Abstract
Glatiramer acetate (GA; Copaxone) is a random copolymer of glutamic acid, lysine, alanine, and tyrosine that is used therapeutically in patients with multiple sclerosis (MS). To investigate the mechanism of the drug’s immunomodulatory effect, we used immunophenotypic approaches to characterize the precise nature of GA-induced T cell responses. We demonstrate here that healthy individuals and untreated MS patients exhibit prominent T cell proliferative responses to GA. However, these responses are different in distinct subsets of T cells. Whereas GA-induced CD4+ T cell responses are comparable in healthy individuals and MS patients, CD8+ T cell responses are significantly lower in untreated MS patients. Treatment with GA results in upregulation of these CD8+ responses with restoration to levels observed in healthy individuals. Both CD4+ and CD8+ GA-specific responses are HLA-restricted. GA therapy also induces a change in the cytokine profile of GA-specific CD4+ and CD8+ T cells. This study provides the first direct immunophenotypic evidence, to our knowledge, of GA-specific CD8+ T cell responses and their upregulation during the course of therapy, which may suggest a role for these responses in the immunomodulatory effects of the drug.
Introduction
Multiple sclerosis (MS) is an inflammatory, demyelinating disorder of the CNS with varied clinical presentations that range from clearly defined relapses and remissions (RRMS) to slow accumulation of disability with or without punctuated exacerbation (progressive MS) (1–3). Progressive MS may be either primary in its presentation (PPMS) or secondary (SPMS) after a period of RRMS. Although the etiology of this diverse group of disorders is unknown, MS is thought to be immune-mediated, as indicated by its characteristic pathology (2) and the presence of autoreactive T cell responses against myelin-related antigens (4–7). Several immunomodulatory strategies are thus being actively pursued for therapeutic intervention in MS.
Glatiramer acetate (GA; Copaxone, registered trademark of Teva Pharmaceutical Industries Ltd.) is a random copolymer of glutamic acid, lysine, alanine, and tyrosine that emerged almost 30 years ago through experiments in an animal model of MS, experimental autoimmune encephalomyelitis (EAE). The peptide copolymer was initially synthesized to mimic the encephalitogenic components of myelin basic protein (MBP). However, it was found that GA inhibited EAE in rodents and primates (8–11). More importantly, in clinical trials (12–15), GA also reduced the rate of exacerbation and decreased the number of new lesions in patients with RRMS.
The mechanism by which GA exerts its immunomodulatory effect on human disease is presently unclear. Several mechanisms, including HLA blockade, T cell receptor antagonism, tolerance induction, and immune deviation, have been proposed and tested, predominantly in EAE (reviewed in refs. 16, 17). Studies in EAE suggest that GA induces a potent Th2-type CD4+ T cell response, which may cause bystander suppression of the Th1-type pathogenic response (18–20). More recent human studies have also shown a Th0/Th2 bias in GA-induced T cell responses in MS patients treated with the drug (21–27). Antigenic specificity (28, 29) and cross-reactivity with MBP (25) have also been suggested. Thus, it is currently proposed that GA induces an immune deviation in CD4+ T cells toward the Th2 phenotype resulting in bystander suppression of myelin-reactive Th1 responses. Previous reports have shown binding of GA to several MHC class II molecules (HLA-DR, in particular) (30–32). Based on these observations and studies using CD8-depleted PBMCs (23), it has been presumed that responses to GA are restricted to the CD4+ T cells. Thus, reports addressing the mechanism of GA immunotherapy have focused predominantly on the CD4+ T cell subset.
However, all previous studies have used either conventional in vitro proliferation assays, ELISPOT assays, or long-term T cell lines and clones for mechanistic evaluation of GA. Direct immunophenotypic characterization of GA-induced responses is lacking. In fact, one recent report used fractionated populations of CD8+ T cells in ELISPOT assays and showed GA-induced production of IFN-γ in one MS patient (26). Such studies emphasize the importance of immunophenotypic evaluation of the responding cells to address the mechanism of the drug’s immunomodulatory effect.
The present study was conducted in order to characterize the phenotype and functional profile of GA-responsive T cells from MS patients by using flow cytometric proliferation assays and molecular characterization of flow-sorted T cell subsets.
Methods
Patients and healthy subjects.
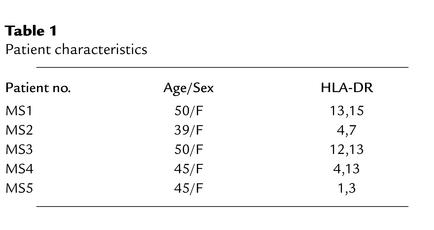
Healthy volunteers and patients with MS were recruited at the University of Texas Southwestern Medical Center. For experiments demonstrated in Figure 1, we recruited 9 healthy subjects (5 female, 4 male; age range 22–38 years) and 23 patients with MS, including 12 patients with RRMS, 6 with PPMS, and 5 with SPMS (18 female, 5 male; age range 28–47 years). At this time, none of these patients had ever been treated with IFN or GA. A few patients had received steroid therapy, but no treatment was received for at least 3 months preceding the day of pheresis; none were suffering from acute relapses. Five of these patients (Table 1) were subsequently put on open-label GA therapy and were studied longitudinally.
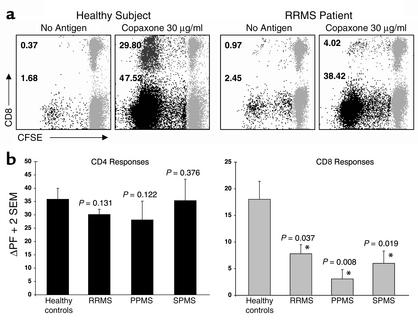
Figure 1.
Untreated MS patients have a deficient CD8+ T cell response to GA. CFSE-based proliferation assays were performed on PBMC specimens from 9 healthy individuals and 23 MS patients. (a) Representative responses from one healthy individual and one RRMS patient are shown. The data represent gated CD3+ T cells, further gated for CD4+/CD8– or CD8+/CD4– T cells. CFSE staining is shown on the x axis and CD8 staining on the y axis (CD8– populations represent gated CD4+ T cells). The gray populations represent nondividing cells. The numbers next to the darker populations represent the proliferating fraction of CD4+ T cells and CD8+ T cells. ΔPF is the difference between specific proliferation and the background (no antigen). Thus, the healthy control had a GA-specific ΔPF of 45.84% for the CD4+ T cells and 29.43% for the CD8+ T cells. The RRMS patient had a CD4+ ΔPF of 35.97% and 3.05% for the CD8+ response. The mean ΔPF was calculated from duplicate cultures in every experiment. (b) The graphs represent mean ΔPF (+ 2 SEM) of GA-specific CD4+ (left panel) and CD8+ (right panel) responses from 9 healthy individuals and 23 untreated MS patients (12 RRMS, 6 PPMS, and 5 SPMS). The responses from the MS patients were compared to those from the healthy individuals. The P values are indicated above the corresponding bars. *Significant differences (P < 0.05).
