Abstract
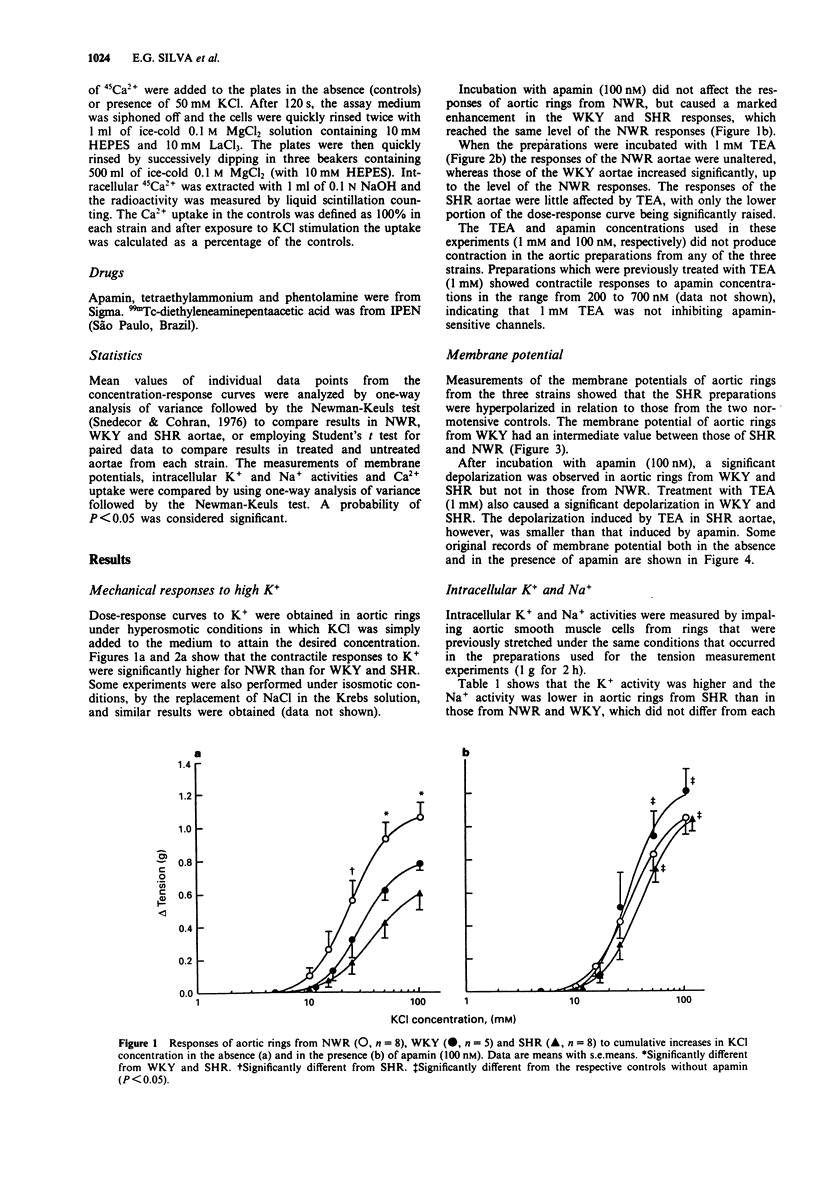
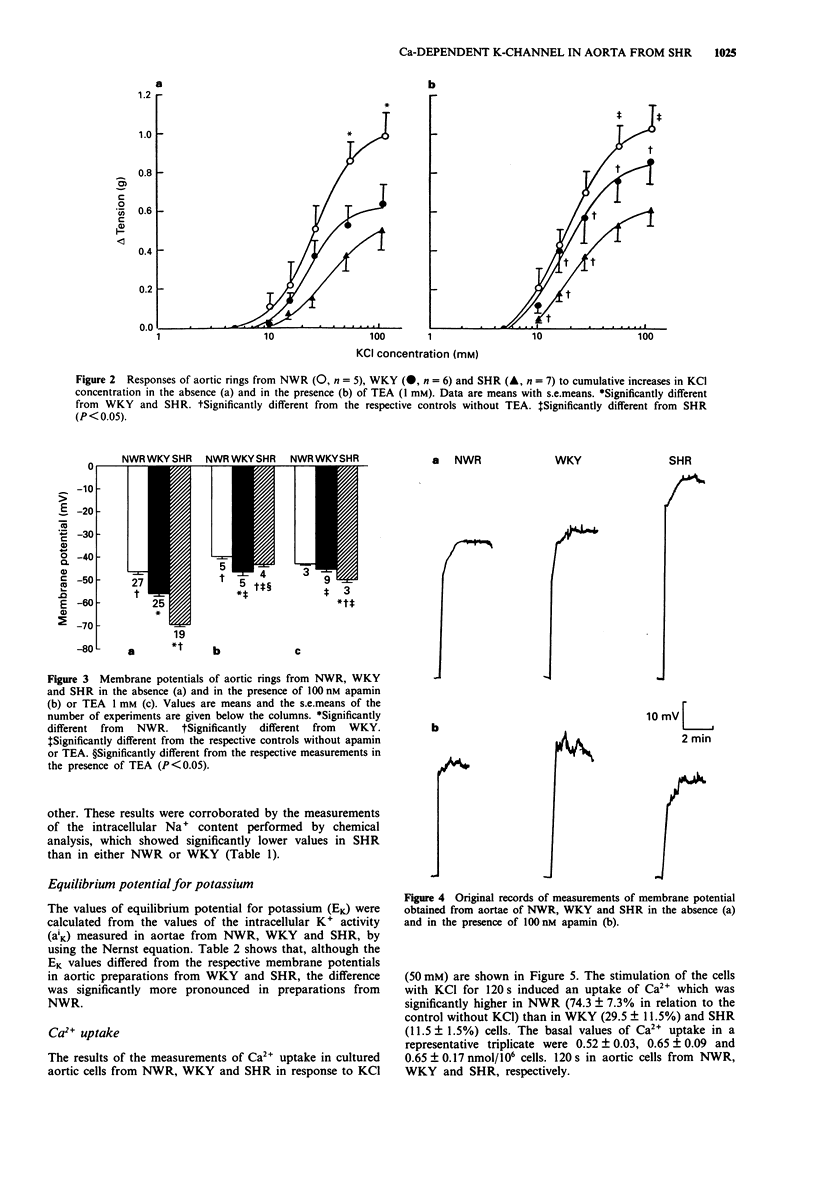
1. Contractile responses to KCl and membrane potentials were determined in aortic rings from spontaneously hypertensive rats (SHR), normotensive Wistar rats (NWR) and Wistar Kyoto rats (WKY) both in the absence and in the presence of the Ca(2+)-dependent K-channel blockers, apamin and tetraethylammonium (TEA). 2. Compared to NWR, aortic rings from WKY and SHR were less reactive and their Ca2+ uptake after stimulation with K+ was decreased. 3. Smooth muscle cell membrane potentials were higher in aortae from SHR and WKY than in NWR aortae, whereas SHR had higher K+ and lower Na+ intracellular activities than WKY and NWR, suggesting overactivity of the Na+/K+ pump in the hypertensive animals. 4. Treatment with apamin caused depolarization of WKY and SHR aortae, and increased their contractile responses to the same level as those of the NWR. Treatment with TEA also caused depolarization of aortae from WKY and SHR, but in the SHR the depolarization induced by TEA was smaller than that produced by apamin and the contractile responses to KCl did not reach the level of those of aortae from NWR. 5. It is concluded that overactivity of Ca(2+)-dependent K-channels in aortae of WKY and SHR contributes to their higher membrane potentials and lower responsiveness to vasoconstrictor stimuli. In SHR, an overactive Na+/K+ pump is also present, and the contribution of apamin-sensitive Ca(2+)-dependent K-channels to the membrane potential and reactivity appears to be more relevant than that of TEA-sensitive channels.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blatz A. L., Magleby K. L. Single apamin-blocked Ca-activated K+ channels of small conductance in cultured rat skeletal muscle. Nature. 1986 Oct 23;323(6090):718–720. doi: 10.1038/323718a0. [DOI] [PubMed] [Google Scholar]
- Bohr D. F., Webb R. C. Vascular smooth muscle membrane in hypertension. Annu Rev Pharmacol Toxicol. 1988;28:389–409. doi: 10.1146/annurev.pa.28.040188.002133. [DOI] [PubMed] [Google Scholar]
- Brayden J. E., Nelson M. T. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992 Apr 24;256(5056):532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- Cecchi X., Alvarez O., Wolff D. Characterization of a calcium-activated potassium channel from rabbit intestinal smooth muscle incorporated into planar bilayers. J Membr Biol. 1986;91(1):11–18. doi: 10.1007/BF01870210. [DOI] [PubMed] [Google Scholar]
