Abstract
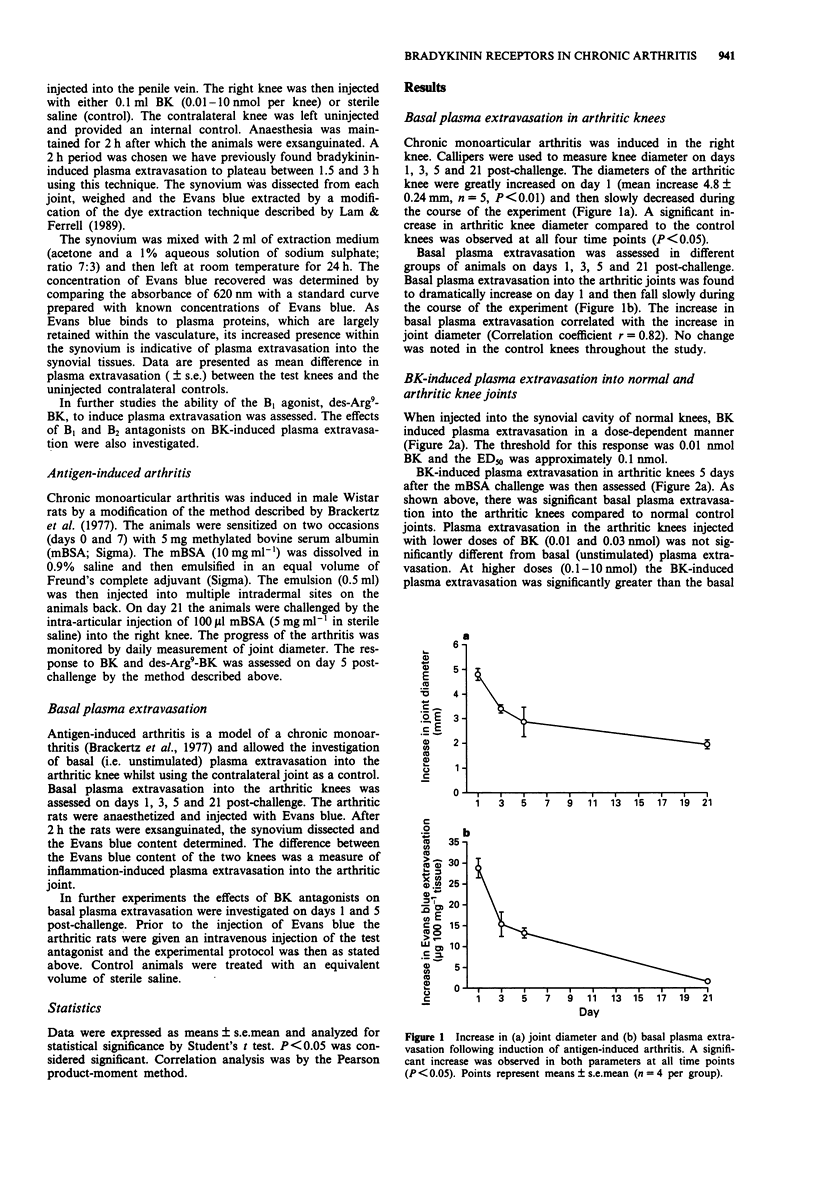
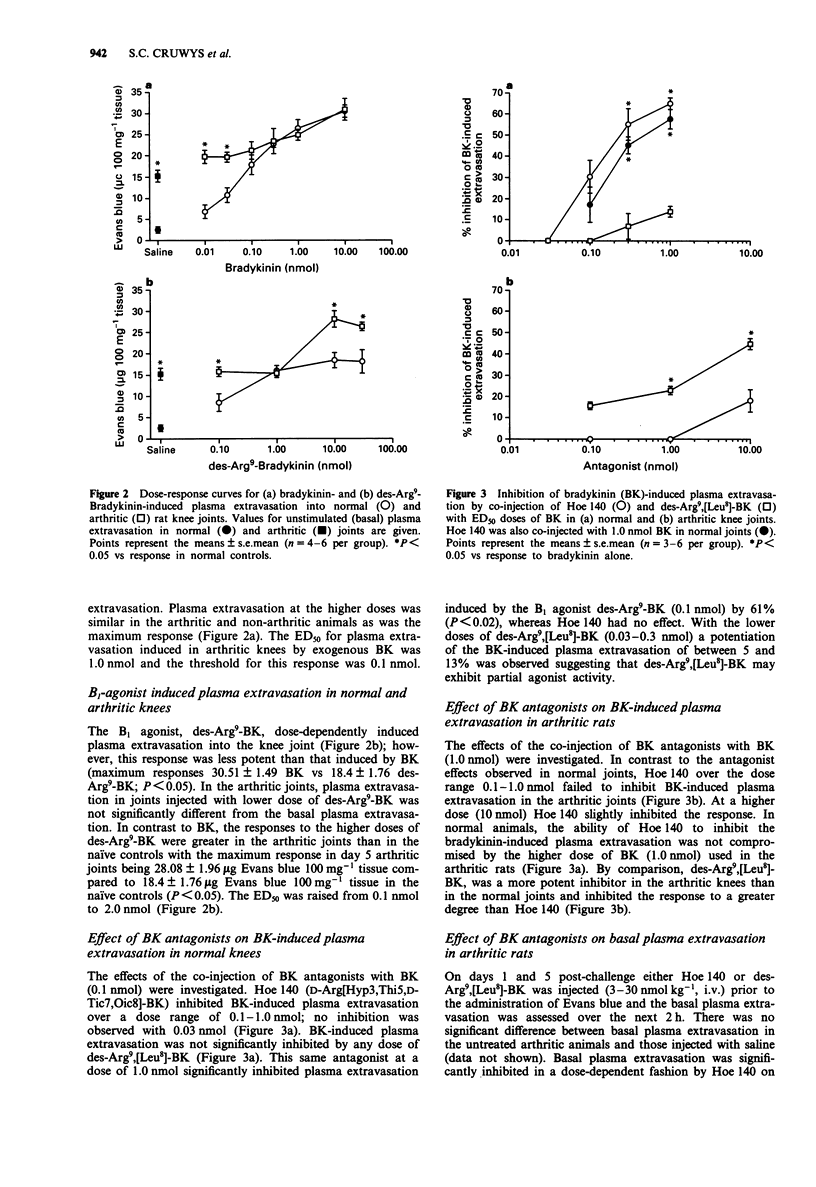
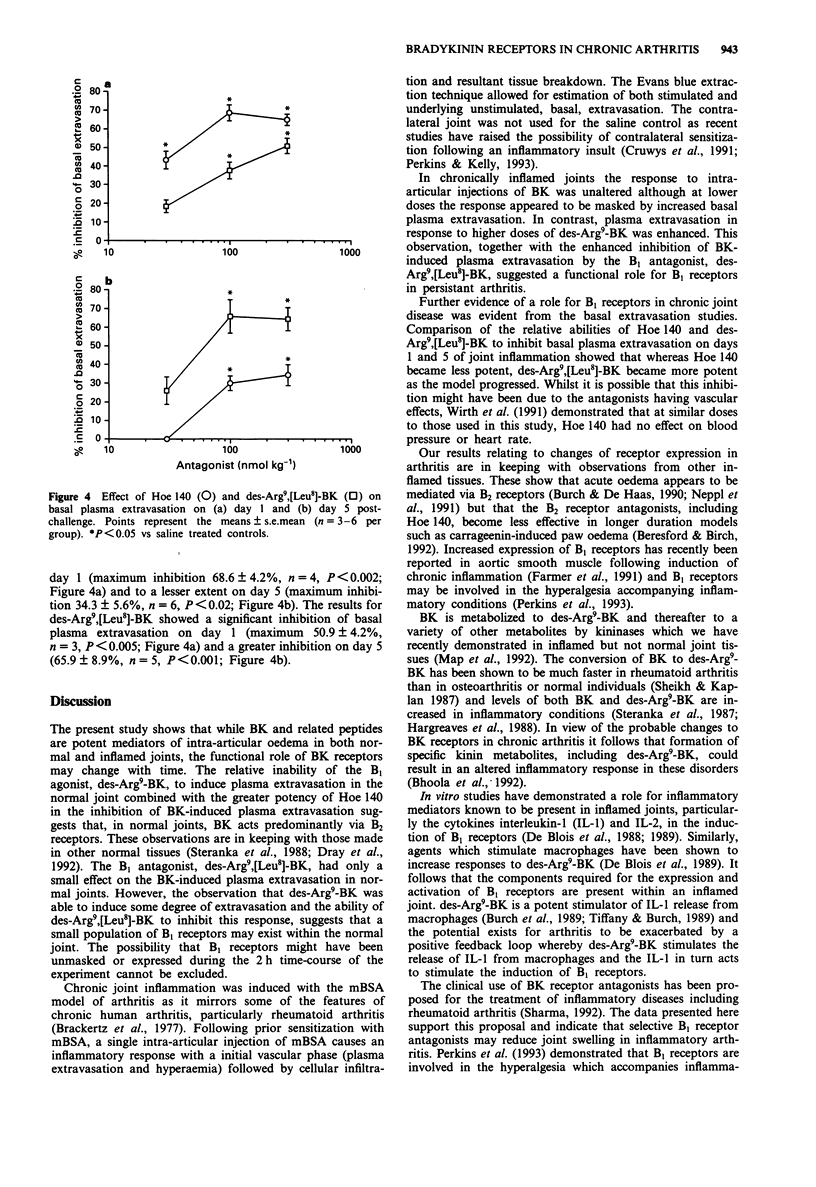
1. The role of bradykinin B1 and B2 receptors in bradykinin- and des-Arg9-bradykinin-induced plasma extravasation in normal and inflamed rat knee joints was investigated by use of an antigen-induced model of chronic arthritis. A modification of an Evans blue extraction technique allowed the unstimulated (basal) plasma extravasation to be assessed in this model. The contributions of bradykinin B1 and B2 receptors towards basal synovial plasma extravasation were determined. 2. In normal knees, intra-articular injection of bradykinin (BK) induced plasma extravasation in a potent, dose-dependent manner with a threshold of 0.01 nmol and an ED50 of 0.1 nmol. In day 5 arthritic knees, basal plasma extravasation was substantially enhanced. Lower doses of BK had no demonstrable effect and increases above basal extravasation were first observed at 0.1 nmol. Thereafter the dose-response mirrored the response in normal knees and the maximal response was unaltered. 3. The B1 agonist, des-Arg9-BK, induced slight but significant plasma extravasation in normal knees but was less potent than bradykinin. This response was inhibited by the B1 receptor antagonist, des-Arg9, [Leu8]-BK. Lower doses of des-Arg9-BK bradykinin did not significantly increase basal extravasation in day 5 arthritic knees but, in contrast to BK, the maximal response was significantly enhanced. 4. The B2 antagonist, Hoe 140, inhibited BK-induced plasma extravasation in normal joints over a dose-range of 0.1-1.0 nmol but was relatively inactive in day 5 inflamed knees.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhoola K. D., Elson C. J., Dieppe P. A. Kinins--key mediators in inflammatory arthritis? Br J Rheumatol. 1992 Aug;31(8):509–518. doi: 10.1093/rheumatology/31.8.509. [DOI] [PubMed] [Google Scholar]
