differentiation, and homeostasis of the hematopoietic system. Cells of all types share the common cell cycle regulators. The different expression patterns of common regulators, in a broad sense, define cell-type or lineage specificity. However, there remains the possibility of hematopoietic cell cycle regulators tailored to the demands of the hematopoietic system. Here we describe a novel protein, HTm4, which serves as a hematopoietic cell cycle regulator. Our data indicate that HTm4 is expressed in hematopoietic tissues and is tightly regulated during the differentiation of hematopoietic stem cells. It binds to cyclin-dependent kinase–associated (CDK-associated) phosphatase-CDK2 (KAP-CDK2) complexes, and the three proteins demonstrate similar patterns of cellular expression in human lymphoid tissues. HTm4 stimulates the phosphatase activity of KAP, and its C-terminal region is required for binding to KAP-CDK2 complexes and the modulation of KAP activity. Overexpression of HTm4 can cause cell cycle arrest at the G0/G1 phase. Thus, HTm4 is a novel hematopoietic modulator for the G1-S cell cycle transition.
Introduction
Cell cycle progression and hematopoiesis are tightly coordinated (1, 2). The maintenance of proper hematopoiesis requires a balance between the proproliferative and antiproliferative properties of hematopoietic stem cells (HSCs). This balance can be biologically translated into the known capabilities of HSCs to self-renew, differentiate, and generate the entire population of hematopoietic cells of all lineages. During cell cycle progression, several antiproliferative signals are activated (3–6). Antiproliferative properties of HSCs serve important biological functions that, in addition to the preservation of integrity of genetic materials, include the preservation of multipotential, early progenitor cells (7, 8), their differentiation to the committed late progenitor cells (9, 10), and subsequent maturation to terminally differentiated hematopoietic cells.
The temporal expression of various cyclins and cyclin-dependent kinases (CDKs) is the foundation upon which many cell cycle modulators exert their influence (3–6). The inhibition of the kinase activities of CDK2, 4, and 6 is known to cause G0/G1 cell cycle arrest (reviewed in ref.11), which is believed to be important for the differentiation and maturation of hematopoietic cells. This inhibition is achieved primarily through the binding of inhibitors to CDKs. The cyclin D-CDK4/6 complexes play an important role in the progression or restriction of cell cycle progression at the G1 phase. Inhibition of cyclin D-CDK4/6 kinase activity by p21Cip1/Waf1/Sdi1 and p27Kip1 inhibitors at this restriction point causes G0/G1 cell cycle arrest. Cyclin E-CDK2 and cyclin A-CDK2 are responsible for the G1-S phase transition (12) and S phase progression of the cell cycle, respectively. Both complexes are susceptible to negative regulation by p21Cip1/Waf1/Sdi1 and p27Kip1. Interference with cyclin E-CDK2 activity can cause G0/G1 cell cycle arrest. With the involvement of cyclin D-CDK4/6, the interplay between p27Kip1 and cyclin E-CDK2 is complex, varying according to the different physiological conditions of the cell (reviewed in ref.3). In a rapidly cycling cell, most of p27Kip1 is degraded, and the residual amount of p27Kip1 is sequestered by cyclin D-CDK4/6, thus alleviating its negative regulation upon cyclin E-CDK2. However, in the absence of mitogen, the concentration of p27Kip1 protein increases while that of cyclin D decreases. This combined effect can result in rapid inactivation of cyclin E-CDK2 kinase and bring about G0/G1 cell cycle arrest in just one cell cycle. Due to their ability to modulate the kinase activities of CDKs, both p21Cip1/Waf1/Sdi1 and p27Kip1 have been implicated to be involved in many aspects of hematopoiesis (7–10), from stem cell kinetics to terminal differentiation and maturation of hematopoietic cells.
The monomeric, phosphorylated CDK2 has only a basal level of kinase activity. Activation of CDK2 kinase activity requires both the phosphorylation of threonine residue (Thr160) by CDK-activating kinase (CAK) and its binding to the cyclins (13–15). CAK can phosphorylate both CDK2 monomer and cyclins-CDK2 complex. Dephosphorylation of the active phosphorylthreonine residue of CDK2 inactivates its intrinsic kinase activity, representing yet another control mechanism for cell cycle progression. KAP, a CDK-associated phosphatase, can dephosphorylate Thr160 in human CDK2 (16). It binds to CDK2 both in the presence and absence of cyclins (16–18), but can only dephosphorylate CDK2 in the absence of cyclins. The binding of KAP to the cyclins-CDK2 complex does not interfere with the kinase activity. It seems the function of KAP is to counter CAK in two ways: (a) dephosphorylation of monomeric CDK2 after it is phosphorylated by CAK and (b) interference with CAK kinase activity by binding to CDK2 or the cyclins-CDK2 complex. The biological significance of KAP is not as well established as that of CDK inhibitors. Nevertheless, it has been shown that exogenous expression of KAP slows the G1 phase cell cycle progression in HeLa cells (17) and that aberrant KAP transcripts are detected in some hepatocellular carcinomas (19). These observations suggest that KAP has the same biological effects as that of CDK inhibitors, although their modes of action are different.
Although the expression patterns of some common cell cycle regulators vary among hematopoietic cells of different lineages (10, 20), all the components of cell cycle regulation thus far discussed are common to all cell types. Here we report a novel protein, HTm4, which acts as a hematopoietic cell cycle regulator. HTm4 is a member of HTm4/CD20/FcεRIβ subfamily with four transmembrane domains (21). It is expressed in hematopoietic cells, including bone marrow cells. It has been found to be associated with atopic asthma (22). HTm4, KAP, and CDK2 form a physiologic complex, the in vivo presence of which is confirmed by their similar patterns of cellular expression in human lymphoid tissue. Our data demonstrate that HTm4 modulates the level of phosphorylation of CDK2 through its direct binding to KAP. The exogenous expression of HTm4 in U937 cells causes G0/G1 cell cycle arrest. Thus, the cell cycle regulatory pathway involving the dephosphorylation of CDK2 has been expanded to include HTm4, and the participation of KAP in hematopoiesis is implicated.
