Abstract
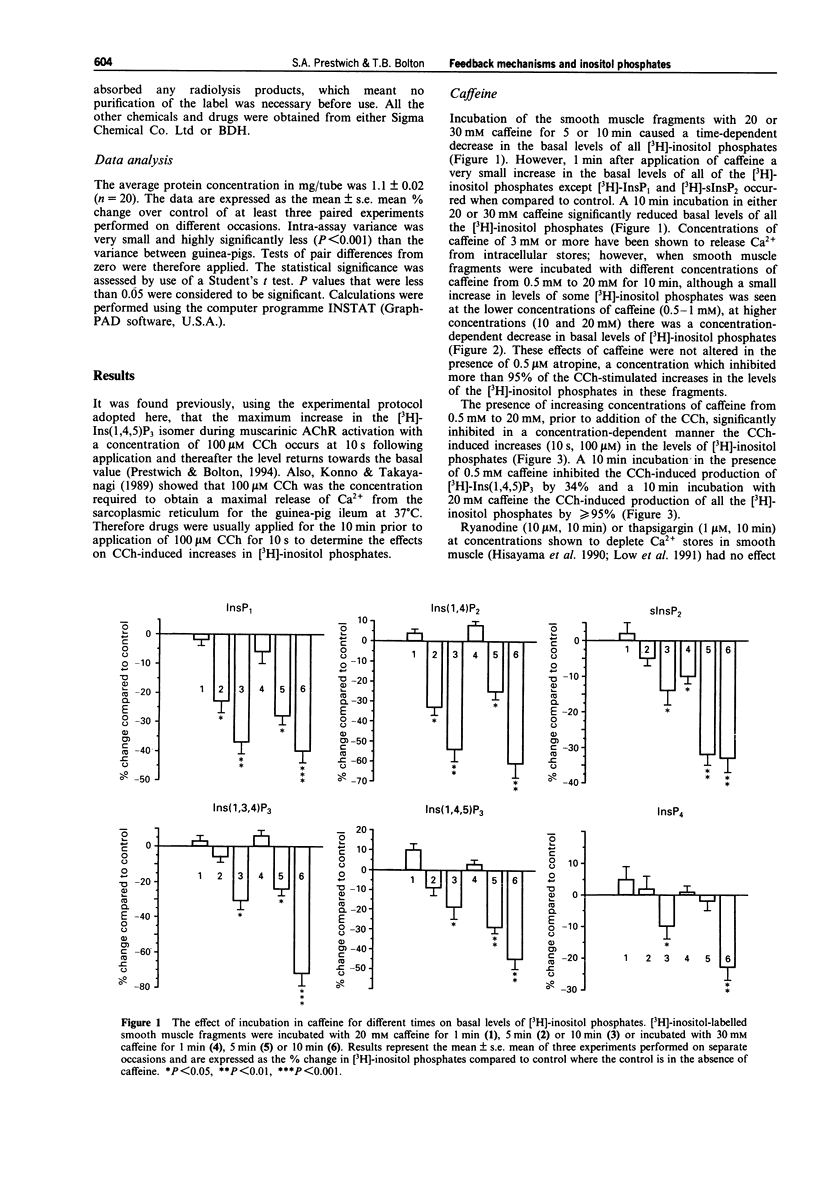
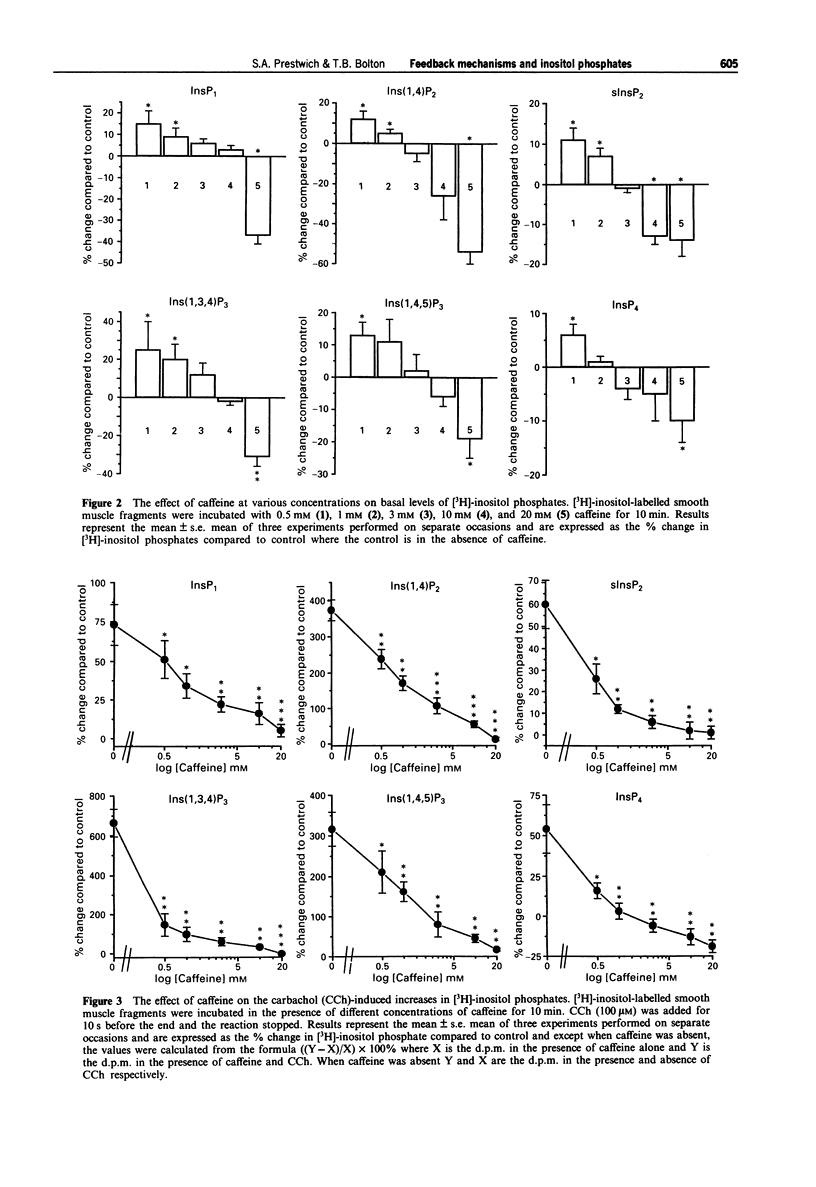
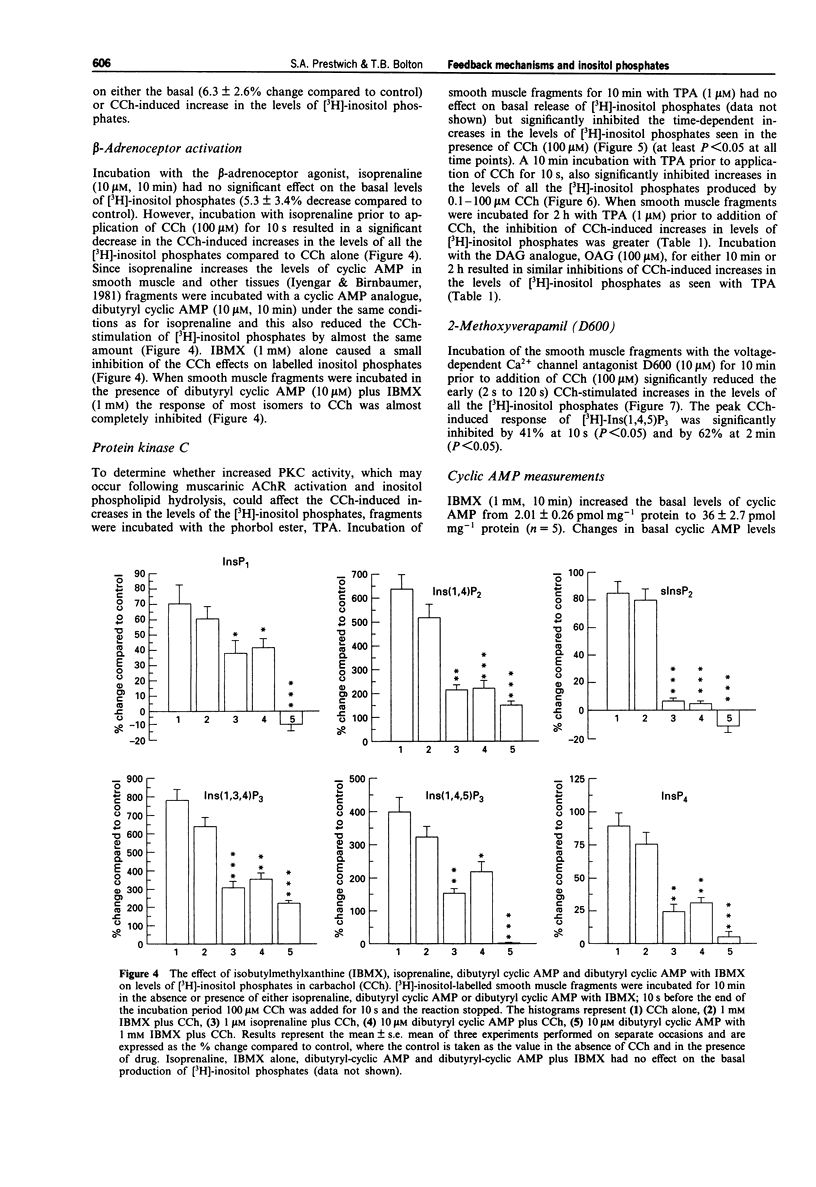
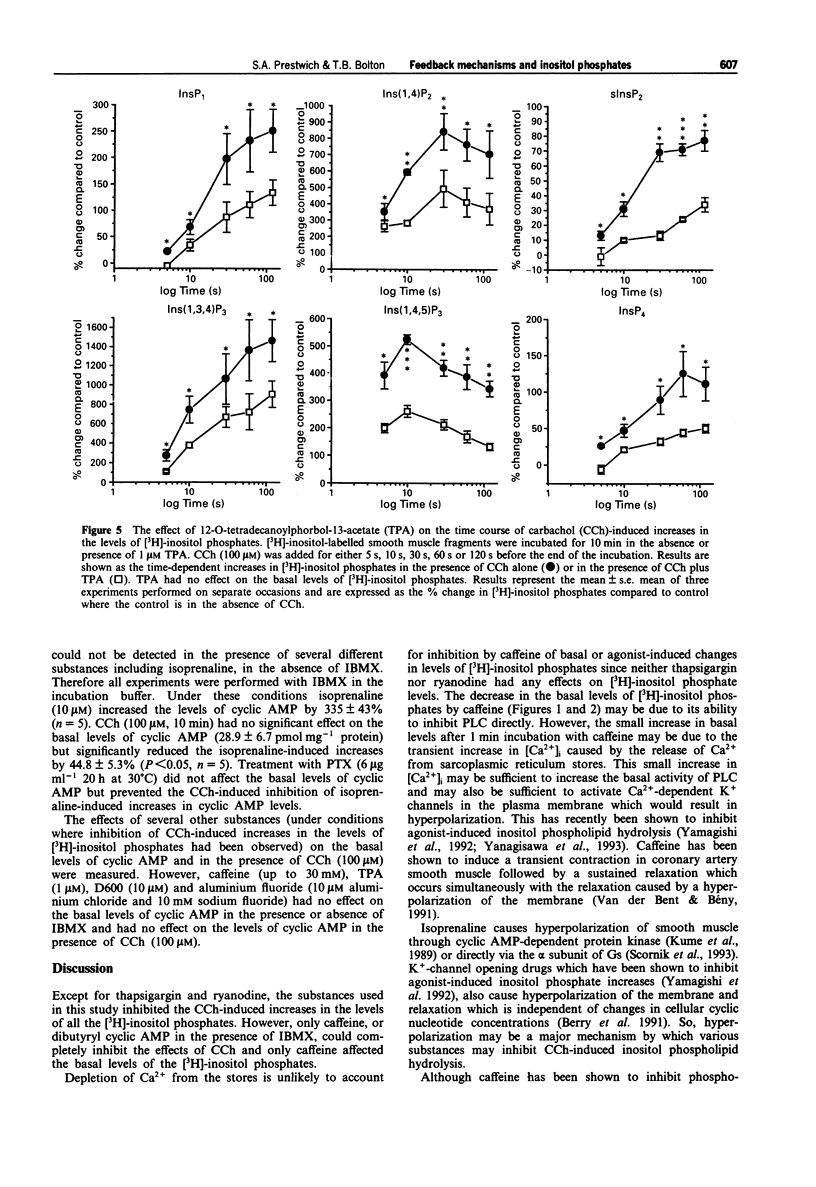
1. The effects of caffeine, isoprenaline, dibutyryl cyclic AMP, isobutylmethylxanthine (IBMX), 12-O-tetradecanoylphorbol-13-acetate (TPA) or 1-oleoyl-2-acetylglycerol (OAG), (protein kinase C (PKC) activators), 2-methoxy verapamil (D600), thapsigargin and ryanodine on muscarinic acetylcholine receptor (AChR)-stimulated inositol phospholipid hydrolysis were studied in smooth muscle fragments from the longitudinal layer of the small intestine of the guinea-pig. 2. Incubation of the fragments with the muscarinic agonist, carbachol (CCh) (100 microM) resulted in rapid increases in the levels of all the inositol phosphate isomers with maximal increases in the [3H]-inositol (1,4,5) trisphosphate ([3H]-Ins(1,4,5)P3) isomer occurring 10 s following incubation. 