Abstract
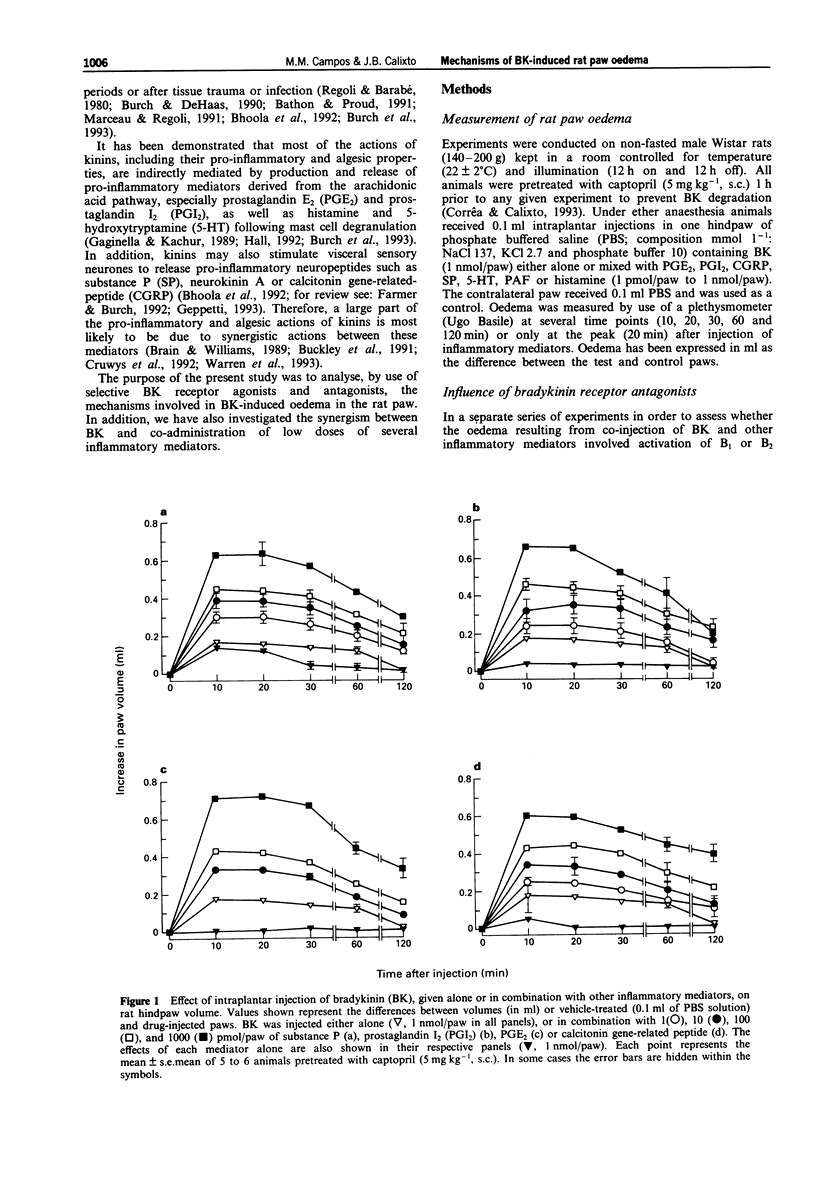
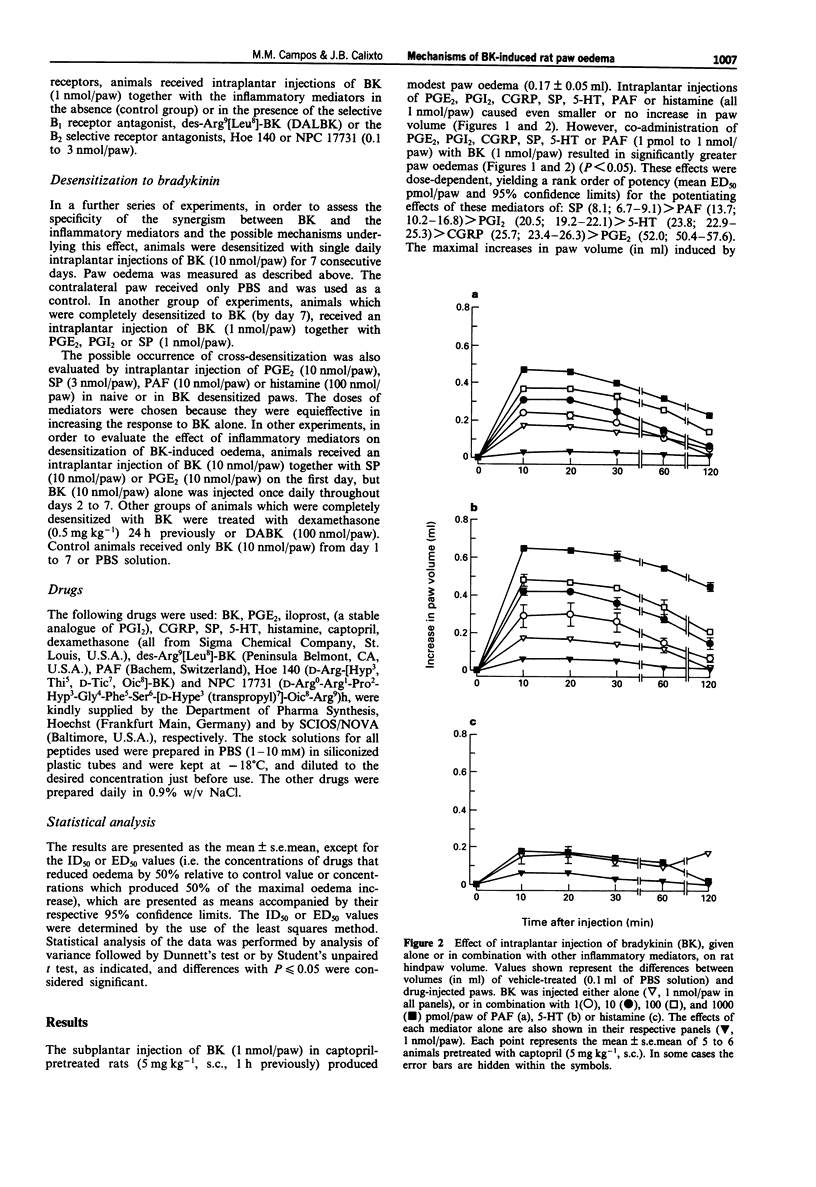
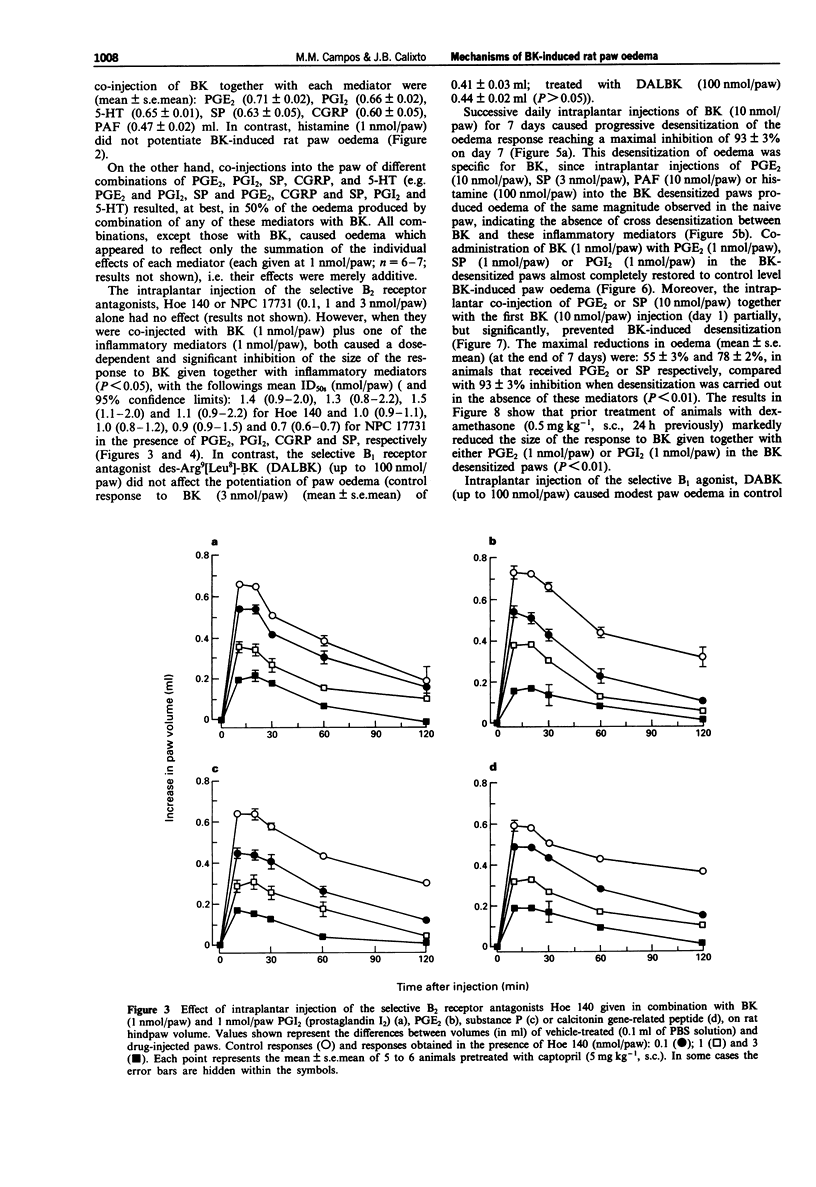
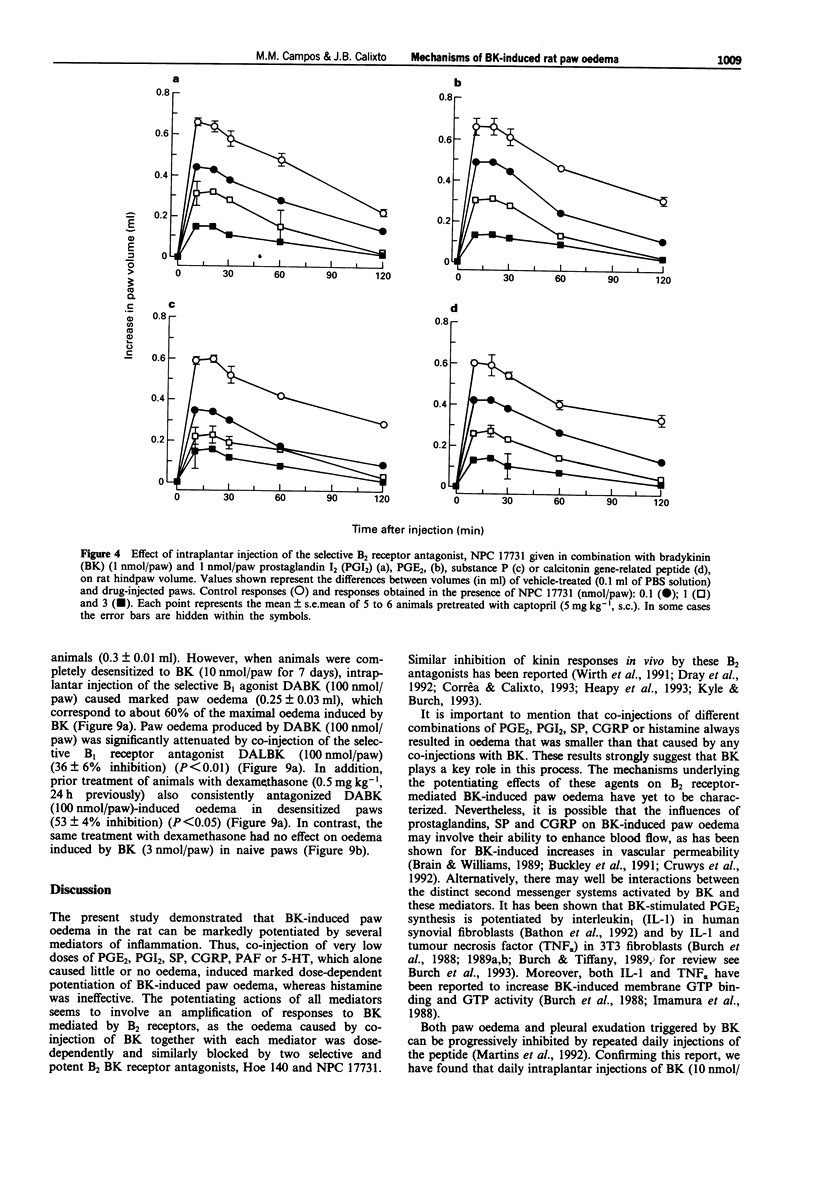
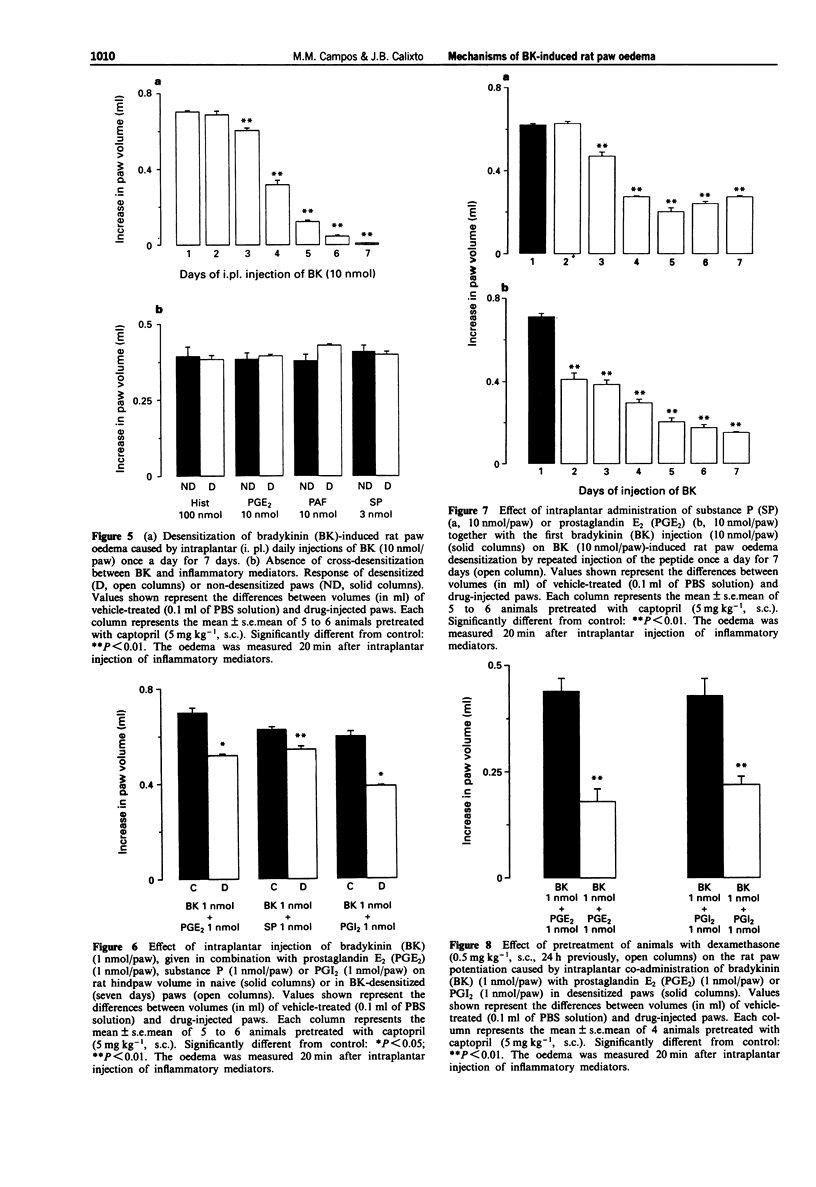
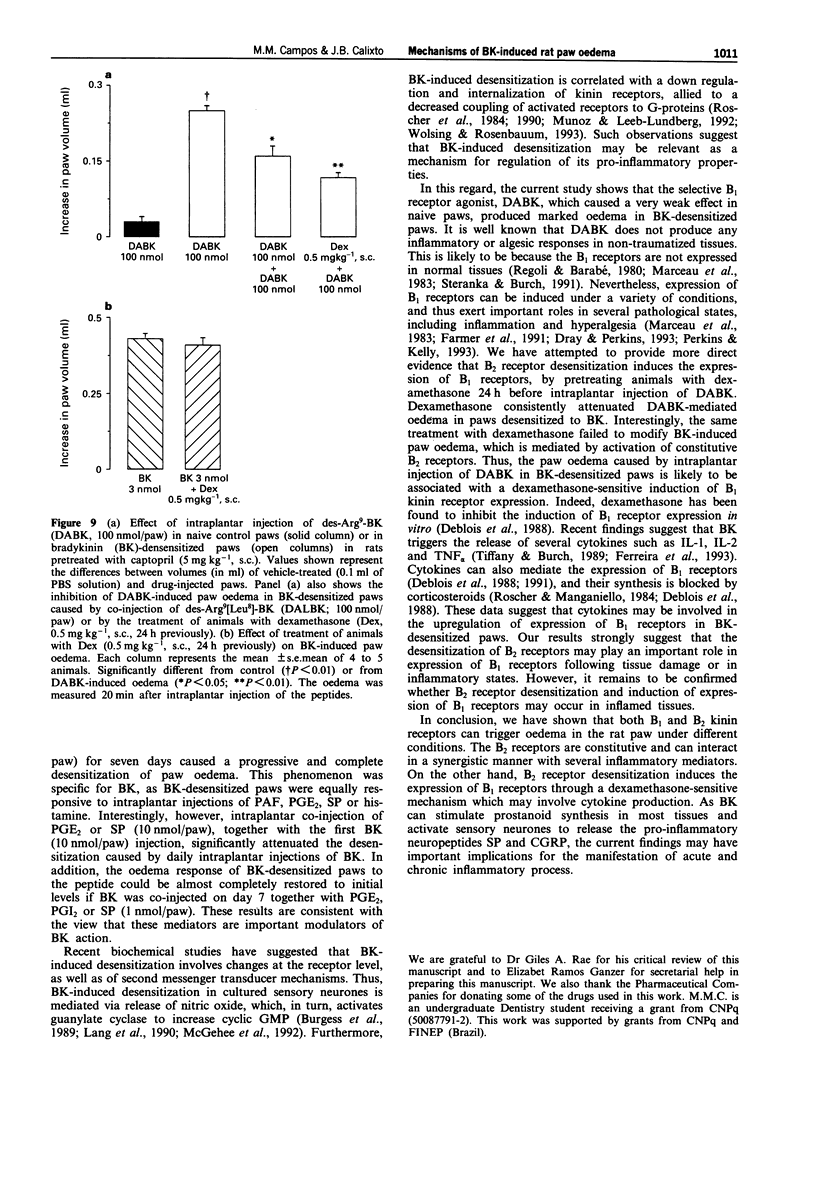
1. The mechanisms involved in bradykinin (BK)-induced oedema in the rat paw as well as the interactions between BK and several inflammatory mediators, have been investigated. 2. Intraplantar injection of BK (1 nmol/paw) in rats pretreated with captopril (5 mg kg-1, s.c.) caused a small amount of oedema formation (0.17 +/- 0.05 ml). Des-Arg9-BK (DABK, a selective B1 receptor agonist) up to 300 nmol/paw caused minimal oedema (0.03 +/- 0.01 ml). 3. Co-administration of prostaglandin E2 (PGE2), prostaglandin I2 (PGI2), calcitonin gene-related peptide (CGRP), 5-hydroxytryptamine (5-HT), substance P (SP) or platelet activating factor (PAF) (1 pmol-1 nmol/paw) with BK (1 nmol/paw) dose-dependently potentiated BK-induced paw oedema. The rank order of potency (mean ED50, pmol/paw) for this effect was: SP (8.1) > PAF (13.7) > PGI2 (20.5) > 5-HT (23.8) > CGRP (25.7) > PGE2 (52.0). Co-administration of BK with the various inflammatory mediators resulted in maximal paw oedemas (ml) of: PGE2 (0.71 +/- 0.02); PGI2 (0.66 +/- 0.02); 5-HT (0.65 +/- 0.01); SP (0.63 +/- 0.05); CGRP (0.60 +/- 0.05) and PAF (0.47 +/- 0.02) ml. Histamine (up to 1 nmol/paw) was ineffective