Abstract
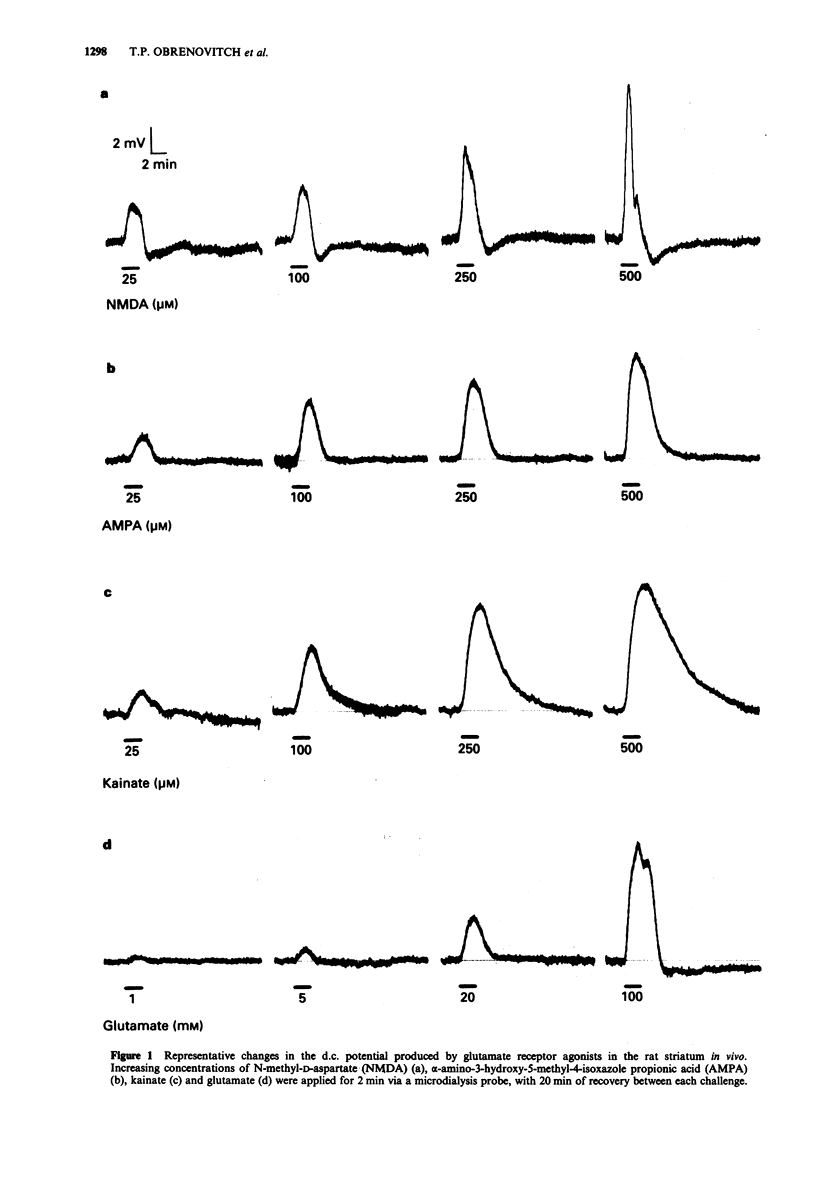
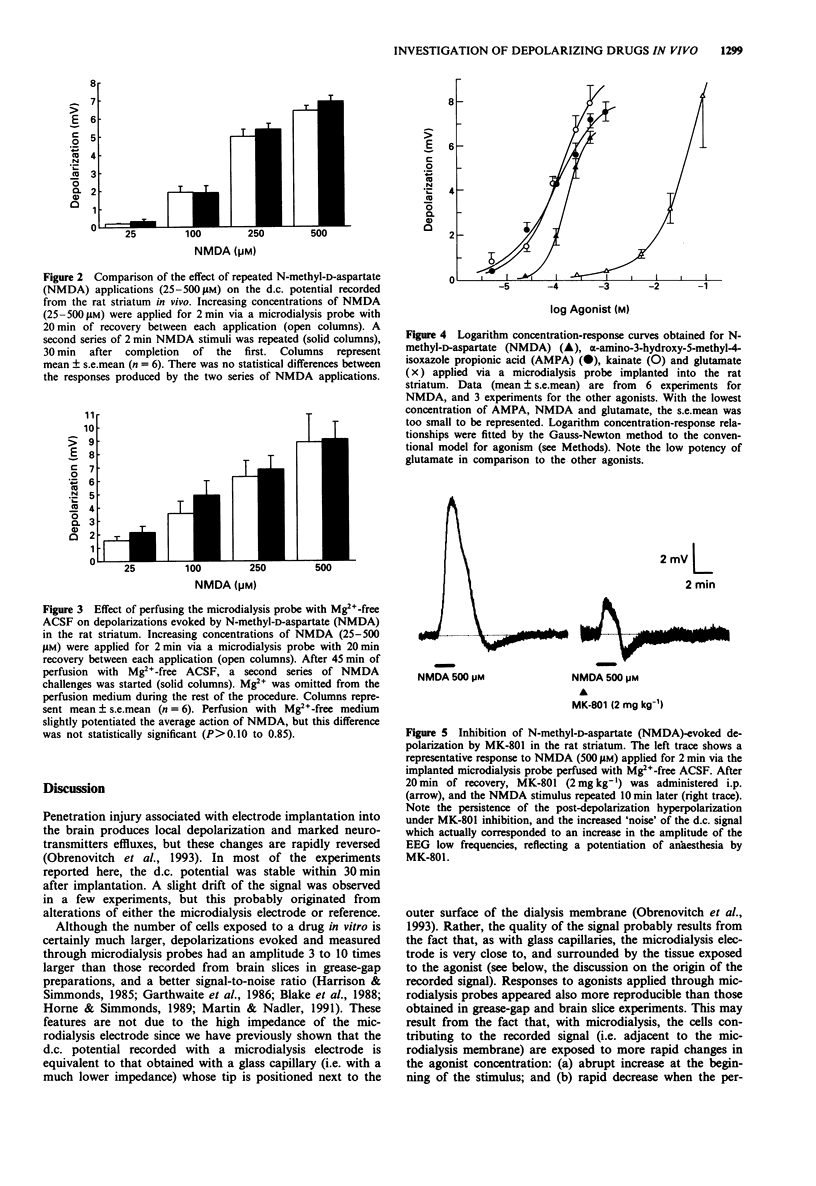
1. The purpose of this study was to examine whether depolarizations evoked by excitatory amino acids can be recorded quantitatively, in vivo, with a microelectrode incorporated within a microdialysis probe. 2. Microdialysis probes incorporating a chlorided silver wire were implanted in the striatum of anaesthetized rats and perfused with artificial cerebrospinal fluid (ACSF). Increasing concentrations of excitatory amino acids were applied for 2 min via the microdialysis probe, and the extracellular direct current (d.c.) potential was recorded between the microdialysis electrode and a reference electrode placed under the scalp. 3. N-methyl-D-aspartate (NMDA, 25-500 microM), alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA, 5-1000 microM), kainate (5-500 microM), and glutamate (0.25-100 mM) evoked concentration-dependent depolarizations with maxima ranging from 7 to 10 mV, i.e. 3 to 10 times larger than those recorded from brain slices in vitro. Depolarizations evoked by glutamate receptor agonists applied by microdialysis shared several features with those recorded from brain slices. The most characteristic were: steep onset and recovery of NMDA and glutamate responses; marked post-depolarization hyperpolarization with NMDA; and very slow recovery after kainate application. At high concentrations (500 microM), NMDA occasionally initiated spreading depression. The relative potency of glutamate and NMDA was of the same order of magnitude to that obtained with the cortical wedge and hippocampal slices, glutamate being 100 to 400 times less potent than NMDA. 4. Two consecutive series of NMDA-stimuli within the same procedure evoked comparable depolarizations, indicating that reliable quantitative analysis of drug action can be performed, with each animal serving as its own control.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beal M. F., Ferrante R. J., Swartz K. J., Kowall N. W. Chronic quinolinic acid lesions in rats closely resemble Huntington's disease. J Neurosci. 1991 Jun;11(6):1649–1659. doi: 10.1523/JNEUROSCI.11-06-01649.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste H., Hüttemeier P. C. Microdialysis--theory and application. Prog Neurobiol. 1990;35(3):195–215. doi: 10.1016/0301-0082(90)90027-e. [DOI] [PubMed] [Google Scholar]