Methods
In vitro cell culture and transfection.
The isolation of bone marrow cells and the subsequent manipulations of the isolated stem cells were carried out as described previously (23). Cells at various stages of differentiation were isolated as single cells and then subjected to RT-PCR analysis for the expression of HTm4. The induction of differentiation of CD34+ stem cells was also done in the presence of 300 U/ml of granulocyte colony stimulating factor (G-CSF) or 3 U/ml of erythropoietin (Epo) for the period of time as specified (24). Transient expression of hemagglutinin-KAP (HA-KAP) was done as follows: 5 μg per 107 cells of pCMV-HA-KAP (18) and the control pCMV vectors were transfected into U937 and KU812, using Superfect reagent (QIAGEN, Valencia, California, USA). Twenty-four hours after transfection, cell lysates were prepared from both samples. U937 and KU812 were routinely maintained in RPMI-1640 medium supplemented with 10% (vol/vol) FBS, 2 mM glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin in an atmosphere of 5% CO2, at 37°C.
Cell fractionation and preparation of cell lysates.
Fractionation of the subcellular components of U937 or KU812 was done as follows: cells were resuspended in ice-cold PBS with Protease Cocktail Tablets (Roche Molecular Biochemicals, Mannheim, Germany) and homogenized in a Douce glass homogenizer. The extract was centrifuged at 200 g for 10 minutes at 4°C to pellet the nuclei. The supernatant was clarified further by centrifugation at 15,000 g for 30 minutes at 4°C. The pellet was considered to be the membrane fraction. Total lysate, nuclear, and membrane fractions were used for the Western blot analysis. For the production of U937 and KU812 cell lysates, we extracted cells (106 cells/ml) in PBS containing 0.5% Triton X-100 and Protease Cocktail Tablets at 4°C for 30 minutes. The clear lysates were collected after centrifugation at 4°C, 10,000 g for 30 minutes and kept on ice before use.
Expression analysis in single cells using RT-PCR.
Individual cells were obtained by means of a Becton Dickinson Immunocytometry Systems (San Jose, California, USA) FACS cell sorter, by limiting dilution analysis as described by Taswell (25). cDNAs representative of the total mRNA isolated from individual cells were synthesized by a micro-reverse transcription reaction using oligo-dT primers as described by Berardi et al. (23). The PCR products were analyzed by Southern blot technique (26) using α32P-dCTP–labeled HTm4 cDNA probe.
Yeast two-hybrid system.
Bait consisting of the last 25 amino acids of HTm4 (CHTm4), from amino acid 190 to 214, was cloned into the vector pVJLII (27). The yeast strain L40 containing two reporter genes, HIS3 and LacZ, was used as the host for a two-hybrid assay. Plasmid vector was cotransformed with the human bone marrow cDNA-pGADGH library (CLONTECH, San Diego, California, USA) into L40. Transformants were plated onto synthetic medium lacking histidine, leucine, and tryptophan. After 10 days of growth, His+ colonies were patched onto selection plates and tested for β-galactosidase activity. Plasmid DNAs were isolated from positive clones and introduced by electroporation into Escherichia coli MH4 plated on leucine-free medium for the selection of the pGADGH construct. The putative positive cDNAs in pGADGH plasmid were then tested in a two-hybrid assay with irrelevant, negative controls. The negative controls used were MEK (28), byr (29), and lamin (27). The identified cDNA clones in pGADGH constructs were considered true positive when they were tested negative with the negative controls. To make sure that the interactions between HTm4 and its target proteins were not permutation dependent, the entire two-hybrid assay was repeated with constructs that had their expression vectors exchanged between two groups. In this case, HTm4 was subcloned into pGADGH while KAP into the vector pVJLII.
Generation of anti-HTm4 polyclonal Ab.
A peptide, GSLQYPYHFQKHF, from the HTm4 sequence between the first and the second predicted transmembrane domains (amino acids 70 to 82), was synthesized with the amino-terminal cysteine added to facilitate coupling and purification procedures. The antigen was prepared and injected into 3- to 9-month-old rabbits to raise antiserum following established protocols (Zymed Laboratories Inc., South San Francisco, California, USA). The specific Ab’s were purified using an epoxy-activated Sepharose 6B coupled with the peptide as described by the manufacturer (Amersham Pharmacia Biotech Europe GmbH, Uppsala, Sweden). Please note that our HTm4 Ab is only suitable for Western blot analysis and cell staining. Thus, for the immunoprecipitation of both the full-length and truncated HTm4 that are exogenously expressed in Flag-tagged forms, we used anti-Flag from Sigma Chemical Co. (St. Louis, Missouri, USA).
Flow cytometry analysis.
To determine the cellular expression (cell surface versus intracellular localization) of HTm4, intact U937 (107 cells/ml) was labeled with 2 μg/ml of polyclonal anti-HTm4 for 20 minutes, followed by the incubation with goat anti-rabbit IgG-FITC–conjugated Ab. For the staining of intracellular proteins, cells were permeabilized with the Fix & Cell Permeabilization Kit, as described by the manufacturer (Caltag Laboratories Inc., Burlingame, California, USA). The flow cytometry analysis was performed on a FACScan flow cytometer (Becton Dickinson Immunocytometry Systems). For the absorption with the peptide (amino acids 70–82) described above, 2 μg/ml of HTm4 Ab was treated with 10 μg of peptide at room temperature with continuous rotation for 30 minutes before the staining of cells.
