Abstract
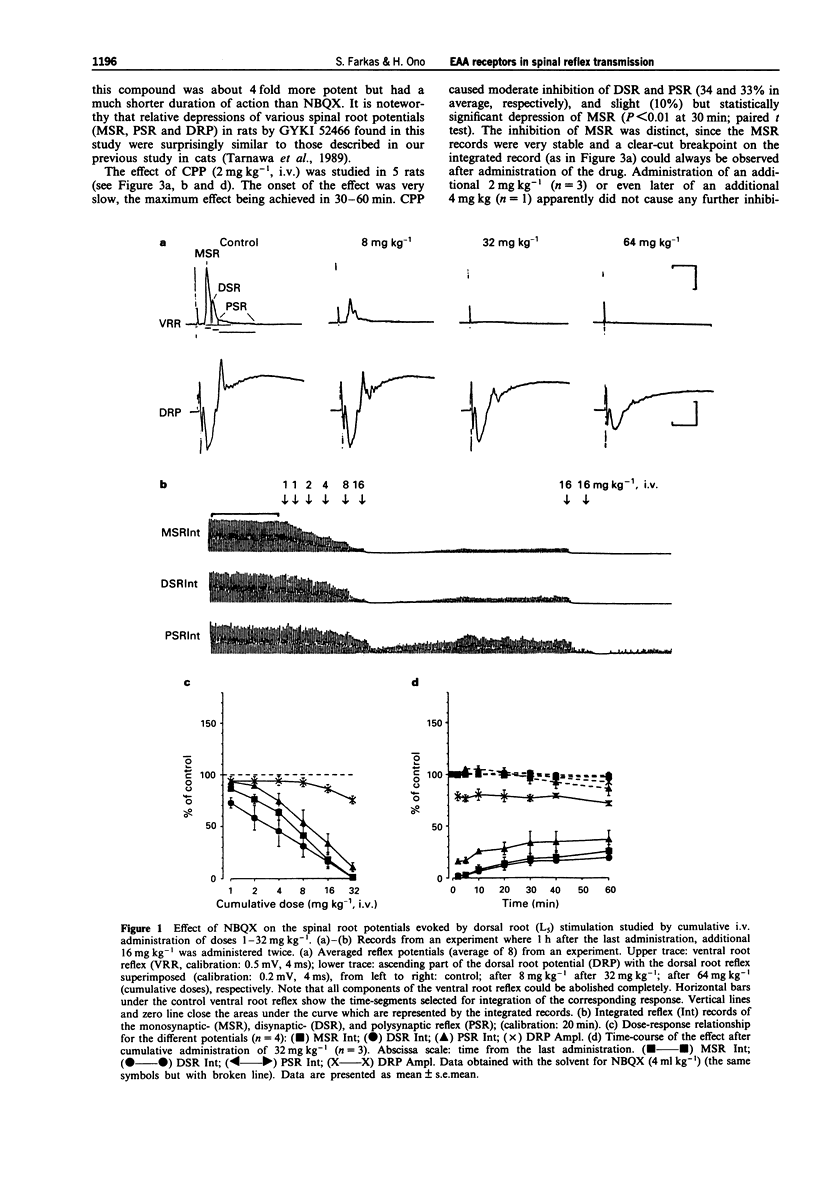
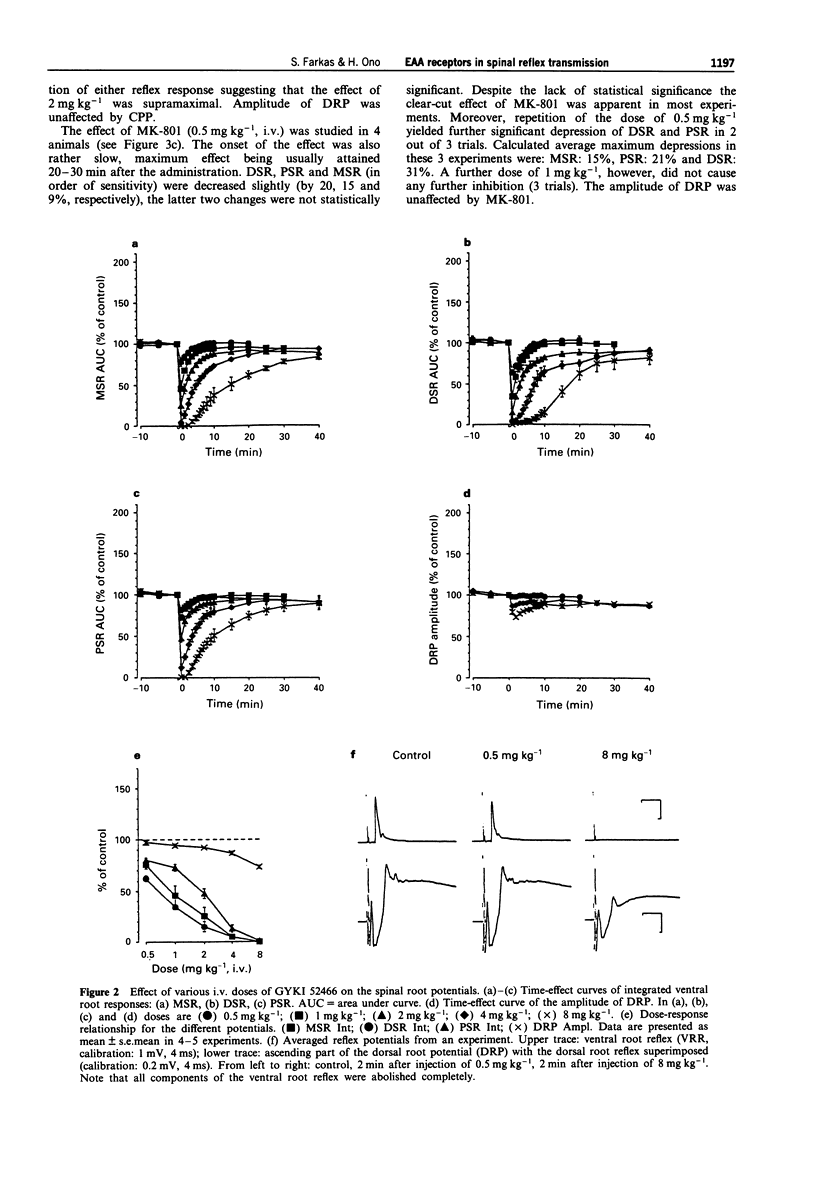
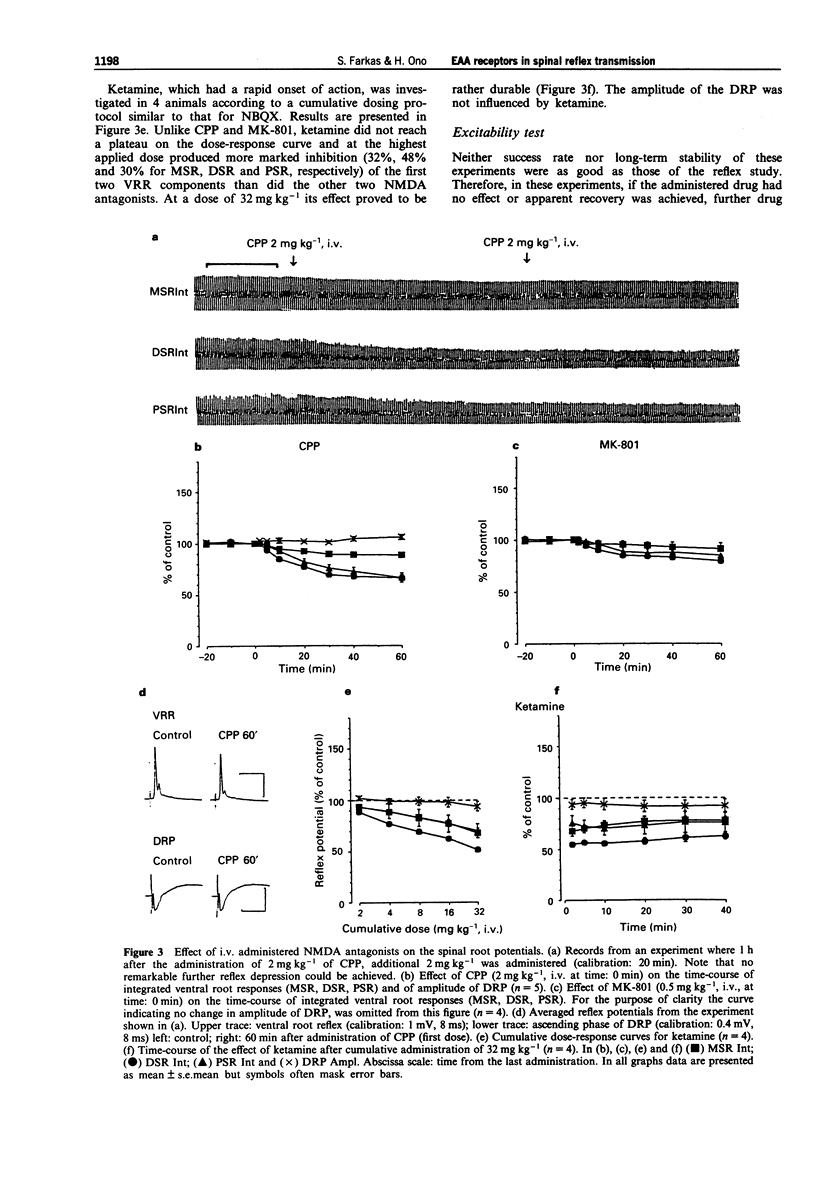
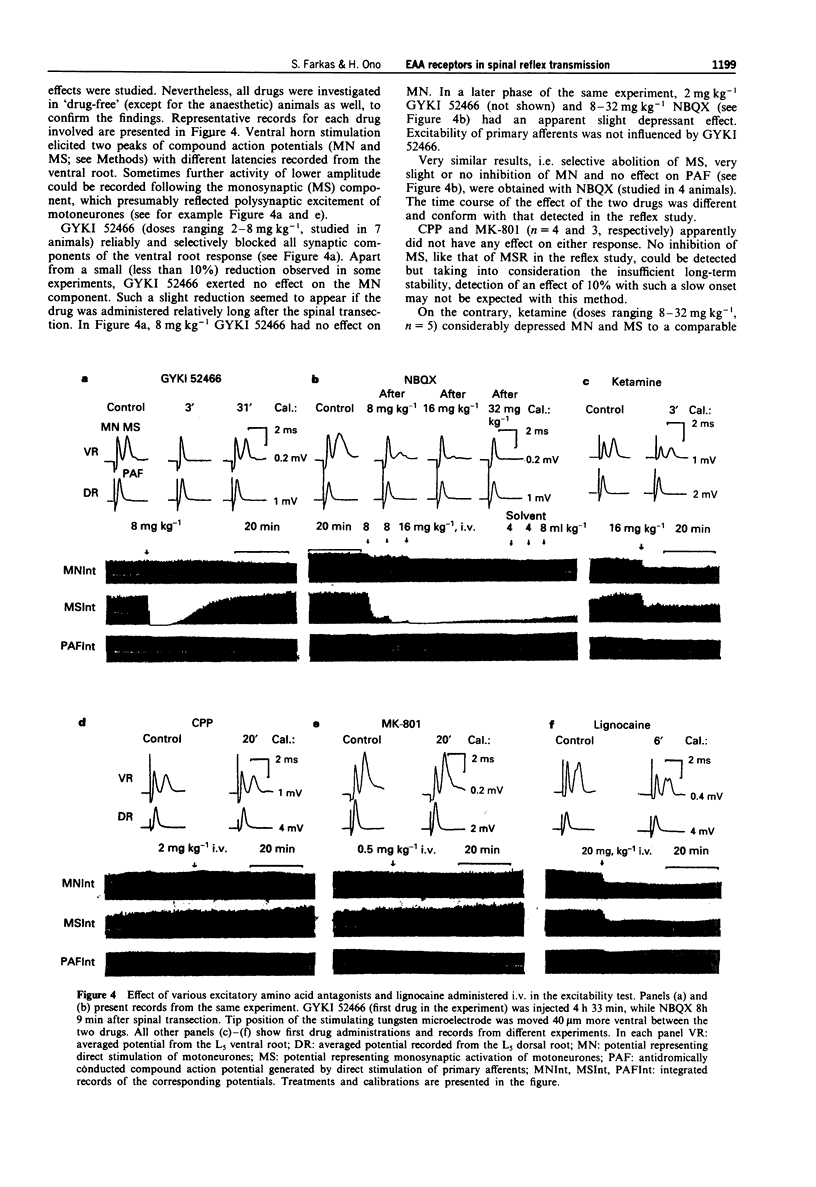
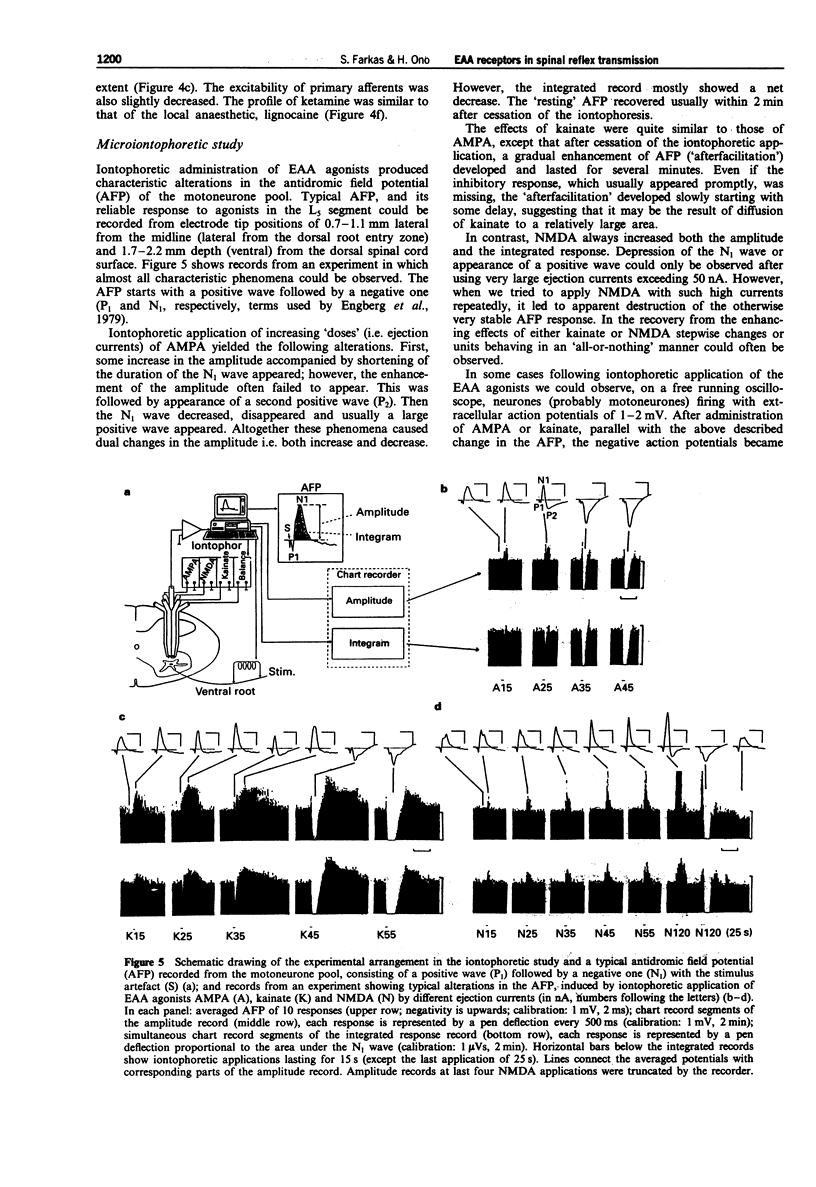
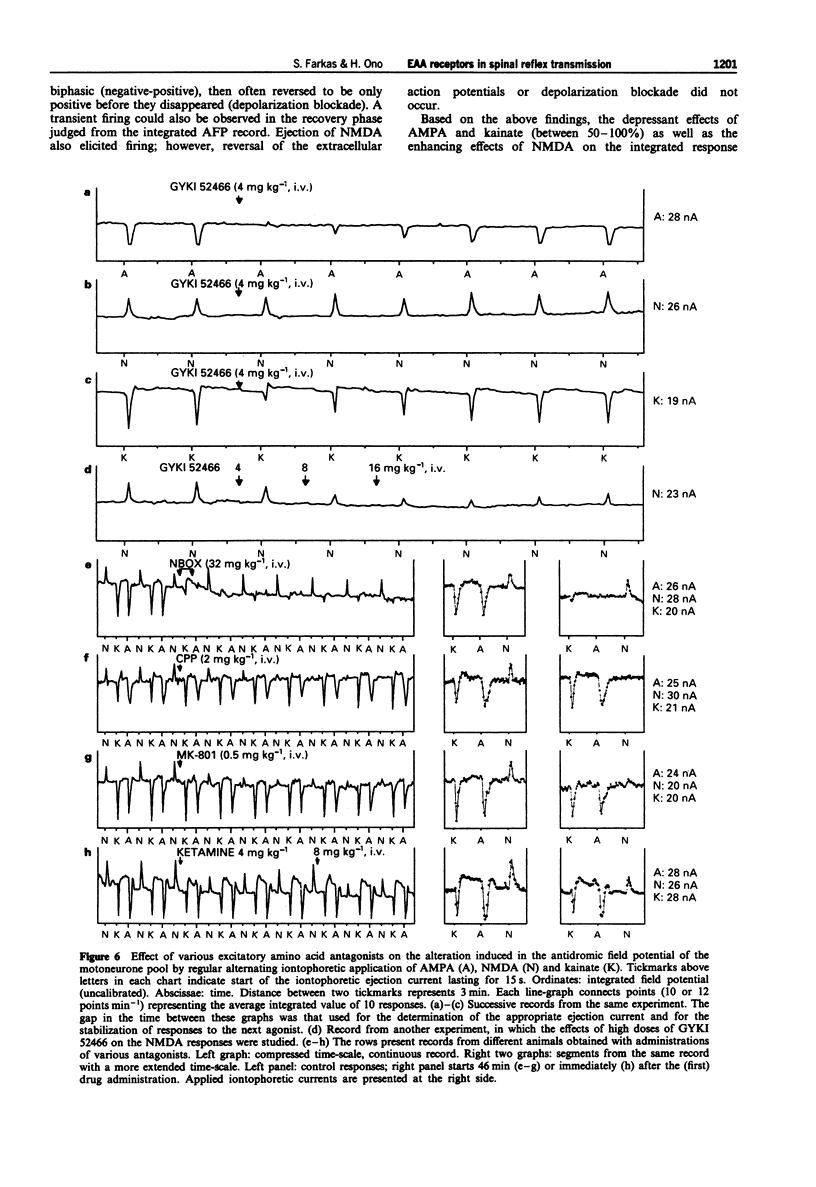
1. The effect of various intravenously administered excitatory amino acid (EAA) antagonists on the dorsal root stimulation-evoked, short latency (up to 10 ms) spinal root reflex potentials of chloralose-urethane anaesthetized C1 spinal rats was studied, in order to gain information on the involvement of non-NMDA (AMPA/kainate; AMPA = alpha-amino-3-hydroxy-5-methyl-isoxazole-4-propionate) and NMDA (N-methyl-D-aspartate) receptors in their mediation. The competitive non-NMDA antagonist, 2,3-dihydroxy-6-nitro-7-sulphamoyl-benzo(F)quinoxaline (NBQX; 1-32 mg kg-1), the non-competitive non-NMDA antagonist, 1-(amino)phenyl-4-methyl-7,8-methylendioxy-5H-2,3-benzodiazepine (GYKI 52466; 0.5-8 mg kg-1), the competitive NMDA antagonist 3-((+/-)-2-carboxypiperazin-4-yl)-propyl-l-phosphonic acid (CPP, 2-8 mg kg-1) and two non-competitive NMDA antagonists: MK-801 (0.5-2 mg kg-1) and ketamine (2-32 mg kg-1) were used as pharmacological tools. 2. Validating the applied pharmacological tools regarding selectivity at the applied doses, their effects were tested on direct (electrical) as well as on synaptic excitability of motoneurones evoked by intraspinal stimulation. Furthermore, their effect was investigated on the responses elicited by microiontophoretic application of EAA agonists (AMPA, kainate and NMDA) into the motoneurone pool, where the extracellular field potential evoked by antidromic stimulation of the ventral root was recorded to detect the effects of EAA agonists. 