Abstract
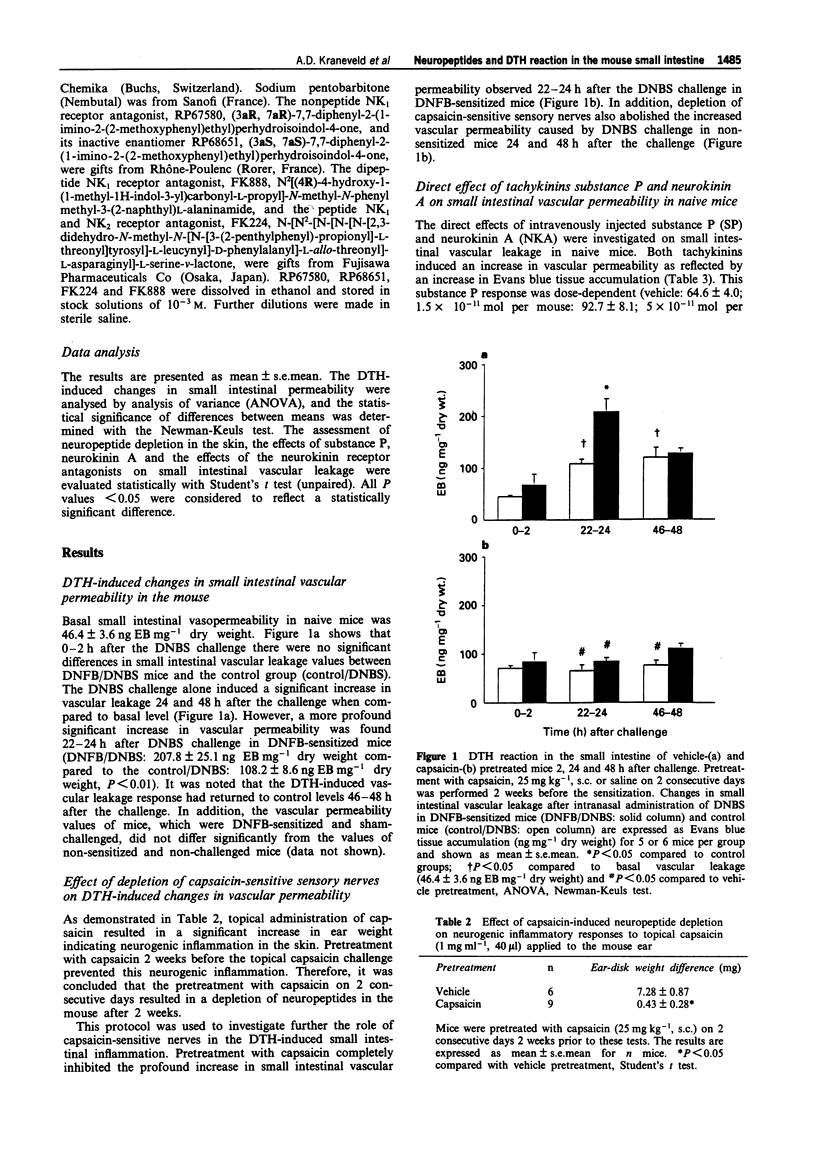
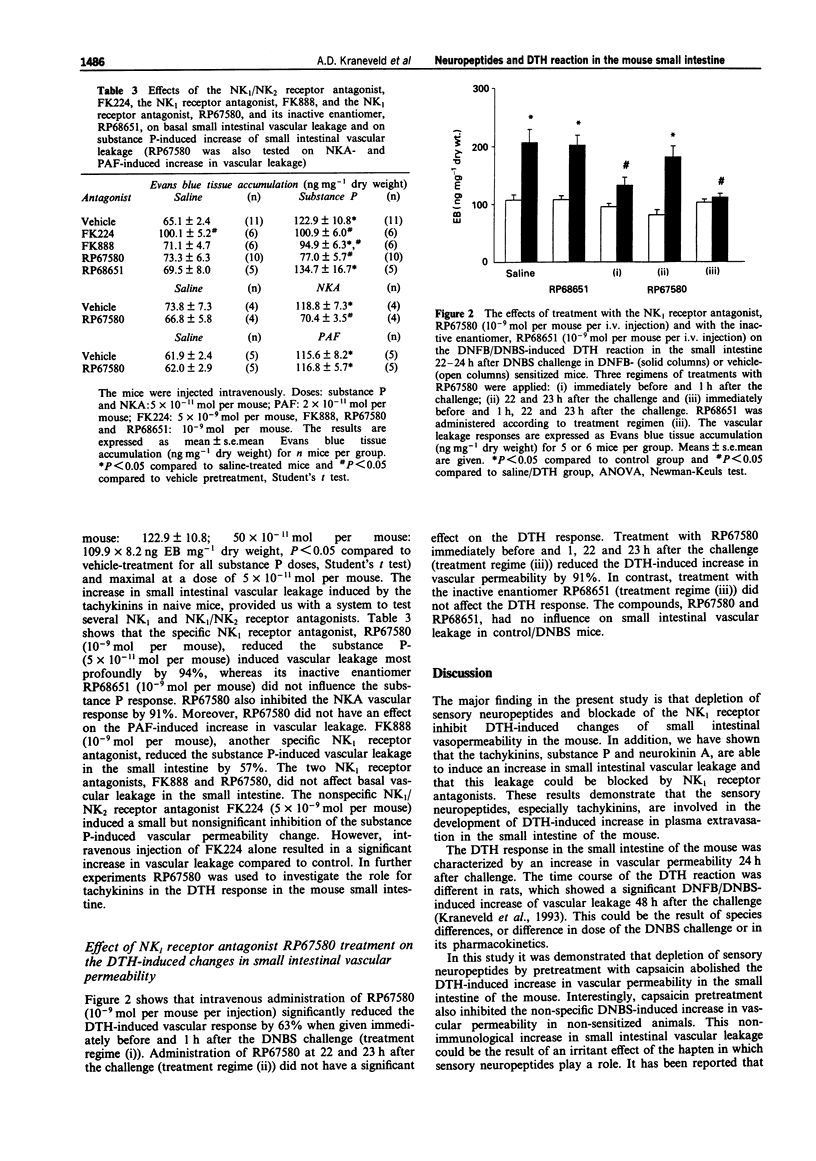
1. This study investigates the effects of capsaicin-induced depletion of sensory neuropeptides and of neurokinin1 (NK1) receptor blockade on delayed-type hypersensitivity (DTH)-induced changes of vascular permeability in the small intestine of the mouse. 2. The DTH reaction in the small intestine was elicited by dinitrofluorobenzene (DNFB)-contact sensitization followed by oral dinitrobenzene sulphonic acid (DNBS) challenge. To assess vascular leakage the accumulation of the plasma marker, Evans blue (EB), was measured 2, 24 and 48 h after the challenge. 3. The small intestinal DTH reaction was characterized by a significant increase in vascular permeability 24 h after the challenge of previously sensitized mice when compared to vehicle-sensitized mice (P < 0.05, ANOVA). Capsaicin-induced depletion of sensory neuropeptides, two weeks before the sensitization, completely inhibited the DTH-induced increase in small intestinal vascular permeability at 24 h (P < 0.05, ANOVA). Vehicle/control: 108.2 +/- 8.6 ng EB mg-1 dry weight; vehicle/DTH 207.8 +/- 25.1 ng EB mg-1 dry weight; capsaicin/control: 65.8 +/- 11.9 ng EB mg-1 dry weight; capsaicin/DTH: 84.3 +/- 7.6 ng EB mg-1 dry weight. 4. The tachykinins, substance P and neurokinin A (1.5 to 50 x 10(-11) mol per mouse, i.v.), induced an increase in vascular leakage in the small intestine of naive mice. The specific NK1 receptor antagonist, RP67580 (10(-9) mol per mouse, i.v.) was the most effective in reducing the substance P-induced plasma extravasation when compared with other NK receptor antagonists, FK224 and FK888.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arizono N., Matsuda S., Hattori T., Kojima Y., Maeda T., Galli S. J. Anatomical variation in mast cell nerve associations in the rat small intestine, heart, lung, and skin. Similarities of distances between neural processes and mast cells, eosinophils, or plasma cells in the jejunal lamina propria. Lab Invest. 1990 May;62(5):626–634. [PubMed] [Google Scholar]
- Assem E. S., Ghanem N. S., Abdullah N. A., Repke H., Foreman J. C., Hayes N. A. Substance P and Arg-Pro-Lys-Pro-NH-C12-H25-induced mediator release from different mast cell subtypes of rat and guinea-pig. Immunopharmacology. 1989 Mar-Apr;17(2):119–128. doi: 10.1016/0162-3109(89)90057-x. [DOI] [PubMed] [Google Scholar]