- England S. K., Wooldridge T. A., Stekiel W. J., Rusch N. J. Enhanced single-channel K+ current in arterial membranes from genetically hypertensive rats. Am J Physiol. 1993 May;264(5 Pt 2):H1337–H1345. doi: 10.1152/ajpheart.1993.264.5.H1337. [DOI] [PubMed] [Google Scholar]
- Feres T., Paiva A. C., Paiva T. B. BK1 and BK2 bradykinin receptors in the rat duodenum smooth muscle. Br J Pharmacol. 1992 Dec;107(4):991–995. doi: 10.1111/j.1476-5381.1992.tb13396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feres T., Vianna L. M., Paiva A. C., Paiva T. B. Effect of treatment with vitamin D3 on the responses of the duodenum of spontaneously hypertensive rats to bradykinin and to potassium. Br J Pharmacol. 1992 Apr;105(4):881–884. doi: 10.1111/j.1476-5381.1992.tb09072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth D. R., DeFelice L. J. Electrical resistance and volume flow in glass microelectrodes. Can J Physiol Pharmacol. 1971 May;49(5):436–447. doi: 10.1139/y71-053. [DOI] [PubMed] [Google Scholar]
- Frediani-Neto E., Silva E. G., Paiva T. B., Paiva A. C. Increased intracellular Na+ and depolarization favor angiotension tachyphylaxis in rabbit aorta. Am J Physiol. 1991 Feb;260(2 Pt 2):H373–H378. doi: 10.1152/ajpheart.1991.260.2.H373. [DOI] [PubMed] [Google Scholar]
- French R. J., Wells J. B. Sodium ions as blocking agents and charge carriers in the potassium channel of the squid giant axon. J Gen Physiol. 1977 Dec;70(6):707–724. doi: 10.1085/jgp.70.6.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason M. M., Ratz P. H., Flaim S. F. Measurement of calcium influx in tethered rings of rabbit aorta under tension. Am J Physiol. 1985 Sep;249(3 Pt 2):H470–H476. doi: 10.1152/ajpheart.1985.249.3.H470. [DOI] [PubMed] [Google Scholar]
- Harder D. R., Hermsmeyer K. Membrane mechanisms in arterial hypertension. Hypertension. 1983 Jul-Aug;5(4):404–408. doi: 10.1161/01.hyp.5.4.404. [DOI] [PubMed] [Google Scholar]