3. The beta-adrenoceptor agonist, isoprenaline (10 microM) and dibutyryl cyclic AMP (10 microM), a membrane permeant analogue of cyclic AMP both reduced the CCh stimulation, but not the basal levels of [3H]-inositol phosphates. This inhibition by dibutyryl cyclic AMP was enhanced in the presence of the phosphodiesterase inhibitor, IBMX. CCh inhibited the isoprenaline-induced increases in the levels of cyclic AMP and this was via a pertussi toxin (PTX)-sensitive G-protein mechanism. 4. TPA (1 microM) and OAG (100 microM) a 1,2-diacylglycerol (DAG) analogue both reduced the CCh-induced increases in [3H]-inositol phosphates levels but neither affected basal values nor the basal levels of cyclic AMP. 5. D600 (10 microM), which blocks voltage-dependent Ca2+ channels, also reduced the CCh-stimulated levels of [3H]-inositol phosphates suggesting that some of the agonist-induced increases are due to a potentiating effect of Ca2+ entering the cell. 6. Caffeine (0.5-30 mM) significantly inhibited both the basal and CCh-induced increases in all the [3H]-inositol phosphate isomers. Its inhibitory action was not due to increases in cyclic AMP since caffeine had no effect on the levels of cyclic AMP at concentrations up to 30 mM. 7. Incubation with thapsigargin (1 microM) and ryanodine (10 microM) had no effect on either basal or CCh-induced inositol phospholipid hydrolysis or cyclic AMP levels. 8. The results indicate a reciprocal inhibition by beta-adrenoceptors and muscarinic AChRs of their effects on cyclic AMP and inositol phosphate levels respectively. Ca2+ entering the cell (but not the action of ryanodine or thapsigargin) potentiates while caffeine inhibits muscarinic AChR-induced rises in inositol phosphate levels. Diacylglycerols may exert a negative feedback inhibition on inositol phosphate production.
