Abstract
1. Our previous work has shown that injection into mice of lipopolysaccharide (LPS) and the cytokines interleukin 1 (IL-1) and tumour necrosis factor (TNF) induces histidine decarboxylase (HDC), the enzyme forming histamine, in various tissues such as liver, lung, spleen and bone marrow, but not in the blood. The induction of HDC also occurs in nude mice and mast cell-deficient mice. On the other hand, haematopoietic cytokines such as IL-3, granulocyte colony-stimulating factor (G-CSF) and granulocyte-macrophage CSF (GM-CSF) only induce HDC in the haematopoietic organs, i.e. bone marrow and spleen. In the present study, the effect of macrophage depletion on the induction of HDC was examined. 2. On day 1 after a single intravenous injection of a macrophage depletor (liposomes encapsulating dichloromethylene diphosphonate, which is toxic when ingested into macrophages), macrophages were almost completely depleted in the liver and reduced by about 50% in the spleen and bone marrow, but not significantly affected in the lung. On day 3, the degrees of the depletion were similar to those of day 1. In the spleen, macrophages were depleted in the red pulp, and there was a structural destruction. 3. In macrophage-depleted mice, the induction of HDC by LPS, IL-1 alpha or TNF-alpha was not impaired in the liver, and was potentiated in the lung and bone marrow. The induction of HDC was decreased only in the spleen at day 3.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

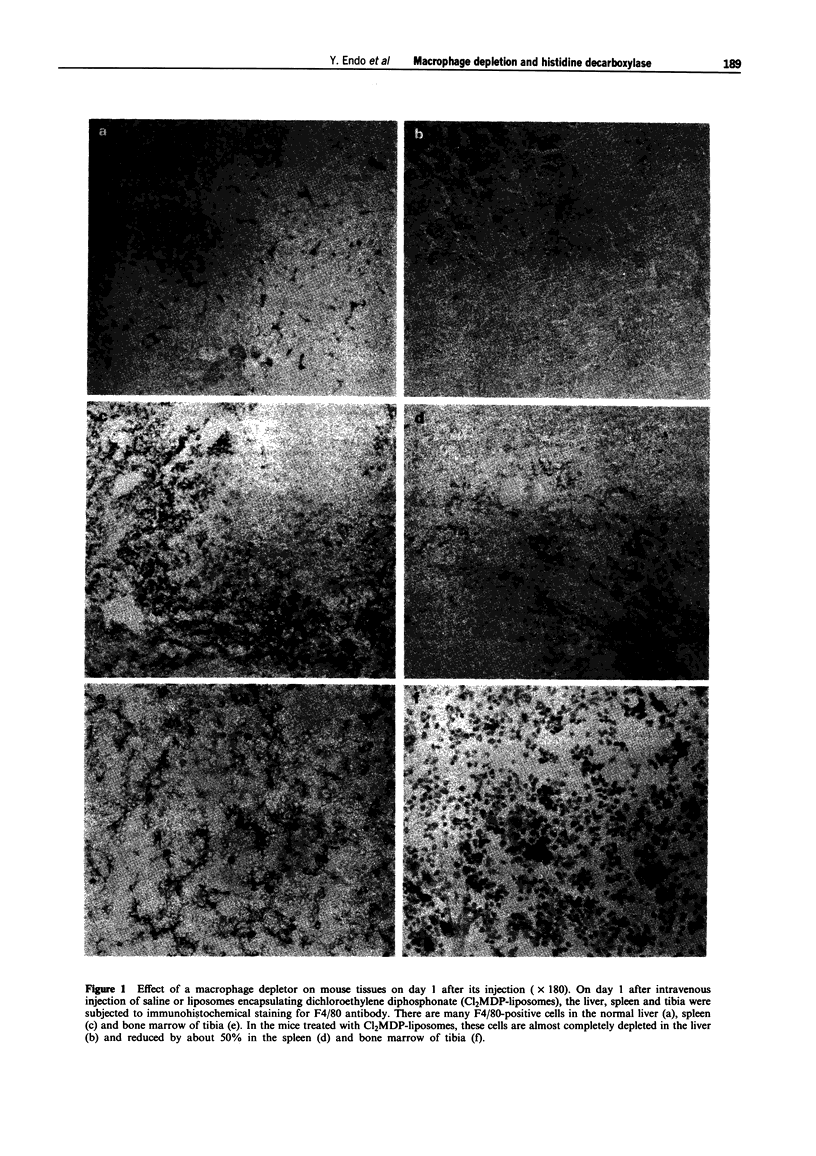
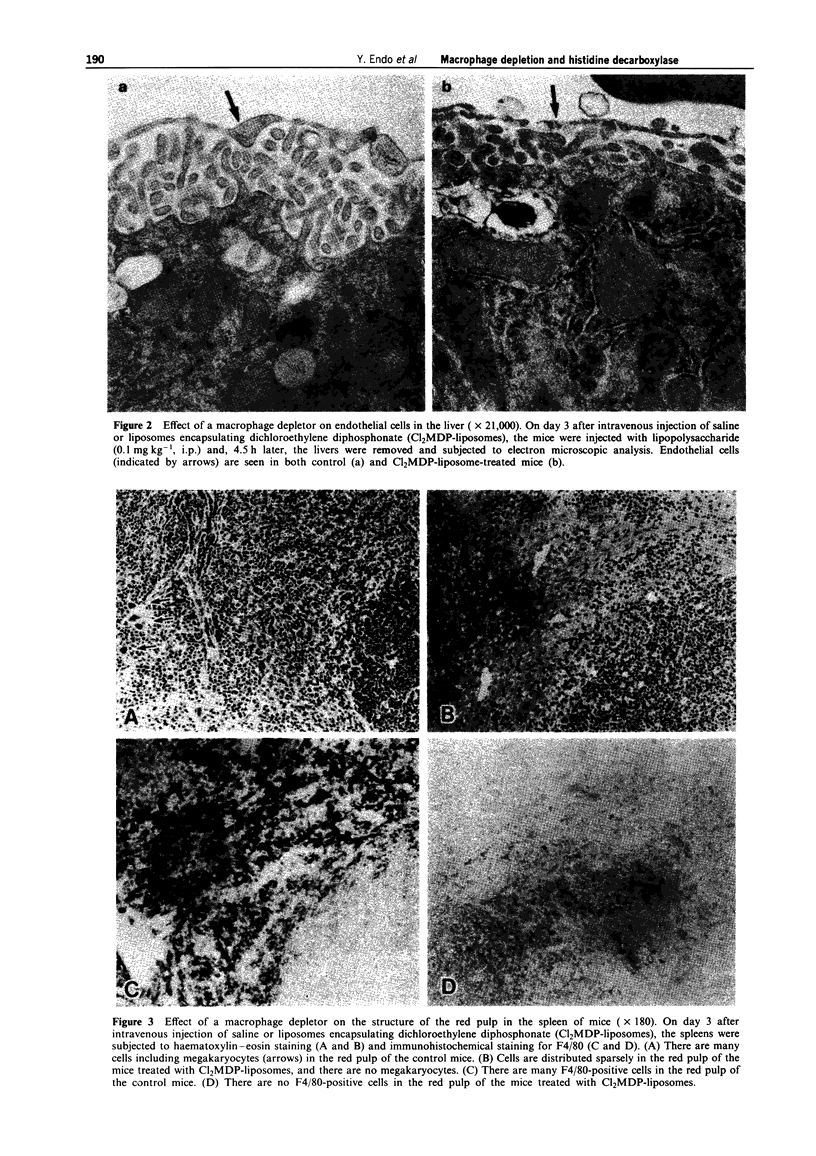
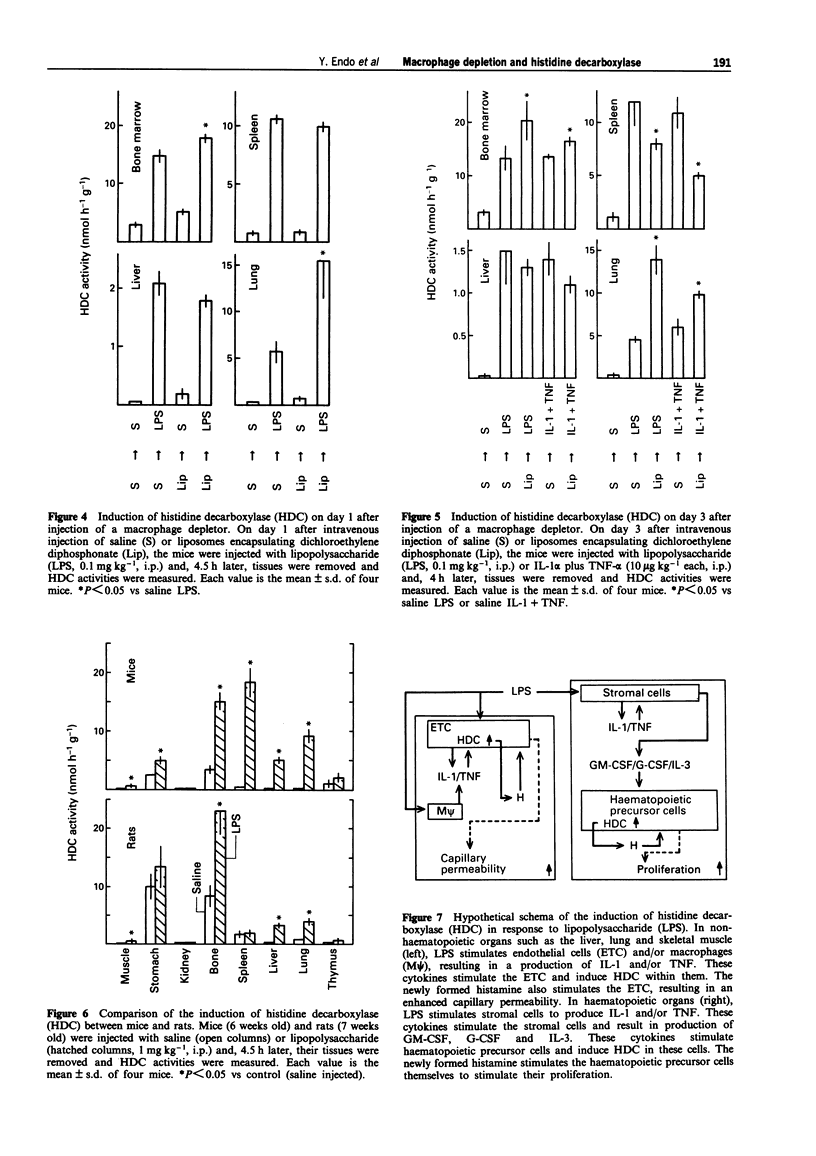


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austyn J. M., Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981 Oct;11(10):805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- Beer D. J., Matloff S. M., Rocklin R. E. The influence of histamine on immune and inflammatory responses. Adv Immunol. 1984;35:209–268. doi: 10.1016/s0065-2776(08)60577-5. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. The endogenous mediator of endotoxic shock. Clin Res. 1987 Apr;35(3):192–197. [PubMed] [Google Scholar]