3. NBQX and GYKI 52466 were able to abolish completely the mono-, di- and polysynaptic ventral root reflexes (MSR, DSR, PSR) and the synaptic excitability of motoneurones, while hardly influencing direct excitability of motoneurones. They markedly attenuated AMPA and kainate responses whilst having little or no effect on NMDA responses. 4. Apparently 'supramaximal' doses of CPP and MK-801 slightly inhibited MSR (by about 10%) moderately reduced DSR and PSR (by about 20-30%) and did not influence excitability of motoneurones. They selectively blocked responses to NMDA. 5. Ketamine dose-dependently inhibited MSR, DSR and PSR. Nevertheless, diminution of none of the responses exceeded 50%. It reduced both direct and synaptic excitability of motoneurones, thus displaying a local anaesthetic-like effect, which may contribute to its reflex inhibitory action. It depressed responses to NMDA whilst having negligible effects on responses to AMPA and kainate. 6. We conclude that non-NMDA receptors play a substantial role in the mediation of MSR, DSR and PSR, while NMDA receptors contribute little to this. Neither MSR nor PSR is mediated exclusively by non-NMDA or NMDA receptors, respectively.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anis N. A., Berry S. C., Burton N. R., Lodge D. The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. Br J Pharmacol. 1983 Jun;79(2):565–575. doi: 10.1111/j.1476-5381.1983.tb11031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barasi S., Roberts M. H. The modification of lumbar motoneurone excitability by stimulation of a putative 5-hydroxytryptamine pathway. Br J Pharmacol. 1974 Nov;52(3):339–348. doi: 10.1111/j.1476-5381.1974.tb08601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barasi S., Roberts M. T. Responses of motoneurones to electrophoretically applied dopamine. Br J Pharmacol. 1977 May;60(1):29–34. doi: 10.1111/j.1476-5381.1977.tb16743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOMBS J. S., ECCLES J. C., FATT P. The electrical properties of the motoneurone membrane. J Physiol. 1955 Nov 28;130(2):291–325. doi: 10.1113/jphysiol.1955.sp005411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Lester R. A. Excitatory amino acid receptors in the vertebrate central nervous system. Pharmacol Rev. 1989 Jun;41(2):143–210. [PubMed] [Google Scholar]
- Davies J., Evans R. H., Herrling P. L., Jones A. W., Olverman H. J., Pook P., Watkins J. C. CPP, a new potent and selective NMDA antagonist. Depression of central neuron responses, affinity for [3H]D-AP5 binding sites on brain membranes and anticonvulsant activity. Brain Res. 1986 Sep 10;382(1):169–173. doi: 10.1016/0006-8993(86)90127-7. [DOI] [PubMed] [Google Scholar]
- Davies J., Watkins J. C. Role of excitatory amino acid receptors in mono- and polysynaptic excitation in the cat spinal cord. Exp Brain Res. 1983;49(2):280–290. doi: 10.1007/BF00238587. [DOI] [PubMed] [Google Scholar]
- Donevan S. D., Rogawski M. A. GYKI 52466, a 2,3-benzodiazepine, is a highly selective, noncompetitive antagonist of AMPA/kainate receptor responses. Neuron. 1993 Jan;10(1):51–59. doi: 10.1016/0896-6273(93)90241-i. [DOI] [PubMed] [Google Scholar]