Full text
PDF

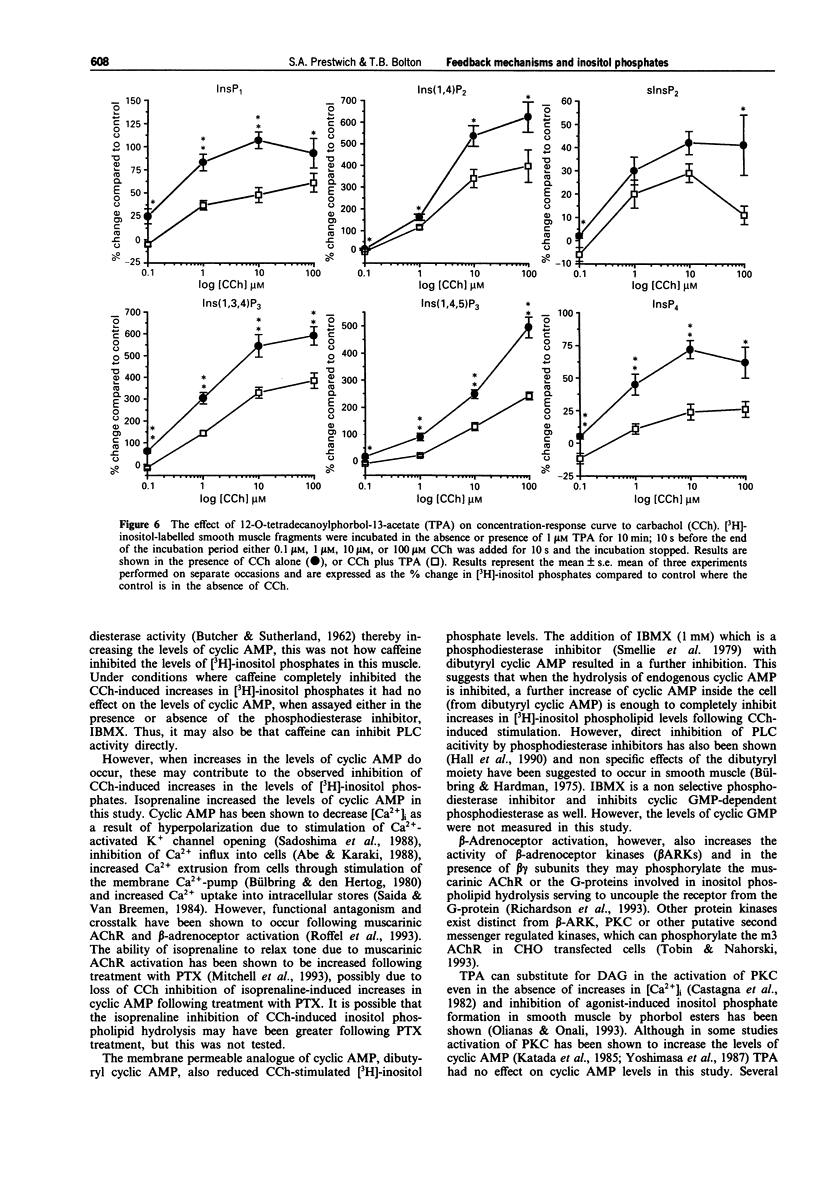
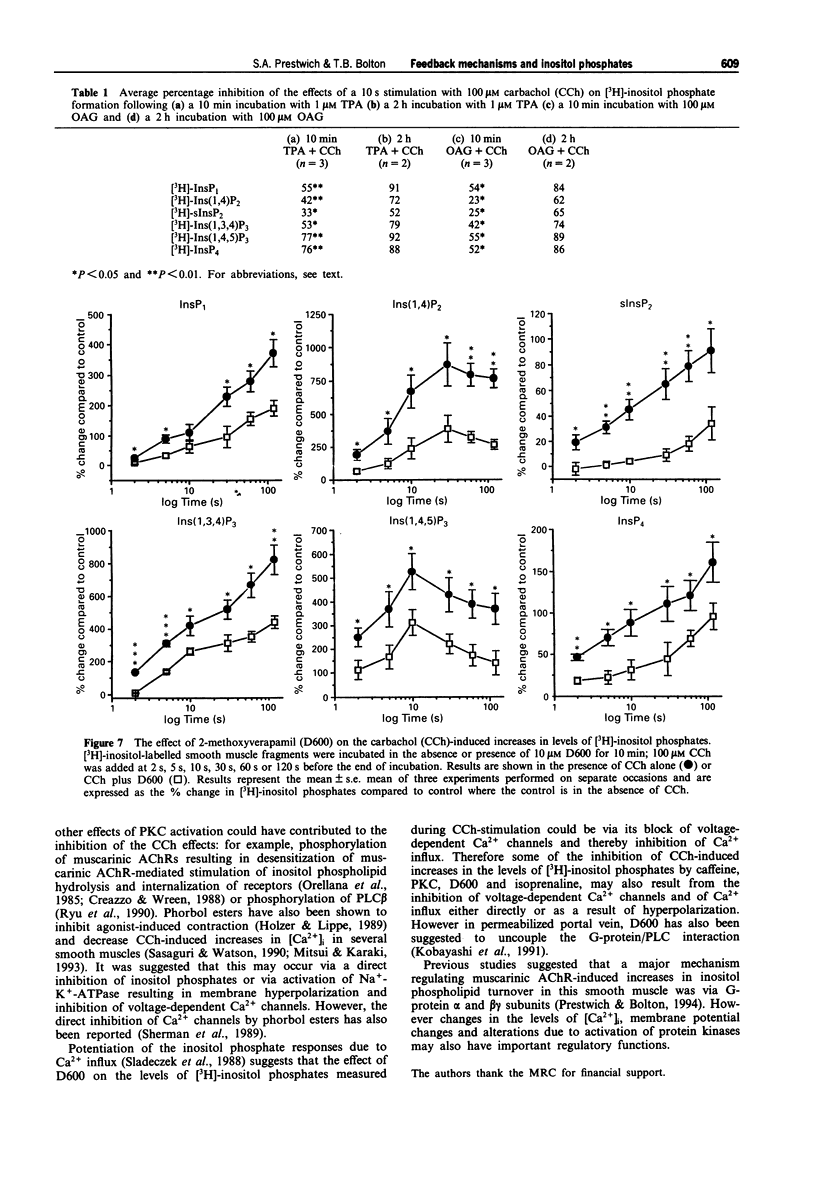


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe A., Karaki H. Mechanisms underlying the inhibitory effect of dibutyryl cyclic AMP in vascular smooth muscle. Eur J Pharmacol. 1992 Feb 18;211(3):305–311. doi: 10.1016/0014-2999(92)90385-h. [DOI] [PubMed] [Google Scholar]
- BUTCHER R. W., SUTHERLAND E. W. Adenosine 3',5'-phosphate in biological materials. I. Purification and properties of cyclic 3',5'-nucleotide phosphodiesterase and use of this enzyme to characterize adenosine 3',5'-phosphate in human urine. J Biol Chem. 1962 Apr;237:1244–1250. [PubMed] [Google Scholar]
- Batty I. R., Nahorski S. R., Irvine R. F. Rapid formation of inositol 1,3,4,5-tetrakisphosphate following muscarinic receptor stimulation of rat cerebral cortical slices. Biochem J. 1985 Nov 15;232(1):211–215. doi: 10.1042/bj2320211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry J. L., Elliott K. R., Foster R. W., Green K. A., Murray M. A., Small R. C. Mechanical, biochemical and electrophysiological studies of RP 49356 and cromakalim in guinea-pig and bovine trachealis muscle. Pulm Pharmacol. 1991;4(2):91–98. doi: 10.1016/0952-0600(91)90058-b. [DOI] [PubMed] [Google Scholar]
- Best L., Bolton T. B. Depolarisation of guinea-pig visceral smooth muscle causes hydrolysis of inositol phospholipids. Naunyn Schmiedebergs Arch Pharmacol. 1986 May;333(1):78–82. doi: 10.1007/BF00569664. [DOI] [PubMed] [Google Scholar]
- Bolton T. B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979 Jul;59(3):606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Broadley K. J., Grassby P. F. Alpha- and beta-adrenoceptor-mediated responses of the guinea-pig ileum and the effects of neuronal uptake inhibition. Naunyn Schmiedebergs Arch Pharmacol. 1985 Dec;331(4):316–323. doi: 10.1007/BF00500813. [DOI] [PubMed] [Google Scholar]
- Bülbring E., Tomita T. Catecholamine action on smooth muscle. Pharmacol Rev. 1987 Mar;39(1):49–96. [PubMed] [Google Scholar]
- Bülbring E., den Hertog A. The action of isoprenaline on the smooth muscle of the guinea-pig taenia coli. J Physiol. 1980 Jul;304:277–296. doi: 10.1113/jphysiol.1980.sp013324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Creazzo T. L., Wrenn R. W. Increased muscarinic receptor binding in heart membranes by an inhibitor of protein kinase C. FEBS Lett. 1988 Dec 19;242(1):175–177. doi: 10.1016/0014-5793(88)81010-x. [DOI] [PubMed] [Google Scholar]
- Ehrlich B. E., Watras J. Inositol 1,4,5-trisphosphate activates a channel from smooth muscle sarcoplasmic reticulum. Nature. 1988 Dec 8;336(6199):583–586. doi: 10.1038/336583a0. [DOI] [PubMed] [Google Scholar]