- Byron J. W. Mechanism for histamine H2-receptor induced cell-cycle changes in the bone marrow stem cell. Agents Actions. 1977 Jul;7(2):209–213. doi: 10.1007/BF01969974. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and interleukin-1 antagonism. Blood. 1991 Apr 15;77(8):1627–1652. [PubMed] [Google Scholar]
- Dorshkind K. Regulation of hemopoiesis by bone marrow stromal cells and their products. Annu Rev Immunol. 1990;8:111–137. doi: 10.1146/annurev.iy.08.040190.000551. [DOI] [PubMed] [Google Scholar]
- Endo Y. A simple method for the determination of polyamines and histamine and its application to the assay of ornithine and histidine decarboxylase activities. Methods Enzymol. 1983;94:42–47. doi: 10.1016/s0076-6879(83)94008-9. [DOI] [PubMed] [Google Scholar]
- Endo Y. Induction of histidine and ornithine decarboxylase activities in mouse tissues by recombinant interleukin-1 and tumor necrosis factor. Biochem Pharmacol. 1989 Apr 15;38(8):1287–1292. doi: 10.1016/0006-2952(89)90335-3. [DOI] [PubMed] [Google Scholar]
- Endo Y. Induction of histidine decarboxylase in mouse tissues by mitogens in vivo. Biochem Pharmacol. 1983 Dec 15;32(24):3835–3838. doi: 10.1016/0006-2952(83)90157-0. [DOI] [PubMed] [Google Scholar]
- Endo Y., Kikuchi T., Nakamura M. Ornithine and histidine decarboxylase activities in mice sensitized to endotoxin, interleukin-1 or tumour necrosis factor by D-galactosamine. Br J Pharmacol. 1992 Nov;107(3):888–894. doi: 10.1111/j.1476-5381.1992.tb14542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo Y., Kikuchi T., Nakamura M., Shinoda H. Determination of histamine and polyamines in calcified tissues of mice: contribution of mast cells and histidine decarboxylase to the amount of histamine in the bone. Calcif Tissue Int. 1992 Jul;51(1):67–71. doi: 10.1007/BF00296220. [DOI] [PubMed] [Google Scholar]
- Endo Y., Kikuchi T., Takeda Y., Nitta Y., Rikiishi H., Kumagai K. GM-CSF and G-CSF stimulate the synthesis of histamine and putrescine in the hematopoietic organs in vivo. Immunol Lett. 1992 Jun;33(1):9–13. doi: 10.1016/0165-2478(92)90086-4. [DOI] [PubMed] [Google Scholar]