- Engberg I., Flatman J. A., Lambert J. D. A comparison of extracellular and intracellular recording during extracellular microiontophoresis. J Neurosci Methods. 1979 Oct;1(3):219–233. doi: 10.1016/0165-0270(79)90033-5. [DOI] [PubMed] [Google Scholar]
- Engberg I., Flatman J. A., Lambert J. D. The action of N-methyl-D-aspartic and kainic acids on motoneurones with emphasis on conductance changes [proceedings]. Br J Pharmacol. 1978 Nov;64(3):384P–385P. [PMC free article] [PubMed] [Google Scholar]
- Engberg I., Tarnawa I., Durand J., Ouardouz M. An analysis of synaptic transmission to motoneurones in the cat spinal cord using a new selective receptor blocker. Acta Physiol Scand. 1993 Jun;148(2):97–100. doi: 10.1111/j.1748-1716.1993.tb09537.x. [DOI] [PubMed] [Google Scholar]
- Evans R. H. The pharmacology of segmental transmission in the spinal cord. Prog Neurobiol. 1989;33(4):255–279. doi: 10.1016/0301-0082(89)90003-8. [DOI] [PubMed] [Google Scholar]
- Farkas S., Tarnawa I., Berzsenyi P. Effects of some centrally acting muscle relaxants on spinal root potentials: a comparative study. Neuropharmacology. 1989 Feb;28(2):161–173. doi: 10.1016/0028-3908(89)90053-1. [DOI] [PubMed] [Google Scholar]
- Fukuda H., Kudo Y., Ono H. Effects of beta-(p-chlorophenyl)-GABA (baclofen) on spinal synaptic activity. Eur J Pharmacol. 1977 Jul 1;44(1):17–24. doi: 10.1016/0014-2999(77)90111-x. [DOI] [PubMed] [Google Scholar]