- Fleischer S., Ogunbunmi E. M., Dixon M. C., Fleer E. A. Localization of Ca2+ release channels with ryanodine in junctional terminal cisternae of sarcoplasmic reticulum of fast skeletal muscle. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7256–7259. doi: 10.1073/pnas.82.21.7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerthoffer W. T., Murphy R. A. Ca2+, myosin phosphorylation, and relaxation of arterial smooth muscle. Am J Physiol. 1983 Sep;245(3):C271–C277. doi: 10.1152/ajpcell.1983.245.3.C271. [DOI] [PubMed] [Google Scholar]
- Hall I. P., Donaldson J., Hill S. J. Modulation of carbachol-induced inositol phosphate formation in bovine tracheal smooth muscle by cyclic AMP phosphodiesterase inhibitors. Biochem Pharmacol. 1990 Apr 15;39(8):1357–1363. doi: 10.1016/0006-2952(90)90013-b. [DOI] [PubMed] [Google Scholar]
- Hisayama T., Takayanagi I., Okamoto Y. Ryanodine reveals multiple contractile and relaxant mechanisms in vascular smooth muscle: simultaneous measurements of mechanical activity and of cytoplasmic free Ca2+ level with fura-2. Br J Pharmacol. 1990 Aug;100(4):677–684. doi: 10.1111/j.1476-5381.1990.tb14075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P., Lippe I. T. Protein kinase C may regulate the tonic component of intestinal smooth muscle contraction in response to substance P. Naunyn Schmiedebergs Arch Pharmacol. 1989 Jan-Feb;339(1-2):214–220. doi: 10.1007/BF00165146. [DOI] [PubMed] [Google Scholar]
- Hughes A. D., Hering S., Bolton T. B. The action of caffeine on inward barium current through voltage-dependent calcium channels in single rabbit ear artery cells. Pflugers Arch. 1990 Jun;416(4):462–466. doi: 10.1007/BF00370755. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Anggård E. E., Letcher A. J., Downes C. P. Metabolism of inositol 1,4,5-trisphosphate and inositol 1,3,4-trisphosphate in rat parotid glands. Biochem J. 1985 Jul 15;229(2):505–511. doi: 10.1042/bj2290505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar R., Birnbaumer L. Hysteretic activation of adenylyl cyclases. I. Effect of Mg ion on the rate of activation by guanine nucleotides and fluoride. J Biol Chem. 1981 Nov 10;256(21):11036–11041. [PubMed] [Google Scholar]
- Karaki H., Ahn H. Y., Urakawa N. Caffeine-induced contraction in vascular smooth muscle. Arch Int Pharmacodyn Ther. 1987 Jan;285(1):60–71. [PubMed] [Google Scholar]
- Katada T., Gilman A. G., Watanabe Y., Bauer S., Jakobs K. H. Protein kinase C phosphorylates the inhibitory guanine-nucleotide-binding regulatory component and apparently suppresses its function in hormonal inhibition of adenylate cyclase. Eur J Biochem. 1985 Sep 2;151(2):431–437. doi: 10.1111/j.1432-1033.1985.tb09120.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Gong M. C., Somlyo A. V., Somlyo A. P. Ca2+ channel blockers distinguish between G protein-coupled pharmacomechanical Ca2+ release and Ca2+ sensitization. Am J Physiol. 1991 Feb;260(2 Pt 1):C364–C370. doi: 10.1152/ajpcell.1991.260.2.C364. [DOI] [PubMed] [Google Scholar]
