Abstract
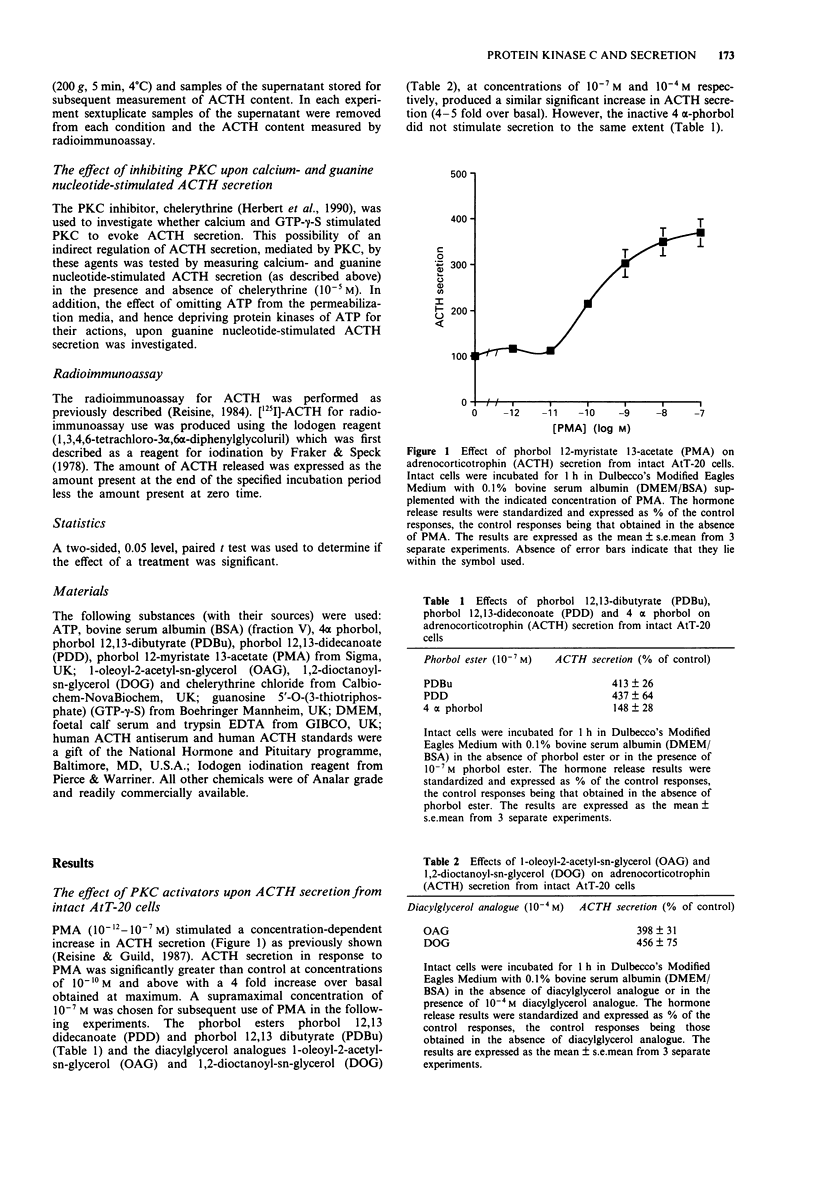
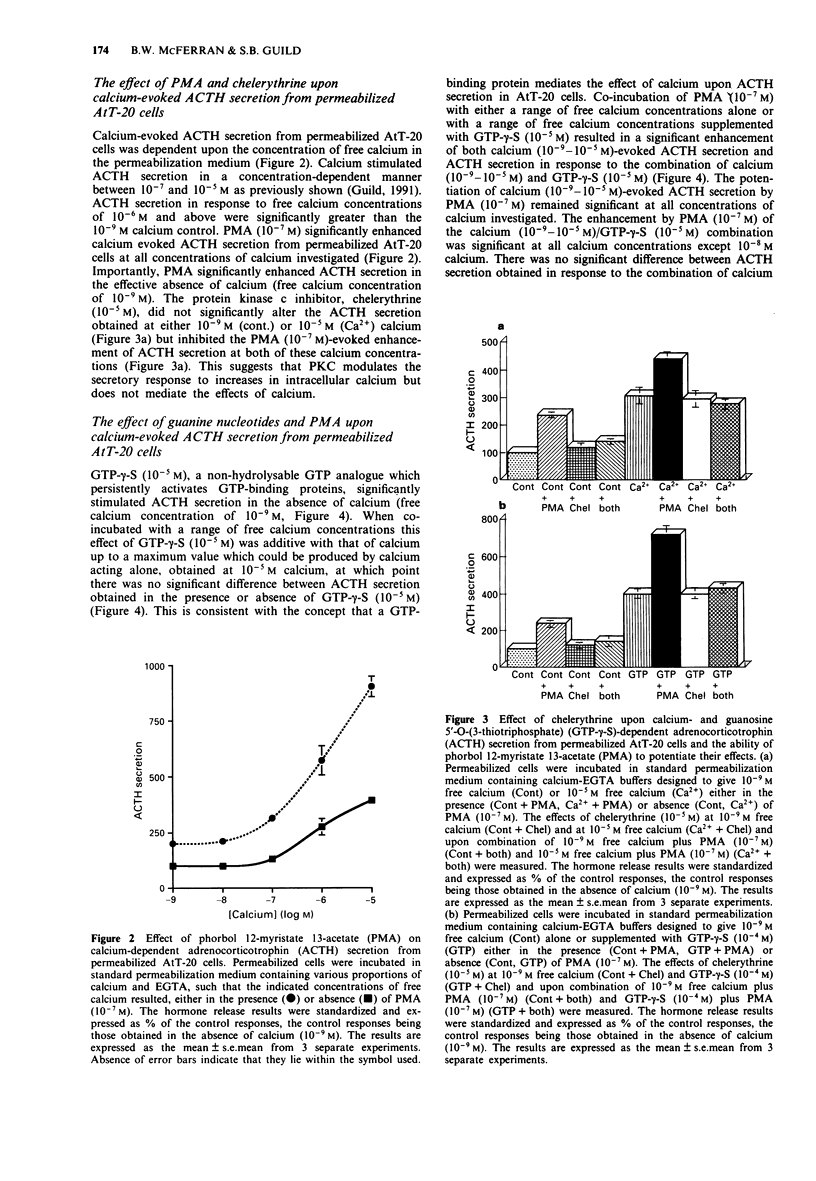
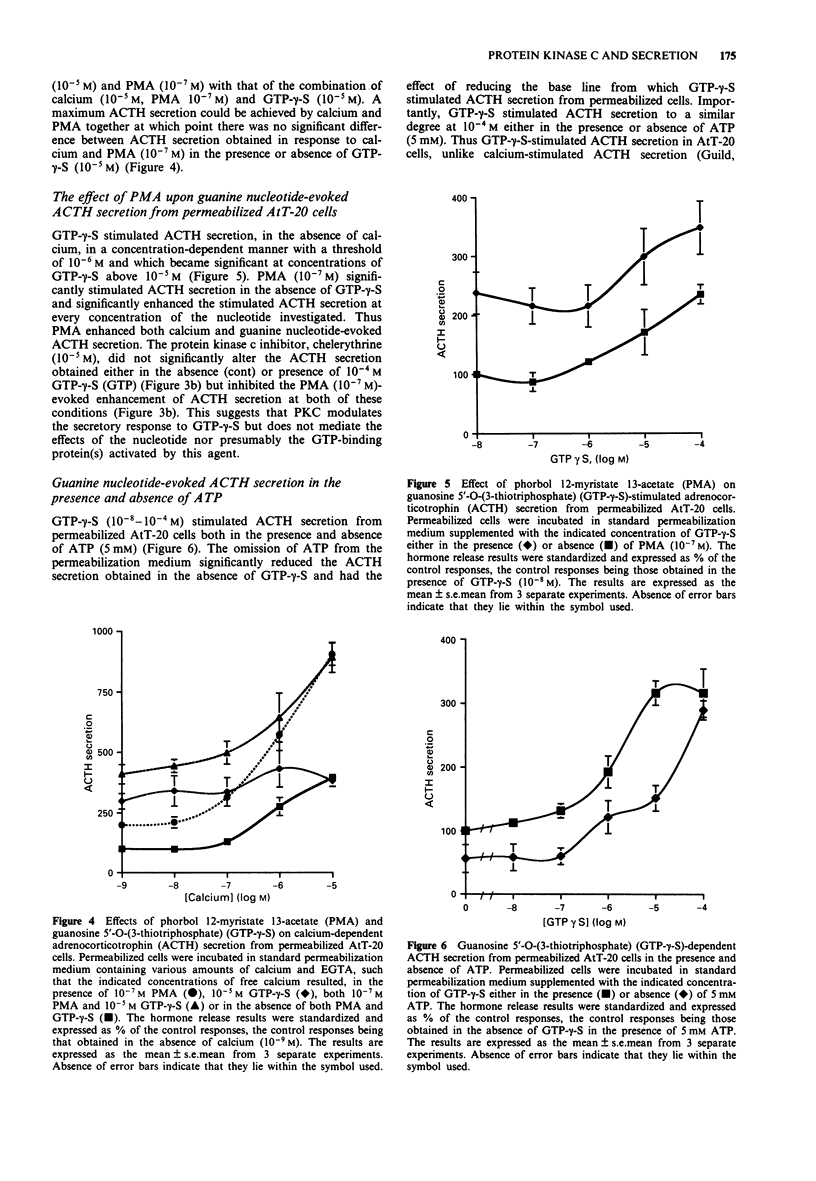
1. The mouse AtT-20/D16-16 anterior pituitary tumour cell line was used as a model system for the study of phorbol 12-myristate 13-acetate (PMA)-mediated enhancement of calcium-evoked adrenocorticotrophin (ACTH) secretion. 2. PMA stimulated ACTH secretion from intact cells in a concentration-dependent manner. Other phorbol esters; phorbol 12,13-dibutyrate (PDBu) and phorbol 12,13-didecanoate (PDD) and diacylglycerol analogues; 1-oleoyl-2-acetyl-sn-glycerol (OAG) and 1,2-dioctanoyl-sn-glycerol (DOG) also stimulated ACTH release from intact AtT-20 cells. This would suggest that activation of protein kinase C (PKC) stimulates ACTH secretion from AtT-20 cells. 3. Calcium stimulated ACTH secretion from electrically-permeabilized cells over the concentration-range of 10(-7) M to 10(-5) M. PMA (10(-7) M) enhanced the amount of ACTH secreted at every concentration of calcium investigated. The PKC inhibitor, chelerythrine (10(-5) M) blocked the PMA (10(-7) M)-evoked enhancement of calcium (10(-5) M)-stimulated ACTH secretion but did not alter significantly the calcium (10(-5) M)-evoked secretion itself. This suggests that PKC modulates the secretory response to increases in intracellular calcium but does not mediate the effects of calcium. 4. Guanosine 5'-O-(3-thiotriphosphate) (GTP-gamma-S, 10(-5) M) stimulated ACTH secretion from permeabilized cells in the absence of calcium and was additive with calcium-evoked ACTH secretion up to a maximum value which could be achieved by calcium acting alone. This suggests that a GTP-binding protein mediates the secretory response to increases in the intracellular calcium.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Samra A. B., Catt K. J., Aguilera G. Involvement of protein kinase C in the regulation of adrenocorticotropin release from rat anterior pituitary cells. Endocrinology. 1986 Jan;118(1):212–217. doi: 10.1210/endo-118-1-212. [DOI] [PubMed] [Google Scholar]