Immunohistochemistry and immunoprecipitation.
The cell preparation for immunohistochemistry study was performed as described (30) with some modifications. Briefly, U937 cells were allowed to adhere to polylysine precoated coverslips for 30 minutes, fixed with 2% formaldehyde in PBS, and permeabilized in 0.1% saponin and 1× PBS. All subsequent washes and Ab hybridization were carried out in the presence of saponin-PBS buffer. Cells were prehybridized with 2% BSA, incubated with 0.2 μg/ml anti-KAP followed by 1 μg/ml anti-HTm4 for 20 minutes at room temperature. The positive staining was visualized with goat anti-mouse IgG Texas Red–conjugated or goat anti-rabbit IgG FITC–conjugated secondary Ab (Jackson ImmunoResearch Laboratories Inc., West Grove, Pennsylvania, USA). For immunoprecipitation, cell lysates equivalent to 5 × 105 cells in 0.5 ml of lysis buffer, 5 μg of Ab, and 40 μl of protein G Sepharose beads (Sigma Chemical Co.) were used per reaction.
Western blot analysis.
Enhanced chemiluminescence detection reagents were purchased from Amersham Pharmacia Biotech (Piscataway, New Jersey, USA). Primary mAb’s used were anti-KAP, anti-CDK2, both from BD Transduction Laboratories (San Diego, California, USA), anti-HA from Covance Research Products Inc. (Richmond, California, USA), and anti-Flag from Sigma Chemical Co. Secondary Ab’s, horseradish peroxidase–conjugated (HRP-conjugated) goat anti-rabbit and rabbit anti-mouse Ab’s, were from Amersham Pharmacia Biotech (Piscataway, New Jersey, USA). All primary Ab’s were used at 1 μg/ml and the secondary Ab’s at 1:5,000 dilution.
Isolation of KAP using GST-CHTm4 fusion protein.
pGEX-4T- CHTm4 vector was used for the production of GST-CHTm4. This expression vector contained the C-terminal region of HTm4 that coded for the amino acid 190 to 214 (CHTm4), with in-frame reading for GST protein at the 5′ end, using pGEX-4T-1 vector (Amersham Pharmacia Biotech, Piscataway, New Jersey, USA). The production and purification of GST-CHTm4 was done according to the protocols specified by the manufacturer (Amersham Pharmacia Biotech, Piscataway, New Jersey, USA). GST protein without the fusion partner was used as control. One milliliter each of U937 and KU812 lysates was incubated with 5 μg of GST-CHTm4 or GST coupled to glutathione Sepharose beads for 4 hours at 4°C and analyzed by Western blot technique.
Tet-Off expression and induction of cell cycle arrest.
The induction of HTm4 expression was done using the Tet-Off expression system of CLONTECH for the studies of its interaction with KAP, Cdk2, and induced cell cycle arrest. HTm4 proteins in both full-length and C-terminal truncated (without the last 22 amino acids, denoted as HTm4-C) forms were used for side-by-side comparison studies. The maintenance and induction of cell culture were carried out according to manufacturer’s protocols. In brief, to generate cell clones expressing the inducible HTm4 constructs we constructed HTm4 and its variants in the pTRE2 vector with an in-frame C-terminal Flag epitope tag. U937 cells expressing the tetracycline-controlled transactivator were used as the host. Cells were cotransfected by electroporation with 5 μg of expression vector and 0.25 μg of pHyg plasmid containing the hygromycine resistance gene per 107 cells. Positive clones were maintained in medium containing 200 μg/ml hygromycin and 1 μg/ml of doxycycline (Dox). In the Tet-Off system, the expression of HTm4 and HTm4-C proteins were induced in the absence of Dox, while their suppressions were achieved in the presence of Dox (1 μg/ml). Cells were synchronized as described by Ling et al. (31). Briefly, 106/ml of cells was treated with two rounds of incubation with 2 mM thymidine for 16 hours each, separated by 10 hours of resting period in the absence of thymidine. The first thymidine treatment was done in the presence of 10% FBS and the second in 0.5% FBS. The stimulation of cell growth, after synchronization, was done in the presence of 15% FBS. Cell samples analyzed at the time points indicated were fixed with 70% alcohol at 4°C for 30 minutes, stained with propidium iodide (50 μg/ml), and then analyzed by flow cytometry.
Immunohistochemical studies.
All staining was performed by standard immunoperoxidase methods. Briefly, slides were deparaffinized and were either not pretreated (HTm4), pretreated in 1 mM EDTA, pH 8.0, for 20 minutes at 95°C (Ki-67), or pretreated in 10 mM sodium citrate, pH 6.0, for 20 minutes at 95°C (CDK2 and KAP). All further steps were performed at room temperature in a hydrated chamber. Slides were pretreated with Peroxidase Block (DAKO Corp., Carpinteria, California, USA) for 5 minutes to quench endogenous peroxidase activity, and a 1:5 dilution of goat serum in 50 Mm Tris-Cl, pH 7.4, for 20 minutes to block nonspecific binding sites. Either affinity-purified rabbit anti-HTm4 Ab (1:1,000 dilution in 50 mM Tris-Cl, pH 7.4, with 3% goat serum); murine anti-CDK2 Ab (1:500 dilution in 50 mM Tris-Cl, pH 7.4, with 3% goat serum; BD Biosciences, PharMingen-Transduction Laboratories, San Diego, California, USA); murine anti-KAP AB (1:1,000 dilution in 50 mM Tris-Cl, pH 7.4, with 3% goat serum; BD Biosciences, Pharmingen-Transduction Laboratories); or murine anti–Ki-67 Ab (MIB-1 clone; 1:100 dilution in 50 mM Tris-Cl, pH 7.4, with 3% goat serum; Coulter Corp., Miami, Florida, USA) was applied at room temperature for 1 hour. After washing in 50 mM Tris-Cl, pH 7.4, secondary goat anti-rabbit or goat anti-mouse HRP-conjugated Ab (Envision detection kit; DAKO Corp.) was applied for 30 minutes. After further washing, immunoperoxidase staining was developed using a 3,3-diaminobenzidine chromogen kit (DAKO, USA) per the manufacturer and counterstaining was done with hematoxylin.