- Komori S., Bolton T. B. Calcium release induced by inositol 1,4,5-trisphosphate in single rabbit intestinal smooth muscle cells. J Physiol. 1991 Feb;433:495–517. doi: 10.1113/jphysiol.1991.sp018440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konno F., Takayanagi I. Relationship between the contractile responses and their coupling second messenger systems for muscarinic drugs in the guinea-pig ileal longitudinal muscle. Arch Int Pharmacodyn Ther. 1989 Sep-Oct;301:15–29. [PubMed] [Google Scholar]
- Kume H., Takai A., Tokuno H., Tomita T. Regulation of Ca2+-dependent K+-channel activity in tracheal myocytes by phosphorylation. Nature. 1989 Sep 14;341(6238):152–154. doi: 10.1038/341152a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Low A. M., Gaspar V., Kwan C. Y., Darby P. J., Bourreau J. P., Daniel E. E. Thapsigargin inhibits repletion of phenylephrine-sensitive intracellular Ca++ pool in vascular smooth muscles. J Pharmacol Exp Ther. 1991 Sep;258(3):1105–1113. [PubMed] [Google Scholar]
- Matsumoto T., Kanaide H., Shogakiuchi Y., Nakamura M. Characteristics of the histamine-sensitive calcium store in vascular smooth muscle. Comparison with norepinephrine- or caffeine-sensitive stores. J Biol Chem. 1990 Apr 5;265(10):5610–5616. [PubMed] [Google Scholar]
- Mitchell R. W., Koenig S. M., Popovich K. J., Kelly E., Tallet J., Leff A. R. Pertussis toxin augments beta-adrenergic relaxation of muscarinic contraction in canine trachealis. Am Rev Respir Dis. 1993 Feb;147(2):327–331. doi: 10.1164/ajrccm/147.2.327. [DOI] [PubMed] [Google Scholar]
- Mitsui M., Karaki H. Contractile and relaxant effects of phorbol ester in the intestinal smooth muscle of guinea-pig taenia caeci. Br J Pharmacol. 1993 May;109(1):229–233. doi: 10.1111/j.1476-5381.1993.tb13558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992 Oct 23;258(5082):607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Olianas M. C., Onali P. Stimulation of phosphoinositide hydrolysis by muscarinic receptor activation in the rat olfactory bulb. Biochem Pharmacol. 1993 Jan 26;45(2):281–287. doi: 10.1016/0006-2952(93)90062-2. [DOI] [PubMed] [Google Scholar]
- Orellana S. A., Solski P. A., Brown J. H. Phorbol ester inhibits phosphoinositide hydrolysis and calcium mobilization in cultured astrocytoma cells. J Biol Chem. 1985 May 10;260(9):5236–5239. [PubMed] [Google Scholar]