- Aguilera G., Harwood J. P., Wilson J. X., Morell J., Brown J. H., Catt K. J. Mechanisms of action of corticotropin-releasing factor and other regulators of corticotropin release in rat pituitary cells. J Biol Chem. 1983 Jul 10;258(13):8039–8045. [PubMed] [Google Scholar]
- Axelrod J., Reisine T. D. Stress hormones: their interaction and regulation. Science. 1984 May 4;224(4648):452–459. doi: 10.1126/science.6143403. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Morgan A. Low molecular mass GTP-binding proteins of adrenal chromaffin cells are present on the secretory granule. FEBS Lett. 1989 Mar 13;245(1-2):122–126. doi: 10.1016/0014-5793(89)80204-2. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Morgan A. Regulated exocytosis. Biochem J. 1993 Jul 15;293(Pt 2):305–316. doi: 10.1042/bj2930305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannies P. S. Prolactin: multiple intracellular processing routes plus several potential mechanisms for regulation. Biochem Pharmacol. 1982 Sep 15;31(18):2845–2849. doi: 10.1016/0006-2952(82)90253-2. [DOI] [PubMed] [Google Scholar]
- Darchen F., Zahraoui A., Hammel F., Monteils M. P., Tavitian A., Scherman D. Association of the GTP-binding protein Rab3A with bovine adrenal chromaffin granules. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5692–5696. doi: 10.1073/pnas.87.15.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer von Mollard G., Südhof T. C., Jahn R. A small GTP-binding protein dissociates from synaptic vesicles during exocytosis. Nature. 1991 Jan 3;349(6304):79–81. doi: 10.1038/349079a0. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Gomperts B. D. GE: a GTP-binding protein mediating exocytosis. Annu Rev Physiol. 1990;52:591–606. doi: 10.1146/annurev.ph.52.030190.003111. [DOI] [PubMed] [Google Scholar]