- Beaujouan J. C., Heuillet E., Petitet F., Saffroy M., Torrens Y., Glowinski J. Higher potency of RP 67580, in the mouse and the rat compared with other nonpeptide and peptide tachykinin NK1 antagonists. Br J Pharmacol. 1993 Mar;108(3):793–800. doi: 10.1111/j.1476-5381.1993.tb12880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck S. H., Burks T. F. The neuropharmacology of capsaicin: review of some recent observations. Pharmacol Rev. 1986 Sep;38(3):179–226. [PubMed] [Google Scholar]
- Costa M., Brookes S., Steele P., Vickers J. Chemical coding of neurons in the gastrointestinal tract. Adv Exp Med Biol. 1991;298:17–27. doi: 10.1007/978-1-4899-0744-8_2. [DOI] [PubMed] [Google Scholar]
- Crivellato E., Damiani D., Mallardi F., Travan L. Suggestive evidence for a microanatomical relationship between mast cells and nerve fibres containing substance P, calcitonin gene related peptide, vasoactive intestinal polypeptide, and somatostatin in the rat mesentery. Acta Anat (Basel) 1991;141(2):127–131. doi: 10.1159/000147111. [DOI] [PubMed] [Google Scholar]
- Crowe S. E., Soda K., Stanisz A. M., Perdue M. H. Intestinal permeability in allergic rats: nerve involvement in antigen-induced changes. Am J Physiol. 1993 Apr;264(4 Pt 1):G617–G623. doi: 10.1152/ajpgi.1993.264.4.G617. [DOI] [PubMed] [Google Scholar]
- Dvorak A. M., McLeod R. S., Onderdonk A. B., Monahan-Earley R. A., Cullen J. B., Antonioli D. A., Morgan E., Blair J. E., Estrella P., Cisneros R. L. Human gut mucosal mast cells: ultrastructural observations and anatomic variation in mast cell-nerve associations in vivo. Int Arch Allergy Immunol. 1992;98(2):158–168. doi: 10.1159/000236180. [DOI] [PubMed] [Google Scholar]
- Fais S., Pallone F., Squarcia O., Biancone L., Ricci F., Paoluzi P., Boirivant M. HLA-DR antigens on colonic epithelial cells in inflammatory bowel disease: I. Relation to the state of activation of lamina propria lymphocytes and to the epithelial expression of other surface markers. Clin Exp Immunol. 1987 Jun;68(3):605–612. [PMC free article] [PubMed] [Google Scholar]
- Gates T. S., Zimmerman R. P., Mantyh C. R., Vigna S. R., Maggio J. E., Welton M. L., Passaro E. P., Jr, Mantyh P. W. Substance P and substance K receptor binding sites in the human gastrointestinal tract: localization by autoradiography. Peptides. 1988 Nov-Dec;9(6):1207–1219. doi: 10.1016/0196-9781(88)90184-2. [DOI] [PubMed] [Google Scholar]
