Abstract
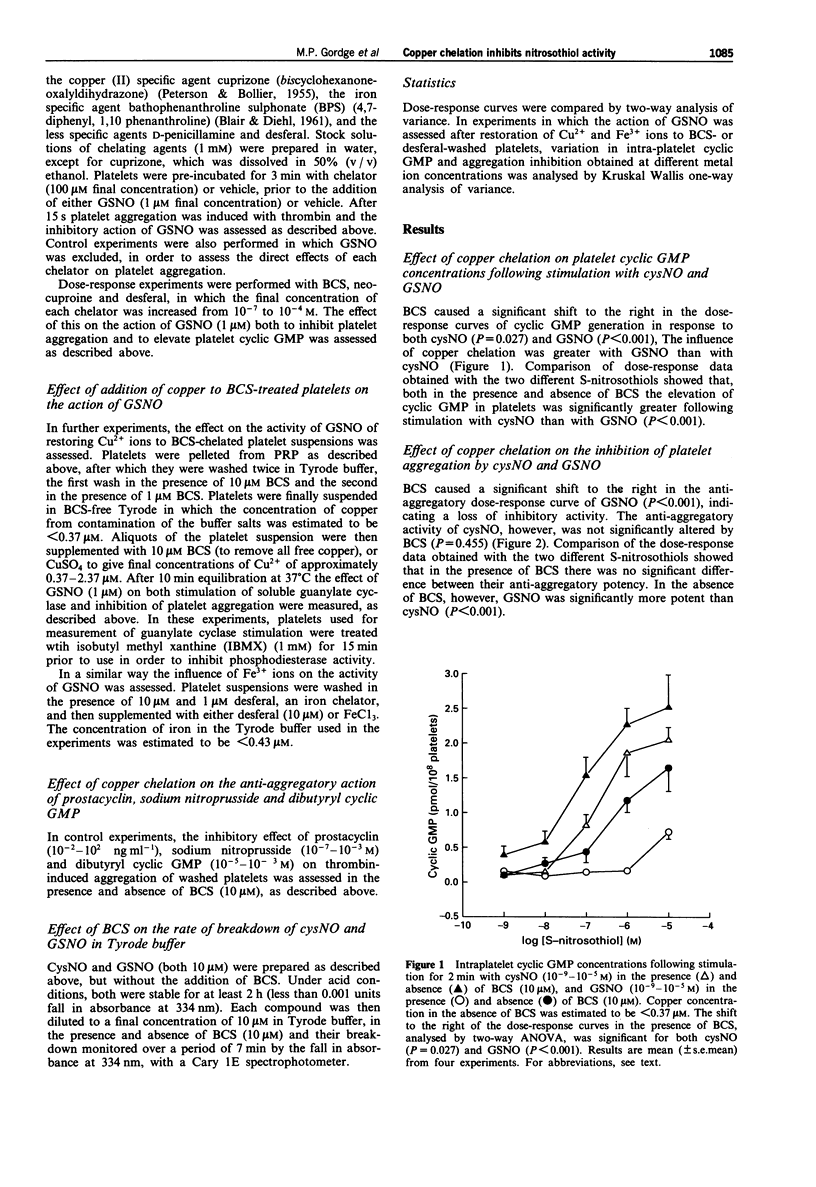
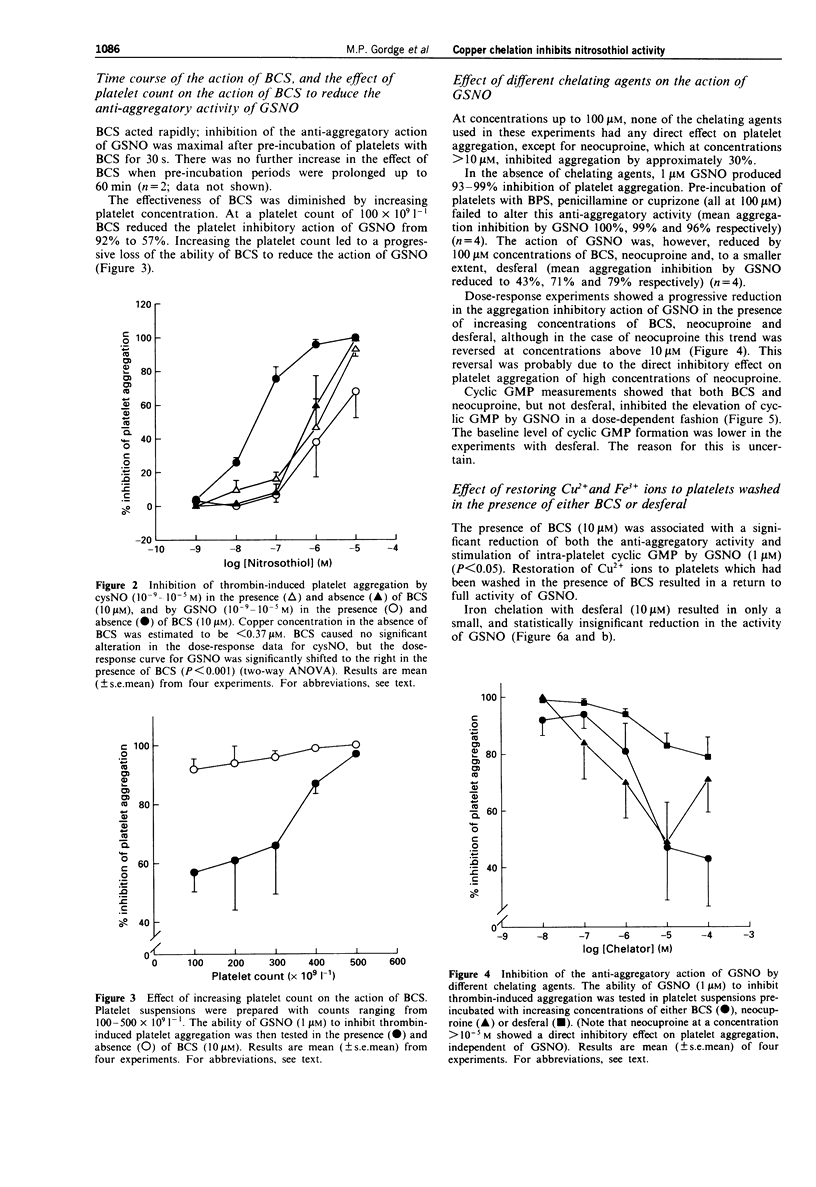
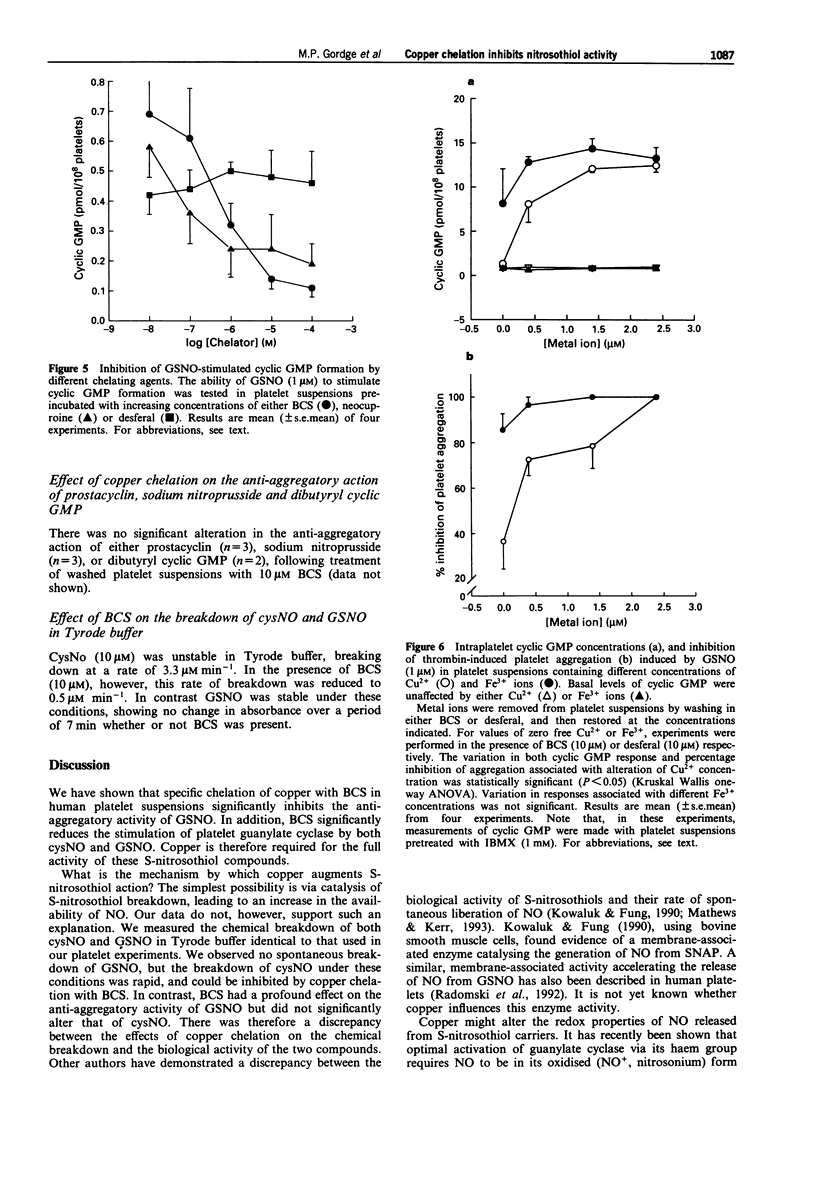
1. The effect of copper on the activity of the S-nitrosothiol compounds S-nitrosocysteine (cysNO) and S-nitrosoglutathione (GSNO) was investigated, using the specific copper chelator bathocuproine sulphonate (BCS), and human washed platelets as target cells. 2. Chelation of trace copper with BCS (10 microM) in washed platelet suspensions reduced the inhibition of thrombin-induced platelet aggregation by GSNO; however, BCS had no significant effect on the anti-aggregatory action of cysNO. BCS inhibited cyclic GMP generation in response to both cysNO and GSNO. 3. The effect of BCS was rapid (within 30 s), and could be abolished by increasing the platelet concentration to 500 x 10(9) l-1. 4. In BCS-treated platelet suspensions, the addition of Cu2+ ions (0.37-2.37 microM) led to a restoration of both guanylate cyclase activation and platelet aggregation inhibition by GSNO. 5. The anti-aggregatory activity of GSNO was reduced in a concentration-dependent manner by the copper (I)-specific chelators BCS and neocuproine, and to a smaller extent by desferal. No effect was observed with the copper (II) specific chelator, cuprizone, the iron-specific chelator, bathophenanthroline sulphonate, or the broader-specificity copper chelator, D-penicillamine. 6. In both BCS-treated and -untreated platelet suspensions, cys NO was more potent than GSNO as a stimulator of guanylate cyclase. In BCS-treated platelet suspensions there was no significant difference between the anti-aggregatory potency of cysNO and GSNO; however, in untreated suspensions, GSNO was significantly more potent than cysNO. Thus, when copper was available, GSNO produced a greater inhibition of aggregation than cysNO, despite being a less potent activator of guanylate cyclase.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Feelisch M., te Poel M., Zamora R., Deussen A., Moncada S. Understanding the controversy over the identity of EDRF. Nature. 1994 Mar 3;368(6466):62–65. doi: 10.1038/368062a0. [DOI] [PubMed] [Google Scholar]