Results
Yeast two-hybrid and fusion protein assays.
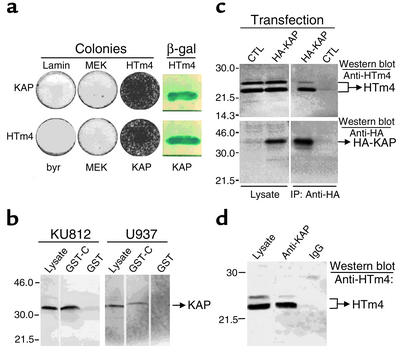
Using the yeast two-hybrid assay, we have detected the binding of HTm4 to KAP (Figure 2a). The specificity of this binding has been confirmed by the isolation of KAP from U937 and KU812 lysates, using a fusion protein of GST and the last 25 amino acids of the C-terminal region of HTm4 (Figure 2b).
Figure 2.
(a) Binding of HTm4 to KAP in yeast two-hybrid assay. Constructs listed in the upper and lower rows were in pGADGH, whereas those to the left of the first column were in pVJLII. HTm4 listed here represents the CHTm4. MEK, byr, and lamin are negative controls. The positive results are shown as colony growth on selective medium and blue color reaction in the presence of β-gal. (b) Isolation of KAP from U937 and KU812 lysates using GST-CHTm4 fusion protein. GST-C represents GST-CHTm4. Lysates derived from U937 and KU812 are absorbed with either GST-CHTm4 or GST-coupled beads, and bound proteins were analyzed in a Western blot assay for the presence of KAP. (c) Coimmunoprecipitation of HA-tagged KAP and HTm4. CTL, pCMV control vector. The source of the samples is listed on the top. The first two columns on the left are lysates alone and the remaining two are after immunoprecipitation with anti-HA. Samples are analyzed by the Western blot technique using anti-HTm4 for the top two panels and anti-HA for the bottom two. The identified proteins are listed on the right. (d) HTm4 and KAP form a physiological complex. The source of the samples is denoted on the top; the first column from the left is U937 lysate alone; the second and third are after immunoprecipitation with anti-KAP and IgG control, respectively. The presence of HTm4 is indicated on the right after Western blot analysis. Representative figures of at least five experiments.
HTm4-KAP immunoprecipitation.
The binding of HTm4 to KAP under physiologic conditions has been established by two different approaches. First, the binding has been detected between the endogenous HTm4 of KU812 and the exogenous HA-tagged KAP after the immunoprecipitation with anti-HA mAb (Figure 2c). Second, to eliminate the possibility that the binding between HTm4 and HA-KAP was spurious, due to the overexpression of the exogenous HA-KAP, we performed a direct immunoprecipitation of endogenous KAP in KU812 cells with anti-KAP Ab (Figure 2d). The resulting immune complexes have been determined to contain the endogenous HTm4, indicating the interaction between KAP and HTm4 is physiologic.
HTm4-KAP Immunohistochemistry.
KAP is located predominantly in the perinuclear area (34), and in U937 HTm4 colocalizes with KAP principally in the same region (Figure 3a). This observation substantiates the physiologic interaction between HTm4 and KAP. The subcellular location for HTm4 has been determined to be in the microsomal membrane fraction (Figure 3b). Our anti-HTm4 polyclonal Ab recognizes the native HTm4 as a doublet. The intracellular staining pattern (Figure 3c) is consistent across the many cells types thus far examined.
Figure 3.
(a) Colocalization of HTm4 and KAP. Staining was visualized with goat anti-mouse IgG Texas Red–conjugated (left) or goat anti-rabbit IgG FITC–conjugated (middle) secondary Ab’s. Merged image is given on the right showing that HTm4 and KAP colocalized predominately in the perinuclear area. (b) HTm4 is located in the intracellular membrane fraction. Source of the samples are denoted on the top row. Samples shown are in duplicates. The presence of HTm4 is indicated on the right after Western blot analysis. (c) Staining of U937 with anti-HTm4 Ab. The manipulations of samples are denoted on the top. The filled peaks represent the staining with anti-HTm4, and the open peaks represent IgG control. x axis, cell counts; y axis, signal intensity of FITC fluorescence. Representative figures of at least five experiments.
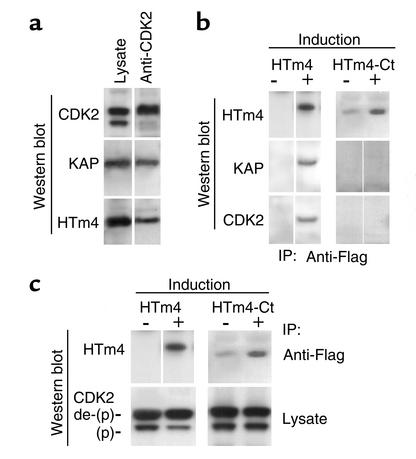
HTm4-KAP-CDK2 Immunoprecipitation.
