Abstract
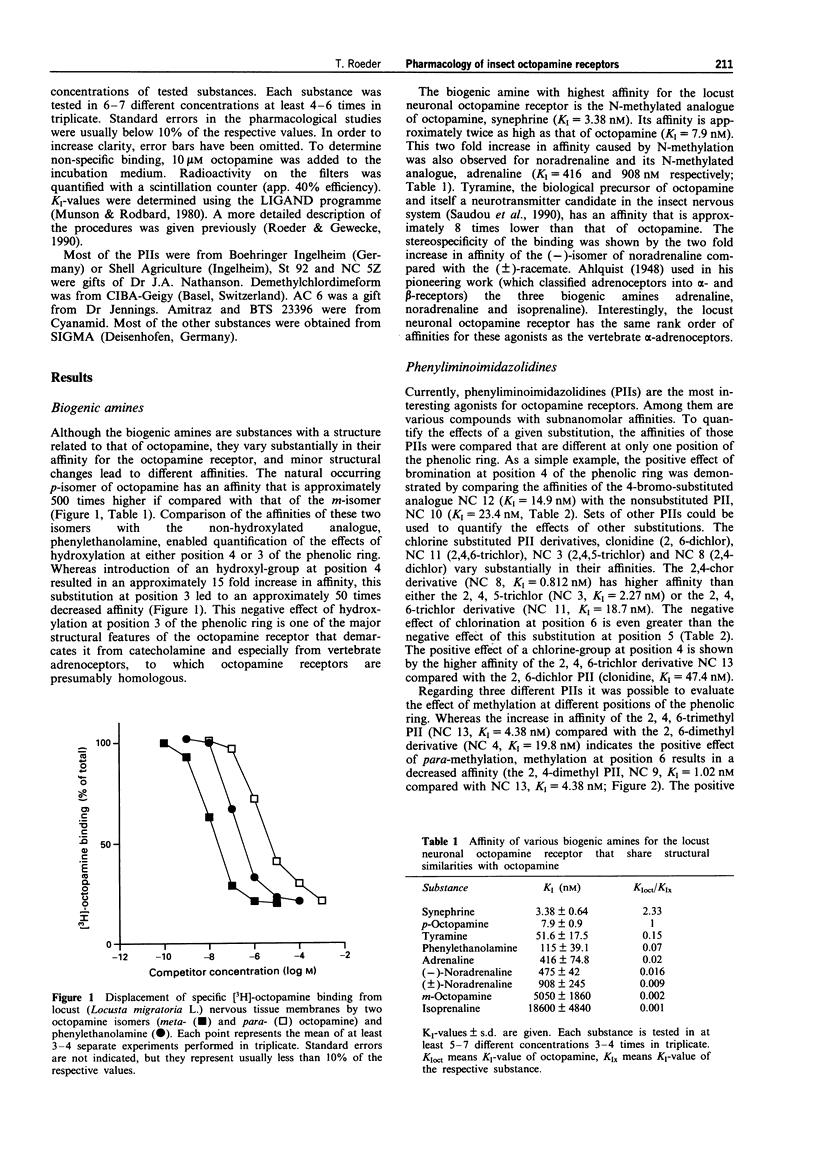
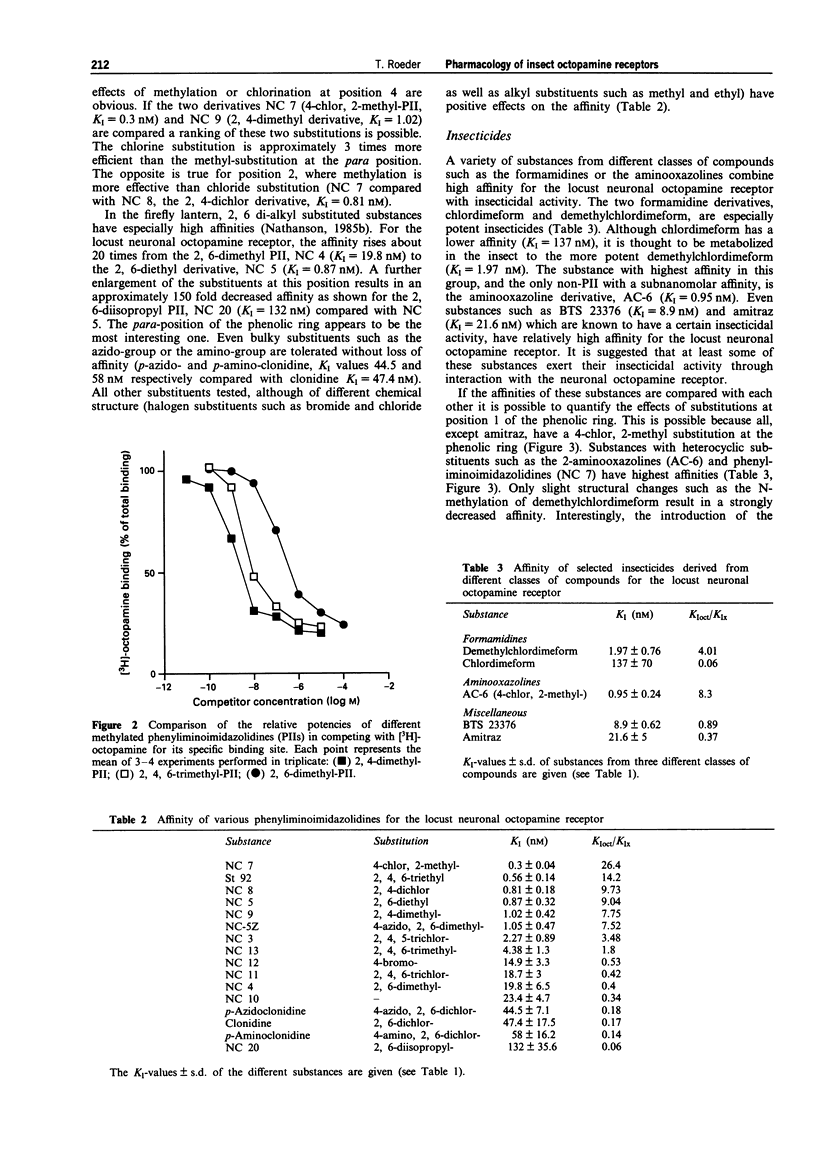
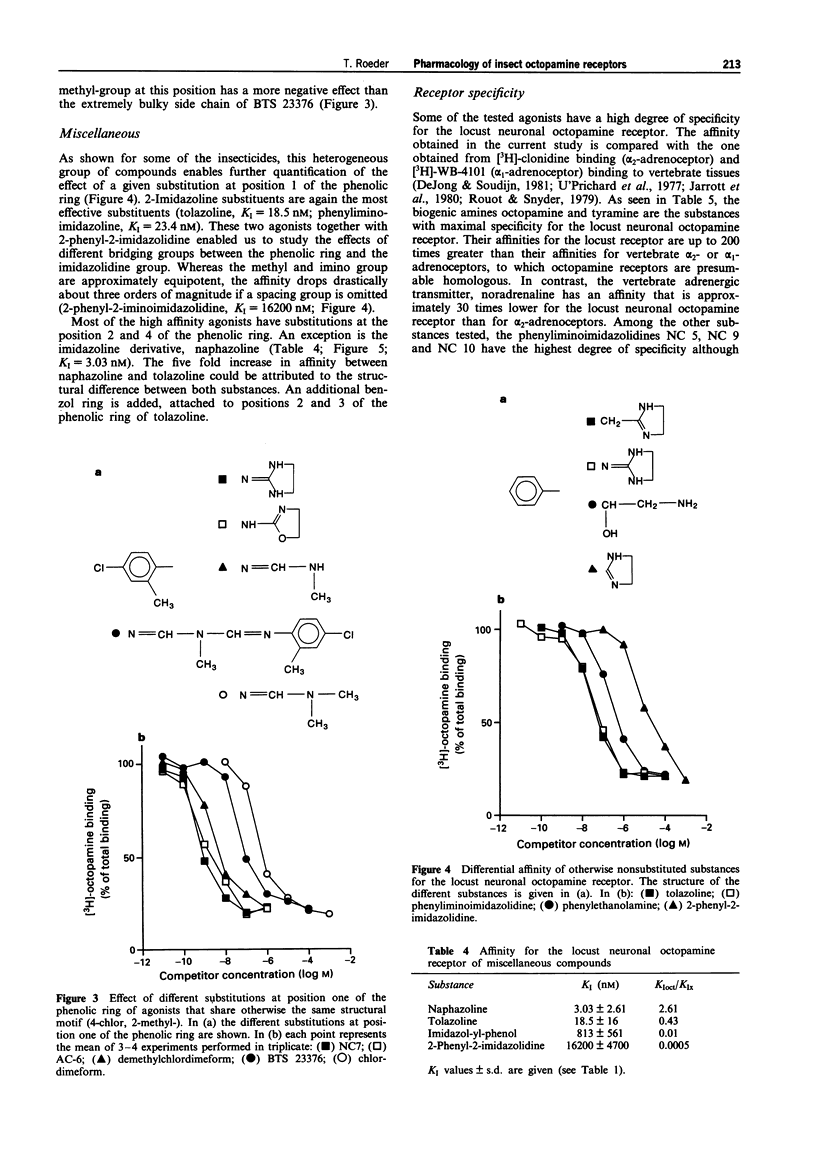
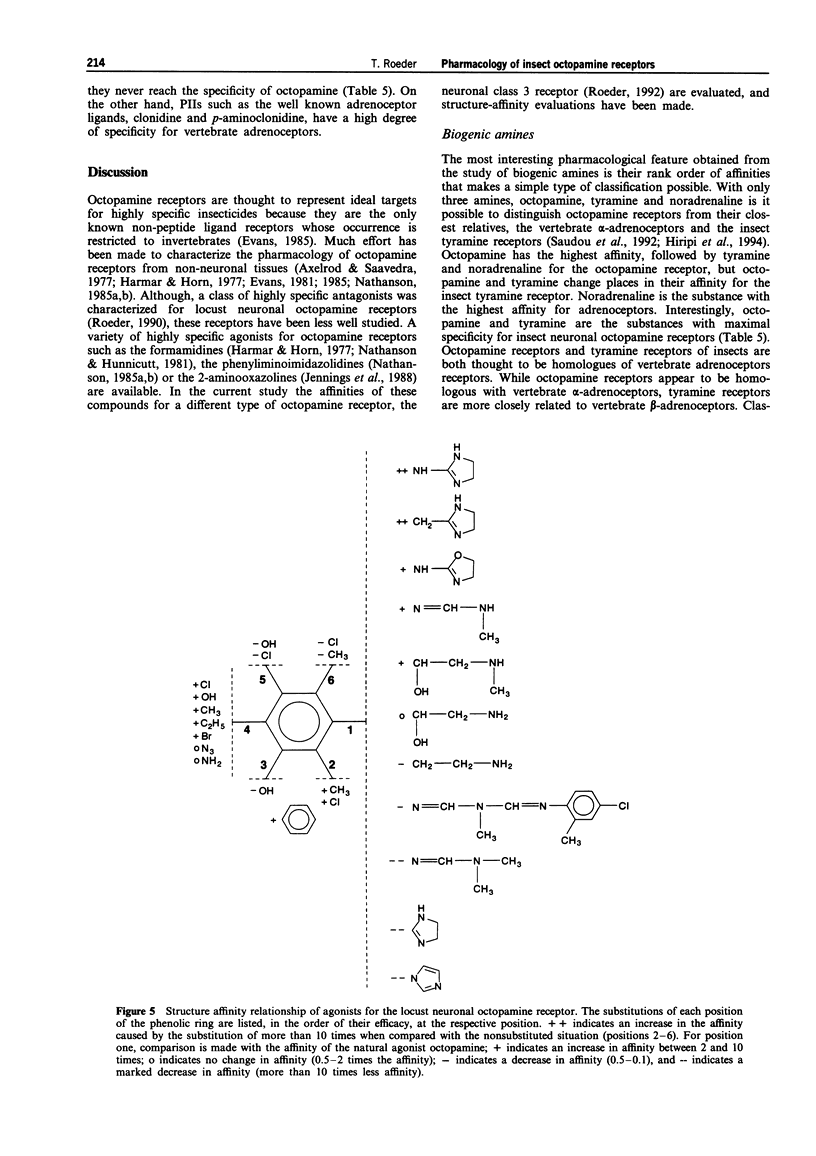
1. The present study characterized highly effective agonists from different classes of compounds for the neuronal octopamine receptor (OAR3) of the migratory locust (Locusta migratoria L.). Biogenic amines and phenyliminoimidazolidines (PIIs) were employed for the study of structure-activity relationships. 2. The highest affinity PIIs were predominantly those with substitutions at the positions 2 and 4 of the phenolic ring (e.g. NC 7, KI = 0.3 nM, NC 8, KI = 0.81 nM). Substitutions at these positions always had positive effects on the affinity of the respective agonists. 3. Substitutions at the positions 3, 5 and 6, however, always had negative effects on the affinity. At the position one of the phenolic ring, heterocyclic substituents are preferred. 4. Some PIIs had a more than 30 times higher affinity for OARs than for alpha-adrenoceptors which are the vertebrate homologues of the insect octopamine receptors. 5. The only non-PII with subnanomolar affinity was the aminooxazoline derivative AC 6 (KI = 0.92 nM). 6. A variety of substances with known insecticidal activity such as chlordimeform, demethylchlor-dimeform, amitraz or AC 6 had high affinity for the locust neuronal octopamine receptor.