Abstract
1. In the present study, the inhibitory effects of the selective beta 2-adrenoceptor agonists, salmeterol, formoterol and salbutamol, have been investigated on contractions of ferret trachea induced both by endogenous and exogenous acetylcholine. The aim of the study was to evaluate quantitative and/or qualitative differences in response which may indicate both pre- and post-junctional sites of action. The non-selective beta-antagonist, sotalol, was used to estimate beta-adrenoceptor involvement. 2. Isometric tension was measured in ferret isolated tracheal strips. The inhibitory effects of the drugs were studied on tonic contractions induced by pre-junctional activation with electrical field stimulation (EFS) (2 Hz, 700 mA) or post-junctional activation with exogenous acetylcholine (ACh) (0.5 microM, about EC80), giving a similar degree of smooth muscle response. 3. Concentration-response experiments were performed with formoterol (0.3 nM-0.3 microM) and salmeterol and salbutamol (10 nM-10 microM). The experiments ended with the addition of sotalol (10 microM). 4. All three beta-agonists inhibited the contractions in a concentration-dependent manner. Salbutamol, formoterol and salmeterol inhibited the EFS-induced contractions by 66(8)%, 105(5)% and 103(8)% (mean(s.e. mean)) respectively. ACh-induced contractions were inhibited by 37(6)%, 72(11)% and 33(8)%. Theophylline (10 nM-3 mM) inhibited the contractions to the same degree. 5. beta-Adrenoceptor blockade by sotalol significantly antagonized the inhibitory effects of salbutamol and formoterol on both EFS- and ACh-induced contractions. The effect of salmeterol on ACh-induced contraction was also significantly antagonized, whereas the inhibition of EFS-induced contraction was virtually unaffected.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF
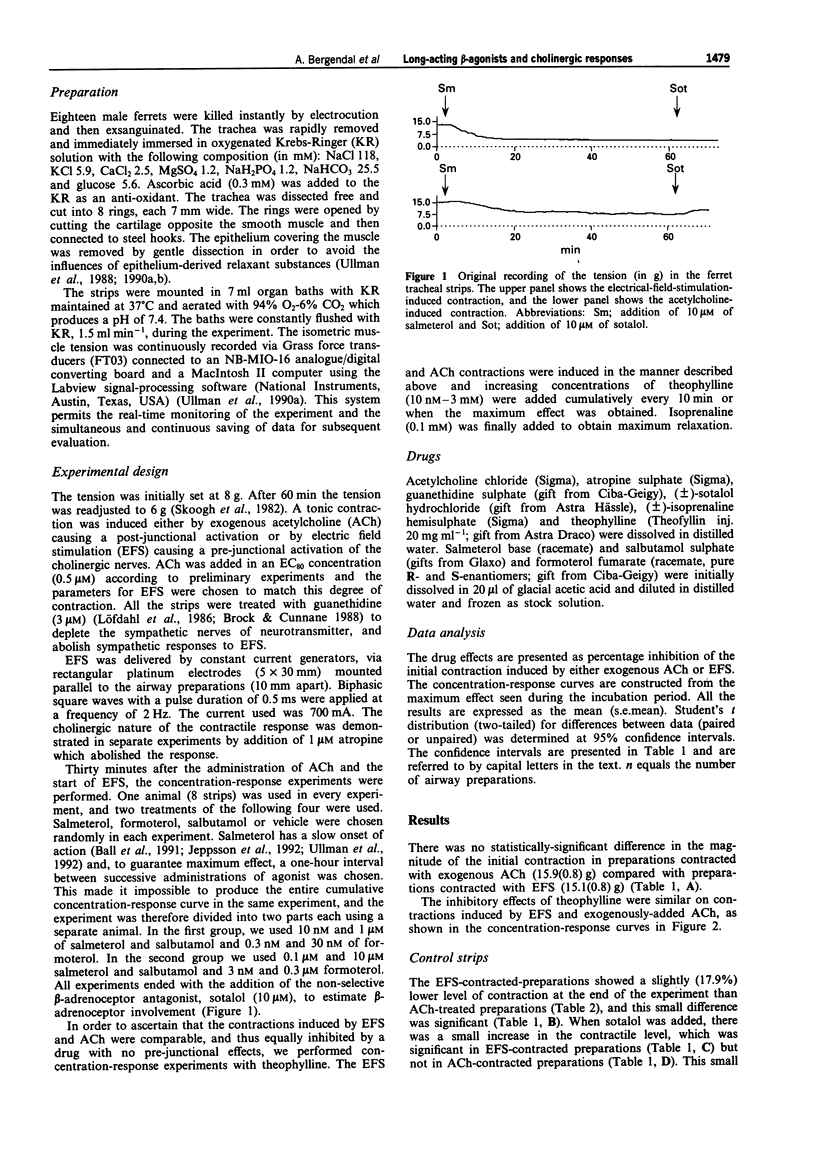
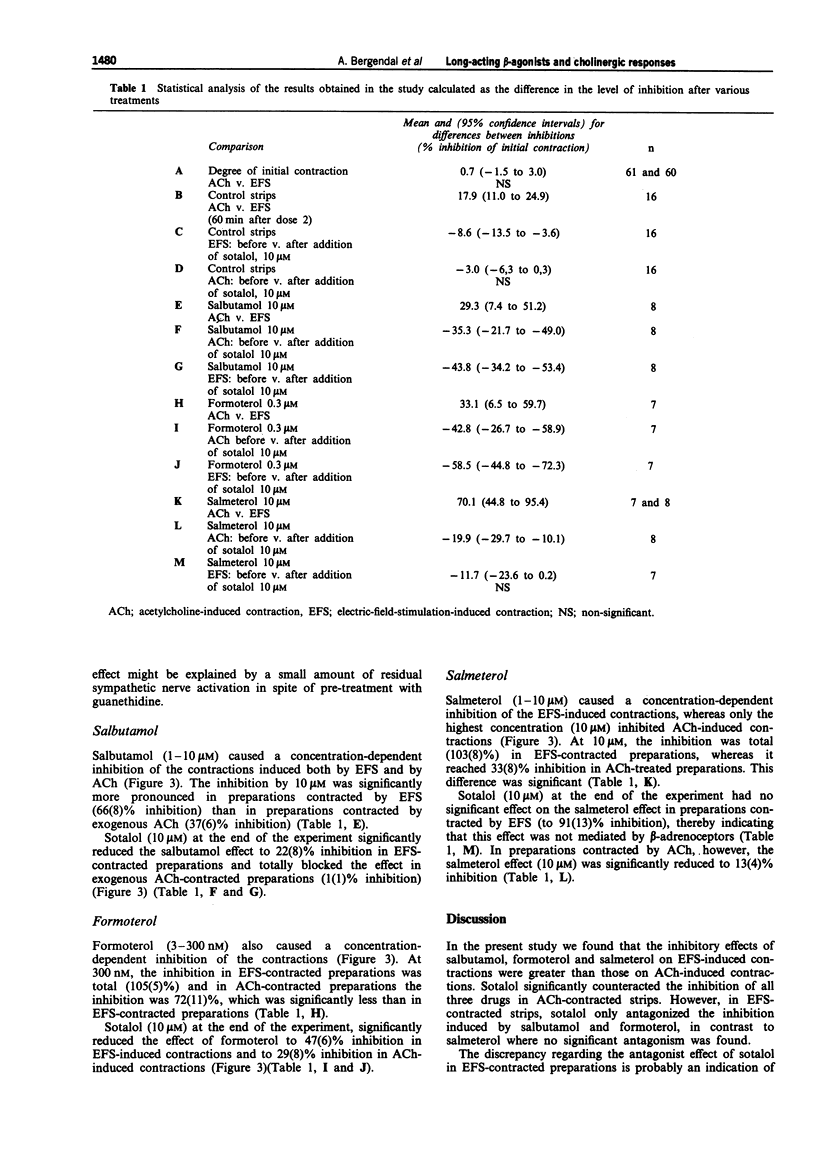
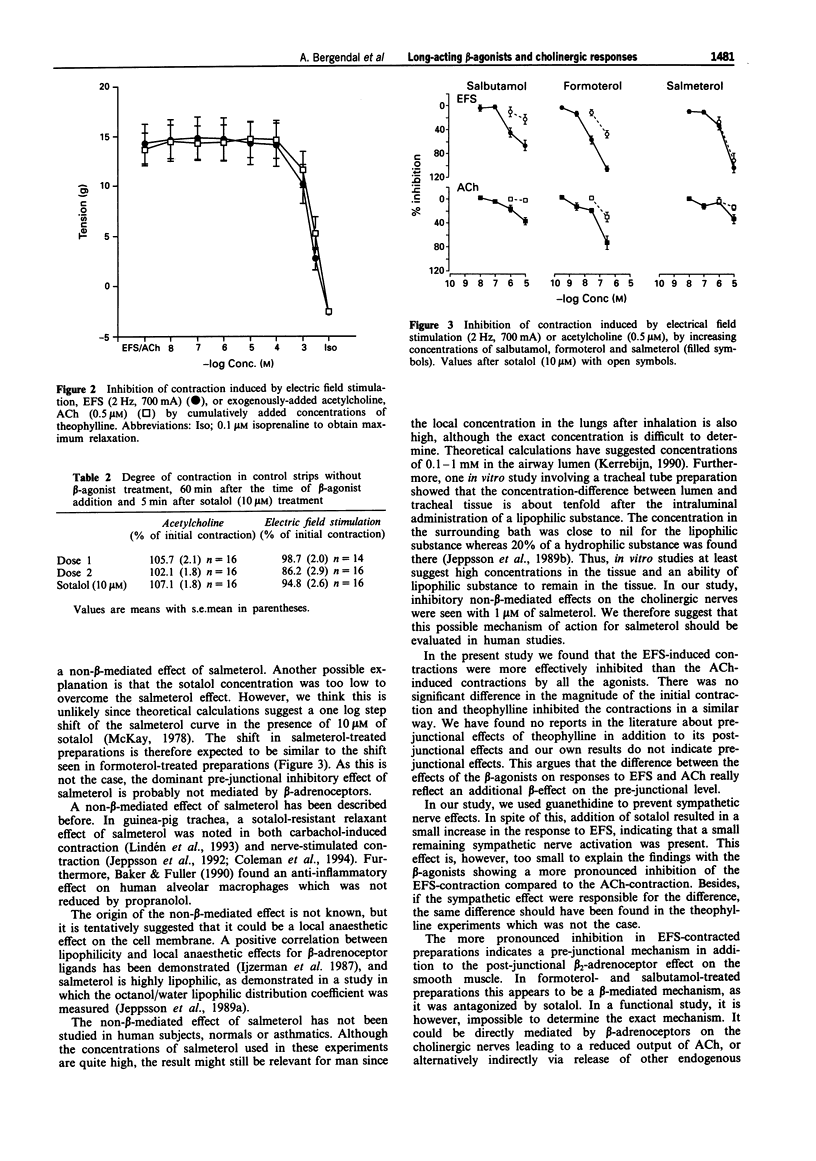