Table 1.
Patient characteristics
PBMCs.
Leukapheresis was performed as approved by the University of Texas Southwestern Institutional Review Board. PBMCs, obtained by Ficoll separation, were frozen on the day of collection, as described (7). For longitudinal studies, leukapheresis was performed every 4 months. Experiments involving longitudinal analysis on a single MS patient were performed on cells thawed and cultured simultaneously.
CFSE-based proliferation assays.
This methodology detects dividing cells by using the green fluorescent dye 5- (and 6-) carboxyfluorescien diacetate succinimidyl ester (CFSE) (33). The cells are first stained with CFSE and then cultured in the presence of different antigens. Dividing cells are then detected by sequential halving of their fluorescence and can be immunophenotyped with cell surface markers.
Thus, PBMCs were first suspended at 1 × 106 per milliliter in PBS and incubated at 37°C for 7 minutes with 0.25 μM CFSE. Following addition of serum and two PBS washes, cells were suspended at 2 × 106 per milliliter in human media (RPMI 1640 supplemented with glutamine, 5% human AB serum, penicillin, and streptomycin). Duplicate 1-ml cultures were set up for each condition. On day 7, cells were washed with FACS buffer (PBS with 1% BSA and 0.1% Na-azide) and stained with phycoerythrin-conjugated (PE-conjugated) anti-CD8, peridinin chlorophyll protein–anti-CD3 (PerCP–anti-CD3), and allophycocyanin–anti-CD4 (APC–anti-CD4). Duplicates were stained with PE–anti-CD4, PerCP–anti-CD3, and APC–anti-CD8 for confirmation (all antibodies from BD Biosciences, San Diego, California, USA). Cells were washed and fixed in 1% paraformaldehyde (BD Biosciences). Flow cytometric data (100,000 non-gated events) were acquired on a BD FACSCalibur four-color flow cytometer using BD CellQuest software. For analysis, BD Paint-A-Gate was used to gate on CD3+ T cells and further on the CD4+/CD8– or CD8+/CD4– populations. The mean background proliferation was calculated based on the proliferating fractions in media alone. The mean change in proliferating fraction (ΔPF) was calculated by subtracting the mean background proliferation from the mean proliferating fraction in response to antigen.
Antigens.
The antigens included GA (Copaxone; 30 μg/ml; provided by Teva Neuroscience Inc., Kansas City, Missouri, USA), bovine MBP (20 μg/ml; Sigma-Aldrich Chemical Co., St. Louis, Missouri, USA), and tetanus toxoid (TT; 20 μg/ml; Accurate Chemical & Scientific Corp., Westbury, New York, USA). Initial experiments revealed a dose-dependent response to GA with an optimal concentration of 30 μg/ml (data not shown), which was used in all experiments. In addition, kinetic experiments revealed that GA-specific responses could be reliably and reproducibly detected after 5–7 days of proliferation. For MBP-specific responses, at least 6–7 days of culture was essential (data not shown). Hence, for standardization and comparison, all further experiments were conducted on day 7 of culture.
HLA blockade.
Purified anti–HLA class I (HLA-A, -B, and -C) and anti–class II (HLA-DP, -DQ, and -DR) antibodies and their appropriate control antibodies (IgG1 and IgG2a) were purchased from BD Biosciences. The antibodies were dialyzed in PBS to remove the azide. CFSE-stained PBMCs were incubated in media with 10 μg/ml of the indicated antibodies at 37°C for 30 minutes before antigens were added.
3H-thymidine incorporation assays.
Conventional 3H-thymidine incorporation assays were conducted as described (7). Briefly, PBMCs were plated in 96-well tissue culture plates at a concentration of 5 × 105 cells per well in 200 μl of media. Quadruplicate cultures were set up with the indicated doses of antigens. Cells were pulsed with 0.25 μCi per well of 3H-thymidine for the last 18 hours of culture. On day 5 of culture, cells were harvested and analyzed for incorporation of radioactivity using a Wallac Betaplate liquid scintillation counter (PerkinElmer Life Sciences, Wellesley, Massachusetts, USA). Results are expressed in cpm or Δcpm (background subtracted).
Intracellular cytokine staining.
CFSE-stained PBMCs were cultured as described above. On day 7, cells were stimulated for 4 hours with a mixture of PMA (0.25 ng/ml) and ionomycin (1 μg/ml; Sigma-Aldrich Chemical Co.) with the Golgi inhibitor brefeldin A (10 μg/ml) added for the retention of intracellular cytokines (34). Cells were then stained with a protocol modified from the one described previously (35). Briefly, cells were first stained for surface markers using PerCP- and APC-conjugated antibodies (CD4 and CD8), as described above. This was followed by permeabilization with FACSLyse solution (BD Biosciences), followed by staining for the indicated intracellular cytokines using PE-conjugated antibodies.
CFSE-based flow sorting.
CFSE-stained PBMCs were suspended at 2 × 106 per milliliter and cultured in 30 ml media in T75 flasks (BD Biosciences). On day 7, cells were stained with PE-, CyChrome-, and APC-conjugated antibodies and sorted by electronic gating into dividing and nondividing, CD4+ and CD8+ T cell populations using a BD FACStar or FACSVantage SE sorter. On populations with adequate yields (>200,000 cells), a “post-sort” run was performed revealing greater than 90% purity. Sorted cells were collected in 1.5-ml Sarstedt tubes, pelleted, and frozen at –80°C for subsequent molecular analyses.