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod J., Saavedra J. M. Octopamine. Nature. 1977 Feb 10;265(5594):501–504. doi: 10.1038/265501a0. [DOI] [PubMed] [Google Scholar]
- Bicker G., Menzel R. Chemical codes for the control of behaviour in arthropods. Nature. 1989 Jan 5;337(6202):33–39. doi: 10.1038/337033a0. [DOI] [PubMed] [Google Scholar]
- De Jong A. P., Soudijn W. Relationships between structure and alpha-adrenergic receptor affinity of clonidine and some related cyclic amidines. Eur J Pharmacol. 1981 Jan 16;69(2):175–188. doi: 10.1016/0014-2999(81)90412-x. [DOI] [PubMed] [Google Scholar]
- Dudai Y. High-affinity octopamine receptors revealed in Drosophila by binding or [3H]octopamine. Neurosci Lett. 1982 Feb 12;28(2):163–167. doi: 10.1016/0304-3940(82)90146-x. [DOI] [PubMed] [Google Scholar]
- ERSPAMER V., BORETTI G. Identification and characterization, by paper chromatography, of enteramine, octopamine, tyramine, histamine and allied substances in extracts of posterior salivary glands of octopoda and in other tissue extracts of vertebrates and invertebrates. Arch Int Pharmacodyn Ther. 1951 Dec;88(3):296–332. [PubMed] [Google Scholar]
