Abstract
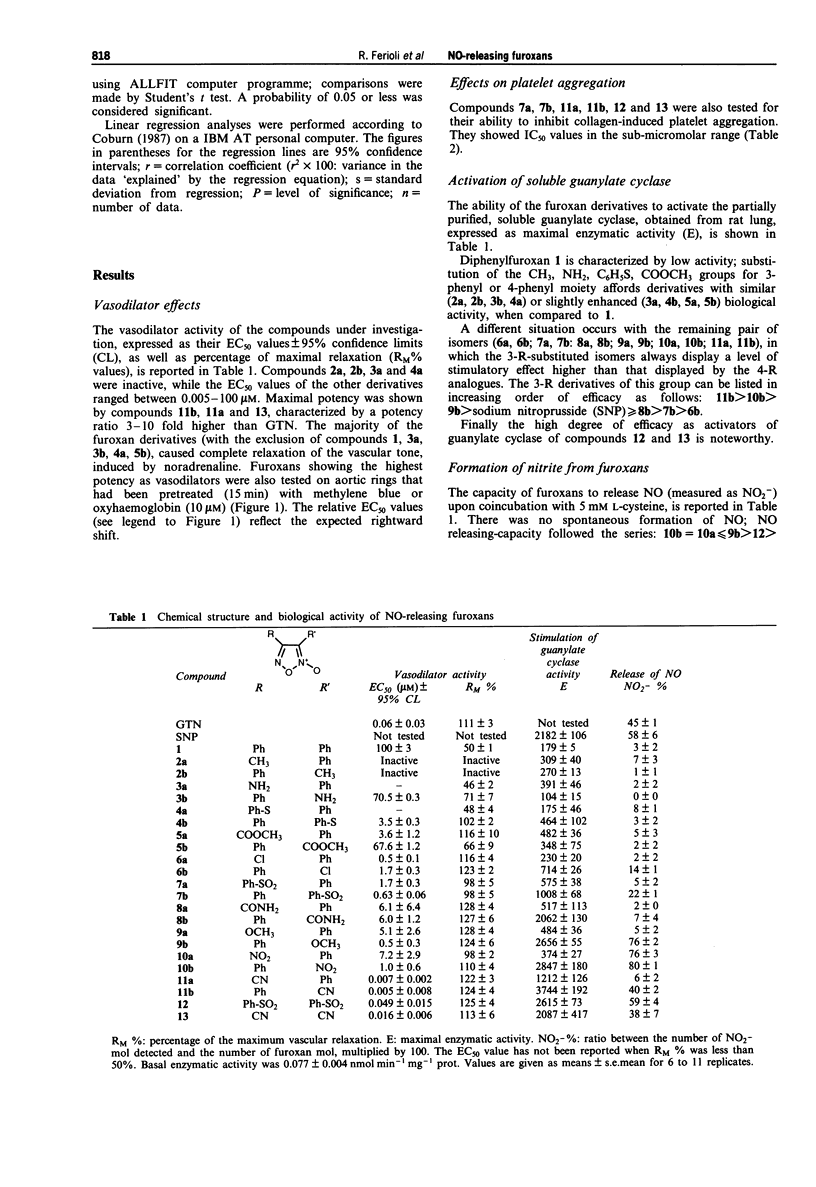
1. The mechanism of action and biological activity of a series of R-substituted and di-R-substituted phenylfuroxans is reported. 2. Maximal potency as vasodilators on rabbit aortic rings, precontracted with noradrenaline (1 microM), was shown by phenyl-cyano isomers and by the 3,4-dicyanofuroxan, characterized by a potency ratio 3-10 fold higher than glyceryl trinitrate (GTN). This effect was reduced upon coincubation with methylene blue or oxyhaemoglobin (10 microM). 3. The furoxan derivatives showing maximal potency as vasodilators were also able to inhibit collagen-induced platelet aggregation, with IC50 values in the sub-micromolar range. 4. The furoxan derivatives were able to stimulate partially purified, rat lung soluble guanylate cyclase; among the most active compounds, the 3-R-substituted isomers displayed a higher level of stimulatory effect than the 4-R analogues. 5. Solutions (0.1 mM) of all the tested furoxans, prepared using 50 mM phosphate buffer, pH 7.4, (diluting 10 mM DMSO stock solutions) did not release nitric oxide (NO) spontaneously; however in presence of 5 mM L-cysteine, a significant NO-releasing capacity was observed, which correlated significantly with their stimulation of the guanylate cyclase activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anggård E. E. Endogenous nitrates--implications for treatment and prevention. Eur Heart J. 1991 May;12 (Suppl A):5–8. [PubMed] [Google Scholar]
- BORN G. V. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature. 1962 Jun 9;194:927–929. doi: 10.1038/194927b0. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Glatt C. E., Lowenstein C., Reed R. R., Snyder S. H. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991 Jun 27;351(6329):714–718. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990 Jan;87(2):682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvino R., Fruttero R., Ghigo D., Bosia A., Pescarmona G. P., Gasco A. 4-Methyl-3-(arylsulfonyl)furoxans: a new class of potent inhibitors of platelet aggregation. J Med Chem. 1992 Aug 21;35(17):3296–3300. doi: 10.1021/jm00095a028. [DOI] [PubMed] [Google Scholar]
- Feelisch M., Schönafinger K., Noack E. Thiol-mediated generation of nitric oxide accounts for the vasodilator action of furoxans. Biochem Pharmacol. 1992 Sep 25;44(6):1149–1157. doi: 10.1016/0006-2952(92)90379-w. [DOI] [PubMed] [Google Scholar]
- Ferioli R., Fazzini A., Folco G. C., Fruttero R., Calvino R., Gasco A., Bongrani S., Civelli M. No-mimetic furoxans: arylsulphonylfuroxans and related compounds. Pharmacol Res. 1993 Oct-Nov;28(3):203–212. doi: 10.1006/phrs.1993.1123. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Ghigo D., Heller R., Calvino R., Alessio P., Fruttero R., Gasco A., Bosia A., Pescarmona G. Characterization of a new compound, S35b, as a guanylate cyclase activator in human platelets. Biochem Pharmacol. 1992 Mar 17;43(6):1281–1288. doi: 10.1016/0006-2952(92)90504-c. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Vavrin Z., Taintor R. R. L-arginine is required for expression of the activated macrophage effector mechanism causing selective metabolic inhibition in target cells. J Immunol. 1987 Jan 15;138(2):550–565. [PubMed] [Google Scholar]