in potentiating the response to BK. 4. Hoe 140 or NPC 17731 (two selective B2 receptor antagonists, 0.1-3 nmol/paw) produced dose-dependent inhibition of paw oedema potentiation induced by co-injection of BK with other mediators with the following mean ID50s (nmol/paw): Hoe 140-1.4; 1.3; 1.5 and 1.1 and NPC 17731-1.0; 1.0; 0.9 and 0.7; in the presence of PGE2, PGI2, CGRP and SP, respectively.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bathon J. M., Manning D. C., Goldman D. W., Towns M. C., Proud D. Characterization of kinin receptors on human synovial cells and upregulation of receptor number by interleukin-1. J Pharmacol Exp Ther. 1992 Jan;260(1):384–392. [PubMed] [Google Scholar]
- Bathon J. M., Proud D. Bradykinin antagonists. Annu Rev Pharmacol Toxicol. 1991;31:129–162. doi: 10.1146/annurev.pa.31.040191.001021. [DOI] [PubMed] [Google Scholar]
- Bhoola K. D., Figueroa C. D., Worthy K. Bioregulation of kinins: kallikreins, kininogens, and kininases. Pharmacol Rev. 1992 Mar;44(1):1–80. [PubMed] [Google Scholar]
- Brain S. D., Williams T. J. Interactions between the tachykinins and calcitonin gene-related peptide lead to the modulation of oedema formation and blood flow in rat skin. Br J Pharmacol. 1989 May;97(1):77–82. doi: 10.1111/j.1476-5381.1989.tb11926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley T. L., Brain S. D., Rampart M., Williams T. J. Time-dependent synergistic interactions between the vasodilator neuropeptide, calcitonin gene-related peptide (CGRP) and mediators of inflammation. Br J Pharmacol. 