Abstract
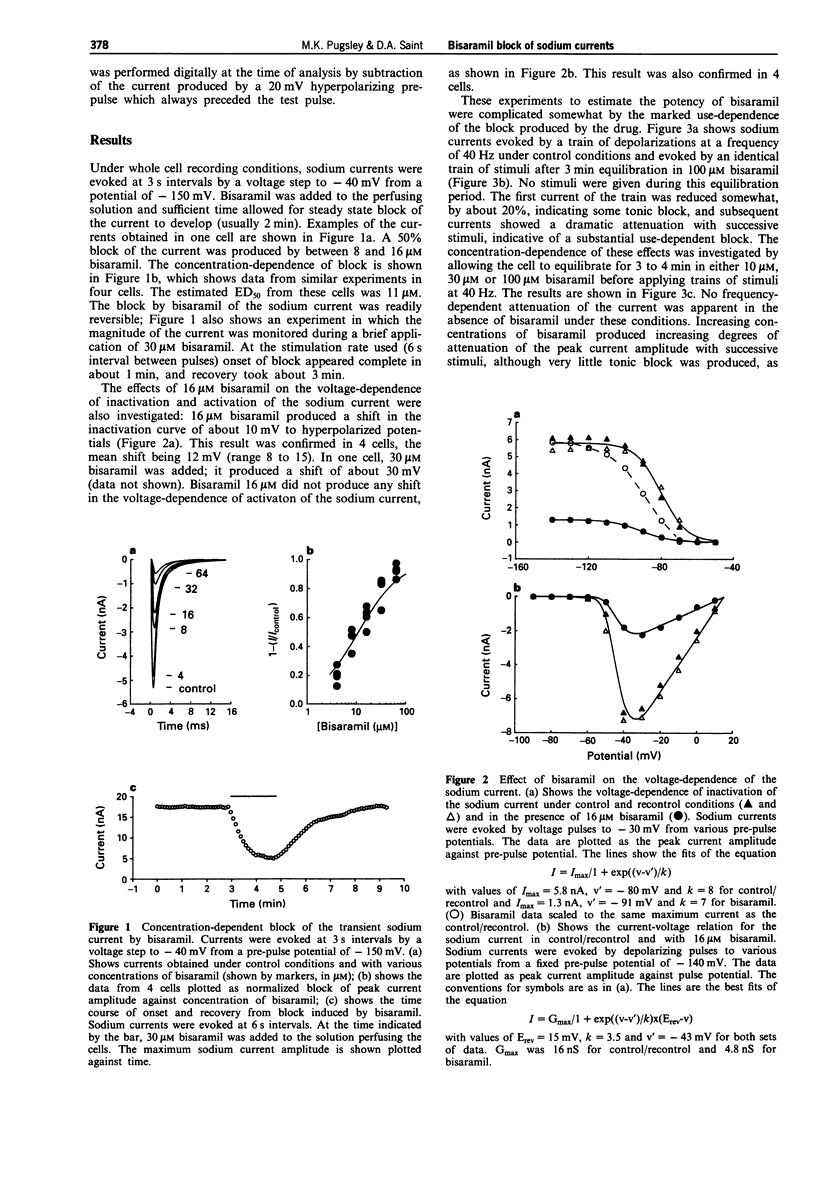
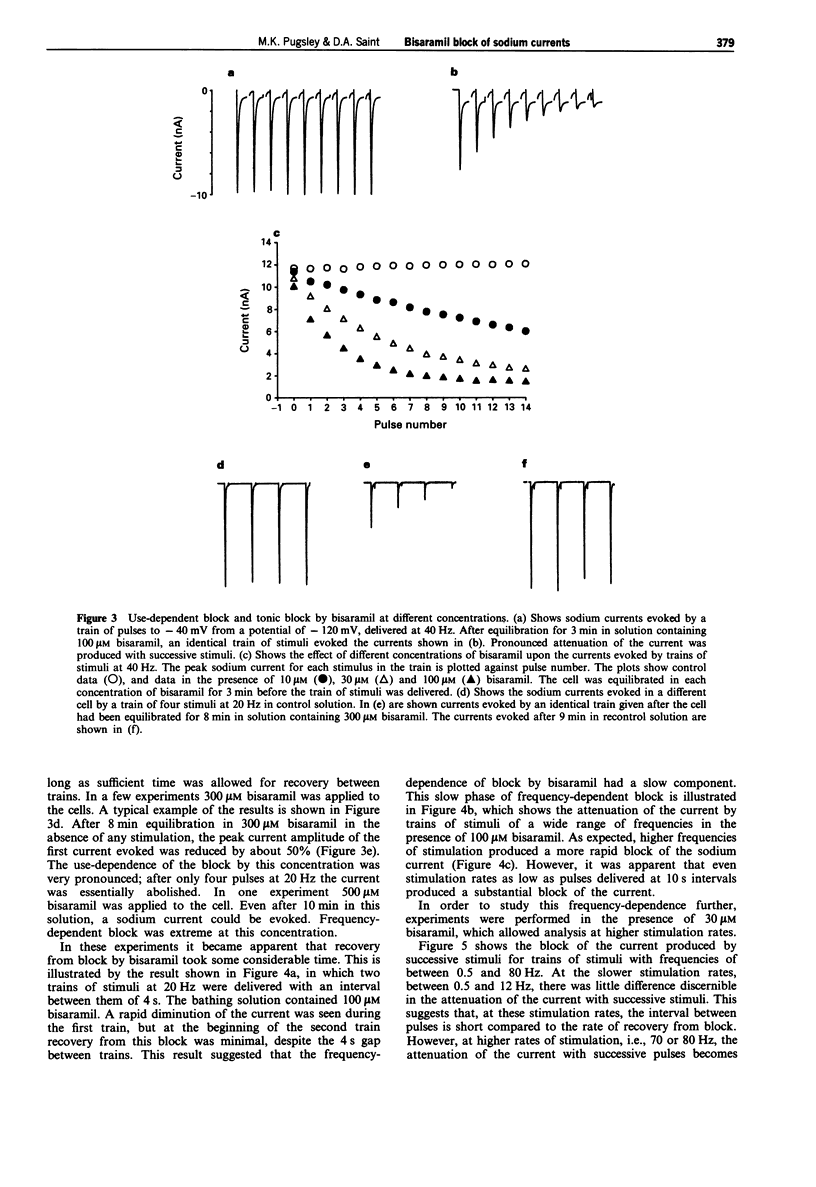
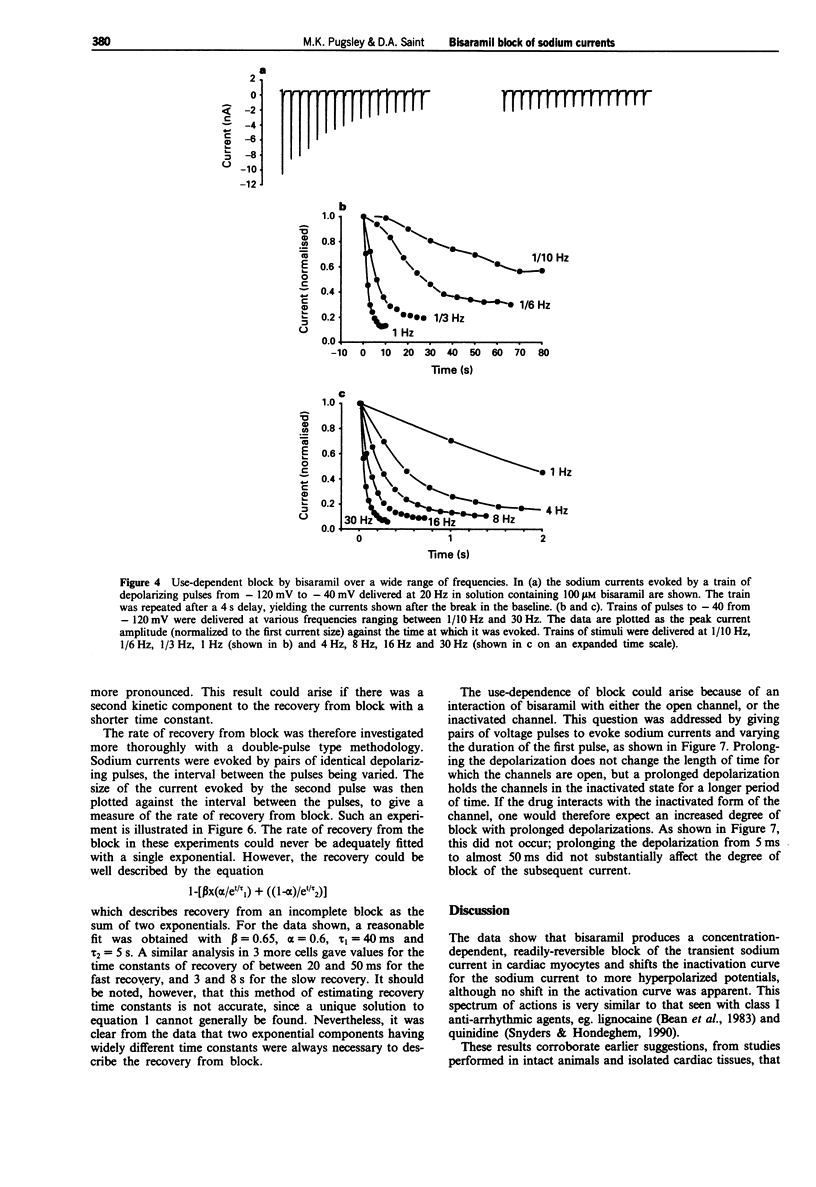
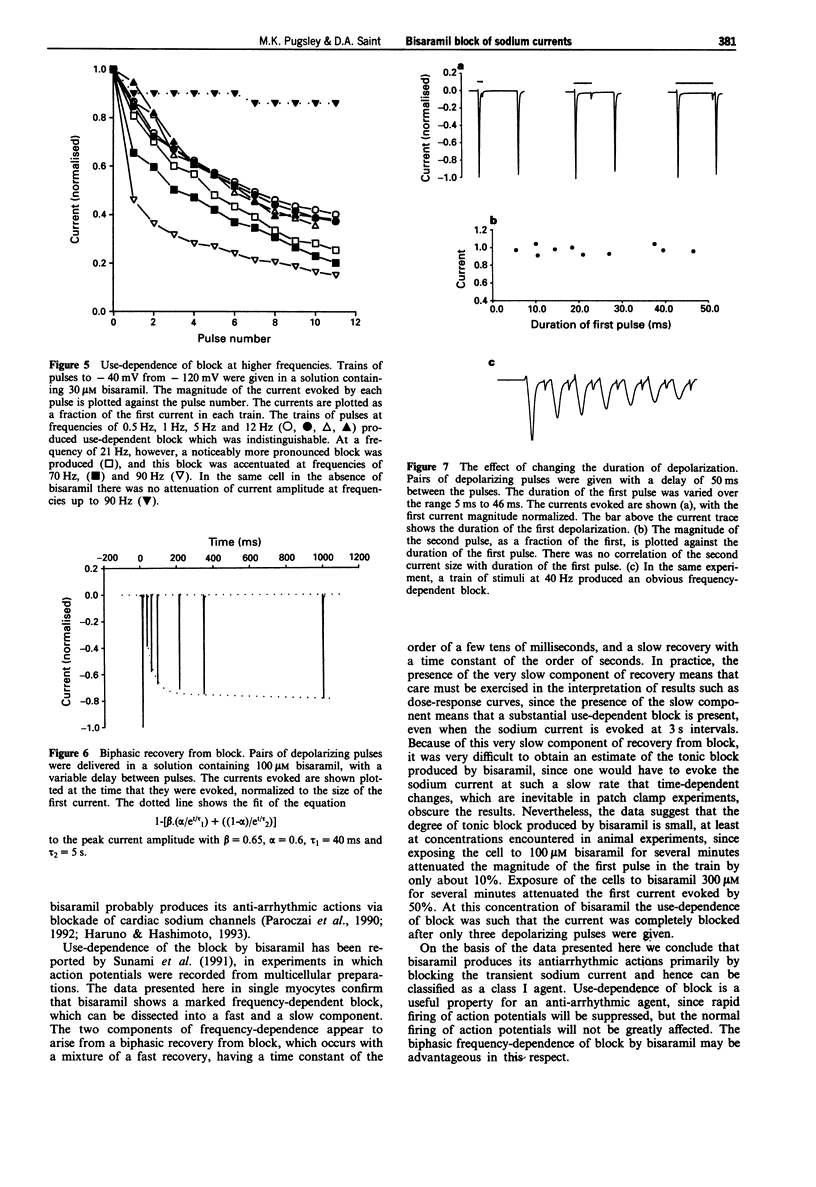
1. The effects of bisaramil on sodium currents in rat isolated cardiac myocytes were examined by use of tight-seal, whole-cell patch clamp techniques. Bisaramil produced a concentration-dependent, readily reversible reduction in peak transient sodium current. When the sodium current was evoked at 3 s intervals the estimated ED50 for bisaramil was about 11 microM. 2. Bisaramil (16 microM) produced a shift in the inactivation curve to hyperpolarized potentials of about 10 mV, but produced no change in the voltage-dependence of activation. 