- Black J. W., Leff P. Operational models of pharmacological agonism. Proc R Soc Lond B Biol Sci. 1983 Dec 22;220(1219):141–162. doi: 10.1098/rspb.1983.0093. [DOI] [PubMed] [Google Scholar]
- Blake J. F., Brown M. W., Collingridge G. L. A quantitative study of the actions of excitatory amino acids and antagonists in rat hippocampal slices. Br J Pharmacol. 1988 Sep;95(1):291–299. doi: 10.1111/j.1476-5381.1988.tb16576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Lester R. A. Excitatory amino acid receptors in the vertebrate central nervous system. Pharmacol Rev. 1989 Jun;41(2):143–210. [PubMed] [Google Scholar]
- Dingledine R., McBain C. J., McNamara J. O. Excitatory amino acid receptors in epilepsy. Trends Pharmacol Sci. 1990 Aug;11(8):334–338. doi: 10.1016/0165-6147(90)90238-4. [DOI] [PubMed] [Google Scholar]
- Garthwaite J., Garthwaite G., Hajós F. Amino acid neurotoxicity: relationship to neuronal depolarization in rat cerebellar slices. Neuroscience. 1986 Jun;18(2):449–460. doi: 10.1016/0306-4522(86)90165-x. [DOI] [PubMed] [Google Scholar]
- Harrison N. L., Simmonds M. A. Quantitative studies on some antagonists of N-methyl D-aspartate in slices of rat cerebral cortex. Br J Pharmacol. 1985 Feb;84(2):381–391. doi: 10.1111/j.1476-5381.1985.tb12922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne A. L., Simmonds M. A. The pharmacology of quisqualate and AMPA in the cerebral cortex of the rat in vitro. Neuropharmacology. 1989 Oct;28(10):1113–1118. doi: 10.1016/0028-3908(89)90125-1. [DOI] [PubMed] [Google Scholar]
- Kelso S. R., Nelson T. E., Leonard J. P. Protein kinase C-mediated enhancement of NMDA currents by metabotropic glutamate receptors in Xenopus oocytes. J Physiol. 1992 Apr;449:705–718. doi: 10.1113/jphysiol.1992.sp019110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum B., Garthwaite J. Excitatory amino acid neurotoxicity and neurodegenerative disease. Trends Pharmacol Sci. 1990 Sep;11(9):379–387. doi: 10.1016/0165-6147(90)90184-a. [DOI] [PubMed] [Google Scholar]
- Meldrum B. Protection against ischaemic neuronal damage by drugs acting on excitatory neurotransmission. Cerebrovasc Brain Metab Rev. 1990 Spring;2(1):27–57. [PubMed] [Google Scholar]
