Abstract
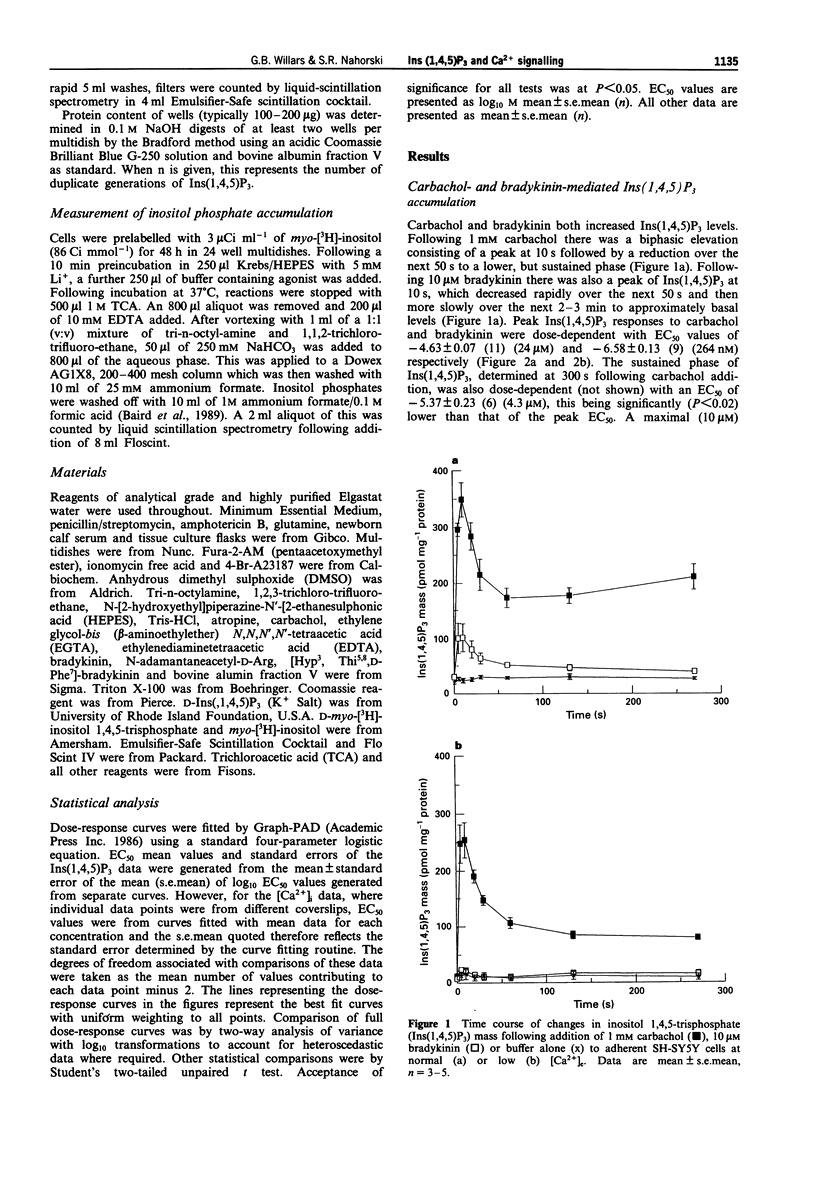
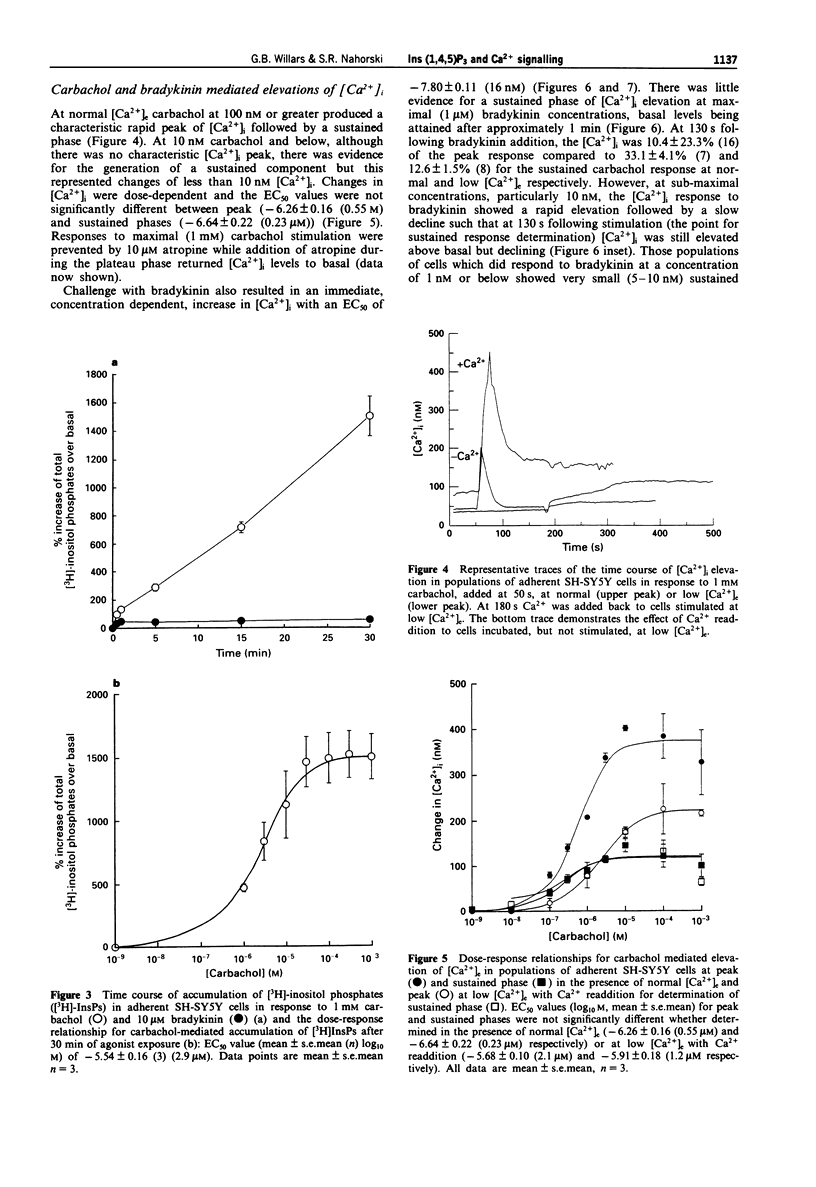
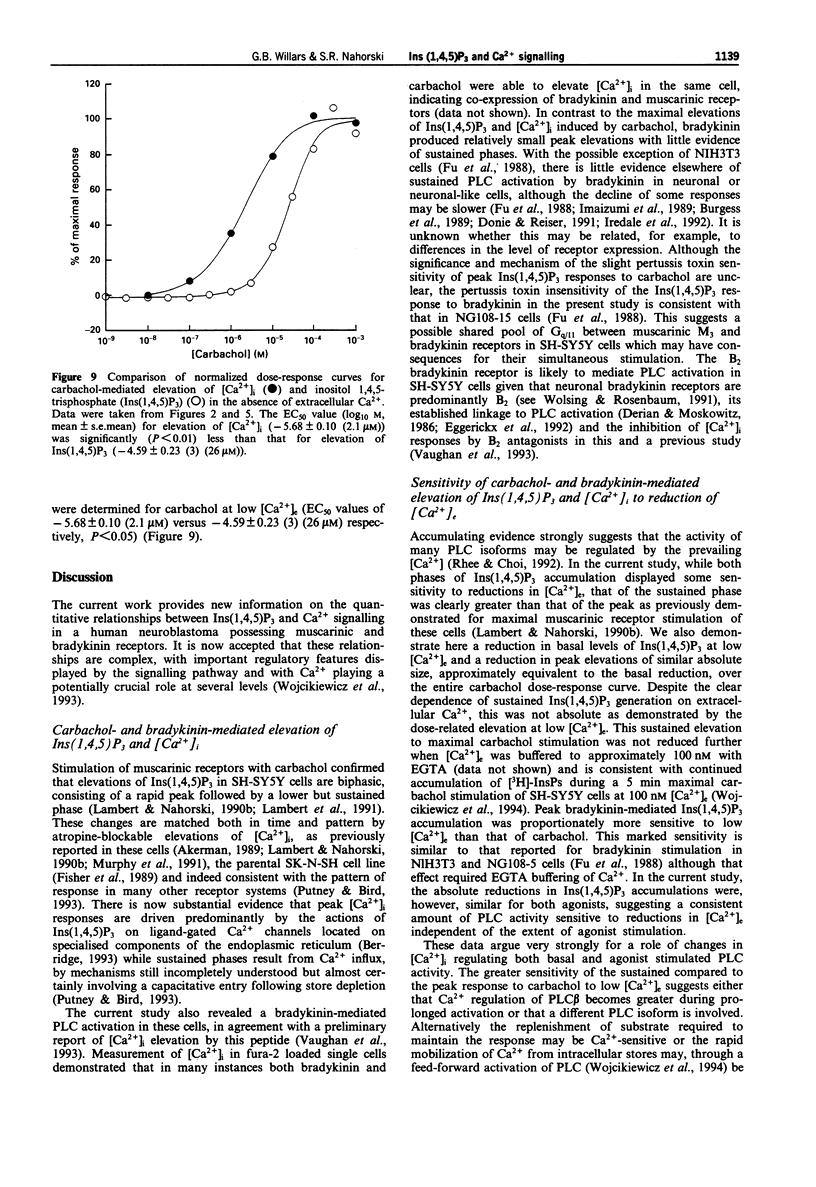
1. Muscarinic and bradykinin receptor-mediated Ins(1,4,5)P3 accumulation, Ca2+ mobilization and Ca2+ entry have been examined in human SH-SY5Y neuroblastoma cells. This has allowed both direct comparison of signalling events by two receptor types potentially linked to the same transduction pathway and an investigation of the interactions between the components of this pathway. 2. Stimulation of muscarinic receptors with carbachol produced biphasic accumulations of Ins(1,4,5)P3 consisting of a rapid peak followed by a lower sustained phase. Both phases were dose-dependent but the potency of elevation at peak was significantly less than that of the sustained phase. Bradykinin also dose-dependently stimulated Ins(1,4,5)P3 accumulation but responses were smaller and not sustained. 3. Lowering of [Ca2+]e reduced basal Ins(1,4,5)P3 levels. Peak Ins(1,4,5)P3 elevation in response to carbachol and bradykinin were lowered by an amount approximating this reduction over the entire dose-response curves. Sustained Ins(1,4,5)P3 elevation in response to carbachol showed a more marked absolute reduction. Agonist potencies were unaffected by lowering [Ca2+]e. Thus, a consistent but small amount of PLC activity during rapid activation appears to be sensitive to lowered [Ca2+]e, whilst activity during sustained stimulation is greatly facilitated by external Ca2+, probably through Ca2+ entry. 4. The temporal- and dose-dependency of carbachol-mediated Ins(1,4,5)P3 accumulations were unaffected by loading cells with fura-2, thus allowing direct comparison of Ins(1,4,5)P3 and [Ca2+]i changes monitored by fura-2. 5. Changes in [Ca2+]i by both agonists revealed temporal patterns that were similar to Ins(1,4,5)P3 accumulations. Only carbachol stimulated a marked sustained [Ca2+]i signal and this was fully dependent on external Ca2+. 6. All agonist-mediated [Ca2+]i elevations occurred with significantly greater potency than that of the respective Ins(1,4,5)P3 accumulations. Further examination of peak elevations in response to carbachol indicated that this was independent of Ca2+ entry. Thus, a major site for amplification of the potency of rapid agonist-mediated responses lies at the level of the Ins(1,4,5)P3 receptor. 