- Guild S. Effects of adenosine 3':5'-cyclic monophosphate and guanine nucleotides on calcium-evoked ACTH release from electrically permeabilized AtT-20 cells. Br J Pharmacol. 1991 Sep;104(1):117–122. doi: 10.1111/j.1476-5381.1991.tb12394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guild S., Itoh Y., Kebabian J. W., Luini A., Reisine T. Forskolin enhances basal and potassium-evoked hormone release from normal and malignant pituitary tissue: the role of calcium. Endocrinology. 1986 Jan;118(1):268–279. doi: 10.1210/endo-118-1-268. [DOI] [PubMed] [Google Scholar]
- Guild S., Reisine T. Molecular mechanisms of corticotropin-releasing factor stimulation of calcium mobilization and adrenocorticotropin release from anterior pituitary tumor cells. J Pharmacol Exp Ther. 1987 Apr;241(1):125–130. [PubMed] [Google Scholar]
- Heisler S., Reisine T. Forskolin stimulates adenylate cyclase activity, cyclic AMP accumulation, and adrenocorticotropin secretion from mouse anterior pituitary tumor cells. J Neurochem. 1984 Jun;42(6):1659–1666. doi: 10.1111/j.1471-4159.1984.tb12757.x. [DOI] [PubMed] [Google Scholar]
- Herbert J. M., Augereau J. M., Gleye J., Maffrand J. P. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1990 Nov 15;172(3):993–999. doi: 10.1016/0006-291x(90)91544-3. [DOI] [PubMed] [Google Scholar]
- Hook V. Y., Heisler S., Sabol S. L., Axelrod J. Corticotropin releasing factor stimulates adrenocorticotropin and beta-endorphin release from AtT-20 mouse pituitary tumor cells. Biochem Biophys Res Commun. 1982 Jun 30;106(4):1364–1371. doi: 10.1016/0006-291x(82)91264-5. [DOI] [PubMed] [Google Scholar]
- Hug H., Sarre T. F. Protein kinase C isoenzymes: divergence in signal transduction? Biochem J. 1993 Apr 15;291(Pt 2):329–343. doi: 10.1042/bj2910329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight D. E., Baker P. F. Calcium-dependence of catecholamine release from bovine adrenal medullary cells after exposure to intense electric fields. J Membr Biol. 1982;68(2):107–140. doi: 10.1007/BF01872259. [DOI] [PubMed] [Google Scholar]