3. The block of the sodium current by bisaramil showed a profound use-dependence. A concentration of 10 microM produced a considerable block of the current with repeated stimulation. The recovery from block was biphasic, showing fast and slow components which had time constants of about 40 ms and 5 s respectively. 4. Bisaramil produced little tonic block of the sodium current at concentrations of 100 microM; at 300 microM it produced tonic block of around 50%, with extreme use-dependence. 5. Bisaramil appeared not to interact primarily with the inactivated form of the channel, since lengthening the depolarizing pulses did not affect the degree of block produced.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bean B. P., Cohen C. J., Tsien R. W. Lidocaine block of cardiac sodium channels. J Gen Physiol. 1983 May;81(5):613–642. doi: 10.1085/jgp.81.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Haruno A., Hashimoto K. Antiarrhythmic effects of bisaramil in canine models of ventricular arrhythmia. Eur J Pharmacol. 1993 Mar 16;233(1):1–6. doi: 10.1016/0014-2999(93)90341-e. [DOI] [PubMed] [Google Scholar]
- Paroczai M., Karpati E., Marko R., Kecskemeti V. Electrophysiological actions of a new antiarrhythmic drug, bisaramil, on isolated heart preparations. Pharmacol Res. 1992 Jan;25(1):75–85. doi: 10.1016/s1043-6618(05)80066-0. [DOI] [PubMed] [Google Scholar]
- Paróczai M., Kárpáti E., Solti F. The effects of bisaramil on experimental arrhythmias. Pharmacol Res. 1990 Jul-Aug;22(4):463–480. doi: 10.1016/1043-6618(90)90753-z. [DOI] [PubMed] [Google Scholar]
- Saint D. A., Ju Y. K., Gage P. W. A persistent sodium current in rat ventricular myocytes. J Physiol. 1992;453:219–231. doi: 10.1113/jphysiol.1992.sp019225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyders D. J., Hondeghem L. M. Effects of quinidine on the sodium current of guinea pig ventricular myocytes. Evidence for a drug-associated rested state with altered kinetics. Circ Res. 1990 Feb;66(2):565–579. doi: 10.1161/01.res.66.2.565. [DOI] [PubMed] [Google Scholar]
- Sunami A., Sawanobori T., Adaniya H., Hiraoka M. Electrophysiological properties of a new antiarrhythmic agent, bisaramil on guinea-pig, rabbit and canine cardiac preparations. Naunyn Schmiedebergs Arch Pharmacol. 1991 Sep;344(3):323–330. doi: 10.1007/BF00183007. [DOI] [PubMed] [Google Scholar]