1991 Jun;103(2):1515–1519. doi: 10.1111/j.1476-5381.1991.tb09819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch R. M., Connor J. R., Axelrod J. Interleukin 1 amplifies receptor-mediated activation of phospholipase A2 in 3T3 fibroblasts. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6306–6309. doi: 10.1073/pnas.85.17.6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch R. M., Connor J. R., Tiffany C. W. The kallikrein-kininogen-kinin system in chronic inflammation. Agents Actions. 1989 Jun;27(3-4):258–260. doi: 10.1007/BF01972790. [DOI] [PubMed] [Google Scholar]
- Burch R. M., DeHaas C. A bradykinin antagonist inhibits carrageenan edema in rats. Naunyn Schmiedebergs Arch Pharmacol. 1990 Aug;342(2):189–193. doi: 10.1007/BF00166963. [DOI] [PubMed] [Google Scholar]
- Burch R. M., Tiffany C. W. Tumor necrosis factor causes amplification of arachidonic acid metabolism in response to interleukin 1, bradykinin, and other agonists. J Cell Physiol. 1989 Oct;141(1):85–89. doi: 10.1002/jcp.1041410113. [DOI] [PubMed] [Google Scholar]
- Burch R. M., White M. F., Connor J. R. Interleukin 1 stimulates prostaglandin synthesis and cyclic AMP accumulation in Swiss 3T3 fibroblasts: interactions between two second messenger systems. J Cell Physiol. 