- Bhoola K. D., Figueroa C. D., Worthy K. Bioregulation of kinins: kallikreins, kininogens, and kininases. Pharmacol Rev. 1992 Mar;44(1):1–80. [PubMed] [Google Scholar]
- Brackertz D., Mitchell G. F., Mackay I. R. Antigen-induced arthritis in mice. I. Induction of arthritis in various strains of mice. Arthritis Rheum. 1977 Apr;20(3):841–850. doi: 10.1002/art.1780200314. [DOI] [PubMed] [Google Scholar]
- Burch R. M., Connor J. R., Tiffany C. W. The kallikrein-kininogen-kinin system in chronic inflammation. Agents Actions. 1989 Jun;27(3-4):258–260. doi: 10.1007/BF01972790. [DOI] [PubMed] [Google Scholar]
- Burch R. M., DeHaas C. A bradykinin antagonist inhibits carrageenan edema in rats. Naunyn Schmiedebergs Arch Pharmacol. 1990 Aug;342(2):189–193. doi: 10.1007/BF00166963. [DOI] [PubMed] [Google Scholar]
- Cruwys S. C., Kidd B. L., Mapp P. I., Walsh D. A., Blake D. R. The effects of calcitonin gene-related peptide on formation of intra-articular oedema by inflammatory mediators. Br J Pharmacol. 1992 Sep;107(1):116–119. doi: 10.1111/j.1476-5381.1992.tb14472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deblois D., Bouthillier J., Marceau F. Effect of glucocorticoids, monokines and growth factors on the spontaneously developing responses of the rabbit isolated aorta to des-Arg9-bradykinin. Br J Pharmacol. 1988 Apr;93(4):969–977. doi: 10.1111/j.1476-5381.1988.tb11487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dray A., Patel I. A., Perkins M. N., Rueff A. Bradykinin-induced activation of nociceptors: receptor and mechanistic studies on the neonatal rat spinal cord-tail preparation in vitro. Br J Pharmacol. 