7. The transient nature of Ins(1,4,5)P3 and [Ca2+]i peaks followed by either lower but sustained levels with carbachol or a return to basal levels with bradykinin suggests rapid but partial desensitization of the muscarinic receptor and complete desensitization of the bradykinin receptor. This indicates receptor-specific desensitization. Further analysis of this was provided by detecting accumulations of [3H]-inositol phosphates ([3H]-InsPs) in Li(+)-blocked, myo-[3H]-inositol labelled cells. Carbachol produced a rapid accumulation over the first minute, followed by a slower linear accumulation for at least 29 min.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerman K. E. Depolarization of human neuroblastoma cells as a result of muscarinic receptor-induced rise in cytosolic Ca2+. FEBS Lett. 1989 Jan 2;242(2):337–340. doi: 10.1016/0014-5793(89)80497-1. [DOI] [PubMed] [Google Scholar]
- Baird J. G., Lambert D. G., McBain J., Nahorski S. R. Muscarinic receptors coupled to phosphoinositide hydrolysis and elevated cytosolic calcium in a human neuroblastoma cell line SK-N-SH. Br J Pharmacol. 1989 Dec;98(4):1328–1334. doi: 10.1111/j.1476-5381.1989.tb12681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I., Watras J., Ehrlich B. E. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991 Jun 27;351(6329):751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Bootman M. D., Berridge M. J., Taylor C. W. All-or-nothing Ca2+ mobilization from the intracellular stores of single histamine-stimulated HeLa cells. J Physiol. 1992 May;450:163–178. doi: 10.1113/jphysiol.1992.sp019121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess G. M., Mullaney I., McNeill M., Dunn P. M., Rang H. P. Second messengers involved in the mechanism of action of bradykinin in sensory neurons in culture. J Neurosci. 1989 Sep;9(9):3314–3325. doi: 10.1523/JNEUROSCI.09-09-03314.