1989 Apr;139(1):29–33. doi: 10.1002/jcp.1041390106. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., Mullaney I., McNeill M., Coote P. R., Minhas A., Wood J. N. Activation of guanylate cyclase by bradykinin in rat sensory neurones is mediated by calcium influx: possible role of the increase in cyclic GMP. J Neurochem. 1989 Oct;53(4):1212–1218. doi: 10.1111/j.1471-4159.1989.tb07417.x. [DOI] [PubMed] [Google Scholar]
- Corrêa C. R., Calixto J. B. Evidence for participation of B1 and B2 kinin receptors in formalin-induced nociceptive response in the mouse. Br J Pharmacol. 1993 Sep;110(1):193–198. doi: 10.1111/j.1476-5381.1993.tb13791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruwys S. C., Kidd B. L., Mapp P. I., Walsh D. A., Blake D. R. The effects of calcitonin gene-related peptide on formation of intra-articular oedema by inflammatory mediators. Br J Pharmacol. 1992 Sep;107(1):116–119. doi: 10.1111/j.1476-5381.1992.tb14472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damas J., Bourdon V., Remacle-Volon G., Adam A. Kinins and peritoneal exudates induced by carrageenin and zymosan in rats. Br J Pharmacol. 1990 Oct;101(2):418–422. doi: 10.1111/j.1476-5381.1990.tb12724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deblois D., Bouthillier J., Marceau F. Effect of glucocorticoids, monokines and growth factors on the spontaneously developing responses of the rabbit isolated aorta to des-Arg9-bradykinin. Br J Pharmacol. 