- Girolomoni G., Tigelaar R. E. Capsaicin-sensitive primary sensory neurons are potent modulators of murine delayed-type hypersensitivity reactions. J Immunol. 1990 Aug 15;145(4):1105–1112. [PubMed] [Google Scholar]
- Jancsó N., Jancsó-Gábor A., Szolcsányi J. Direct evidence for neurogenic inflammation and its prevention by denervation and by pretreatment with capsaicin. Br J Pharmacol Chemother. 1967 Sep;31(1):138–151. doi: 10.1111/j.1476-5381.1967.tb01984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janković B. D., Isaković K. Neuro-endocrine correlates of immune response. I. Effects of brain lesions on antibody production, Arthus reactivity and delayed hypersensitivity in the rat. Int Arch Allergy Appl Immunol. 1973;45(3):360–372. [PubMed] [Google Scholar]
- Joos G. F., Pauwels R. A. The in vivo effect of tachykinins on airway mast cells of the rat. Am Rev Respir Dis. 1993 Oct;148(4 Pt 1):922–926. doi: 10.1164/ajrccm/148.4_Pt_1.922. [DOI] [PubMed] [Google Scholar]
- Josefsson E., Carlsten H., Lange S., Tarkowski A. Peripheral denervation suppresses the late phase of delayed-type hypersensitivity. Int Arch Allergy Appl Immunol. 1991;95(1):58–63. doi: 10.1159/000235455. [DOI] [PubMed] [Google Scholar]
- Karmeli F., Eliakim R., Okon E., Rachmilewitz D. Gastric mucosal damage by ethanol is mediated by substance P and prevented by ketotifen, a mast cell stabilizer. Gastroenterology. 1991 May;100(5 Pt 1):1206–1216. [PubMed] [Google Scholar]
- Macris N. T., Schiavi R. C., Camerino M. S., Stein M. Effect of hypothalamic lesions on immune processes in the guinea pig. Am J Physiol. 1970 Nov;219(5):1205–1209. doi: 10.1152/ajplegacy.1970.219.5.1205. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Evangelista S., Abelli L., Somma V., Meli A. Capsaicin-sensitive mechanisms and experimentally induced duodenal ulcers in rats. J Pharm Pharmacol. 1987 Jul;39(7):559–561. doi: 10.1111/j.2042-7158.1987.tb03179.x. [DOI] [PubMed] [Google Scholar]
- Mantyh C. R., Gates T. S., Zimmerman R. P., Welton M. L., Passaro E. P., Jr, Vigna S. R., Maggio J. E., Kruger L., Mantyh P. W. Receptor binding sites for substance P, but not substance K or neuromedin K, are expressed in high concentrations by arterioles, venules, and lymph nodules in surgical specimens obtained from patients with ulcerative colitis and Crohn disease. Proc Natl Acad Sci U S A. 1988 May;85(9):3235–3239. doi: 10.1073/pnas.85.9.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marić D., Janković B. D. Enkephalins and immunity. II: In vivo modulation of cell-mediated immunity. Ann N Y Acad Sci. 1987;496:126–136. doi: 10.1111/j.1749-6632.1987.tb35755.x. [DOI] [PubMed] [Google Scholar]