- Holtz J., Förstermann U., Pohl U., Giesler M., Bassenge E. Flow-dependent, endothelium-mediated dilation of epicardial coronary arteries in conscious dogs: effects of cyclooxygenase inhibition. J Cardiovasc Pharmacol. 1984 Nov-Dec;6(6):1161–1169. [PubMed] [Google Scholar]
- Ignarro L. J. Biosynthesis and metabolism of endothelium-derived nitric oxide. Annu Rev Pharmacol Toxicol. 1990;30:535–560. doi: 10.1146/annurev.pa.30.040190.002535. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J., Lippton H., Edwards J. C., Baricos W. H., Hyman A. L., Kadowitz P. J., Gruetter C. A. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther. 1981 Sep;218(3):739–749. [PubMed] [Google Scholar]
- Kruszyna H., Kruszyna R., Smith R. P., Wilcox D. E. Red blood cells generate nitric oxide from directly acting, nitrogenous vasodilators. Toxicol Appl Pharmacol. 1987 Dec;91(3):429–438. doi: 10.1016/0041-008x(87)90064-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Nakane M., Arai K., Saheki S., Kuno T., Buechler W., Murad F. Molecular cloning and expression of cDNAs coding for soluble guanylate cyclase from rat lung. J Biol Chem. 1990 Oct 5;265(28):16841–16845. [PubMed] [Google Scholar]
- Nakane M., Saheki S., Kuno T., Ishii K., Murad F. Molecular cloning of a cDNA coding for 70 kilodalton subunit of soluble guanylate cyclase from rat lung. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1139–1147. doi: 10.1016/s0006-291x(88)80992-6. [DOI] [PubMed] [Google Scholar]
- Needleman P., Jakschik B., Johnson E. M., Jr Sulfhydryl requirement for relaxation of vascular smooth muscle. J Pharmacol Exp Ther. 1973 Nov;187(2):324–331. [PubMed] [Google Scholar]
- Needleman P., Johnson E. M., Jr Mechanism of tolerance development to organic nitrates. J Pharmacol Exp Ther. 1973 Mar;184(3):709–715. [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Comparative pharmacology of endothelium-derived relaxing factor, nitric oxide and prostacyclin in platelets. Br J Pharmacol. 1987 Sep;92(1):181–187. doi: 10.1111/j.1476-5381.1987.tb11310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubanyi G. M., Vanhoutte P. M. Nature of endothelium-derived relaxing factor: are there two relaxing mediators? Circ Res. 1987 Nov;61(5 Pt 2):II61–II67. [PubMed] [Google Scholar]
- Seth P., Fung H. L. Biochemical characterization of a membrane-bound enzyme responsible for generating nitric oxide from nitroglycerin in vascular smooth muscle cells. Biochem Pharmacol. 1993 Oct 19;46(8):1481–1486. doi: 10.1016/0006-2952(93)90115-d. [DOI] [PubMed] [Google Scholar]
- Yamada H., Yoneyama F., Satoh K., Taira N. Comparison of the effects of the novel vasodilator FK409 with those of nitroglycerin in isolated coronary artery of the dog. Br J Pharmacol. 1991 Jul;103(3):1713–1718. doi: 10.1111/j.1476-5381.1991.tb09852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