RT-PCR assays.
mRNA was obtained from sorted populations of cells using the Oligotex mRNA kit (QIAGEN Inc., Valencia, California, USA) and was reverse-transcribed to cDNA using the Ready-To-Go First Strand cDNA kit (Amersham Pharmacia Biotech, Piscataway, New Jersey, USA). Qualitative PCR assays were performed on cDNA using the following primers (all 5′–3′; purchased from Life Technologies Inc., Gaithersburg, Maryland, USA):
β-actin: forward, CCTGGACTTCGAGCAAGAGA; reverse, ACTTGCGCTCAGGAGGAGCA
IFN-γ: forward, TTTAATGCAGGTCATTCAGATGTA; reverse, CACTTGGATGAGTTCATGTATTGC
IL-4: forward, AACTTTGTCCACGGACACAAGTGC; reverse, GAATCGGATCAGCTGCTTGTGCCT
TNF-α: forward, CGAGTCTGGGCAGGTCTACTTT; reverse, AAGCTGTAGGCCCCAGTGAGTT
TGF-β1: forward, GCCCTGGACACCAACTATTGC; reverse, GCTGCACTTGCAGGAGCGCAC
Fifty-microliter PCR reactions were performed in PCR buffer, 1.5 mM MgCl2, 0.2 mM dNTP, 2.5 U Taq (Life Technologies Inc.), and 0.4 mM forward and reverse primers. PCR was performed on the Applied Biosystems (Foster City, California, USA) GeneAmp 9700 thermocycler with the following cycles (in °C): (95 × 7 min; 50 × 1 min; 72 × 1 min) × 1 cycle; (94 × 1 min; 50 × 1 min; 72 × 1 min) × 35 cycles; hold at 4°C.
Statistical analyses.
Unpaired (for cross-sectional studies) and paired (for longitudinal studies) t tests were used to compare the CD4+ and CD8+ T cell responses between different groups. P values of less than 0.05 were considered significant.
Results
Deficient CD8+ T cell responses to GA in patients with MS.
Previous reports have demonstrated GA-induced proliferative responses in both healthy individuals and patients with MS (23, 24). In order to characterize the nature of these responses, we performed CFSE-based flow cytometric proliferation assays on 9 healthy control subjects and 23 untreated MS patients (12 RRMS, 6 PPMS, and 5 SPMS). Figure 1a demonstrates typical responses from one healthy individual and one patient with RRMS, in the presence (or absence) of 30 μg/ml of GA. The data represent gated CD3+ T cells, further gated for CD4+/CD8– or CD8+/CD4– T cells. There was a robust proliferative response to GA in both the healthy control and the MS patient. In the healthy individual, 47.52% of the CD4+ T cells were in the proliferating fraction, with a background of 1.68 (ΔPF = 45.84%), while the ΔPF for CD8+ T cells was 29.43% (29.80% – 0.37%). The RRMS patient showed a prominent CD4+ GA-specific response (ΔPF = 35.97%) but a significantly lower CD8+ response (ΔPF = 3.05%). Figure 1b demonstrates the mean ΔPF values for GA-induced CD4+ (left panel) and CD8+ (right panel) responses from 9 healthy individuals and 23 MS patients. There were no statistical differences in the GA-specific CD4+ responses between healthy controls and MS patients. In contrast, the CD8+ T cell responses from untreated patients with various types of MS were significantly lower compared with healthy controls (Figure 1b).
Differential upregulation and restoration of GA-specific CD8+ T cell responses following GA therapy.
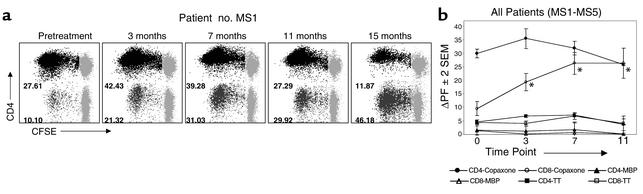
To investigate whether GA therapy had an effect on T cell responses, we studied five MS patients who received open-label GA therapy daily for more than 6 months (Table 1). PBMCs from these patients were collected by leukapheresis at 4-month intervals. All patients began daily GA injections approximately 1 month following their first pheresis. Figure 2 demonstrates the results from longitudinal CFSE-based assays conducted on these patients. Figure 2a shows the dynamics of GA-specific T cell responses from patient no. MS1 over 15 months of therapy. The GA-specific CD4+ T cell proliferative responses showed an initial increase in this patient followed by a gradual decline to values below pretreatment levels. In contrast, the CD8+ T cell responses showed a sustained increase over the course of GA therapy.
Figure 2.
GA-specific CD8+ T cell responses are differentially upregulated following GA therapy. CFSE-based proliferation assays were performed on longitudinal PBMC specimens obtained from five MS patients on daily GA therapy. (a) GA-specific proliferative responses from a representative patient (no. MS1) are shown at the pretreatment time point and at 3, 7, 11, and 15 months after the initiation of GA therapy. The data represent gated CD4+/CD8– or CD8+/CD4– T cells. CFSE staining is shown on the x axis and CD4 staining on the y axis. The gray populations represent nondividing cells. The numbers next to the darker populations represent the proliferating fraction of CD4+ and CD8+ T cells. The mean background proliferation ranged from 0.29 to 4.36 in various experiments. (b) This graph represents longitudinal mean ΔPF values (± 2 SEM) of CD4+ and CD8+ T cell responses to GA, MBP, and TT from all five MS patients on GA therapy (Table 1). The pretreatment time point (0) and the 3-, 7-, and 11-month time points are shown. *Statistically significant increase of GA-specific CD8+ T cell responses, compared with those in the pretreatment specimens (P < 0.05).
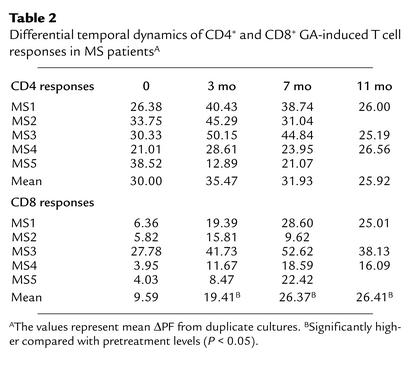
Figure 2b shows the mean ΔPF values of CD4+ and CD8+ T cell responses from all five patients over an 11-month course. The overall pattern of CD4+ GA-specific responses was a transient increase at the 3-month time point, followed by a gradual decline. This pattern was seen in four of five patients (Table 2). However, at the cohort level, none of these alterations reached statistical significance. In contrast, following GA therapy, GA-specific CD8+ T cell responses significantly increased (Figure 2 and Table 2) to levels observed in healthy individuals (Figure 1). Such variations in GA-specific CD4+ or CD8+ T cell responses were not observed in untreated MS patients (data not shown).
Table 2.