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challiss R. A., Chilvers E. R., Willcocks A. L., Nahorski S. R. Heterogeneity of [3H]inositol 1,4,5-trisphosphate binding sites in adrenal-cortical membranes. Characterization and validation of a radioreceptor assay. Biochem J. 1990 Jan 15;265(2):421–427. doi: 10.1042/bj2650421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derian C. K., Moskowitz M. A. Polyphosphoinositide hydrolysis in endothelial cells and carotid artery segments. Bradykinin-2 receptor stimulation is calcium-independent. J Biol Chem. 1986 Mar 15;261(8):3831–3837. [PubMed] [Google Scholar]
- Donié F., Reiser G. Mass measurements of inositol 1,4,5-trisphosphate and inositol 1,3,4,5-tetrakisphosphate in a neuronal cell line stimulated with bradykinin: inositolphosphate response shows desensitization. Biochem Biophys Res Commun. 1991 Dec 31;181(3):997–1003. doi: 10.1016/0006-291x(91)92035-i. [DOI] [PubMed] [Google Scholar]
- Eggerickx D., Raspe E., Bertrand D., Vassart G., Parmentier M. Molecular cloning, functional expression and pharmacological characterization of a human bradykinin B2 receptor gene. Biochem Biophys Res Commun. 1992 Sep 30;187(3):1306–1313. doi: 10.1016/0006-291x(92)90445-q. [DOI] [PubMed] [Google Scholar]
- Fisher S. K., Domask L. M., Roland R. M. Muscarinic receptor regulation of cytoplasmic Ca2+ concentrations in human SK-N-SH neuroblastoma cells: Ca2+ requirements for phospholipase C activation. Mol Pharmacol. 1989 Feb;35(2):195–204. [PubMed] [Google Scholar]
- Fisher S. K., Heacock A. M. A putative M3 muscarinic cholinergic receptor of high molecular weight couples to phosphoinositide hydrolysis in human SK-N-SH neuroblastoma cells. J Neurochem. 1988 Mar;50(3):984–987. doi: 10.1111/j.1471-4159.1988.tb03008.x. [DOI] [PubMed] [Google Scholar]
- Fu T., Okano Y., Nozawa Y. Bradykinin-induced generation of inositol 1,4,5-trisphosphate in fibroblasts and neuroblastoma cells: effect of pertussis toxin, extracellular calcium, and down-regulation of protein kinase C. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1429–1435. doi: 10.1016/s0006-291x(88)81035-0. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hausdorff W. P., Caron M. G., Lefkowitz R. J. Turning off the signal: desensitization of beta-adrenergic receptor function. FASEB J. 1990 Aug;4(11):2881–2889. [PubMed] [Google Scholar]
