Abstract
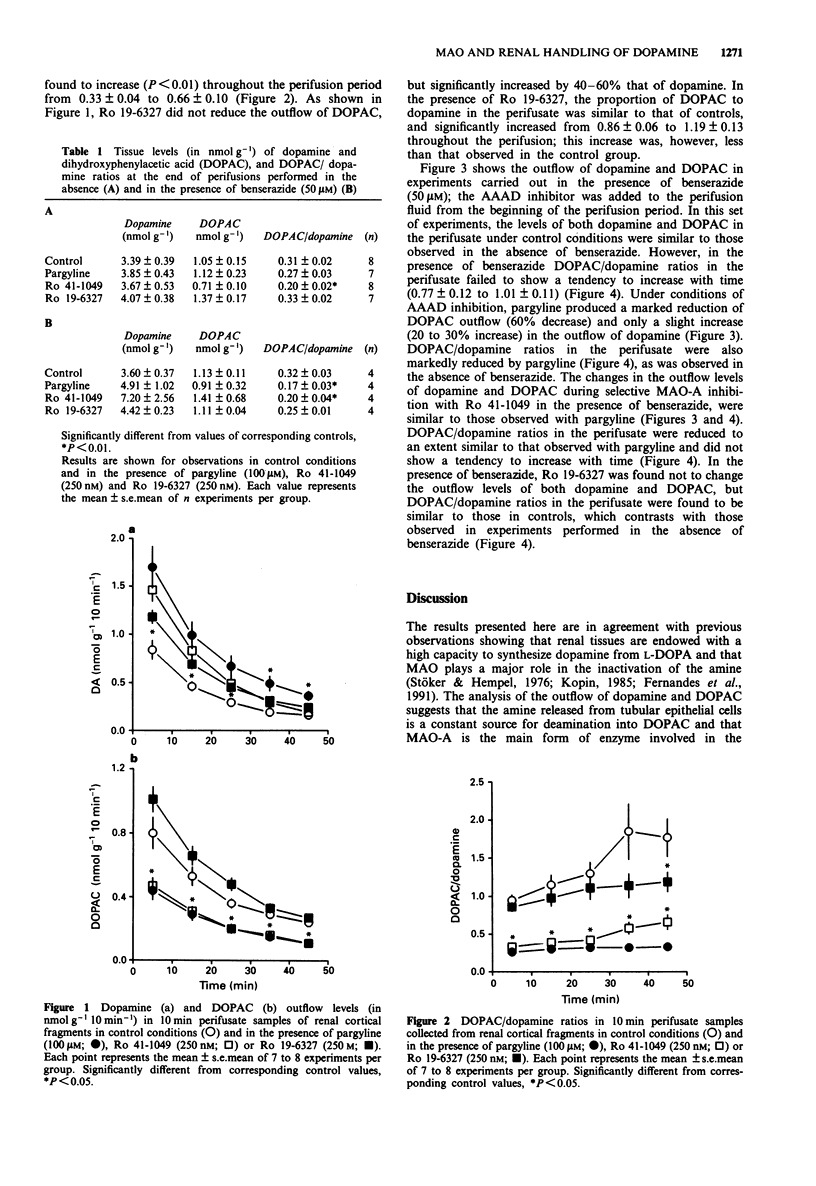
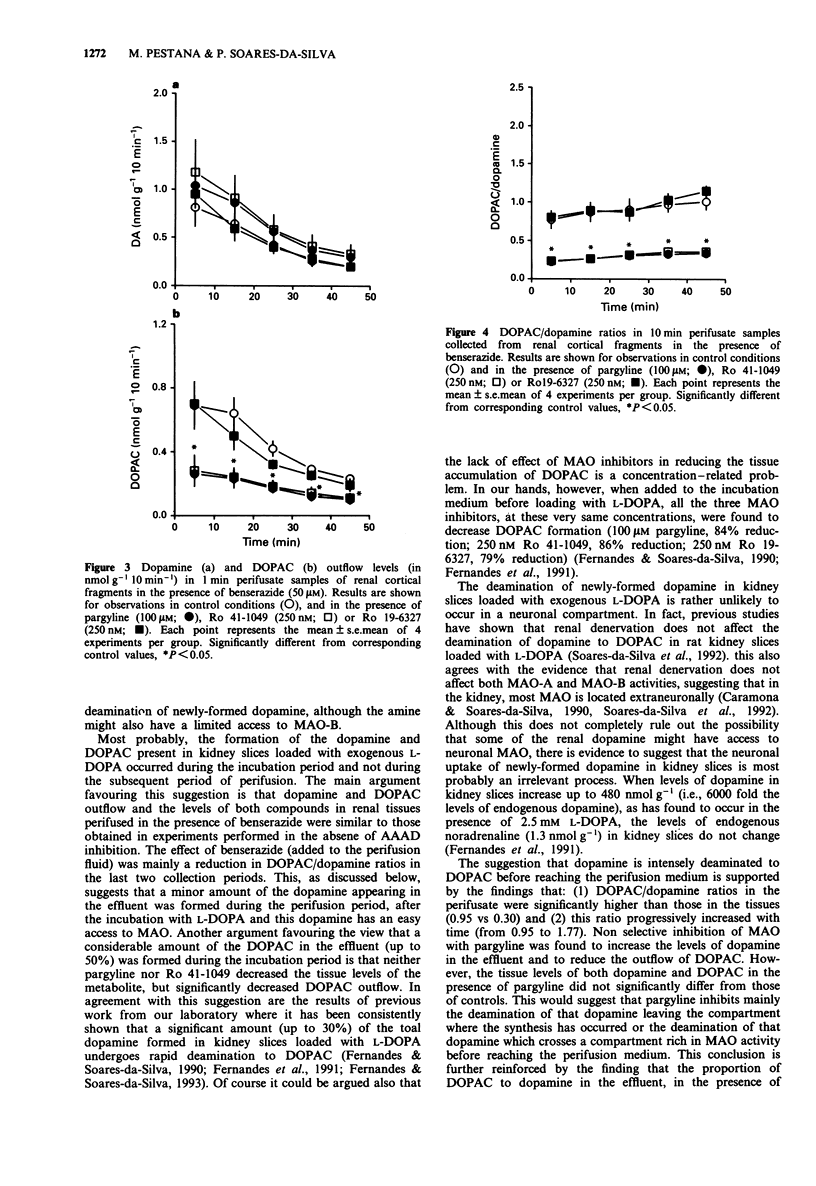
1. The influence of pargyline and of selective inhibitors of type A and B monoamine oxidase (MAO), Ro 41-1049 and Ro 19-6327 respectively, on the outflow of dopamine and 3,4-dihydroxyphenylacetic acid (DOPAC) in slices of rat renal cortex loaded with exogenous L-3,4-dihydroxyphenylalanine (L-DOPA) was examined. Dopamine and DOPAC in the tissues and in the effluent were assayed by means of h.p.l.c. with electrochemical detection. 2. The levels of newly-formed dopamine and DOPAC in the perifusate decreased progressively with time. In control conditions, DOPAC/dopamine ratios in the perifusate were 3 to 5 fold those in the tissue and were found to increase progressively with time. The addition of pargyline (100 microM), produced a marked decrease in the outflow levels of DOPAC (45 to 54% reduction) and significantly increased the levels of dopamine in the effluent (102 to 158% increase); DOPAC/dopamine ratios in the perifusate remained stable throughout the perifusion and were similar to those found in the tissues. The addition of the MAO-A inhibitor Ro 41-1049 to the perifusion fluid also significantly decreased DOPAC outflow (41% to 54% reduction) and increased dopamine outflow (19% to 80% increase). In the presence of Ro 41-1049 DOPAC/dopamine ratios in the perifusate were lower (P < 0.01) than in controls; in contrast with the effect of pargyline, this ratio was found to increase (P < 0.01) throughout the perifusion period. Ro 19-6327 did not reduce the outflow of DOPAC, but significantly increased (by 40-60%) that of dopamine.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aperia A., Bertorello A., Seri I. Dopamine causes inhibition of Na+-K+-ATPase activity in rat proximal convoluted tubule segments. Am J Physiol. 1987 Jan;252(1 Pt 2):F39–F45. doi: 10.1152/ajprenal.1987.252.1.F39. [DOI] [PubMed] [Google Scholar]