KAP is known to bind to CDK2 and Cdc2 (17, 18). It is demonstrated here that HTm4 binds to KAP-CDK2 complexes (Figure 4a) in immune complexes derived from U937 lysate using anti-CDK2 mAb. CDK2 is detected as doublets, with the upper band being the dephosphorylated form and the lower the phosphorylated form (Figure 4c). The immunoprecipitated complexes may be composed of solely HTm4-KAP-CDK2 or as a mixture with CDK2-KAP. In light of our previous yeast two-hybrid assay, which showed only the binding between HTm4 and KAP, it is unlikely that HTm4 interacts directly with CDK2. The C-terminus is responsible for the binding of HTm4 to KAP in the yeast two-hybrid assay. Therefore, we examined whether the C-terminus of HTm4 is required for its binding to KAP-CDK2. As shown in Figure 4b, the C-terminal region indeed is required for the binding of HTm4 to KAP-CDK2 complexes.
Figure 4.
(a) HTm4 binds to KAP-CDK2 under normal physiological conditions. Samples analyzed are shown on the top. The lane marked anti-CDK2 includes the samples immunoprecipitated with anti-CDK2 mAb and analyzed by Western blot technique using Ab’s as indicated on the left. (b) The C-terminal region of HTm4 is required for its binding to KAP-CDK2. HTm4-Ct, HTm4 without the last 22 amino acids. Ab’s used for the Western blot analysis, after immunoprecipitation with anti-Flag, are listed on the left. The induction of protein expression in the absence of Dox is marked as +, and no induction in the presence of Dox is marked as –. (c) The C-terminal region of HTm4 is required for the enhancement of the phosphatase activity of KAP. The descriptions for c are the same as Figure 4b, except the top panels show the immunoprecipitation with anti-Flag Ab and the bottom panels are derived from 50 μg of total lysate per sample. The upper bands in the lower panels are the dephosphorylated form of CDK2, marked as de-(P), and the lower bands are the phosphorylated (P) form. A reduction in intensity can be seen in the phosphorylated CDK2 in the presence of overexpressed Flag-HTm4. Representative figures of at least five experiments.
CDK2 phosphorylation status.
KAP is known to dephosphorylate Thr160 in the human CDK2 in the absence of cyclin A (16), acting as a negative regulator. It can also bind to CDK2 in the presence of cyclin A; however, the formation of cyclin A-CDK2 complexes does preclude the dephosphorylation of CDK2 by KAP. The phosphatase activity of KAP is enhanced upon the binding of HTm4 as demonstrated in Figure 4c, which shows that the exogenous expression of full-length HTm4 promotes the dephosphorylation of CDK2, whereas, in the presence of C-terminal truncated HTm4, the level of phosphorylated CDK2 remains unchanged. This observation implicates the modulation of CDK2 by HTm4 through its direct binding to KAP.
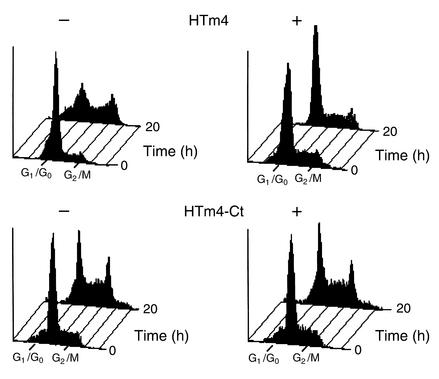
Induction of cell cycle arrest.
We here evaluate the effects of HTm4 on cell cycle progression through the overexpression of intact HTm4 in synchronized U937 cells (Figure 5). After 20 hours of serum stimulation, 60.8% (n = 5; the percentage given is an average derived from five experiments) of cells, in the presence of exogenous HTm4, accumulate at G0/G1 phase of cell cycle in contrast to 35% (n = 5) in the absence of exogenous HTm4. This effect is not detected in U937 cells with exogenous C-terminal truncated HTm4, 36.3% versus 34.4% (induced vs. noninduced; n = 5 for both).
Figure 5.
Cell cycle arrest is induced by the exogenous expression of HTm4. The upper panels are U937 with inducible Flag-HTm4 expression vector and the lower panels are with Flag-HTm4-Ct. +, induction of expression in the absence of Dox; –, no induction in the presence of Dox. Time intervals are given on the right. 0 hours, before the addition of FBS into the cultures; 20 hours, cells were cultured in the presence of serum for 20 hours after synchronization. X axis shows cell cycle phase analyzed. Marked here are G0/G1 and G2/M; in between (not marked) is S phase. Representative figures of at least five experiments.
Immunohistochemical studies.
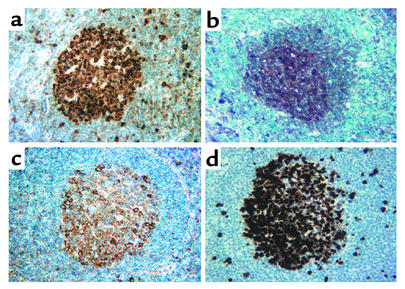
We then studied the expression of CDK2, KAP, and HTm4 in human tissues using immunostaining with anti-CDK2, anti-KAP, and anti-HTm4 Ab’s. Figure 6 shows dense expression of all three proteins in the proliferating cell populations of germinal centers within secondary follicles of human tonsillar tissue. No significant expression of CDK2, KAP, or HTm4 was seen in the surrounding mantle zones of the follicles. All three proteins also demonstrated similar patterns of cellular colocalization with predominantly paranuclear/cytoplasmic staining, and to a lesser degree, nuclear staining, most evident with CDK2. This pattern of expression coincides exactly with cells that are in the active cell cycle, as evidenced by an identical staining pattern seen for Ki-67, a nuclear cell proliferation-associated antigen, expressed in all active stages of the cell cycle (Figure 6d). Taken together, these findings suggest that these proteins are expressed specifically in actively cycling cells of the germinal centers within secondary follicles of human lymphoid tissues.
Figure 6.