1992 Dec;107(4):1129–1134. doi: 10.1111/j.1476-5381.1992.tb13418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dray A., Perkins M. Bradykinin and inflammatory pain. Trends Neurosci. 1993 Mar;16(3):99–104. doi: 10.1016/0166-2236(93)90133-7. [DOI] [PubMed] [Google Scholar]
- Farmer S. G., McMillan B. A., Meeker S. N., Burch R. M. Induction of vascular smooth muscle bradykinin B1 receptors in vivo during antigen arthritis. Agents Actions. 1991 Sep;34(1-2):191–193. doi: 10.1007/BF01993275. [DOI] [PubMed] [Google Scholar]
- Hargreaves K. M., Troullos E. S., Dionne R. A., Schmidt E. A., Schafer S. C., Joris J. L. Bradykinin is increased during acute and chronic inflammation: therapeutic implications. Clin Pharmacol Ther. 1988 Dec;44(6):613–621. doi: 10.1038/clpt.1988.202. [DOI] [PubMed] [Google Scholar]
- Henry P. J., Rigby P. J., Self G. J., Preuss J. M., Goldie R. G. Endothelin-1-induced [3H]-inositol phosphate accumulation in rat trachea. Br J Pharmacol. 1992 Jan;105(1):135–141. doi: 10.1111/j.1476-5381.1992.tb14224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Håkanson R., Beding B., Ekman R., Heilig M., Wahlestedt C., Sundler F. Multiple tachykinin pools in sensory nerve fibres in the rabbit iris. Neuroscience. 1987 Jun;21(3):943–950. doi: 10.1016/0306-4522(87)90049-2. [DOI] [PubMed] [Google Scholar]
- Kidd B. L., Mapp P. I., Blake D. R., Gibson S. J., Polak J. M. Neurogenic influences in arthritis. Ann Rheum Dis. 1990 Aug;49(8):649–652. doi: 10.1136/ard.49.8.