- Knight D. E., Scrutton M. C. Gaining access to the cytosol: the technique and some applications of electropermeabilization. Biochem J. 1986 Mar 15;234(3):497–506. doi: 10.1042/bj2340497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffer A., Churcher Y. Calcium and GTP-gamma-S as single effectors of secretion from permeabilized rat mast cells: requirements for ATP. Biochim Biophys Acta. 1993 Apr 16;1176(3):222–230. doi: 10.1016/0167-4889(93)90048-t. [DOI] [PubMed] [Google Scholar]
- Lillie T. H., Gomperts B. D. Nucleotides and divalent cations as effectors and modulators of exocytosis in permeabilized rat mast cells. Philos Trans R Soc Lond B Biol Sci. 1992 Apr 29;336(1276):25–34. doi: 10.1098/rstb.1992.0040. [DOI] [PubMed] [Google Scholar]
- Luini A., De Matteis M. A. Dual regulation of ACTH secretion by guanine nucleotides in permeabilized AtT-20 cells. Cell Mol Neurobiol. 1988 Mar;8(1):129–138. doi: 10.1007/BF00712918. [DOI] [PubMed] [Google Scholar]
- Luini A., De Matteis M. A. Evidence that receptor-linked G protein inhibits exocytosis by a post-second-messenger mechanism in AtT-20 cells. J Neurochem. 1990 Jan;54(1):30–38. doi: 10.1111/j.1471-4159.1990.tb13279.x. [DOI] [PubMed] [Google Scholar]
- Luini A., Lewis D., Guild S., Corda D., Axelrod J. Hormone secretagogues increase cytosolic calcium by increasing cAMP in corticotropin-secreting cells. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8034–8038. doi: 10.1073/pnas.82.23.8034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki K., Reisine T., Kebabian J. W. Adenosine 3',5'-monophosphate (cAMP)-dependent protein kinase activity in rodent pituitary tissue: possible role in cAMP-dependent hormone secretion. Endocrinology. 1984 Nov;115(5):1933–1945. doi: 10.1210/endo-115-5-1933. [DOI] [PubMed] [Google Scholar]
- Ngsee J. K., Fleming A. M., Scheller R. H. A rab protein regulates the localization of secretory granules in AtT-20 cells. Mol Biol Cell. 1993 Jul;4(7):747–756. doi: 10.1091/mbc.4.7.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S., Akita Y., Hata A., Osada S., Kubo K., Konno Y., Akimoto K., Mizuno K., Saido T., Kuroki T. Structural and functional diversities of a family of signal transducing protein kinases, protein kinase C family; two distinct classes of PKC, conventional cPKC and novel nPKC. Adv Enzyme Regul. 1991;31:287–303. doi: 10.1016/0065-2571(91)90018-h. [DOI] [PubMed] [Google Scholar]
