Abstract
1. The electrophysiological actions of the P2-purinoceptor agonists, adenosine 5'-triphosphate (ATP), 2-methylthioATP (2-meSATP), alpha, beta-methyleneATP (alpha, beta-meATP) and uridine 5'-triphosphate (UTP) were studied under concentration and voltage-clamp conditions in acutely dissociated rat tail artery smooth muscle cells. For comparison, their actions as vasoconstrictors were studied in intact ring preparations. 2. Rapid application of ATP (100 nM-1 microM) via a U-tube superfusion system activated concentration-dependent inward currents with a latency to onset of less than 3 ms. The inward current decayed by more than 95% during a 2 s application of 300 nM and 1 microM ATP. 3. 2-meSATP (100 mM-1 microM) and alpha, beta-meATP (100 nM-1 microM) also evoked transient inward currents. The agonist order of potency was ATP = 2-meSATP > or = alpha, beta-meATP. UTP (300 nM-1 microM) did not produce a change in the holding current. 4. A second application of ATP (300 nM and 1 microM) 10 min after the first, evoked currents which were one third of the initial amplitude. This decline was dependent upon activation of the P2-purinoceptor. Similar results were seen with 2-meSATP and alpha, beta-meATP (both 300 nM and 1 microM). Cross-desensitization was seen between ATP and 2-meSATP or alpha, beta-meATP. 5. Inward currents evoked by ATP, 2-meSATP and alpha, beta-meATP (all 1 microM) were abolished by the P2-purinoceptor antagonist suramin (100 microM).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

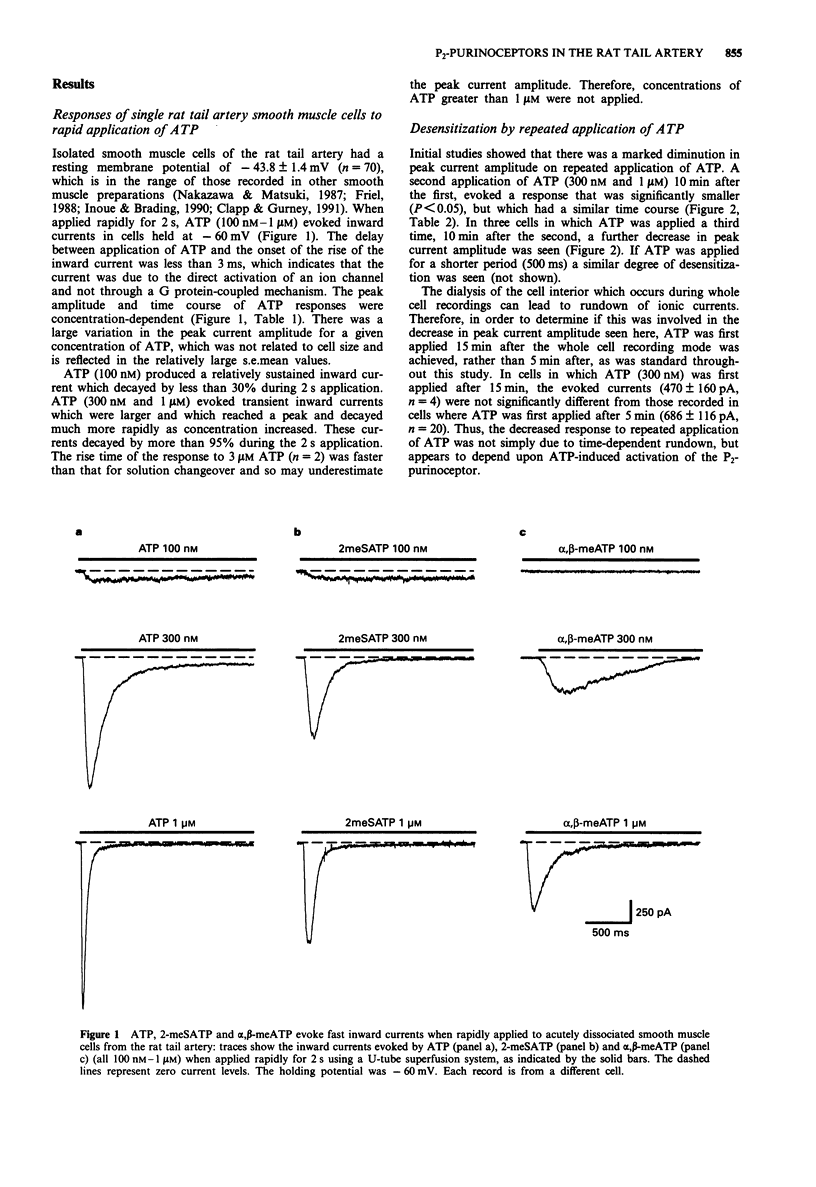
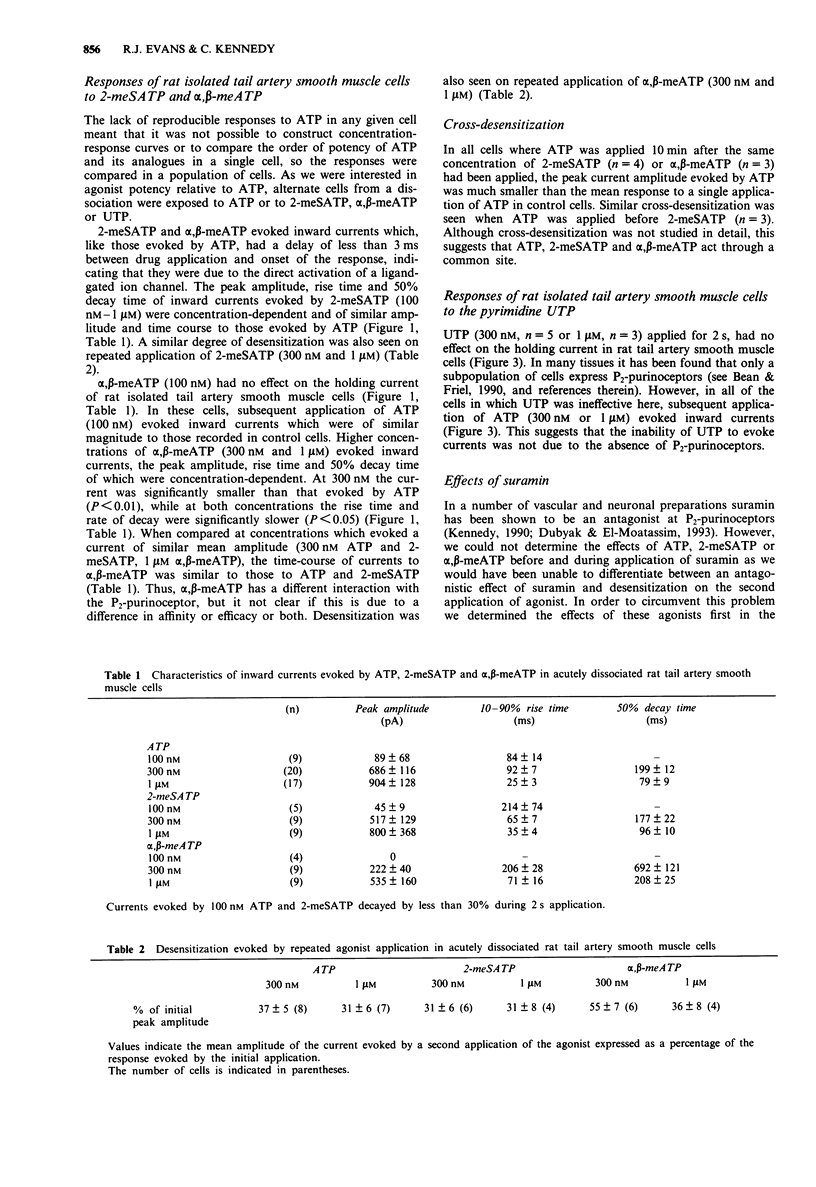
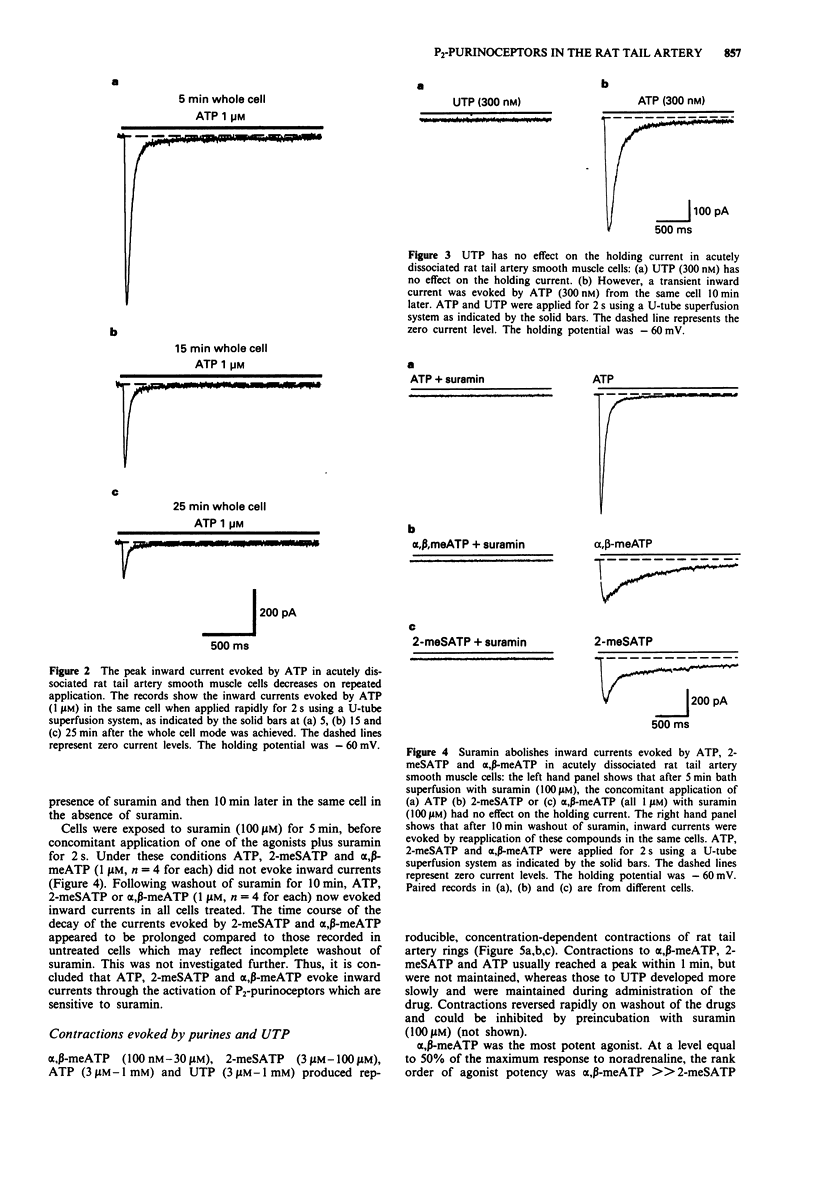