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson G. P., Lindén A., Rabe K. F. Why are long-acting beta-adrenoceptor agonists long-acting? Eur Respir J. 1994 Mar;7(3):569–578. doi: 10.1183/09031936.94.07030569. [DOI] [PubMed] [Google Scholar]
- Baker D. G., Don H. Catecholamines abolish vagal but not acetylcholine tone in the intact cat trachea. J Appl Physiol (1985) 1987 Dec;63(6):2490–2498. doi: 10.1152/jappl.1987.63.6.2490. [DOI] [PubMed] [Google Scholar]
- Ball D. I., Brittain R. T., Coleman R. A., Denyer L. H., Jack D., Johnson M., Lunts L. H., Nials A. T., Sheldrick K. E., Skidmore I. F. Salmeterol, a novel, long-acting beta 2-adrenoceptor agonist: characterization of pharmacological activity in vitro and in vivo. Br J Pharmacol. 1991 Nov;104(3):665–671. doi: 10.1111/j.1476-5381.1991.tb12486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock J. A., Cunnane T. C. Studies on the mode of action of bretylium and guanethidine in post-ganglionic sympathetic nerve fibres. Naunyn Schmiedebergs Arch Pharmacol. 1988 Nov;338(5):504–509. doi: 10.1007/BF00179321. [DOI] [PubMed] [Google Scholar]
- Danser A. H., van den Ende R., Lorenz R. R., Flavahan N. A., Vanhoutte P. M. Prejunctional beta 1-adrenoceptors inhibit cholinergic transmission in canine bronchi. J Appl Physiol (1985) 1987 Feb;62(2):785–790. doi: 10.1152/jappl.1987.62.2.785. [DOI] [PubMed] [Google Scholar]
- Ijzerman A. P., Nagesser A., Garritsen A. The membrane stabilizing activity of beta-adrenoceptor ligands. Quantitative evaluation of the interaction of phenoxypropanolamines with the [3H]batrachotoxinin A 20-alpha-benzoate binding site on voltage-sensitive sodium channels in rat brain. Biochem Pharmacol. 1987 Dec 15;36(24):4239–4244. doi: 10.1016/0006-2952(87)90664-2. [DOI] [PubMed] [Google Scholar]