Immunoperoxidase staining of secondary follicles of the tonsil with CDK2, HTm4, KAP, and Ki-67. Sections of reactive tonsillar tissue are stained for expression of (a) CDK2, (b) HTm4, (c) KAP, and (d) Ki-67. Note the strong staining of the proliferating cells of the germinal center for all Ab’s with absence of staining of the surrounding mantle zones. The cellular protein localization is predominantly nuclear for CDK2 and Ki-67 (a and d, respectively) with nuclear and paranuclear staining noted for HTm4 and KAP (b and c, respectively). Magnification ×400, HRP stain; hematoxylin was used as counterstain.
Discussion
We have shown that HTm4 is expressed in hematopoietic cells and tissues and highly regulated during the differentiation of hematopoietic stem cells. Our data also indicate that HTm4 expression is regulated during HSC progression through the cell cycle. This regulation seems to be both stage- and lineage-specific. Under certain cellular conditions, the expression of HTm4 is likely to be associated with the exit from cell cycle progression. As presented here, HTm4 is detected in G0 quiescent stem cells and terminally differentiated hematopoietic cells of certain lineages. The biological role of HTm4 we elucidated is consistent with this observation.
Of particular interest to hematopoietic cell cycle control, we explored the interaction between HTm4 and KAP and the effects on CDK2 phosphorylation and cell cycle progression. Our data indicate a physiologic HTm4-KAP interaction. HTm4 binds to KAP-CDK2 complexes through the binding of KAP to HTm4 and can promote detectably the phosphatase activity of KAP. The C-terminal region of HTm4 is required for these actions. The overexpression of HTm4 can cause cell cycle arrest at G0/G1 phase. We thus identify HTm4 as a novel hematopoietic modulator for the G1-S cell cycle transition. We here put our findings in the context of previous pertinent research.
Our observations are consistent with the functional role of CDK2, which has been shown to be required for the transition from G1 to S phase of cell cycle (12). Previous research demonstrated that disruption of CDK2 activity by deactivation or overexpression may cause either G1 cell cycle arrest (35) or uncontrollable cell growth (36), respectively. Creating a situation similar to the deactivation of CDK2, the exogenous expression of HTm4 causes the progression of cell cycle to slow and therefore implicates a role for HTm4 in cell cycle regulation. There are two possible modes of action, which might work in concert, for HTm4 to accomplish its functional role in vivo. First, the binding of HTm4 to KAP-CDK2 might enhance the phosphatase activity of KAP, which in turn would deactivate CDK2 at a faster rate. CDK2 is known to be such a potent kinase that it can inactivate the exogenously expressed retinoblastoma protein endogenously, and thus, prevent cell cycle arrest (37–39). The fine-tuning of CDK2 kinase activity may be essential for the balanced cell cycle progression. Second, because HTm4 is a transmembrane protein located in the perinuclear area, it might function to sequester KAP-CDK2 or KAP-CDK2-cyclin E/A. This would keep these complexes out of their functional milieu and impact the transition from G1 to S phase and S phase progression of the cell cycle. Put in the context of previous findings, our data support the biological role of HTm4 as a regulator that modulates and possibly maintains a critical level of cyclin E/A-CDK2 kinase activity during the G1-S transition and S phase progression.
Our data on cell cycle arrest caused by the overexpression of exogenous HTm4 are consistent with the findings of Yeh et al. (19). We have demonstrated that expression of HTm4 stimulates the phosphatase activity of KAP and possibly sequesters CDK2 from its functional pathway, attenuating cell cycle progression at G0/G1. Yeh et al. found that the KAP produced by a hepatoma was defective in its ability to bind CDK2. This would result in a persistently activated CDK2 and uncontrollable cell growth. Interestingly, though, it has been shown that KAP is upregulated in both breast and prostate cancers (34). This apparent contradiction can be explained by the fact that in this study the integrity of KAP and its associated pathway were not confirmed. In addition, it should not be assumed that the same abnormality would yield an identical outcome in different cell contexts, because the status of other key cell cycle regulators may vary (3). When the downstream components are impaired, the resulting phenotypes cannot be overcome even when an upstream antagonist is overexpressed (40). The accompanying upregulation of regulators, along the same pathway as the damaged signaling component, might have been compensatory rather than causative (41).
Interestingly, our confirmation of the in vivo coexpression of KAP, CDK2, and HTm4 in the proliferating cell populations of germinal centers within secondary follicles of human tonsils showed that these proteins are highly expressed in the actively cycling cells. As we have described both in the Discussion and Introduction, the observation that HTm4 is expressed in both quiescent and actively cycling cells has basis and precedent. As described, p21 and p27 have been reported to have dual functions in cell cycle progression (42, 43). The same explanations for expression in proliferating and nonproliferating states that apply to p21 and p27 apply to HTm4: (a) expression of an inhibitory cell cycle regulator in response to proliferation or (b) an effect that is modified by the concentrations of other cell cycle regulators, both known and unknown (3, 42, 43).
In conclusion, our research has provided substantial evidence for HTm4 as a novel hematopoietic modulator for the G1-S cell-cycle transition. We have identified that HTm4 binds to KAP and that HTm4 forms a physiologic complex with KAP and CDK2. Exogenous expression of HTm4 promotes the dephosphorylation of CDK2, leading to cell cycle arrest at G0/G1 phase. The immunohistochemistry data showing expression of HTm4, KAP, and CDK2 in the germinal centers of lymphoid tissues highlight the potential biological relevance of our observations in vivo.
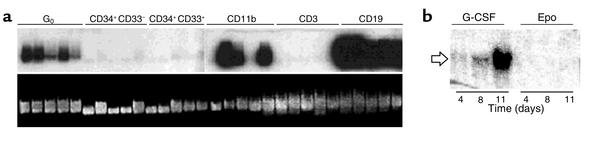
Figure 1.