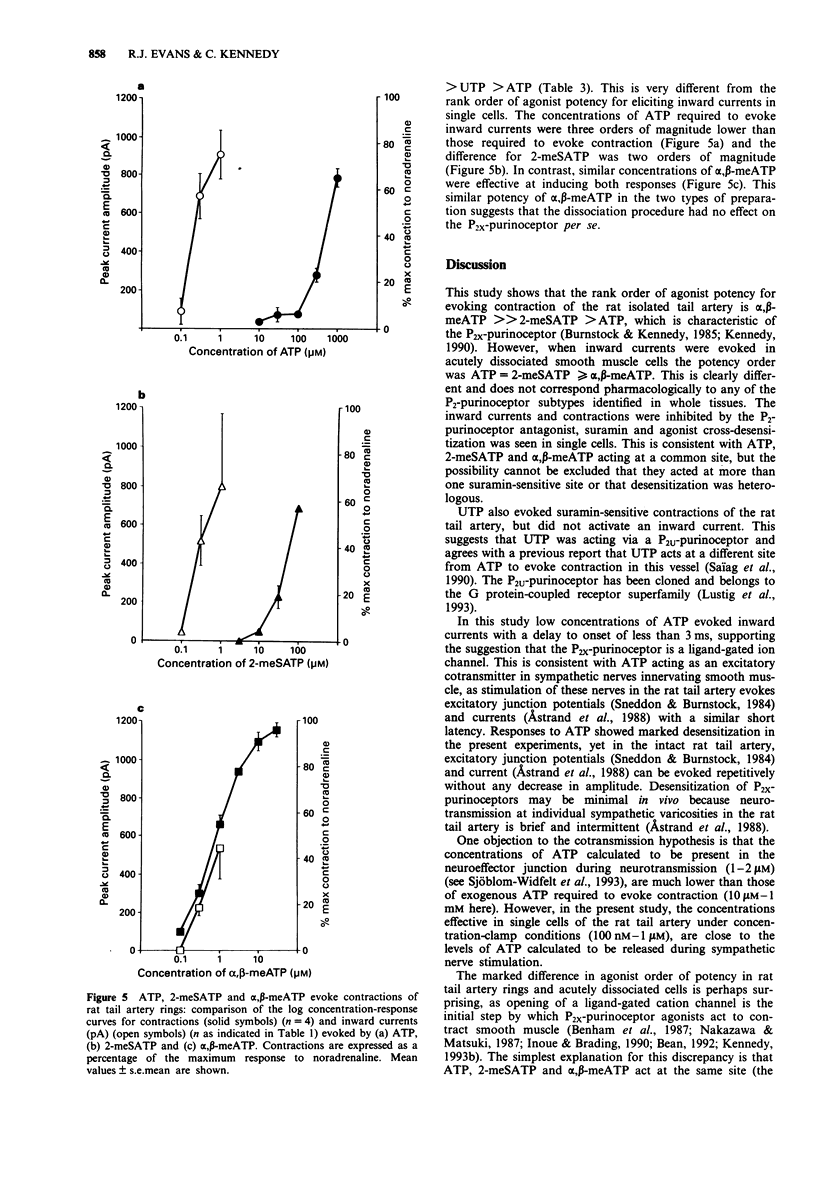


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astrand P., Brock J. A., Cunnane T. C. Time course of transmitter action at the sympathetic neuroeffector junction in rodent vascular and non-vascular smooth muscle. J Physiol. 1988 Jul;401:657–670. doi: 10.1113/jphysiol.1988.sp017185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P., Friel D. D. ATP-activated channels in excitable cells. Ion Channels. 1990;2:169–203. doi: 10.1007/978-1-4615-7305-0_5. [DOI] [PubMed] [Google Scholar]
- Bean B. P. Pharmacology and electrophysiology of ATP-activated ion channels. Trends Pharmacol Sci. 1992 Mar;13(3):87–90. doi: 10.1016/0165-6147(92)90032-2. [DOI] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Byrne N. G., Large W. A. Action of externally applied adenosine triphosphate on single smooth muscle cells dispersed from rabbit ear artery. J Physiol. 1987 Jun;387:473–488. doi: 10.1113/jphysiol.1987.sp016585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Tsien R. W. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987 Jul 16;328(6127):275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Bevan J. A., Osher J. V. A direct method for recording tension changes in the wall of small blood vessels in vitro. Agents Actions. 1972;2(5):257–260. doi: 10.1007/BF02087051. [DOI] [PubMed] [Google Scholar]
- Bo X., Burnstock G. Heterogeneous distribution of [3H]alpha,beta-methylene ATP binding sites in blood vessels. J Vasc Res. 1993 Mar-Apr;30(2):87–101. doi: 10.1159/000158980. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol. 1985;16(5):433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Overview. Purinergic mechanisms. Ann N Y Acad Sci. 1990;603:1–18. doi: 10.1111/j.1749-6632.1990.tb37657.x. [DOI] [PubMed] [Google Scholar]