- Imaizumi T., Osugi T., Misaki N., Uchida S., Yoshida H. Heterologous desensitization of bradykinin-induced phosphatidylinositol response and Ca2+ mobilization by neurotensin in NG108-15 cells. Eur J Pharmacol. 1989 Feb 28;161(2-3):203–208. doi: 10.1016/0014-2999(89)90844-3. [DOI] [PubMed] [Google Scholar]
- Iredale P. A., Martin K. F., Hill S. J., Kendall D. A. Agonist-induced changes in [Ca2+]i in N1E-115 cells: differential effects of bradykinin and carbachol. Eur J Pharmacol. 1992 Jun 5;226(2):163–168. doi: 10.1016/0922-4106(92)90178-x. [DOI] [PubMed] [Google Scholar]
- Klueppelberg U. G., Gates L. K., Gorelick F. S., Miller L. J. Agonist-regulated phosphorylation of the pancreatic cholecystokinin receptor. J Biol Chem. 1991 Feb 5;266(4):2403–2408. [PubMed] [Google Scholar]
- Kwatra M. M., Schwinn D. A., Schreurs J., Blank J. L., Kim C. M., Benovic J. L., Krause J. E., Caron M. G., Lefkowitz R. J. The substance P receptor, which couples to Gq/11, is a substrate of beta-adrenergic receptor kinase 1 and 2. J Biol Chem. 1993 May 5;268(13):9161–9164. [PubMed] [Google Scholar]
- Lambert D. G., Challiss R. A., Nahorski S. R. Accumulation and metabolism of Ins(1,4,5)P3 and Ins(1,3,4,5)P4 in muscarinic-receptor-stimulated SH-SY5Y neuroblastoma cells. Biochem J. 1991 Feb 1;273(Pt 3):791–794. doi: 10.1042/bj2730791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert D. G., Ghataorre A. S., Nahorski S. R. Muscarinic receptor binding characteristics of a human neuroblastoma SK-N-SH and its clones SH-SY5Y and SH-EP1. Eur J Pharmacol. 1989 Jun 8;165(1):71–77. doi: 10.1016/0014-2999(89)90771-1. [DOI] [PubMed] [Google Scholar]
- Lambert D. G., Nahorski S. R. Muscarinic-receptor-mediated changes in intracellular Ca2+ and inositol 1,4,5-trisphosphate mass in a human neuroblastoma cell line, SH-SY5Y. Biochem J. 1990 Jan 15;265(2):555–562. doi: 10.1042/bj2650555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert D. G., Nahorski S. R. Pertussis toxin inhibits alpha 2-adrenoceptor-mediated inhibition of adenylate cyclase without affecting muscarinic regulation of [Ca2+]i or inositol phosphate generation in SH-SY5Y human neuroblastoma cells. Biochem Pharmacol. 1990 Nov 15;40(10):2291–2295. doi: 10.1016/0006-2952(90)90725-z. [DOI] [PubMed] [Google Scholar]