- Evans P. D. Multiple receptor types for octopamine in the locust. J Physiol. 1981 Sep;318:99–122. doi: 10.1113/jphysiol.1981.sp013853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmar A. J., Horn A. S. Octopamine-sensitive adenylate cyclase in cockroach brain: effects of agonists, antagonists, and guanylyl nucleotides. Mol Pharmacol. 1977 May;13(3):512–520. [PubMed] [Google Scholar]
- Hiripi L., Juhos S., Downer R. G. Characterization of tyramine and octopamine receptors in the insect (Locusta migratoria migratorioides) brain. Brain Res. 1994 Jan 7;633(1-2):119–126. doi: 10.1016/0006-8993(94)91530-x. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Nathanson J. A. Characterization of octopamine-sensitive adenylate cyclase: elucidation of a class of potent and selective octopamine-2 receptor agonists with toxic effects in insects. Proc Natl Acad Sci U S A. 1985 Jan;82(2):599–603. doi: 10.1073/pnas.82.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanson J. A., Greengard P. Octopamine-sensitive adenylate cyclse: evidence for a biological role of octopamine in nervous tissue. Science. 1973 Apr 20;180(4083):308–310. doi: 10.1126/science.180.4083.308. [DOI] [PubMed] [Google Scholar]
- Nathanson J. A., Hunnicutt E. J. N-demethylchlordimeform: a potent partial agonist of octopamine-sensitive adenylate cyclase. Mol Pharmacol. 1981 Jul;20(1):68–75. [PubMed] [Google Scholar]