Differential temporal dynamics of CD4+ and CD8+ GA-induced T cell responses in MS patientsA
In addition to GA-specific responses, we also evaluated T cell responses to MBP and TT (Figure 2b). Although mild variations were observed in individual patients, at the level of the patient cohort, the responses were stable over the course of treatment. In other patients that were treated with different immunosuppressive therapy, a global reduction was observed in GA-, MBP-, and TT-specific CD4+ and CD8+ T cell responses (data not shown).
HLA restriction of GA-specific responses.
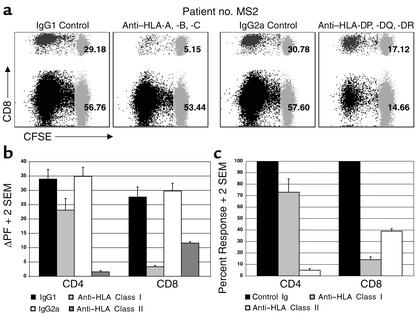
To test whether the CD4+ and CD8+ T cell responses to GA were restricted to classical HLA molecules, we performed CFSE-based proliferation assays in the presence of anti–HLA class I and anti–HLA class II antibodies (or appropriate controls). Blockade of HLA class I molecules resulted in prominent inhibition of the GA-specific CD8+ T cell responses (Figure 3). In three of the five patients, class I blockade also inhibited CD4+ T cell responses, as represented in the means shown in Figure 3, b and c. As expected, HLA class II blockade predominantly inhibited CD4+ T cell responses but also resulted in a significant inhibition of CD8+ T cell responses, most likely due to suppression of CD4+ T cell–mediated help. Taken together, these results demonstrate that both CD4+ and CD8+ GA-specific T cell responses are restricted to classical HLA molecules.
Figure 3.
GA-specific CD4+ and CD8+ T cell responses are HLA-restricted. CFSE-based proliferation assays were performed on PBMC specimens from five MS patients on daily GA therapy for 3 months in the presence of anti-HLA antibodies or appropriate control Ig. (a) These dot plots show CD4+ and CD8+ GA-specific proliferative responses from a representative patient (no. MS2) in the presence of indicated antibodies. The two left panels demonstrate the effect of HLA class I blockade (compared with the IgG1 control), whereas the two right panels demonstrate the effect of HLA class II blockade (compared with IgG2a control). CFSE staining is shown on the x axis and CD8 staining on the y axis. The numbers represent the proliferating fraction of CD4+ and CD8+ T cells. (b) This graph represents mean ΔPF (+ 2 SEM) values of CD4+ and CD8+ T cell responses to GA from all five patients in the presence of indicated antibodies. (c) The same data are expressed in the form of percent response, where the proliferation in the presence of appropriate control Ig was assigned a 100% value.
Kinetics of 3H-thymidine–based proliferative assays during treatment course.
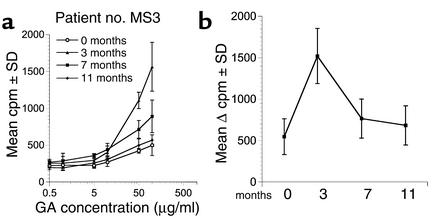
Previous studies that have used 3H-thymidine incorporation assays have shown characteristic kinetics of GA-specific proliferation during GA therapy. Following an initial enhancement around 2–3 months of treatment, most patients show a gradual decline in GA-induced 3H-thymidine incorporation through the course of therapy (23–26). Since we observed a temporal enhancement of CD8+ T cell responses using the CFSE-based assays, we conducted parallel 3H-thymidine incorporation on PBMCs from the same patients to test whether this increase in responses could be detected by the conventional assay. As shown in Figure 4, the GA-treated MS patients showed kinetics very similar to that previously described in the literature. The same pattern was observed in 3H-thymidine–based limiting dilution assays that were conducted over a 7-day time frame (data not shown). Thus, the kinetic curves from both of the 3H-thymidine–based assays paralleled those of CD4+ T cell responses (Figure 2) and were unable to sensitively detect the enhanced CD8+ response, which constituted a very small proportion of the overall bulk proliferative response.
Figure 4.
Kinetics of conventional 3H-thymidine–based GA-specific proliferation during therapy. Conventional 3H-thymidine–based proliferation assays were performed on longitudinal PBMC specimens obtained from MS patients on daily GA therapy. (a) The GA-specific dose-response curves are from a representative patient (no. MS3) at the pretreatment time point and at 3, 7, and 11 months after the initiation of GA therapy, as indicated. The cpm ± SD are plotted on the y axis and the concentration of GA is plotted on the x axis (logarithmic scale). (b) The mean Δ cpm (background subtracted) ± SEM from four GA-treated patients are shown at the time points indicated on the x axis (GA at 50 μg/ml).
Cytokine profiles of GA-specific responses.
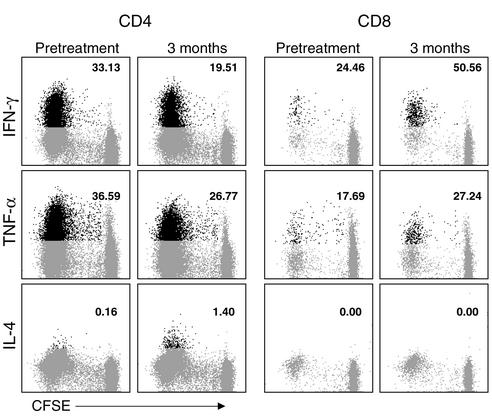
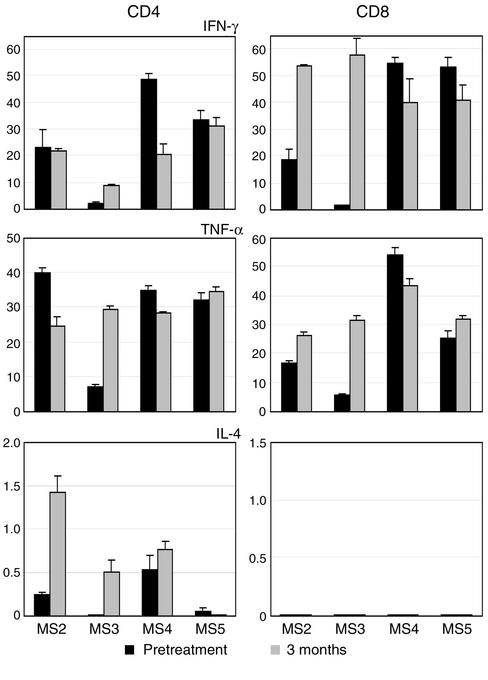
Previous reports have demonstrated that treatment with GA induces immune deviation of GA-specific CD4+ T cells toward the Th2 phenotype in MS (21–27). To characterize the cytokine profiles of GA-specific CD4+ and CD8+ T cells separately, we used two approaches. First, we performed intracellular cytokine staining for the flow cytometric detection of IFN-γ, TNF-α, and IL-4 in GA-specific CD4+ and CD8+ T cells. For this we used a novel assay combining CFSE-based proliferation and intracellular cytokine flow cytometry (Figures 5 and 6). On day 7 of a CFSE-stained culture, we restimulated the cells in the presence of brefeldin A, which is a Golgi inhibitor and causes retention of cytokine within the cytoplasm (34, 35). The cells were stained first for surface markers (CD4 and CD8), and then permeabilized and stained for intracellular cytokines. Figure 5 demonstrates the dot plots from one patient. Figure 6 shows the results from four MS patients, plotted as the percentage of GA-responsive cells that produced the indicated cytokine. The GA-specific CD8+ T cells showed prominent production of IFN-γ and TNF-α. No IL-4 was detected in this subset. The pattern of cytokine production by GA-specific CD4+ T cells showed variability within the different patients. Some patients appeared to demonstrate a mild shift toward the Th0/Th2 phenotype, as suggested by previous reports (21–27).