- Gaston B., Reilly J., Drazen J. M., Fackler J., Ramdev P., Arnelle D., Mullins M. E., Sugarbaker D. J., Chee C., Singel D. J. Endogenous nitrogen oxides and bronchodilator S-nitrosothiols in human airways. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):10957–10961. doi: 10.1073/pnas.90.23.10957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerzer R., Böhme E., Hofmann F., Schultz G. Soluble guanylate cyclase purified from bovine lung contains heme and copper. FEBS Lett. 1981 Sep 14;132(1):71–74. doi: 10.1016/0014-5793(81)80429-2. [DOI] [PubMed] [Google Scholar]
- Gordge M. P., Neild G. H. Platelets from patients on haemodialysis show impaired responses to nitric oxide. Clin Sci (Lond) 1992 Sep;83(3):313–318. doi: 10.1042/cs0830313. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Protection against tissue damage in vivo by desferrioxamine: what is its mechanism of action? Free Radic Biol Med. 1989;7(6):645–651. doi: 10.1016/0891-5849(89)90145-7. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J. Biosynthesis and metabolism of endothelium-derived nitric oxide. Annu Rev Pharmacol Toxicol. 1990;30:535–560. doi: 10.1146/annurev.pa.30.040190.002535. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Lippton H., Edwards J. C., Baricos W. H., Hyman A. L., Kadowitz P. J., Gruetter C. A. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther. 1981 Sep;218(3):739–749. [PubMed] [Google Scholar]
- Jansen A., Drazen J., Osborne J. A., Brown R., Loscalzo J., Stamler J. S. The relaxant properties in guinea pig airways of S-nitrosothiols. J Pharmacol Exp Ther. 1992 Apr;261(1):154–160. [PubMed] [Google Scholar]
- Kowaluk E. A., Fung H. L. Spontaneous liberation of nitric oxide cannot account for in vitro vascular relaxation by S-nitrosothiols. J Pharmacol Exp Ther. 1990 Dec;255(3):1256–1264. [PubMed] [Google Scholar]
- Landgraf W., Regulla S., Meyer H. E., Hofmann F. Oxidation of cysteines activates cGMP-dependent protein kinase. J Biol Chem. 1991 Sep 5;266(25):16305–16311. [PubMed] [Google Scholar]