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam F. Y., Ferrell W. R. Inhibition of carrageenan induced inflammation in the rat knee joint by substance P antagonist. Ann Rheum Dis. 1989 Nov;48(11):928–932. doi: 10.1136/ard.48.11.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapp P. I., Walsh D. A., Kidd B. L., Cruwys S. C., Polak J. M., Blake D. R. Localization of the enzyme neutral endopeptidase to the human synovium. J Rheumatol. 1992 Dec;19(12):1838–1844. [PubMed] [Google Scholar]
- Neppl H., Neuhof H., Afflerbach F., Llach Puig Neppl J., Berghöfer A. Bradykinin-induced oedema formation proceeds from B2 receptor stimulation and is potentiated by concomitantly released prostaglandins. Acta Physiol Scand. 1991 Jun;142(2):141–147. doi: 10.1111/j.1748-1716.1991.tb09141.x. [DOI] [PubMed] [Google Scholar]
- Perkins M. N., Campbell E., Dray A. Antinociceptive activity of the bradykinin B1 and B2 receptor antagonists, des-Arg9, [Leu8]-BK and HOE 140, in two models of persistent hyperalgesia in the rat. Pain. 1993 May;53(2):191–197. doi: 10.1016/0304-3959(93)90080-9. [DOI] [PubMed] [Google Scholar]
- Perkins M. N., Kelly D. Induction of bradykinin B1 receptors in vivo in a model of ultra-violet irradiation-induced thermal hyperalgesia in the rat. Br J Pharmacol. 1993 Dec;110(4):1441–1444. doi: 10.1111/j.1476-5381.1993.tb13982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proud D., Kaplan A. P. Kinin formation: mechanisms and role in inflammatory disorders. Annu Rev Immunol. 1988;6:49–83. doi: 10.1146/annurev.iy.06.040188.000405. [DOI] [PubMed] [Google Scholar]
- Regoli D., Barabé J. Pharmacology of bradykinin and related kinins. Pharmacol Rev. 1980 Mar;32(1):1–46. [PubMed] [Google Scholar]
- Sharma J. N. Involvement of the kinin-forming system in the physiopathology of rheumatoid inflammation. Agents Actions Suppl. 1992;38(Pt 3):343–361. [PubMed] [Google Scholar]