1988 Apr;93(4):969–977. doi: 10.1111/j.1476-5381.1988.tb11487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dray A., Patel I. A., Perkins M. N., Rueff A. Bradykinin-induced activation of nociceptors: receptor and mechanistic studies on the neonatal rat spinal cord-tail preparation in vitro. Br J Pharmacol. 1992 Dec;107(4):1129–1134. doi: 10.1111/j.1476-5381.1992.tb13418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dray A., Perkins M. Bradykinin and inflammatory pain. Trends Neurosci. 1993 Mar;16(3):99–104. doi: 10.1016/0166-2236(93)90133-7. [DOI] [PubMed] [Google Scholar]
- Farmer S. G., Burch R. M. Biochemical and molecular pharmacology of kinin receptors. Annu Rev Pharmacol Toxicol. 1992;32:511–536. doi: 10.1146/annurev.pa.32.040192.002455. [DOI] [PubMed] [Google Scholar]
- Farmer S. G., McMillan B. A., Meeker S. N., Burch R. M. Induction of vascular smooth muscle bradykinin B1 receptors in vivo during antigen arthritis. Agents Actions. 1991 Sep;34(1-2):191–193. doi: 10.1007/BF01993275. [DOI] [PubMed] [Google Scholar]
- Ferreira S. H., Lorenzetti B. B., Poole S. Bradykinin initiates cytokine-mediated inflammatory hyperalgesia. Br J Pharmacol. 1993 Nov;110(3):1227–1231. doi: 10.1111/j.1476-5381.1993.tb13946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppetti P. Sensory neuropeptide release by bradykinin: mechanisms and pathophysiological implications. Regul Pept. 1993 Aug 13;47(1):1–23. doi: 10.1016/0167-0115(93)90268-d. [DOI] [PubMed] [Google Scholar]