- Pacaud P., Bolton T. B. Relation between muscarinic receptor cationic current and internal calcium in guinea-pig jejunal smooth muscle cells. J Physiol. 1991 Sep;441:477–499. doi: 10.1113/jphysiol.1991.sp018763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestwich S. A., Bolton T. B. Measurement of picomole amounts of any inositol phosphate isomer separable by h.p.l.c. by means of a bioluminescence assay. Biochem J. 1991 Mar 15;274(Pt 3):663–672. doi: 10.1042/bj2740663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quast U. Do the K+ channel openers relax smooth muscle by opening K+ channels? Trends Pharmacol Sci. 1993 Sep;14(9):332–337. doi: 10.1016/0165-6147(93)90006-6. [DOI] [PubMed] [Google Scholar]
- Rana R. S., Hokin L. E. Role of phosphoinositides in transmembrane signaling. Physiol Rev. 1990 Jan;70(1):115–164. doi: 10.1152/physrev.1990.70.1.115. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Takuwa Y., Park S. Protein kinase C in the regulation of smooth muscle contraction. FASEB J. 1987 Sep;1(3):177–185. [PubMed] [Google Scholar]
- Richardson R. M., Kim C., Benovic J. L., Hosey M. M. Phosphorylation and desensitization of human m2 muscarinic cholinergic receptors by two isoforms of the beta-adrenergic receptor kinase. J Biol Chem. 1993 Jun 25;268(18):13650–13656. [PubMed] [Google Scholar]
- Roffel A. F., Meurs H., Elzinga C. R., Zaagsma J. Muscarinic M2 receptors do not participate in the functional antagonism between methacholine and isoprenaline in guinea pig tracheal smooth muscle. Eur J Pharmacol. 1993 Nov 9;249(2):235–238. doi: 10.1016/0014-2999(93)90438-n. [DOI] [PubMed] [Google Scholar]
- Ryu S. H., Kim U. H., Wahl M. I., Brown A. B., Carpenter G., Huang K. P., Rhee S. G. Feedback regulation of phospholipase C-beta by protein kinase C. J Biol Chem. 1990 Oct 15;265(29):17941–17945. [PubMed] [Google Scholar]
- Sadoshima J., Akaike N., Tomoike H., Kanaide H., Nakamura M. Ca-activated K channel in cultured smooth muscle cells of rat aortic media. Am J Physiol. 1988 Sep;255(3 Pt 2):H410–H418. doi: 10.1152/ajpheart.1988.255.3.H410. [DOI] [PubMed] [Google Scholar]
- Saida K., van Breemen C. Characteristics of the norepinephrine-sensitive Ca2+ store in vascular smooth muscle. Blood Vessels. 1984;21(1):43–52. doi: 10.1159/000158493. [DOI] [PubMed] [Google Scholar]
- Sasaguri T., Watson S. P. Phorbol esters inhibit smooth muscle contractions through activation of Na(+)-K(+)-ATPase. Br J Pharmacol. 1990 Feb;99(2):237–242. doi: 10.1111/j.1476-5381.1990.tb14687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scornik F. S., Codina J., Birnbaumer L., Toro L. Modulation of coronary smooth muscle KCa channels by Gs alpha independent of phosphorylation by protein kinase A. Am J Physiol. 1993 Oct;265(4 Pt 2):H1460–H1465. doi: 10.1152/ajpheart.1993.265.4.H1460. [DOI] [PubMed] [Google Scholar]