Figure 5.
Intracellular cytokine staining of GA-specific CD4+ and CD8+ T cells. PBMCs were obtained from patient no. MS3 before treatment and at 3 months after the initiation of GA therapy. The cells were stained with CFSE and cultured in the presence of GA. On day 7 of culture, the cells were restimulated for 4 hours with PMA and ionomycin in the presence of brefeldin A and then stained for surface CD4 and CD8 and intracellular cytokine (IFN-γ, TNF-α, or IL-4), as indicated. The data are gated for either CD4+ (left six panels) or CD8+ T cells (right six panels) and show CFSE staining on the x axis and cytokine staining on the y axis. The cutoff was based on staining with isotypic control antibodies. The population in black represents the cytokine-producing, GA-specific cells (i.e., cells within the proliferating fraction). The number indicates the percentage of the GA-specific (proliferating) cells that were positive for the indicated cytokines. As individual cytokine staining was performed in different tubes, it is not possible to assess whether the same or distinct subsets of cells produced the different cytokines.
Figure 6.
Cytokine profiles of GA-specific CD4+ and CD8+ T cells. As described in Figure 5, cytokine flow cytometry was conducted on GA-specific cells from the indicated patients. The data from gated CD4+ (left panels) and CD8+ (right panels) T cells are plotted as percent positive cells (+ SEM) on the y axis and represent the proportion of gated, GA-responsive cells that produced the indicated cytokine (duplicate cultures).
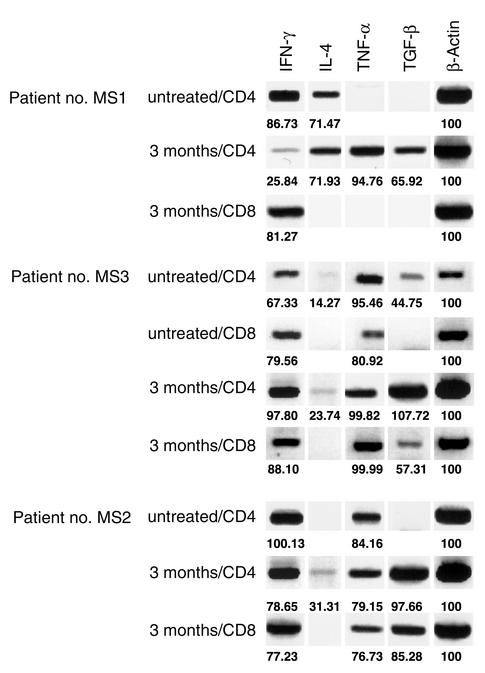
To further verify our observations and evaluate production of another Th2/Th3–type marker, we used a molecular approach. GA-reactive CD4+ and CD8+ T cells from 7-day cultures were flow-sorted, followed by qualitative RT-PCR analysis to detect transcripts for various cytokine genes (Figure 7). Overall, our findings correlated with the cytokine flow cytometry data. After GA treatment, there was an alteration in the cytokine profile of GA-specific CD4+ T cells. IL-4 message was detected in GA-specific CD4+ T cells in post-treatment specimens. GA-specific CD8+ T cells were positive for IFN-γ. Interestingly, TGF-β and TNF-α were detected in both CD4+ and CD8+ GA-specific T cells. Thus, GA-specific CD4+ and CD8+ T cells showed a mixed cytokine profile with some production of regulatory cytokines.
Figure 7.
Functional profile of GA-specific CD4+ and CD8+ T cells. Nonquantitative RT-PCR analyses were performed on the indicated flow-sorted GA-specific CD4+ and CD8+ T cells for qualitative detection of IFN-γ, IL-4, TNF-α, and TGF-β. These assays were performed on PBMCs from three patients before treatment and at 3 months after the initiation of GA therapy. Patients no. MS1 and no. MS2 did not yield adequate GA-reactive CD8+ cells before treatment (no β-actin bands observed). The numbers indicate band density values normalized to β-actin.
Discussion
GA is used as an immunomodulatory treatment in patients with MS. It is thought to deviate GA-specific CD4+ T cell responses toward the Th2 phenotype, thereby causing bystander suppression of the pathogenic myelin-specific Th1 responses (21–27). However, direct immunophenotypic analysis of GA-induced responses has not been carried out thus far. In the present study, we characterized the immunophenotype of GA-induced T cell responses.
Using CFSE-based flow cytometric proliferation assays, we detected T cell responses to GA in both healthy individuals and MS patients, as described in previous studies (23, 24). However, we observed significant differences in the phenotypic subsets of T cells responding to this drug. Whereas the CD4+ GA-specific T cell responses were comparable in all groups, the CD8+ responses were significantly lower in untreated MS patients (Figure 1). To our knowledge, this is the first direct immunophenotypic evidence of a CD8+ proliferative response to GA.
Following treatment with GA, the GA-induced CD4+ and CD8+ T cell responses displayed differential regulation. The overall pattern of CD4+ responses appeared to be a transient upregulation around the 3-month time point followed by a gradual decline (Figure 2; Table 2). The CD8+ T cell responses, however, showed distinct upregulation after treatment and were significantly higher compared with baseline levels, more comparable to those seen in healthy individuals (Figures 1 and 2). Since CD4+ responses to GA show temporal downregulation, in contrast to upregulation of CD8+ responses, it is possible that the immunomodulatory effect of this drug may be mediated, at least in part, by the GA-induced CD8+ T cells.