- Ganong A. H., Lanthorn T. H., Cotman C. W. Kynurenic acid inhibits synaptic and acidic amino acid-induced responses in the rat hippocampus and spinal cord. Brain Res. 1983 Aug 22;273(1):170–174. doi: 10.1016/0006-8993(83)91108-3. [DOI] [PubMed] [Google Scholar]
- Headley P. M., Grillner S. Excitatory amino acids and synaptic transmission: the evidence for a physiological function. Trends Pharmacol Sci. 1990 May;11(5):205–211. doi: 10.1016/0165-6147(90)90116-p. [DOI] [PubMed] [Google Scholar]
- Honoré T., Davies S. N., Drejer J., Fletcher E. J., Jacobsen P., Lodge D., Nielsen F. E. Quinoxalinediones: potent competitive non-NMDA glutamate receptor antagonists. Science. 1988 Aug 5;241(4866):701–703. doi: 10.1126/science.2899909. [DOI] [PubMed] [Google Scholar]
- Jahr C. E., Yoshioka K. Ia afferent excitation of motoneurones in the in vitro new-born rat spinal cord is selectively antagonized by kynurenate. J Physiol. 1986 Jan;370:515–530. doi: 10.1113/jphysiol.1986.sp015948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T., Ono H., Fukuda H. Simultaneous evaluation of drug effects on both the spinal cord and the descending pathways in rats. Arch Int Pharmacodyn Ther. 1987 Jun;287(2):203–210. [PubMed] [Google Scholar]
- King A. E., Lopez-Garcia J. A., Cumberbatch M. Antagonism of synaptic potentials in ventral horn neurones by 6-cyano-7-nitroquinoxaline-2,3-dione: a study in the rat spinal cord in vitro. Br J Pharmacol. 1992 Oct;107(2):375–381. doi: 10.1111/j.1476-5381.1992.tb12754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S. K., Smith D. A., Siarey R. J., Evans R. H. Effect of 6-cyano-2,3-dihydroxy-7-nitro-quinoxaline (CNQX) on dorsal root-, NMDA-, kainate- and quisqualate-mediated depolarization of rat motoneurones in vitro. Br J Pharmacol. 