- Clapp L. H., Gurney A. M. Outward currents in rabbit pulmonary artery cells dissociated with a new technique. Exp Physiol. 1991 Sep;76(5):677–693. doi: 10.1113/expphysiol.1991.sp003535. [DOI] [PubMed] [Google Scholar]
- Dubyak G. R., el-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol. 1993 Sep;265(3 Pt 1):C577–C606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- Edwards F. A., Gibb A. J., Colquhoun D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992 Sep 10;359(6391):144–147. doi: 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- Evans R. J., Derkach V., Surprenant A. ATP mediates fast synaptic transmission in mammalian neurons. Nature. 1992 Jun 11;357(6378):503–505. doi: 10.1038/357503a0. [DOI] [PubMed] [Google Scholar]
- Evans R. J., Surprenant A. Vasoconstriction of guinea-pig submucosal arterioles following sympathetic nerve stimulation is mediated by the release of ATP. Br J Pharmacol. 1992 Jun;106(2):242–249. doi: 10.1111/j.1476-5381.1992.tb14323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friel D. D. An ATP-sensitive conductance in single smooth muscle cells from the rat vas deferens. J Physiol. 1988 Jul;401:361–380. doi: 10.1113/jphysiol.1988.sp017167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon E. L., Pearson J. D., Dickinson E. S., Moreau D., Slakey L. L. The hydrolysis of extracellular adenine nucleotides by arterial smooth muscle cells. Regulation of adenosine production at the cell surface. J Biol Chem. 1989 Nov 15;264(32):18986–18995. [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illes P., Nörenberg W. Neuronal ATP receptors and their mechanism of action. Trends Pharmacol Sci. 1993 Feb;14(2):50–54. doi: 10.1016/0165-6147(93)90030-n. [DOI] [PubMed] [Google Scholar]