- Sheikh I. A., Kaplan A. P. Assessment of kininases in rheumatic diseases and the effect of therapeutic agents. Arthritis Rheum. 1987 Feb;30(2):138–145. doi: 10.1002/art.1780300203. [DOI] [PubMed] [Google Scholar]
- Steranka L. R., DeHaas C. J., Vavrek R. J., Stewart J. M., Enna S. J., Snyder S. H. Antinociceptive effects of bradykinin antagonists. Eur J Pharmacol. 1987 Apr 14;136(2):261–262. doi: 10.1016/0014-2999(87)90723-0. [DOI] [PubMed] [Google Scholar]
- Steranka L. R., Manning D. C., DeHaas C. J., Ferkany J. W., Borosky S. A., Connor J. R., Vavrek R. J., Stewart J. M., Snyder S. H. Bradykinin as a pain mediator: receptors are localized to sensory neurons, and antagonists have analgesic actions. Proc Natl Acad Sci U S A. 1988 May;85(9):3245–3249. doi: 10.1073/pnas.85.9.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany C. W., Burch R. M. Bradykinin stimulates tumor necrosis factor and interleukin-1 release from macrophages. FEBS Lett. 1989 Apr 24;247(2):189–192. doi: 10.1016/0014-5793(89)81331-6. [DOI] [PubMed] [Google Scholar]
- Wirth K., Hock F. J., Albus U., Linz W., Alpermann H. G., Anagnostopoulos H., Henk S., Breipohl G., König W., Knolle J. Hoe 140 a new potent and long acting bradykinin-antagonist: in vivo studies. Br J Pharmacol. 1991 Mar;102(3):774–777. doi: 10.1111/j.1476-5381.1991.tb12249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deBlois D., Bouthillier J., Marceau F. Pharmacological modulation of the up-regulated responses to des-Arg9-bradykinin in vivo and in vitro. Immunopharmacology. 1989 May-Jun;17(3):187–198. doi: 10.1016/0162-3109(89)90047-7. [DOI] [PubMed] [Google Scholar]