- Harvey B. J., Kernan R. P. Intracellular ion activities in frog skin in relation to external sodium and effects of amiloride and/or ouabain. J Physiol. 1984 Apr;349:501–517. doi: 10.1113/jphysiol.1984.sp015170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A. W. Altered ion transport in vascular smooth muscle from spontaneously hypertensive rats. Influences of aldosterone, norepinephrine, and angiotensin. Circ Res. 1973 Nov;33(5):563–572. doi: 10.1161/01.res.33.5.563. [DOI] [PubMed] [Google Scholar]
- McAfee J. G., Gagne G., Atkins H. L., Kirchner P. T., Reba R. C., Blaufox M. D., Smith E. M. Biological distribution and excretion of DTPA labeled with Tc-99m and In-111. J Nucl Med. 1979 Dec;20(12):1273–1278. [PubMed] [Google Scholar]
- Moreland R. S., Major T. C., Webb R. C. Contractile responses to ouabain and K+-free solution in aorta from hypertensive rats. Am J Physiol. 1986 Apr;250(4 Pt 2):H612–H619. doi: 10.1152/ajpheart.1986.250.4.H612. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Nakamura A., Fine B., Aviv A. Blunted cGMP response to ANF in vascular smooth muscle cells of SHR. Am J Physiol. 1988 Nov;255(5 Pt 1):C573–C580. doi: 10.1152/ajpcell.1988.255.5.C573. [DOI] [PubMed] [Google Scholar]
- Nelson M. T., Patlak J. B., Worley J. F., Standen N. B. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol. 1990 Jul;259(1 Pt 1):C3–18. doi: 10.1152/ajpcell.1990.259.1.C3. [DOI] [PubMed] [Google Scholar]
- Romey G., Lazdunski M. The coexistence in rat muscle cells of two distinct classes of Ca2+-dependent K+ channels with different pharmacological properties and different physiological functions. Biochem Biophys Res Commun. 1984 Jan 30;118(2):669–674. doi: 10.1016/0006-291x(84)91355-x. [DOI] [PubMed] [Google Scholar]
- Rusch N. J., De Lucena R. G., Wooldridge T. A., England S. K., Cowley A. W., Jr A Ca(2+)-dependent K+ current is enhanced in arterial membranes of hypertensive rats. Hypertension. 1992 Apr;19(4):301–307. doi: 10.1161/01.hyp.19.4.301. [DOI] [PubMed] [Google Scholar]
- Sada T., Koike H., Ikeda M., Sato K., Ozaki H., Karaki H. Cytosolic free calcium of aorta in hypertensive rats. Chronic inhibition of angiotensin converting enzyme. Hypertension. 1990 Sep;16(3):245–251. doi: 10.1161/01.hyp.16.3.245. [DOI] [PubMed] [Google Scholar]
- Sadoshima J., Akaike N., Tomoike H., Kanaide H., Nakamura M. Ca-activated K channel in cultured smooth muscle cells of rat aortic media. Am J Physiol. 1988 Sep;255(3 Pt 2):H410–H418. doi: 10.1152/ajpheart.1988.255.3.H410. [DOI] [PubMed] [Google Scholar]