1990 Aug;100(4):850–854. doi: 10.1111/j.1476-5381.1990.tb14103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog Neurobiol. 1987;28(3):197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Ono H., Fukuda H., Kudo Y. Mechanisms of depressant action of baclofen on the spinal reflex in the rat. Neuropharmacology. 1979 Aug-Sep;18(8-9):647–653. doi: 10.1016/0028-3908(79)90030-3. [DOI] [PubMed] [Google Scholar]
- Ono H., Fukuda H., Kudo Y. Mechanisms of depressant action of muscle relaxants on spinal reflexes: participation of membrane stabilizing action. J Pharmacobiodyn. 1984 Mar;7(3):171–176. doi: 10.1248/bpb1978.7.171. [DOI] [PubMed] [Google Scholar]
- Onodera K., Takeuchi A. Uneven distribution of excitatory amino acid receptors on ventral horn neurones of newborn rat spinal cord. J Physiol. 1991 Aug;439:257–276. doi: 10.1113/jphysiol.1991.sp018666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouardouz M., Durand J. GYKI 52466 antagonizes glutamate responses but not NMDA and kainate responses in rat abducens motoneurones. Neurosci Lett. 1991 Apr 15;125(1):5–8. doi: 10.1016/0304-3940(91)90115-a. [DOI] [PubMed] [Google Scholar]
- Polc P. 2-Amino-7-phosphonoheptanoic acid depresses gamma-motoneurons and polysynaptic reflexes in the cat spinal cord. Eur J Pharmacol. 1985 Nov 19;117(3):387–389. doi: 10.1016/0014-2999(85)90015-9. [DOI] [PubMed] [Google Scholar]