The expression of HTm4 is highly regulated during the differentiation of hematopoietic stem cells. (a) MicroRT-PCR analysis of HTm4 expression in single hematopoietic cells at various stages of differentiation. Cell surface markers for each sample are denoted on the top row. G0 represents the quiescent CD34+/CD38– HSC. The middle row is a Southern blot analysis of HTm4 cDNA obtained through RT-PCR technique. The bottom row shows the loaded cDNA stained with ethidium bromide dye. Each lane represents a single cell. (b) Northern blot analysis of HTm4 expression in CD34+ cells that were induced to differentiate with the treatment of either G-CSF or Epo for time intervals as indicated. At day 11, cell populations are determined to be 60% neutrophils and 70% erythrocytes when induced with G-CSF and Epo, respectively.
Acknowledgments
This work was supported by NIH grant AI-43663 from the National Institute of Allergy and Infectious Diseases and by grant RSG-01-241-01-LIB from the American Cancer Society (to C.N. Adra); NIH grant AI-33100 (to M.H. Sayegh); RIKEN, Sagawa Foundation for Promotion of Cancer Research, Osaka Cancer Research Foundation, Welfide Medical Research Foundation (to T. Shirakawa); KO8 grant DK 02761 (to T. Cheng); FAPESP, Sao Paulo, Brazil (to J.L. Donato); NIH-P30CA6516 (to J.L. Kutok); NIH-HL44851, NIH-HL65909, and the Richard Saltonstall Charitable Foundation (to D.T. Scadden). This work was also supported by the Adra family and Dr. F. Jdeed, and is dedicated to the memory of Dr. Ramzi S. Cotran. We thank Professor T. Nakahata for his help and support. Assistance with preparation of manuscript was provided by Rachel Badovinac.
Footnotes
Jose L. Donato and Jon Ko contributed equally to this work.
References
- 1.Metcalf D. The molecular control of cell division, differentiation commitment and maturation in haemopoietic cells. Nature. 1989; 339:27–30. doi: 10.1038/339027a0. [DOI] [PubMed] [Google Scholar]
- 2.Ogawa M. Differentiation and proliferation of hematopoietic stem cells. Blood. 1993; 81:2844–2853. [PubMed] [Google Scholar]
- 3.Sherr CJ. Cancer cell cycles. Science. 1996; 274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 4.Morgan DO. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol. 1997; 13:261–291. doi: 10.1146/annurev.cellbio.13.1.261. [DOI] [PubMed] [Google Scholar]
- 5.Johnson DG, et al. Cyclins and cell cycle checkpoints. Annu Rev Pharmacol Toxicol. 1999; 39:295–312. doi: 10.1146/annurev.pharmtox.39.1.295. [DOI] [PubMed] [Google Scholar]
- 6.Israels ED, et al. The cell cycle. Stem Cells. 2001; 19:88–91. doi: 10.1634/stemcells.19-1-88. [DOI] [PubMed] [Google Scholar]
- 7.Cheng T, et al. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000; 287:1804–1808. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- 8.Cheng T, et al. Stem cell repopulation efficiency but not pool size is governed by p27(kip1) Nat Med. 2000; 6:1235–1240. doi: 10.1038/81335. [DOI] [PubMed] [Google Scholar]
- 9.Steinman RA, et al. Regulation of p21(WAF1) expression during normal myeloid differentiation. Blood. 1998; 91:4531–4542. [PubMed] [Google Scholar]
- 10.Taniguchi T, et al. Expression of p21(Cip1/Waf1/Sdi1) and p27(Kip1) cyclin-dependent kinase inhibitors during human hematopoiesis. Blood. 1999; 93:4167–4178. [PubMed] [Google Scholar]
- 11.Sherr CJ, et al. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999; 13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 12.Tsai LH, et al. The cdk2 kinase is required for the G1-to-S transition in mammalian cells. Oncogene. 1993; 8:1593–1602. [PubMed] [Google Scholar]
- 13.Brown NR, et al. Effects of phosphorylation of threonine 160 on cyclin-dependent kinase 2 structure and activity. J Biol Chem. 1999; 274:8746–8756. doi: 10.1074/jbc.274.13.8746. [DOI] [PubMed] [Google Scholar]
- 14.Solomon MJ. Activation of the various cyclin/cdc2 protein kinases. Curr Opin Cell Biol. 1993; 5:180–186. doi: 10.1016/0955-0674(93)90100-5. [DOI] [PubMed] [Google Scholar]
- 15.Morgan DO. Principles of CDK regulation. Nature. 1995; 374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- 16.Poon RY, et al. Dephosphorylation of Cdk2 Thr160 by the cyclin-dependent kinase-interacting phosphatase KAP in the absence of cyclin. Science. 1995; 270:90–93. doi: 10.1126/science.270.5233.90. [DOI] [PubMed] [Google Scholar]
- 17.Gyuris J, et al. Cdi1, a human G1 and S phase protein phosphatase that associates with Cdk2. Cell. 1993; 75:791–803. doi: 10.1016/0092-8674(93)90498-f. [DOI] [PubMed] [Google Scholar]
- 18.Hannon GJ, et al. KAP: a dual specificity phosphatase that interacts with cyclin-dependent kinases. Proc Natl Acad Sci USA. 1994; 91:1731–1735. doi: 10.1073/pnas.91.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeh CT, et al. Aberrant transcripts of the cyclin-dependent kinase-associated protein phosphatase in hepatocellular carcinoma. Cancer Res. 2000; 60:4697–4700. [PubMed] [Google Scholar]
- 20.Furukawa Y, et al. Lineage-specific regulation of cell cycle control gene expression during haematopoietic cell differentiation. Br J Haematol. 2000; 110:663–673. doi: 10.1046/j.1365-2141.2000.02253.x. [DOI] [PubMed] [Google Scholar]
- 21.Adra CN, et al. Cloning of the cDNA for a hematopoietic cell-specific protein related to CD20 and the beta subunit of the high-affinity IgE receptor: evidence for a family of proteins with four membrane-spanning regions. Proc Natl Acad Sci USA. 1994; 91:10178–10152. doi: 10.1073/pnas.91.21.10178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adra CN, et al. Chromosome 11q13 and atopic asthma. Clin Genet. 1999; 55:431–437. doi: 10.1034/j.1399-0004.1999.550606.x. [DOI] [PubMed] [Google Scholar]
- 23.Berardi AC, et al. Functional isolation and characterization of human hematopoietic stem cells. Science. 1995; 267:104–108. doi: 10.1126/science.7528940. [DOI] [PubMed] [Google Scholar]
- 24.Cheng T, et al. Temporal mapping of gene expression levels during the differentiation of individual primary hematopoietic cells. Proc Natl Acad Sci USA. 1996; 93:13158–13163. doi: 10.1073/pnas.93.23.13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J Immunol. 1981; 126:1614–1619. [PubMed] [Google Scholar]
- 26.Sambrook, J., Fritsch, E., and Maniatis, T. 1989. Molecular cloning: a laboratory manual. 2nd edition. Cold Spring Harbor Laboratory Press. Plainview, New York, USA.