- Inoue R., Brading A. F. The properties of the ATP-induced depolarization and current in single cells isolated from the guinea-pig urinary bladder. Br J Pharmacol. 1990 Jul;100(3):619–625. doi: 10.1111/j.1476-5381.1990.tb15856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy C. P1- and P2-purinoceptor subtypes--an update. Arch Int Pharmacodyn Ther. 1990 Jan-Feb;303:30–50. [PubMed] [Google Scholar]
- Lustig K. D., Shiau A. K., Brake A. J., Julius D. Expression cloning of an ATP receptor from mouse neuroblastoma cells. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5113–5117. doi: 10.1073/pnas.90.11.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K., Matsuki N. Adenosine triphosphate-activated inward current in isolated smooth muscle cells from rat vas deferens. Pflugers Arch. 1987 Aug;409(6):644–646. doi: 10.1007/BF00584668. [DOI] [PubMed] [Google Scholar]
- O'Connor S. E., Dainty I. A., Leff P. Further subclassification of ATP receptors based on agonist studies. Trends Pharmacol Sci. 1991 Apr;12(4):137–141. doi: 10.1016/0165-6147(91)90530-6. [DOI] [PubMed] [Google Scholar]
- Saïag B., Milon D., Allaín H., Rault B., Van den Driessche J. Constriction of the smooth muscle of rat tail and femoral arteries and dog saphenous vein is induced by uridine triphosphate via 'pyrimidinoceptors', and by adenosine triphosphate via P2x purinoceptors. Blood Vessels. 1990;27(6):352–364. doi: 10.1159/000158829. [DOI] [PubMed] [Google Scholar]