Prior studies have used traditional 3H-thymidine–based in vitro proliferation assays that measure antigen-induced incorporation of 3H-thymidine in proliferating cell cultures. Using these assay procedures, we found a transient increase in GA-specific bulk proliferation followed by a decline during the treatment course (Figure 4), similar to previously reported observations (24, 25). Thus, the temporal dynamics of 3H-thymidine–based proliferation appears to predominantly mirror the CD4+ GA-specific T cell responses. Whereas dividing CD8+ T cells would undoubtedly incorporate 3H-thymidine during DNA synthesis, it is important to note that the enhanced CD8+ T cell responses are not reflected in bulk proliferation. The CD8+ T cell population forms a minor component of the overall proliferating fraction due to the high CD4/CD8 ratio. This is especially true in patients with MS, who may show a skewed CD4 preponderance (36). As an example, in Figure 2a, at the 3-month time point for patient no. MS1, we observed a prominent GA-specific CD8+ T cell response with a proliferating fraction of 21.32%. However, this represents only 3.08% of the total live cells in culture, whereas the concurrent CD4+ response with a proliferating fraction of 42.43% represents 26.21% of the total live cells. Thus, the CD4+ T cell response contributes a far greater proportion of the bulk proliferation. To detect shifts in the CD8+ responses, it is essential to specifically analyze that population of T cells. Additionally, the CFSE-based proliferation assay measures cumulative proliferation over the entire culture period, whereas the 3H-thymidine–based assay measures incorporation only over the last 12–18 hours of culture. These factors explain why a previous report using comparative bulk proliferation assays before and after depletion of CD8+ T cells from untreated MS patients did not detect significant differences, leading to the interpretation that there are no CD8+ responses to GA (23). However, it is still possible that such a difference may be detected in fractionated cells from healthy individuals or treated MS patients who have a robust CD8+ T cell response. This emphasizes the need to specifically evaluate this subset of cells by using direct phenotypic assays or pre-sorted populations of cells.
Several investigators have reported that MS patients have defective suppressor function in their CD8+ T cell compartment (36–38). A regulatory role for CD8+ T cells has been demonstrated in EAE (39–42). Upregulation of such suppressive ability has also been seen following combined therapy with etretinate and IFN-β1b (43). From these studies, it is tempting to speculate that the diminished CD8+ T cell response to GA in untreated MS patients may be reflective of the global defect of regulatory cells in this subset. As a corollary, treatment with GA may function, in part, through the induction and restoration of this regulatory population of cells.
To investigate the functional profile of GA-induced T cell responses, we performed flow cytometric staining on GA-responsive cells as well as molecular evaluation of flow-sorted populations of GA-reactive CD4+ and CD8+ T cells (Figures 5–7). There were changes in the cytokine profiles of post-treatment GA-specific cells. IL-4 was detected predominantly in post-treatment CD4+ T cells. This finding corroborates previous reports that have shown a shift toward the Th0/Th2 phenotype of long-term T cell lines derived from treated patients and in ELISPOT assays performed on bulk PBMC specimens (24–26).
GA-reactive CD8+ T cells were positive for IFN-γ protein and message (Figures 5–7). One previous report, using ELISPOT assays performed on one GA-treated patient, suggested that GA-specific IFN-γ production may be prominent in the CD8+ subset (26). Using short-term cytokine flow cytometry assays (6–8 hours of culture), we have also observed such GA-specific IFN-γ production in CD8+ T cells at the protein level in some patients (unpublished observations). Thus, GA treatment appears to induce an IFN-γ–secreting CD8+ T cell response. Inter-estingly, the suppressive activity of CD8+ T cells in mitogen-driven in vitro proliferation assays is thought to be mediated by IFN-γ secretion (37).
We also detected the presence of TGF-β message in GA-specific CD4+ and CD8+ T cells (Figure 7). This is a significant finding, as TGF-β has been shown to be an important mediator of bystander suppressive effects of Th2/Th3–type responses in EAE (44–46). However, TNF-α was also detected in these cells, which may be pro- or antiinflammatory in different systems. Thus, the overall cytokine profile of GA-induced CD8+ T cells appears to have a component that may mediate regulatory inhibition of disease-mediating responses.
Of note, we did not observe statistically significant changes in the MBP-specific proliferative responses in these patients during the treatment course, although individual patients showed mild variation. This finding is consistent with previous reports (24). Thus, at least in the PBMC compartment, there does not appear to be an obvious suppression of the MBP-specific response. It is possible that GA-specific T cells exert their regulatory effect locally at the site of tissue damage or through cross-reactive competition in these microenvironments. Further investigation is required to determine the exact mechanisms and the extent to which GA-specific CD4+ and CD8+ T cells may play a role in the modulatory effect of this drug.
To summarize, our observations provide the first direct immunophenotypic evidence, to our knowledge, of the presence of GA-induced CD8+ T cell responses. These responses are deficient in untreated MS patients and are upregulated in patients on GA therapy. The overall cytokine profiles of GA-induced responses are suggestive of, but do not provide conclusive evidence for, a regulatory functional profile. These findings support the notion that GA-induced CD8+ T cells may play an important regulatory role in the immunomodulatory effects of this drug and warrant further characterization. Finally, the upregulation of CD8+ T cell responses may serve as a useful marker in the monitoring of GA therapy.
Acknowledgments
These studies were supported by the following research grants: to N.J. Karandikar, US Public Health Service NIH grant AI-49990 and a grant from the Wadsworth Foundation (Seattle, Washington, USA); and to M.K. Racke, NIH grants NS-37513 and AI-47133, National Multiple Sclerosis Society grant RG2969-A-4, and a grant from the Yellow Rose Foundation (Dallas, Texas, USA). The authors would like to thank Becky Price for performing the leukaphereses.
Footnotes
See the related Commentary beginning on page 581.
Jason M. Brenchley, David R. Ambrozak, Daniel C. Douek, and Richard A. Koup’s present address is: Vaccine Research Center, NIH/National Institute of Allergy and Infectious Diseases, Bethesda, Maryland, USA.