- Shibata S., Kurahashi K., Kuchii M. A possible etiology of contractility impairment of vascular smooth muscle from spontaneously hypertensive rats. J Pharmacol Exp Ther. 1973 May;185(2):406–417. [PubMed] [Google Scholar]
- Shoemaker R. L., Worrell R. T. Ca2(+)-sensitive K+ channel in aortic smooth muscle of rats. Proc Soc Exp Biol Med. 1991 Mar;196(3):325–332. doi: 10.3181/00379727-196-43196. [DOI] [PubMed] [Google Scholar]
- Singer J. J., Walsh J. V. Large conductance ca-activated k channels in smooth muscle cell membrane: reduction in unitary currents due to internal na ions. Biophys J. 1984 Jan;45(1):68–70. doi: 10.1016/s0006-3495(84)84112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector S., Fleisch J. H., Maling H. M., Brodie B. B. Vascular smooth muscle reactivity in normotensive and hypertensive rats. Science. 1969 Dec 5;166(3910):1300–1301. doi: 10.1126/science.166.3910.1300. [DOI] [PubMed] [Google Scholar]
- Tamura H., Hopp L., Kino M., Tokushige A., Searle B. M., Khalil F., Aviv A. Na+-K+ regulation in cultured vascular smooth muscle cell of the spontaneously hypertensive rat. Am J Physiol. 1986 Jun;250(6 Pt 1):C939–C947. doi: 10.1152/ajpcell.1986.250.6.C939. [DOI] [PubMed] [Google Scholar]
- Tomobe Y., Ishikawa T., Yanagisawa M., Kimura S., Masaki T., Goto K. Mechanisms of altered sensitivity to endothelin-1 between aortic smooth muscles of spontaneously hypertensive and Wistar-Kyoto rats. J Pharmacol Exp Ther. 1991 May;257(2):555–561. [PubMed] [Google Scholar]
- Van Breemen C., Farinas B. R., Gerba P., McNaughton E. D. Excitation-contraction coupling in rabbit aorta studied by the lanthanum method for measuring cellular calcium influx. Circ Res. 1972 Jan;30(1):44–54. doi: 10.1161/01.res.30.1.44. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Kanaide H., Nakamura M. Metabolism of glycosaminoglycans of cultured rat aortic smooth muscle cells altered during subculture. Br J Exp Pathol. 1983 Apr;64(2):156–165. [PMC free article] [PubMed] [Google Scholar]
- Yellen G. Relief of Na+ block of Ca2+-activated K+ channels by external cations. J Gen Physiol. 1984 Aug;84(2):187–199. doi: 10.1085/jgp.84.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]