- Matsuda H., Kawakita K., Kiso Y., Nakano T., Kitamura Y. Substance P induces granulocyte infiltration through degranulation of mast cells. J Immunol. 1989 Feb 1;142(3):927–931. [PubMed] [Google Scholar]
- Ohkubo H., Nakanishi S. Molecular characterization of the three tachykinin receptors. Ann N Y Acad Sci. 1991;632:53–62. doi: 10.1111/j.1749-6632.1991.tb33094.x. [DOI] [PubMed] [Google Scholar]
- Rachmilewitz D., Simon P. L., Schwartz L. W., Griswold D. E., Fondacaro J. D., Wasserman M. A. Inflammatory mediators of experimental colitis in rats. Gastroenterology. 1989 Aug;97(2):326–337. doi: 10.1016/0016-5085(89)90068-1. [DOI] [PubMed] [Google Scholar]
- Rogers D. F., Boschetto P., Barnes P. J. Plasma exudation. Correlation between Evans blue dye and radiolabeled albumin in guinea pig airways in vivo. J Pharmacol Methods. 1989 Jul;21(4):309–315. doi: 10.1016/0160-5402(89)90068-5. [DOI] [PubMed] [Google Scholar]
- Schreiber S., MacDermott R. P., Raedler A., Pinnau R., Bertovich M. J., Nash G. S. Increased activation of isolated intestinal lamina propria mononuclear cells in inflammatory bowel disease. Gastroenterology. 1991 Oct;101(4):1020–1030. doi: 10.1016/0016-5085(91)90729-5. [DOI] [PubMed] [Google Scholar]
- Scicchitano R., Biennenstock J., Stanisz A. M. In vivo immunomodulation by the neuropeptide substance P. Immunology. 1988 Apr;63(4):733–735. [PMC free article] [PubMed] [Google Scholar]
- Shanahan F., Anton P. Neuroendocrine modulation of the immune system. Possible implications for inflammatory bowel disease. Dig Dis Sci. 1988 Mar;33(3 Suppl):41S–49S. doi: 10.1007/BF01538130. [DOI] [PubMed] [Google Scholar]
- Shanahan F., Denburg J. A., Fox J., Bienenstock J., Befus D. Mast cell heterogeneity: effects of neuroenteric peptides on histamine release. J Immunol. 1985 Aug;135(2):1331–1337. [PubMed] [Google Scholar]
- Skofitsch G., Savitt J. M., Jacobowitz D. M. Suggestive evidence for a functional unit between mast cells and substance P fibers in the rat diaphragm and mesentery. Histochemistry. 1985;82(1):5–8. doi: 10.1007/BF00502084. [DOI] [PubMed] [Google Scholar]