- Laurie S. H., Prime D. M. The formation and nature of the mixed valence copper-D-penicillamine-chloride cluster in aqueous solution and its relevance to the treatment of Wilson's disease. J Inorg Biochem. 1979 Nov;11(3):229–239. doi: 10.1016/s0162-0134(00)80020-3. [DOI] [PubMed] [Google Scholar]
- Lieberman E. H., O'Neill S., Mendelsohn M. E. S-nitrosocysteine inhibition of human platelet secretion is correlated with increases in platelet cGMP levels. Circ Res. 1991 Jun;68(6):1722–1728. doi: 10.1161/01.res.68.6.1722. [DOI] [PubMed] [Google Scholar]
- Mathews W. R., Kerr S. W. Biological activity of S-nitrosothiols: the role of nitric oxide. J Pharmacol Exp Ther. 1993 Dec;267(3):1529–1537. [PubMed] [Google Scholar]
- McArdle H. J., Gross S. M., Creaser I., Sargeson A. M., Danks D. M. Effect of chelators on copper metabolism and copper pools in mouse hepatocytes. Am J Physiol. 1989 Apr;256(4 Pt 1):G667–G672. doi: 10.1152/ajpgi.1989.256.4.G667. [DOI] [PubMed] [Google Scholar]
- McDonald B., Reep B., Lapetina E. G., Molina y Vedia L. Glyceraldehyde-3-phosphate dehydrogenase is required for the transport of nitric oxide in platelets. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11122–11126. doi: 10.1073/pnas.90.23.11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellion B. T., Ignarro L. J., Myers C. B., Ohlstein E. H., Ballot B. A., Hyman A. L., Kadowitz P. J. Inhibition of human platelet aggregation by S-nitrosothiols. Heme-dependent activation of soluble guanylate cyclase and stimulation of cyclic GMP accumulation. Mol Pharmacol. 1983 May;23(3):653–664. [PubMed] [Google Scholar]
- Mendelsohn M. E., O'Neill S., George D., Loscalzo J. Inhibition of fibrinogen binding to human platelets by S-nitroso-N-acetylcysteine. J Biol Chem. 1990 Nov 5;265(31):19028–19034. [PubMed] [Google Scholar]
- POILLON W. N., DAWSON C. R. ON THE NATURE OF COPPER IN ASCORBATE OXIDASE. I. THE VALENCE STATE OF COPPER IN THE DENATURED AND NATIVE ENZYME. Biochim Biophys Acta. 1963 Sep 3;77:27–36. doi: 10.1016/0006-3002(63)90466-9. [DOI] [PubMed] [Google Scholar]