- Polc P., Haefely W. Effects of intravenous kainic acid, N-methyl-D-aspartate, and (--)-nuciferine on the cat spinal cord. Naunyn Schmiedebergs Arch Pharmacol. 1977 Nov;300(3):199–203. doi: 10.1007/BF00500961. [DOI] [PubMed] [Google Scholar]
- Roberts M. H., Davies M., Girdlestone D., Foster G. A. Effects of 5-hydroxytryptamine agonists and antagonists on the responses of rat spinal motoneurones to raphe obscurus stimulation. Br J Pharmacol. 1988 Oct;95(2):437–448. doi: 10.1111/j.1476-5381.1988.tb11664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan G. P., Boisse N. R. Benzodiazepine tolerance, physical dependence and withdrawal: electrophysiological study of spinal reflex function. J Pharmacol Exp Ther. 1984 Nov;231(2):464–471. [PubMed] [Google Scholar]
- Sheardown M. J., Nielsen E. O., Hansen A. J., Jacobsen P., Honoré T. 2,3-Dihydroxy-6-nitro-7-sulfamoyl-benzo(F)quinoxaline: a neuroprotectant for cerebral ischemia. Science. 1990 Feb 2;247(4942):571–574. doi: 10.1126/science.2154034. [DOI] [PubMed] [Google Scholar]
- Sommer B., Seeburg P. H. Glutamate receptor channels: novel properties and new clones. Trends Pharmacol Sci. 1992 Jul;13(7):291–296. doi: 10.1016/0165-6147(92)90088-n. [DOI] [PubMed] [Google Scholar]
- Tarnawa I., Farkas S., Berzsenyi P., Pataki A., Andrási F. Electrophysiological studies with a 2,3-benzodiazepine muscle relaxant: GYKI 52466. Eur J Pharmacol. 1989 Aug 22;167(2):193–199. doi: 10.1016/0014-2999(89)90579-7. [DOI] [PubMed] [Google Scholar]