- Hall J. M. Bradykinin receptors: pharmacological properties and biological roles. Pharmacol Ther. 1992 Nov;56(2):131–190. doi: 10.1016/0163-7258(92)90016-s. [DOI] [PubMed] [Google Scholar]
- Hargreaves K. M., Troullos E. S., Dionne R. A., Schmidt E. A., Schafer S. C., Joris J. L. Bradykinin is increased during acute and chronic inflammation: therapeutic implications. Clin Pharmacol Ther. 1988 Dec;44(6):613–621. doi: 10.1038/clpt.1988.202. [DOI] [PubMed] [Google Scholar]
- Heapy C. G., Shaw J. S., Farmer S. C. Differential sensitivity of antinociceptive assays to the bradykinin antagonist Hoe 140. Br J Pharmacol. 1993 Jan;108(1):209–213. doi: 10.1111/j.1476-5381.1993.tb13464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura K., Sherman M. L., Spriggs D., Kufe D. Effect of tumor necrosis factor on GTP binding and GTPase activity in HL-60 and L929 cells. J Biol Chem. 1988 Jul 25;263(21):10247–10253. [PubMed] [Google Scholar]
- Lang E., Novak A., Reeh P. W., Handwerker H. O. Chemosensitivity of fine afferents from rat skin in vitro. J Neurophysiol. 1990 Apr;63(4):887–901. doi: 10.1152/jn.1990.63.4.887. [DOI] [PubMed] [Google Scholar]
- Marceau F., Lussier A., Regoli D., Giroud J. P. Pharmacology of kinins: their relevance to tissue injury and inflammation. Gen Pharmacol. 1983;14(2):209–229. doi: 10.1016/0306-3623(83)90001-0. [DOI] [PubMed] [Google Scholar]