- Menniti F. S., Takemura H., Oliver K. G., Putney J. W., Jr Different modes of regulation for receptors activating phospholipase C in the rat pancreatoma cell line AR4-2J. Mol Pharmacol. 1991 Nov;40(5):727–733. [PubMed] [Google Scholar]
- Murphy N. P., Vaughan P. F., Ball S. G., McCormack J. G. The cholinergic regulation of intracellular calcium in the human neuroblastoma, SH-SY5Y. J Neurochem. 1991 Dec;57(6):2116–2123. doi: 10.1111/j.1471-4159.1991.tb06430.x. [DOI] [PubMed] [Google Scholar]
- Ogino Y., Costa T. The epithelial phenotype of human neuroblastoma cells express bradykinin, endothelin, and angiotensin II receptors that stimulate phosphoinositide hydrolysis. J Neurochem. 1992 Jan;58(1):46–56. doi: 10.1111/j.1471-4159.1992.tb09275.x. [DOI] [PubMed] [Google Scholar]
- Oldershaw K. A., Nunn D. L., Taylor C. W. Quantal Ca2+ mobilization stimulated by inositol 1,4,5-trisphosphate in permeabilized hepatocytes. Biochem J. 1991 Sep 15;278(Pt 3):705–708. doi: 10.1042/bj2780705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney J. W., Jr, Bird G. S. The inositol phosphate-calcium signaling system in nonexcitable cells. Endocr Rev. 1993 Oct;14(5):610–631. doi: 10.1210/edrv-14-5-610. [DOI] [PubMed] [Google Scholar]
- Rhee S. G., Choi K. D. Regulation of inositol phospholipid-specific phospholipase C isozymes. J Biol Chem. 1992 Jun 25;267(18):12393–12396. [PubMed] [Google Scholar]
- Ross R. A., Spengler B. A., Biedler J. L. Coordinate morphological and biochemical interconversion of human neuroblastoma cells. J Natl Cancer Inst. 1983 Oct;71(4):741–747. [PubMed] [Google Scholar]
- Shao Y., McCarthy K. D. Quantitative relationship between alpha 1-adrenergic receptor density and the receptor-mediated calcium response in individual astroglial cells. Mol Pharmacol. 1993 Aug;44(2):247–254. [PubMed] [Google Scholar]