References
- 1.Thompson AJ, et al. Primary progressive multiple sclerosis. Brain. 1997;120:1085–1096. doi: 10.1093/brain/120.6.1085. [DOI] [PubMed] [Google Scholar]
- 2.Martin R, McFarland HF, McFarlin DE. Immunological aspects of demyelinating diseases. Annu Rev Immunol. 1992;10:153–187. doi: 10.1146/annurev.iy.10.040192.001101. [DOI] [PubMed] [Google Scholar]
- 3.McFarlin DE, McFarland HF. Multiple sclerosis (first of two parts) N Engl J Med. 1982;307:1183–1188. doi: 10.1056/NEJM198211043071905. [DOI] [PubMed] [Google Scholar]
- 4.Tuohy VK, Yu M, Weinstock-Guttman B, Kinkel RP. Diversity and plasticity of self recognition during the development of multiple sclerosis. J Clin Invest. 1997;99:1682–1690. doi: 10.1172/JCI119331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goebels N, et al. Repertoire dynamics of autoreactive T cells in multiple sclerosis patients and healthy subjects: epitope spreading versus clonal persistence. Brain. 2000;123:508–518. doi: 10.1093/brain/123.3.508. [DOI] [PubMed] [Google Scholar]
- 6.Lehmann PV, Sercarz EE, Forsthuber T, Dayan CM, Gammon G. Determinant spreading and the dynamics of the autoimmune T-cell repertoire. Immunol Today. 1993;14:203–208. doi: 10.1016/0167-5699(93)90163-F. [DOI] [PubMed] [Google Scholar]
- 7.Lovett-Racke AE, et al. Decreased dependence of myelin basic protein-reactive T cells on CD28-mediated costimulation in multiple sclerosis patients. A marker of activated/memory T cells. J Clin Invest. 1998;101:725–730. doi: 10.1172/JCI1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teitelbaum D, Meshorer A, Hirshfeld T, Arnon R, Sela M. Suppression of experimental allergic encephalomyelitis by a synthetic polypeptide. Eur J Immunol. 1971;1:242–248. doi: 10.1002/eji.1830010406. [DOI] [PubMed] [Google Scholar]
- 9.Teitelbaum D, et al. Suppression of experimental allergic encephalomyelitis in Rhesus monkeys by a synthetic basic copolymer. Clin Immunol Immunopathol. 1974;3:256–262. doi: 10.1016/0090-1229(74)90012-9. [DOI] [PubMed] [Google Scholar]
- 10.Keith AB, Arnon R, Teitelbaum D, Caspary EA, Wisniewski HM. The effect of Cop 1, a synthetic polypeptide, on chronic relapsing experimental allergic encephalomyelitis in guinea pigs. J Neurol Sci. 1979;42:267–274. doi: 10.1016/0022-510x(79)90058-3. [DOI] [PubMed] [Google Scholar]
- 11.Teitelbaum D, Webb C, Meshorer A, Arnon R, Sela M. Suppression by several synthetic polypeptides of experimental allergic encephalomyelitis induced in guinea pigs and rabbits with bovine and human basic encephalitogen. Eur J Immunol. 1973;3:273–279. doi: 10.1002/eji.1830030505. [DOI] [PubMed] [Google Scholar]
- 12.Abramsky O, Teitelbaum D, Arnon R. Effect of a synthetic polypeptide (COP 1) on patients with multiple sclerosis and with acute disseminated encephalomeylitis. Preliminary report. J Neurol Sci. 1977;31:433–438. doi: 10.1016/0022-510x(77)90220-9. [DOI] [PubMed] [Google Scholar]
- 13.Bornstein MB, Miller AI, Teitelbaum D, Arnon R, Sela M. Multiple sclerosis: trial of a synthetic polypeptide. Ann Neurol. 1982;11:317–319. doi: 10.1002/ana.410110314. [DOI] [PubMed] [Google Scholar]
- 14.Johnson KP, et al. Sustained clinical benefits of glatiramer acetate in relapsing multiple sclerosis patients observed for 6 years. Copolymer 1 Multiple Sclerosis Study Group. Mult Scler. 2000;6:255–266. doi: 10.1177/135245850000600407. [DOI] [PubMed] [Google Scholar]
- 15.Johnson KP, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology. 1995;45:1268–1276. doi: 10.1212/wnl.45.7.1268. [DOI] [PubMed] [Google Scholar]
- 16.Gran B, et al. Mechanisms of immunomodulation by glatiramer acetate. Neurology. 2000;55:1704–1714. doi: 10.1212/wnl.55.11.1704. [DOI] [PubMed] [Google Scholar]
- 17.Neuhaus O, Farina C, Wekerle H, Hohlfeld R. Mechanisms of action of glatiramer acetate in multiple sclerosis. Neurology. 2001;56:702–708. doi: 10.1212/wnl.56.6.702. [DOI] [PubMed] [Google Scholar]
- 18.Aharoni R, Teitelbaum D, Sela M, Arnon R. Copolymer 1 induces T cells of the T helper type 2 that crossreact with myelin basic protein and suppress experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 1997;94:10821–10826. doi: 10.1073/pnas.94.20.10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aharoni R, Teitelbaum D, Sela M, Arnon R. Bystander suppression of experimental autoimmune encephalomyelitis by T cell lines and clones of the Th2 type induced by copolymer 1. J Neuroimmunol. 1998;91:135–146. doi: 10.1016/s0165-5728(98)00166-0. [DOI] [PubMed] [Google Scholar]
- 20.Aharoni R, et al. Specific Th2 cells accumulate in the central nervous system of mice protected against experimental autoimmune encephalomyelitis by copolymer 1. Proc Natl Acad Sci USA. 2000;97:11472–11477. doi: 10.1073/pnas.97.21.11472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller A, et al. Treatment of multiple sclerosis with copolymer-1 (Copaxone): implicating mechanisms of Th1 to Th2/Th3 immune-deviation. J Neuroimmunol. 1998;92:113–121. doi: 10.1016/s0165-5728(98)00191-x. [DOI] [PubMed] [Google Scholar]
- 22.Brenner T, et al. Humoral and cellular immune responses to copolymer 1 in multiple sclerosis patients treated with Copaxone. J Neuroimmunol. 2001;115:152–160. doi: 10.1016/s0165-5728(01)00250-8. [DOI] [PubMed] [Google Scholar]
- 23.Duda PW, Krieger JI, Schmied MC, Balentine C, Hafler DA. Human and murine CD4 T cell reactivity to a complex antigen: recognition of the synthetic random polypeptide glatiramer acetate. J Immunol. 2000;165:7300–7307. doi: 10.4049/jimmunol.165.12.7300. [DOI] [PubMed] [Google Scholar]