- Nathanson J. A. Identification of octopaminergic agonists with selectivity for octopamine receptor subtypes. J Pharmacol Exp Ther. 1993 May;265(2):509–515. [PubMed] [Google Scholar]
- Nathanson J. A., Kaugars G. A probe for octopamine receptors: synthesis of 2-[(4-azido-2,6-diethylphenyl)imino]imidazolidine and its tritiated derivative, a potent reversible-irreversible activator of octopamine-sensitive adenylate cyclase. J Med Chem. 1989 Aug;32(8):1795–1799. doi: 10.1021/jm00128a022. [DOI] [PubMed] [Google Scholar]
- Nathanson J. A. Phenyliminoimidazolidines. Characterization of a class of potent agonists of octopamine-sensitive adenylate cyclase and their use in understanding the pharmacology of octopamine receptors. Mol Pharmacol. 1985 Sep;28(3):254–268. [PubMed] [Google Scholar]
- Roeder T. A new octopamine receptor class in locust nervous tissue, the octopamine 3 (OA3) receptor. Life Sci. 1992;50(1):21–28. doi: 10.1016/0024-3205(92)90193-s. [DOI] [PubMed] [Google Scholar]
- Roeder T., Gewecke M. Octopamine receptors in locust nervous tissue. Biochem Pharmacol. 1990 Jun 1;39(11):1793–1797. doi: 10.1016/0006-2952(90)90127-7. [DOI] [PubMed] [Google Scholar]