- Ito Y. Pre- and post-junctional actions of procaterol, a beta 2-adrenoceptor stimulant, on dog tracheal tissue. Br J Pharmacol. 1988 Sep;95(1):268–274. doi: 10.1111/j.1476-5381.1988.tb16573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen L. J., Daniel E. E. Characterization of the prejunctional beta adrenoceptors in canine bronchial smooth muscle. J Pharmacol Exp Ther. 1990 Aug;254(2):741–749. [PubMed] [Google Scholar]
- Jeppsson A. B., Källström B. L., Waldeck B. Studies on the interaction between formoterol and salmeterol in guinea-pig trachea in vitro. Pharmacol Toxicol. 1992 Oct;71(4):272–277. doi: 10.1111/j.1600-0773.1992.tb00982.x. [DOI] [PubMed] [Google Scholar]
- Jeppsson A. B., Löfdahl C. G., Waldeck B., Widmark E. On the predictive value of experiments in vitro in the evaluation of the effect duration of bronchodilator drugs for local administration. Pulm Pharmacol. 1989;2(2):81–85. doi: 10.1016/0952-0600(89)90028-8. [DOI] [PubMed] [Google Scholar]
- Jeppsson A. B., Roos C., Waldeck B., Widmark E. Pharmacodynamic and pharmacokinetic aspects on the transport of bronchodilator drugs through the tracheal epithelium of the guinea-pig. Pharmacol Toxicol. 1989 Jan;64(1):58–63. doi: 10.1111/j.1600-0773.1989.tb00602.x. [DOI] [PubMed] [Google Scholar]
- Kerrebijn K. F. Long-term drug treatment of asthma in children. Lung. 1990;168 (Suppl):142–153. doi: 10.1007/BF02718127. [DOI] [PubMed] [Google Scholar]
- Lindén A., Bergendal A., Ullman A., Skoogh B. E., Löfdahl C. G. Salmeterol, formoterol, and salbutamol in the isolated guinea pig trachea: differences in maximum relaxant effect and potency but not in functional antagonism. Thorax. 1993 May;48(5):547–553. doi: 10.1136/thx.48.5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay D. How should values of pA2 and affinity constants for pharmacological competitive antagonists be estimated? J Pharm Pharmacol. 1978 May;30(5):312–313. doi: 10.1111/j.2042-7158.1978.tb13237.x. [DOI] [PubMed] [Google Scholar]
- Martin J. G., Collier B. Acetylcholine release from canine isolated airway is not modulated by norepinephrine. J Appl Physiol (1985) 1986 Sep;61(3):1025–1030. doi: 10.1152/jappl.1986.61.3.1025. [DOI] [PubMed] [Google Scholar]