- Millan M. H., Obrenovitch T. P., Sarna G. S., Lok S. Y., Symon L., Meldrum B. S. Changes in rat brain extracellular glutamate concentration during seizures induced by systemic picrotoxin or focal bicuculline injection: an in vivo dialysis study with on-line enzymatic detection. Epilepsy Res. 1991 Jul;9(2):86–91. doi: 10.1016/0920-1211(91)90017-a. [DOI] [PubMed] [Google Scholar]
- Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992 Oct 23;258(5082):597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- Obrenovitch T. P., Urenjak J., Richards D. A., Ueda Y., Curzon G., Symon L. Extracellular neuroactive amino acids in the rat striatum during ischaemia: comparison between penumbral conditions and ischaemia with sustained anoxic depolarisation. J Neurochem. 1993 Jul;61(1):178–186. doi: 10.1111/j.1471-4159.1993.tb03553.x. [DOI] [PubMed] [Google Scholar]
- Paulsen R. E., Fonnum F. Role of glial cells for the basal and Ca2+-dependent K+-evoked release of transmitter amino acids investigated by microdialysis. J Neurochem. 1989 Jun;52(6):1823–1829. doi: 10.1111/j.1471-4159.1989.tb07263.x. [DOI] [PubMed] [Google Scholar]
- Seeburg P. H. The TINS/TiPS Lecture. The molecular biology of mammalian glutamate receptor channels. Trends Neurosci. 1993 Sep;16(9):359–365. doi: 10.1016/0166-2236(93)90093-2. [DOI] [PubMed] [Google Scholar]
- Tao R., Hjorth S. Differences in the in vitro and in vivo 5-hydroxytryptamine extraction performance among three common microdialysis membranes. J Neurochem. 1992 Nov;59(5):1778–1785. doi: 10.1111/j.1471-4159.1992.tb11010.x. [DOI] [PubMed] [Google Scholar]
- Teitelbaum J. S., Zatorre R. J., Carpenter S., Gendron D., Evans A. C., Gjedde A., Cashman N. R. Neurologic sequelae of domoic acid intoxication due to the ingestion of contaminated mussels. N Engl J Med. 1990 Jun 21;322(25):1781–1787. doi: 10.1056/NEJM199006213222505. [DOI] [PubMed] [Google Scholar]
- Wahl F., Obrenovitch T. P., Hardy A. M., Plotkine M., Boulu R., Symon L. Extracellular glutamate during focal cerebral ischaemia in rats: time course and calcium dependency. J Neurochem. 1994 Sep;63(3):1003–1011. doi: 10.1046/j.1471-4159.1994.63031003.x. [DOI] [PubMed] [Google Scholar]
- Watanabe A., Ishida Y., Honda H., Kobayashi M., Ohizumi Y. Ca(2+)-dependent aggregation of rabbit platelets induced by maitotoxin, a potent marine toxin, isolated from a dinoflagellate. Br J Pharmacol. 1993 May;109(1):29–36. doi: 10.1111/j.1476-5381.1993.tb13527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