- Reisine T., Guild S. Activators of protein kinase C and cyclic AMP-dependent protein kinase regulate intracellular calcium levels through distinct mechanisms in mouse anterior pituitary tumor cells. Mol Pharmacol. 1987 Oct;32(4):488–496. [PubMed] [Google Scholar]
- Reisine T. Phorbol esters and corticotropin releasing factor stimulate calcium influx in the anterior pituitary tumor cell line, AtT-20, through different intracellular sites of action. J Pharmacol Exp Ther. 1989 Mar;248(3):984–990. [PubMed] [Google Scholar]
- Reisine T. Somatostatin desensitization: loss of the ability of somatostatin to inhibit cyclic AMP accumulation and adrenocorticotropin hormone release. J Pharmacol Exp Ther. 1984 Apr;229(1):14–20. [PubMed] [Google Scholar]
- Ryves W. J., Evans A. T., Olivier A. R., Parker P. J., Evans F. J. Activation of the PKC-isotypes alpha, beta 1, gamma, delta and epsilon by phorbol esters of different biological activities. FEBS Lett. 1991 Aug 19;288(1-2):5–9. doi: 10.1016/0014-5793(91)80989-g. [DOI] [PubMed] [Google Scholar]
- Taylor S. S., Buechler J. A., Yonemoto W. cAMP-dependent protein kinase: framework for a diverse family of regulatory enzymes. Annu Rev Biochem. 1990;59:971–1005. doi: 10.1146/annurev.bi.59.070190.004543. [DOI] [PubMed] [Google Scholar]
- Toutant M., Aunis D., Bockaert J., Homburger V., Rouot B. Presence of three pertussis toxin substrates and Go alpha immunoreactivity in both plasma and granule membranes of chromaffin cells. FEBS Lett. 1987 May 11;215(2):339–344. doi: 10.1016/0014-5793(87)80174-6. [DOI] [PubMed] [Google Scholar]
- Vitale N., Mukai H., Rouot B., Thiersé D., Aunis D., Bader M. F. Exocytosis in chromaffin cells. Possible involvement of the heterotrimeric GTP-binding protein G(o). J Biol Chem. 1993 Jul 15;268(20):14715–14723. [PubMed] [Google Scholar]