- Park J. W. Reaction of S-nitrosoglutathione with sulfhydryl groups in protein. Biochem Biophys Res Commun. 1988 Apr 29;152(2):916–920. doi: 10.1016/s0006-291x(88)80127-x. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Rees D. D., Dutra A., Moncada S. S-nitroso-glutathione inhibits platelet activation in vitro and in vivo. Br J Pharmacol. 1992 Nov;107(3):745–749. doi: 10.1111/j.1476-5381.1992.tb14517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuschke D. A., Reed M. W., Saari J. T., Miller F. N. Copper deficiency alters vasodilation in the rat cremaster muscle microcirculation. J Nutr. 1992 Jul;122(7):1547–1552. doi: 10.1093/jn/122.7.1547. [DOI] [PubMed] [Google Scholar]
- Severina I. S., Bussygina O. G., Grigorjev N. B. Effect of nitroso complexes of some transition metals on the activity of soluble guanylate cyclase. Biochem Int. 1992 Mar;26(4):695–705. [PubMed] [Google Scholar]
- Simon D. I., Stamler J. S., Jaraki O., Keaney J. F., Osborne J. A., Francis S. A., Singel D. J., Loscalzo J. Antiplatelet properties of protein S-nitrosothiols derived from nitric oxide and endothelium-derived relaxing factor. Arterioscler Thromb. 1993 Jun;13(6):791–799. doi: 10.1161/01.atv.13.6.791. [DOI] [PubMed] [Google Scholar]
- Stamler J. S., Jaraki O., Osborne J., Simon D. I., Keaney J., Vita J., Singel D., Valeri C. R., Loscalzo J. Nitric oxide circulates in mammalian plasma primarily as an S-nitroso adduct of serum albumin. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7674–7677. doi: 10.1073/pnas.89.16.7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamler J. S., Singel D. J., Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992 Dec 18;258(5090):1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- White A. A., Crawford K. M., Patt C. S., Lad P. J. Activation of soluble guanylate cyclase from rat lung by incubation or by hydrogen peroxide. J Biol Chem. 1976 Dec 10;251(23):7304–7312. [PubMed] [Google Scholar]