- Turski L., Bressler K., Klockgether T., Stephens D. N. Differential effects of the excitatory amino acid antagonists, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and 3-((+-)-2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid (CPP), on spinal reflex activity in mice. Neurosci Lett. 1990 May 18;113(1):66–71. doi: 10.1016/0304-3940(90)90496-v. [DOI] [PubMed] [Google Scholar]
- Turski L., Jacobsen P., Honoré T., Stephens D. N. Relief of experimental spasticity and anxiolytic/anticonvulsant actions of the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate antagonist 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo(F)quinoxaline. J Pharmacol Exp Ther. 1992 Feb;260(2):742–747. [PubMed] [Google Scholar]
- Watkins J. C., Krogsgaard-Larsen P., Honoré T. Structure-activity relationships in the development of excitatory amino acid receptor agonists and competitive antagonists. Trends Pharmacol Sci. 1990 Jan;11(1):25–33. doi: 10.1016/0165-6147(90)90038-a. [DOI] [PubMed] [Google Scholar]
- Wong E. H., Kemp J. A., Priestley T., Knight A. R., Woodruff G. N., Iversen L. L. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7104–7108. doi: 10.1073/pnas.83.18.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki J., Ono H., Nagao T. Stimulatory and inhibitory effects of serotonergic hallucinogens on spinal mono- and polysynaptic reflex pathways in the rat. Neuropharmacology. 1992 Jul;31(7):635–642. doi: 10.1016/0028-3908(92)90141-b. [DOI] [PubMed] [Google Scholar]
- Zeman S., Lodge D. Pharmacological characterization of non-NMDA subtypes of glutamate receptor in the neonatal rat hemisected spinal cord in vitro. Br J Pharmacol. 1992 Jun;106(2):367–372. doi: 10.1111/j.1476-5381.1992.tb14342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