- Martins M. A., Pasquale C. P., Bozza P. T., Silva P. M., Faria Neto H. C., COrdeiro R. S. Homologous tachyphylaxis to bradykinin and its interference with allergic pleurisy in actively sensitized rats. Eur J Pharmacol. 1992 Sep 10;220(1):55–61. doi: 10.1016/0014-2999(92)90011-r. [DOI] [PubMed] [Google Scholar]
- McGehee D. S., Goy M. F., Oxford G. S. Involvement of the nitric oxide-cyclic GMP pathway in the desensitization of bradykinin responses of cultured rat sensory neurons. Neuron. 1992 Aug;9(2):315–324. doi: 10.1016/0896-6273(92)90170-i. [DOI] [PubMed] [Google Scholar]
- Munoz C. M., Leeb-Lundberg L. M. Receptor-mediated internalization of bradykinin. DDT1 MF-2 smooth muscle cells process internalized bradykinin via multiple degradative pathways. J Biol Chem. 1992 Jan 5;267(1):303–309. [PubMed] [Google Scholar]
- Perkins M. N., Kelly D. Induction of bradykinin B1 receptors in vivo in a model of ultra-violet irradiation-induced thermal hyperalgesia in the rat. Br J Pharmacol. 1993 Dec;110(4):1441–1444. doi: 10.1111/j.1476-5381.1993.tb13982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proud D., Kaplan A. P. Kinin formation: mechanisms and role in inflammatory disorders. Annu Rev Immunol. 1988;6:49–83. doi: 10.1146/annurev.iy.06.040188.000405. [DOI] [PubMed] [Google Scholar]