- 27.Van Aelst L, et al. Identification of a novel Rac1-interacting protein involved in membrane ruffling. EMBO J. 1996; 15:3778–3786. [PMC free article] [PubMed] [Google Scholar]
- 28.Crews CM, et al. The primary structure of MEK, a protein kinase that phosphorylates the ERK gene product. Science. 1992; 258:478–480. doi: 10.1126/science.1411546. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, et al. byr2, a Schizosaccharomyces pombe gene encoding a protein kinase capable of partial suppression of the ras1 mutant phenotype. Mol Cell Biol. 1991; 11:3554–3563. doi: 10.1128/mcb.11.7.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adra CN, et al. LAPTM5: a novel lysosomal-associated multispanning membrane protein preferentially expressed in hematopoietic cells. Genomics. 1996; 35:328–337. doi: 10.1006/geno.1996.0364. [DOI] [PubMed] [Google Scholar]
- 31.Ling YH, et al. Cell cycle-dependent cytotoxicity, G2/M phase arrest, and disruption of p34cdc2/cyclin B1 activity induced by doxorubicin in synchronized P388 cells. Mol Pharmacol. 1996; 49:832–841. [PubMed] [Google Scholar]
- 32.Hass R, et al. Characterization of human TUR leukemia cells: continued cell cycle progression in the presence of phorbol ester is associated with resistance to apoptosis. Eur J Cell Biol. 1994; 65:408–416. [PubMed] [Google Scholar]
- 33.Hass R, et al. Resistance to phorbol ester-induced differentiation of a U-937 myeloid leukemia cell variant with a signaling defect upstream to Raf-1 kinase. Cell Growth Differ. 1993; 4:657–663. [PubMed] [Google Scholar]
- 34.Lee SW, et al. Overexpression of kinase-associated phosphatase (KAP) in breast and prostate cancer and inhibition of the transformed phenotype by antisense KAP expression. Mol Cell Biol. 2000; 20:1723–1732. doi: 10.1128/mcb.20.5.1723-1732.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghiani C, et al. Inhibition of cyclin E-cyclin-dependent kinase 2 complex formation and activity is associated with cell cycle arrest and withdrawal in oligodendrocyte progenitor cells. J Neurosci. 2001; 21:1274–1282. doi: 10.1523/JNEUROSCI.21-04-01274.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muller-Tidow C, et al. Cyclin E is the only cyclin-dependent kinase 2-associated cyclin that predicts metastasis and survival in early stage non-small cell lung cancer. Cancer Res. 2001; 61:647–53. [PubMed] [Google Scholar]
- 37.Lukas J, et al. Cyclin E-induced S phase without activation of the pRb/E2F pathway. Genes Dev. 1997; 11:1479–1492. doi: 10.1101/gad.11.11.1479. [DOI] [PubMed] [Google Scholar]
- 38.Knudsen ES, et al. Dual mechanisms for the inhibition of E2F binding to RB by cyclin- dependent kinase-mediated RB phosphorylation. Mol Cell Biol. 1997; 17:5771–5783. doi: 10.1128/mcb.17.10.5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knudsen ES, et al. Inhibition of DNA synthesis by RB: effects on G1/S transition and S-phase progression. Genes Dev. 1998; 12:2278–2292. doi: 10.1101/gad.12.15.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strobeck MW, et al. Restoration of retinoblastoma mediated signaling to Cdk2 results in cell cycle arrest. Oncogene. 2000; 19:1857–1867. doi: 10.1038/sj.onc.1203510. [DOI] [PubMed] [Google Scholar]
- 41.Weinstein IB. Disorders in cell circuitry during multistage carcinogenesis: the role of homeostasis. Carcinogenesis. 2000; 21:857–864. doi: 10.1093/carcin/21.5.857. [DOI] [PubMed] [Google Scholar]
- 42.LaBaer J, et al. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 1997; 11:847–862. doi: 10.1101/gad.11.7.847. [DOI] [PubMed] [Google Scholar]
- 43.Cheng T, et al. TGF-β1 mediates cell cycle arrest of primitive hematopoietic cells independent of p21Cip1/Waf1 or p27Kip1. Blood. 2001; 98:3643–3649. doi: 10.1182/blood.v98.13.3643. [DOI] [PubMed] [Google Scholar]