- Sjöblom-Widfeldt N., Arner A., Nilsson H. Contraction of small mesenteric arteries induced by micromolar concentrations of ATP released from caged ATP. J Vasc Res. 1993 Jan-Feb;30(1):38–42. doi: 10.1159/000158973. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Burnstock G. ATP as a co-transmitter in rat tail artery. Eur J Pharmacol. 1984 Oct 30;106(1):149–152. doi: 10.1016/0014-2999(84)90688-5. [DOI] [PubMed] [Google Scholar]
- Webb T. E., Simon J., Krishek B. J., Bateson A. N., Smart T. G., King B. F., Burnstock G., Barnard E. A. Cloning and functional expression of a brain G-protein-coupled ATP receptor. FEBS Lett. 1993 Jun 14;324(2):219–225. doi: 10.1016/0014-5793(93)81397-i. [DOI] [PubMed] [Google Scholar]
- Welford L. A., Cusack N. J., Hourani S. M. ATP analogues and the guinea-pig taenia coli: a comparison of the structure-activity relationships of ectonucleotidases with those of the P2-purinoceptor. Eur J Pharmacol. 1986 Oct 7;129(3):217–224. doi: 10.1016/0014-2999(86)90431-0. [DOI] [PubMed] [Google Scholar]
- Welford L. A., Cusack N. J., Hourani S. M. The structure-activity relationships of ectonucleotidases and of excitatory P2-purinoceptors: evidence that dephosphorylation of ATP analogues reduces pharmacological potency. Eur J Pharmacol. 1987 Sep 2;141(1):123–130. doi: 10.1016/0014-2999(87)90418-3. [DOI] [PubMed] [Google Scholar]
- Xiong Z. L., Kitamura K., Kuriyama H. ATP activates cationic currents and modulates the calcium current through GTP-binding protein in rabbit portal vein. J Physiol. 1991;440:143–165. doi: 10.1113/jphysiol.1991.sp018701. [DOI] [PMC free article] [PubMed] [Google Scholar]