- Regoli D., Barabé J. Pharmacology of bradykinin and related kinins. Pharmacol Rev. 1980 Mar;32(1):1–46. [PubMed] [Google Scholar]
- Roscher A. A., Klier C., Dengler R., Faussner A., Müller-Esterl W. Regulation of bradykinin action at the receptor level. J Cardiovasc Pharmacol. 1990;15 (Suppl 6):S39–S43. [PubMed] [Google Scholar]
- Roscher A. A., Manganiello V. C., Jelsema C. L., Moss J. Autoregulation of bradykinin receptors and bradykinin-induced prostacyclin formation in human fibroblasts. J Clin Invest. 1984 Aug;74(2):552–558. doi: 10.1172/JCI111452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren J. B., Wilson A. J., Loi R. K., Coughlan M. L. Opposing roles of cyclic AMP in the vascular control of edema formation. FASEB J. 1993 Nov;7(14):1394–1400. doi: 10.1096/fasebj.7.14.7693536. [DOI] [PubMed] [Google Scholar]
- Wirth K., Hock F. J., Albus U., Linz W., Alpermann H. G., Anagnostopoulos H., Henk S., Breipohl G., König W., Knolle J. Hoe 140 a new potent and long acting bradykinin-antagonist: in vivo studies. Br J Pharmacol. 1991 Mar;102(3):774–777. doi: 10.1111/j.1476-5381.1991.tb12249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolsing D. H., Rosenbaum J. S. The mechanism for the rapid desensitization in bradykinin-stimulated inositol monophosphate production in NG108-15 cells involves interaction of a single receptor with multiple signaling pathways. J Pharmacol Exp Ther. 1993 Jul;266(1):253–261. [PubMed] [Google Scholar]