- Endo Y., Nakamura M. Active translocation of platelets into sinusoidal and Disse spaces in the liver in response to lipopolysaccharides, interleukin-1 and tumor necrosis factor. Gen Pharmacol. 1993 Sep;24(5):1039–1053. doi: 10.1016/0306-3623(93)90348-2. [DOI] [PubMed] [Google Scholar]
- Endo Y., Nakamura M., Kikuchi T., Shinoda H., Takeda Y., Nitta Y., Kumagai K. Aminoalkylbisphosphonates, potent inhibitors of bone resorption, induce a prolonged stimulation of histamine synthesis and increase macrophages, granulocytes, and osteoclasts in vivo. Calcif Tissue Int. 1993 Mar;52(3):248–254. doi: 10.1007/BF00298728. [DOI] [PubMed] [Google Scholar]
- Endo Y., Nakamura M. The effect of lipopolysaccharide, interleukin-1 and tumour necrosis factor on the hepatic accumulation of 5-hydroxytryptamine and platelets in the mouse. Br J Pharmacol. 1992 Mar;105(3):613–619. doi: 10.1111/j.1476-5381.1992.tb09028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo Y. Simultaneous induction of histidine and ornithine decarboxylases and changes in their product amines following the injection of Escherichia coli lipopolysaccharide into mice. Biochem Pharmacol. 1982 Apr 15;31(8):1643–1647. doi: 10.1016/0006-2952(82)90394-x. [DOI] [PubMed] [Google Scholar]
- Endo Y., Suzuki R., Kumagai K. Macrophages can produce factors capable of inducing histidine decarboxylase, a histamine-forming enzyme, in vivo in the liver, spleen, and lung of mice. Cell Immunol. 1986 Jan;97(1):13–22. doi: 10.1016/0008-8749(86)90370-9. [DOI] [PubMed] [Google Scholar]
- Furutani Y., Notake M., Yamayoshi M., Yamagishi J., Nomura H., Ohue M., Furuta R., Fukui T., Yamada M., Nakamura S. Cloning and characterization of the cDNAs for human and rabbit interleukin-1 precursor. Nucleic Acids Res. 1985 Aug 26;13(16):5869–5882. doi: 10.1093/nar/13.16.5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch S., Austyn J. M., Gordon S. Expression of the macrophage-specific antigen F4/80 during differentiation of mouse bone marrow cells in culture. J Exp Med. 1981 Sep 1;154(3):713–725. doi: 10.1084/jem.154.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel B., Schneider E., Piquet-Pellorce C., Machavoine F., Kindler V., Luffau G., Dy M. Antigenic challenge of immunized mice induces endogeneous production of IL-3 that increases histamine synthesis in hematopoietic organs. J Immunol. 1990 Aug 15;145(4):1222–1226. [PubMed] [Google Scholar]