- Thompson N. T., Bonser R. W., Tateson J. E., Spacey G. D., Randall R. W., Hodson H. F., Garland L. G. A quantitative investigation into the dependence of Ca2+ mobilisation on changes in inositol 1,4,5-trisphosphate levels in the stimulated neutrophil. Br J Pharmacol. 1991 Jun;103(2):1592–1596. doi: 10.1111/j.1476-5381.1991.tb09832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin A. B., Keys B., Nahorski S. R. Phosphorylation of a phosphoinositidase C-linked muscarinic receptor by a novel kinase distinct from beta-adrenergic receptor kinase. FEBS Lett. 1993 Dec 13;335(3):353–357. doi: 10.1016/0014-5793(93)80418-t. [DOI] [PubMed] [Google Scholar]
- Tobin A. B., Nahorski S. R. Rapid agonist-mediated phosphorylation of m3-muscarinic receptors revealed by immunoprecipitation. J Biol Chem. 1993 May 5;268(13):9817–9823. [PubMed] [Google Scholar]
- Vaughan P. F., Kaye D. F., McDonald R., Reeve H. L., Ball S. G., Peers C. Bradykinin-evoked release of [3H]noradrenaline from the human neuroblastoma SH-SY5Y. Biochem Soc Trans. 1993 Nov;21(4):420S–420S. doi: 10.1042/bst021420s. [DOI] [PubMed] [Google Scholar]
- Wall S. J., Yasuda R. P., Li M., Wolfe B. B. Development of an antiserum against m3 muscarinic receptors: distribution of m3 receptors in rat tissues and clonal cell lines. Mol Pharmacol. 1991 Nov;40(5):783–789. [PubMed] [Google Scholar]
- Wojcikiewicz R. J., Tobin A. B., Nahorski S. R. Desensitization of cell signalling mediated by phosphoinositidase C. Trends Pharmacol Sci. 1993 Jul;14(7):279–285. doi: 10.1016/0165-6147(93)90131-3. [DOI] [PubMed] [Google Scholar]
- Wojcikiewicz R. J., Tobin A. B., Nahorski S. R. Muscarinic receptor-mediated inositol 1,4,5-trisphosphate formation in SH-SY5Y neuroblastoma cells is regulated acutely by cytosolic Ca2+ and by rapid desensitization. J Neurochem. 1994 Jul;63(1):177–185. doi: 10.1046/j.1471-4159.1994.63010177.x. [DOI] [PubMed] [Google Scholar]
- Wolsing D. H., Rosenbaum J. S. Bradykinin-stimulated inositol phosphate production in NG108-15 cells is mediated by a small population of binding sites which rapidly desensitize. J Pharmacol Exp Ther. 1991 May;257(2):621–633. [PubMed] [Google Scholar]
- Yu V. C., Sadée W. Phosphatidylinositol turnover in neuroblastoma cells: regulation by bradykinin, acetylcholine, but not mu- and delta-opioid receptors. Neurosci Lett. 1986 Nov 11;71(2):219–223. doi: 10.1016/0304-3940(86)90562-8. [DOI] [PubMed] [Google Scholar]