- 24.Duda PW, Schmied MC, Cook SL, Krieger JI, Hafler DA. Glatiramer acetate (Copaxone) induces degenerate, Th2-polarized immune responses in patients with multiple sclerosis. J Clin Invest. 2000;105:967–976. doi: 10.1172/JCI8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neuhaus O, et al. Multiple sclerosis: comparison of copolymer-1-reactive T cell lines from treated and untreated subjects reveals cytokine shift from T helper 1 to T helper 2 cells. Proc Natl Acad Sci USA. 2000;97:7452–7457. doi: 10.1073/pnas.97.13.7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farina C, et al. Treatment of multiple sclerosis with Copaxone (COP): Elispot assay detects COP-induced interleukin-4 and interferon-gamma response in blood cells. Brain. 2001;124:705–719. doi: 10.1093/brain/124.4.705. [DOI] [PubMed] [Google Scholar]
- 27.Qin Y, Zhang DQ, Prat A, Pouly S, Antel J. Characterization of T cell lines derived from glatiramer-acetate-treated multiple sclerosis patients. J Neuroimmunol. 2000;108:201–206. doi: 10.1016/s0165-5728(00)00263-0. [DOI] [PubMed] [Google Scholar]
- 28.Teitelbaum D, Milo R, Arnon R, Sela M. Synthetic copolymer 1 inhibits human T-cell lines specific for myelin basic protein. Proc Natl Acad Sci USA. 1992;89:137–141. doi: 10.1073/pnas.89.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aharoni R, Teitelbaum D, Arnon R, Sela M. Copolymer 1 acts against the immunodominant epitope 82-100 of myelin basic protein by T cell receptor antagonism in addition to major histocompatibility complex blocking. Proc Natl Acad Sci USA. 1999;96:634–639. doi: 10.1073/pnas.96.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fridkis-Hareli M, et al. Direct binding of myelin basic protein and synthetic copolymer 1 to class II major histocompatibility complex molecules on living antigen-presenting cells: specificity and promiscuity. Proc Natl Acad Sci USA. 1994;91:4872–4876. doi: 10.1073/pnas.91.11.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fridkis-Hareli M, et al. Binding motifs of copolymer 1 to multiple sclerosis- and rheumatoid arthritis-associated HLA-DR molecules. J Immunol. 1999;162:4697–4704. [PubMed] [Google Scholar]
- 32.Racke M, Martin R, McFarland H, Fritz R. Copolymer-1-induced inhibition of antigen-specific T cell activation: interference with antigen presentation. J Neuroimmunol. 1992;37:75–84. doi: 10.1016/0165-5728(92)90157-g. [DOI] [PubMed] [Google Scholar]
- 33.Lyons AB. Analysing cell division in vivo and in vitro using flow cytometric measurement of CFSE dye dilution. J Immunol Methods. 2000;243:147–154. doi: 10.1016/s0022-1759(00)00231-3. [DOI] [PubMed] [Google Scholar]
- 34.Nylander S, Kalies I. Brefeldin A, but not monensin, completely blocks CD69 expression on mouse lymphocytes: efficacy of inhibitors of protein secretion in protocols for intracellular cytokine staining by flow cytometry. J Immunol Methods. 1999;224:69–76. doi: 10.1016/s0022-1759(99)00010-1. [DOI] [PubMed] [Google Scholar]
- 35.Waldrop SL, Pitcher CJ, Peterson DM, Maino VC, Picker LJ. Determination of antigen-specific memory/effector CD4+ T cell frequencies by flow cytometry: evidence for a novel, antigen-specific homeostatic mechanism in HIV-associated immunodeficiency. J Clin Invest. 1997;99:1739–1750. doi: 10.1172/JCI119338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crucian B, et al. Alterations in levels of CD28-/CD8+ suppressor cell precursor and CD45RO+/CD4+ memory T lymphocytes in the peripheral blood of multiple sclerosis patients. Clin Diagn Lab Immunol. 1995;2:249–252. doi: 10.1128/cdli.2.2.249-252.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balashov KE, Khoury SJ, Hafler DA, Weiner HL. Inhibition of T cell responses by activated human CD8+ T cells is mediated by interferon-gamma and is defective in chronic progressive multiple sclerosis. J Clin Invest. 1995;95:2711–2719. doi: 10.1172/JCI117973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Antel J, Bania M, Noronha A, Neely S. Defective suppressor cell function mediated by T8+ cell lines from patients with progressive multiple sclerosis. J Immunol. 1986;137:3436–3439. [PubMed] [Google Scholar]
- 39.Koh DR, et al. Less mortality but more relapses in experimental allergic encephalomyelitis in CD8–/– mice. Science. 1992;256:1210–1213. doi: 10.1126/science.256.5060.1210. [DOI] [PubMed] [Google Scholar]
- 40.Becher B, et al. Interferon-gamma secretion by peripheral blood T-cell subsets in multiple sclerosis: correlation with disease phase and interferon-beta therapy. Ann Neurol. 1999;45:247–250. [PubMed] [Google Scholar]
- 41.Jiang H, Braunstein NS, Yu B, Winchester R, Chess L. CD8+ T cells control the TH phenotype of MBP-reactive CD4+ T cells in EAE mice. Proc Natl Acad Sci USA. 2001;98:6301–6306. doi: 10.1073/pnas.101123098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wekerle H. Immunopathogenesis of multiple sclerosis. Acta Neurol (Napoli) 1991;13:197–204. [PubMed] [Google Scholar]
- 43.Qu ZX, Pliskin N, Jensen MW, White D, Arnason BG. Etretinate augments interferon beta-1b effects on suppressor cells in multiple sclerosis. Arch Neurol. 2001;58:87–90. doi: 10.1001/archneur.58.1.87. [DOI] [PubMed] [Google Scholar]
- 44.Racke M, et al. Evidence of endogenous regulatory function of transforming growth factor-beta 1 in experimental allergic encephalomyelitis. Int Immunol. 1992;4:615–620. doi: 10.1093/intimm/4.5.615. [DOI] [PubMed] [Google Scholar]
- 45.Hafler D, et al. Oral administration of myelin induces antigen-specific TGF-beta 1 secreting T cells in patients with multiple sclerosis. Ann NY Acad Sci. 1997;835:120–131. doi: 10.1111/j.1749-6632.1997.tb48623.x. [DOI] [PubMed] [Google Scholar]
- 46.Weiner H. Oral tolerance for the treatment of autoimmune diseases. Annu Rev Med. 1997;48:341–351. doi: 10.1146/annurev.med.48.1.341. [DOI] [PubMed] [Google Scholar]