- Roeder T. High-affinity antagonists of the locust neuronal octopamine receptor. Eur J Pharmacol. 1990 Nov 27;191(2):221–224. doi: 10.1016/0014-2999(90)94151-m. [DOI] [PubMed] [Google Scholar]
- Roeder T., Nathanson J. A. Characterization of insect neuronal octopamine receptors (OA3 receptors). Neurochem Res. 1993 Aug;18(8):921–925. doi: 10.1007/BF00998278. [DOI] [PubMed] [Google Scholar]
- Rouot B. R., Snyder S. H. [3H]Para-amino-clonidine: a novel ligand which binds with high affinity to alpha-adrenergic receptors. Life Sci. 1979 Aug 27;25(9):769–774. doi: 10.1016/0024-3205(79)90521-6. [DOI] [PubMed] [Google Scholar]
- Saudou F., Amlaiky N., Plassat J. L., Borrelli E., Hen R. Cloning and characterization of a Drosophila tyramine receptor. EMBO J. 1990 Nov;9(11):3611–3617. doi: 10.1002/j.1460-2075.1990.tb07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sombati S., Hoyle G. Generation of specific behaviors in a locust by local release into neuropil of the natural neuromodulator octopamine. J Neurobiol. 1984 Nov;15(6):481–506. doi: 10.1002/neu.480150607. [DOI] [PubMed] [Google Scholar]
- U'Prichard D. C., Greenberg D. A., Snyder S. H. Binding characteristics of a radiolabeled agonist and antagonist at central nervous system alpha noradrenergic receptors. Mol Pharmacol. 1977 May;13(3):454–473. [PubMed] [Google Scholar]