- Stanisz A. M., Befus D., Bienenstock J. Differential effects of vasoactive intestinal peptide, substance P, and somatostatin on immunoglobulin synthesis and proliferations by lymphocytes from Peyer's patches, mesenteric lymph nodes, and spleen. J Immunol. 1986 Jan;136(1):152–156. [PubMed] [Google Scholar]
- Stead R. H., Bienenstock J., Stanisz A. M. Neuropeptide regulation of mucosal immunity. Immunol Rev. 1987 Dec;100:333–359. doi: 10.1111/j.1600-065x.1987.tb00538.x. [DOI] [PubMed] [Google Scholar]
- Stead R. H., Dixon M. F., Bramwell N. H., Riddell R. H., Bienenstock J. Mast cells are closely apposed to nerves in the human gastrointestinal mucosa. Gastroenterology. 1989 Sep;97(3):575–585. doi: 10.1016/0016-5085(89)90627-6. [DOI] [PubMed] [Google Scholar]
- Stead R. H., Tomioka M., Quinonez G., Simon G. T., Felten S. Y., Bienenstock J. Intestinal mucosal mast cells in normal and nematode-infected rat intestines are in intimate contact with peptidergic nerves. Proc Natl Acad Sci U S A. 1987 May;84(9):2975–2979. doi: 10.1073/pnas.84.9.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain M. G., Agro A., Blennerhassett P., Stanisz A., Collins S. M. Increased levels of substance P in the myenteric plexus of Trichinella-infected rats. Gastroenterology. 1992 Jun;102(6):1913–1919. doi: 10.1016/0016-5085(92)90313-n. [DOI] [PubMed] [Google Scholar]
- Udaka K., Takeuchi Y., Movat H. Z. Simple method for quantitation of enhanced vascular permeability. Proc Soc Exp Biol Med. 1970 Apr;133(4):1384–1387. doi: 10.3181/00379727-133-34695. [DOI] [PubMed] [Google Scholar]
- Umeda Y., Takamiya M., Yoshizaki H., Arisawa M. Inhibition of mitogen-stimulated T lymphocyte proliferation by calcitonin gene-related peptide. Biochem Biophys Res Commun. 1988 Jul 15;154(1):227–235. doi: 10.1016/0006-291x(88)90674-2. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Marshall S., Specian R. D., Grisham M. B. A comparative analysis of two models of colitis in rats. Gastroenterology. 1992 May;102(5):1524–1534. doi: 10.1016/0016-5085(92)91710-l. [DOI] [PubMed] [Google Scholar]
- Yano H., Wershil B. K., Arizono N., Galli S. J. Substance P-induced augmentation of cutaneous vascular permeability and granulocyte infiltration in mice is mast cell dependent. J Clin Invest. 1989 Oct;84(4):1276–1286. doi: 10.1172/JCI114295. [DOI] [PMC free article] [PubMed] [Google Scholar]