- Shima H., Blaustein M. P. Modulation of evoked contractions in rat arteries by ryanodine, thapsigargin, and cyclopiazonic acid. Circ Res. 1992 May;70(5):968–977. doi: 10.1161/01.res.70.5.968. [DOI] [PubMed] [Google Scholar]
- Sladeczek F., Schmidt B. H., Alonso R., Vian L., Tep A., Yasumoto T., Cory R. N., Bockaert J. New insights into maitotoxin action. Eur J Biochem. 1988 Jul 1;174(4):663–670. doi: 10.1111/j.1432-1033.1988.tb14149.x. [DOI] [PubMed] [Google Scholar]
- Smellie F. W., Davis C. W., Daly J. W., Wells J. N. Alkylxanthines: inhibition of adenosine-elicited accumulation of cyclic AMP in brain slices and of brain phosphodiesterase activity. Life Sci. 1979 Jun 25;24(26):2475–2482. doi: 10.1016/0024-3205(79)90458-2. [DOI] [PubMed] [Google Scholar]
- Tobin A. B., Keys B., Nahorski S. R. Phosphorylation of a phosphoinositidase C-linked muscarinic receptor by a novel kinase distinct from beta-adrenergic receptor kinase. FEBS Lett. 1993 Dec 13;335(3):353–357. doi: 10.1016/0014-5793(93)80418-t. [DOI] [PubMed] [Google Scholar]
- Toescu E. C., O'Neill S. C., Petersen O. H., Eisner D. A. Caffeine inhibits the agonist-evoked cytosolic Ca2+ signal in mouse pancreatic acinar cells by blocking inositol trisphosphate production. J Biol Chem. 1992 Nov 25;267(33):23467–23470. [PubMed] [Google Scholar]
- Yamagishi T., Yanagisawa T., Taira N. Activation of phospholipase C by the agonist U46619 is inhibited by cromakalim-induced hyperpolarization in porcine coronary artery. Biochem Biophys Res Commun. 1992 Sep 30;187(3):1517–1522. doi: 10.1016/0006-291x(92)90474-y. [DOI] [PubMed] [Google Scholar]
- Yanagisawa T., Yamagishi T., Okada Y. Hyperpolarization induced by K+ channel openers inhibits Ca2+ influx and Ca2+ release in coronary artery. Cardiovasc Drugs Ther. 1993 Aug;7 (Suppl 3):565–574. doi: 10.1007/BF00877622. [DOI] [PubMed] [Google Scholar]
- Yoshimasa T., Sibley D. R., Bouvier M., Lefkowitz R. J., Caron M. G. Cross-talk between cellular signalling pathways suggested by phorbol-ester-induced adenylate cyclase phosphorylation. Nature. 1987 May 7;327(6117):67–70. doi: 10.1038/327067a0. [DOI] [PubMed] [Google Scholar]
- van der Bent V., Bény J. L. Mechanisms controlling caffeine-induced relaxation of coronary artery of the pig. Br J Pharmacol. 1991 Aug;103(4):1877–1882. doi: 10.1111/j.1476-5381.1991.tb12345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vliet A., Rademaker B., Bast A. A beta adrenoceptor with atypical characteristics is involved in the relaxation of the rat small intestine. J Pharmacol Exp Ther. 1990 Oct;255(1):218–226. [PubMed] [Google Scholar]