- Nakaya N., Tasaka K. The influence of histamine on precursors of granulocytic leukocytes in murine bone marrow. Life Sci. 1988;42(9):999–1010. doi: 10.1016/0024-3205(88)90430-4. [DOI] [PubMed] [Google Scholar]
- Neugebauer E., Lorenz W., Beckurts T., Maroske D., Merte H. Significance of histamine formation and release in the development of endotoxic shock: proof of current concepts by randomized controlled studies in rats. Rev Infect Dis. 1987 Sep-Oct;9 (Suppl 5):S585–S593. doi: 10.1093/clinids/9.supplement_5.s585. [DOI] [PubMed] [Google Scholar]
- SCHAYER R. W. Evidence that induced histamine is an intrinsic regulator of the microcirculatory system. Am J Physiol. 1962 Jan;202:66–72. doi: 10.1152/ajplegacy.1962.202.1.66. [DOI] [PubMed] [Google Scholar]
- Schneider E., Piquet-Pellorce C., Dy M. New role for histamine in interleukin-3-induced proliferation of hematopoietic stem cells. J Cell Physiol. 1990 May;143(2):337–343. doi: 10.1002/jcp.1041430218. [DOI] [PubMed] [Google Scholar]
- Thepen T., Van Rooijen N., Kraal G. Alveolar macrophage elimination in vivo is associated with an increase in pulmonary immune response in mice. J Exp Med. 1989 Aug 1;170(2):499–509. doi: 10.1084/jem.170.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rooijen N., Kors N., vd Ende M., Dijkstra C. D. Depletion and repopulation of macrophages in spleen and liver of rat after intravenous treatment with liposome-encapsulated dichloromethylene diphosphonate. Cell Tissue Res. 1990 May;260(2):215–222. doi: 10.1007/BF00318625. [DOI] [PubMed] [Google Scholar]
- Yi S. N., Xu Y. H. The influence of histamine at various concentrations on the cell cycle state of hematopoietic stem cells (CFU-s). Int J Cell Cloning. 1988 Jul;6(4):290–295. doi: 10.1002/stem.5530060406. [DOI] [PubMed] [Google Scholar]
- van Rooijen N., Kors N., Kraal G. Macrophage subset repopulation in the spleen: differential kinetics after liposome-mediated elimination. J Leukoc Biol. 1989 Feb;45(2):97–104. doi: 10.1002/jlb.45.2.97. [DOI] [PubMed] [Google Scholar]