- Rhoden K. J., Meldrum L. A., Barnes P. J. Inhibition of cholinergic neurotransmission in human airways by beta 2-adrenoceptors. J Appl Physiol (1985) 1988 Aug;65(2):700–705. doi: 10.1152/jappl.1988.65.2.700. [DOI] [PubMed] [Google Scholar]
- Skoogh B. E., Holtzman M. J., Sheller J. R., Nadel J. A. Barbiturates depress vagal motor pathway to ferret trachea at ganglia. J Appl Physiol Respir Environ Exerc Physiol. 1982 Jul;53(1):253–257. doi: 10.1152/jappl.1982.53.1.253. [DOI] [PubMed] [Google Scholar]
- Skoogh B. E., Löthwall J., Löfdahl C. G., Svedmyr N. Classification of beta-adrenoceptors in ferret tracheal smooth muscle by pharmacological responses. Pulm Pharmacol. 1989;1(4):173–177. doi: 10.1016/s0952-0600(89)80014-6. [DOI] [PubMed] [Google Scholar]
- Ullman A., Bergendal A., Lindén A., Waldeck B., Skoogh B. E., Löfdahl C. G. Onset of action and duration of effect of formoterol and salmeterol compared to salbutamol in isolated guinea pig trachea with or without epithelium. Allergy. 1992 Aug;47(4 Pt 2):384–387. doi: 10.1111/j.1398-9995.1992.tb02076.x. [DOI] [PubMed] [Google Scholar]
- Ullman A., Ciabattoni G., Löfdahl C. G., Lindén A., Svedmyr N., Skoogh B. E. Epithelium-derived PGE2 inhibits the contractile response to cholinergic stimulation in isolated ferret trachea. Pulm Pharmacol. 1990;3(3):155–160. doi: 10.1016/0952-0600(90)90047-m. [DOI] [PubMed] [Google Scholar]
- Ullman A., Löfdahl C. G., Svedmyr N., Bernsten L., Skoogh B. E. Mucosal inhibition of cholinergic contractions in ferret trachea can be transferred between organ baths. Eur Respir J. 1988 Dec;1(10):908–912. [PubMed] [Google Scholar]
- Ullman A., Löfdahl C. G., Svedmyr N., Skoogh B. E. Nerve stimulation releases mucosa-derived inhibitory factors, both prostanoids and nonprostanoids, in isolated ferret trachea. Am Rev Respir Dis. 1990 Mar;141(3):748–751. doi: 10.1164/ajrccm/141.3.748. [DOI] [PubMed] [Google Scholar]