- Baines A. D., Chan W. Production of urine free dopamine from DOPA; a micropuncture study. Life Sci. 1980 Jan 28;26(4):253–259. doi: 10.1016/0024-3205(80)90334-3. [DOI] [PubMed] [Google Scholar]
- Caramona M. M., Soares-da-Silva P. Evidence for an extraneuronal location of monoamine oxidase in renal tissues. Naunyn Schmiedebergs Arch Pharmacol. 1990 May;341(5):411–413. doi: 10.1007/BF00176332. [DOI] [PubMed] [Google Scholar]
- Chan Y. L. Cellular mechanisms of renal tubular transport of I-dopa and its derivatives in the rat: microperfusion studies. J Pharmacol Exp Ther. 1976 Oct;199(1):17–24. [PubMed] [Google Scholar]
- Da Prada M., Kettler R., Keller H. H., Cesura A. M., Richards J. G., Saura Marti J., Muggli-Maniglio D., Wyss P. C., Kyburz E., Imhof R. From moclobemide to Ro 19-6327 and Ro 41-1049: the development of a new class of reversible, selective MAO-A and MAO-B inhibitors. J Neural Transm Suppl. 1990;29:279–292. doi: 10.1007/978-3-7091-9050-0_27. [DOI] [PubMed] [Google Scholar]
- Felder C. C., Campbell T., Albrecht F., Jose P. A. Dopamine inhibits Na(+)-H+ exchanger activity in renal BBMV by stimulation of adenylate cyclase. Am J Physiol. 1990 Aug;259(2 Pt 2):F297–F303. doi: 10.1152/ajprenal.1990.259.2.F297. [DOI] [PubMed] [Google Scholar]
- Fernandes M. H., Pestana M., Soares-da-Silva P. Deamination of newly-formed dopamine in rat renal tissues. Br J Pharmacol. 1991 Mar;102(3):778–782. doi: 10.1111/j.1476-5381.1991.tb12250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes M. H., Soares-da-Silva P. Effects of MAO-A and MAO-B selective inhibitors Ro 41-1049 and Ro 19-6327 on the deamination of newly formed dopamine in the rat kidney. J Pharmacol Exp Ther. 1990 Dec;255(3):1309–1313. [PubMed] [Google Scholar]
- Fernandes M. H., Soares-da-Silva P. Type A and B monoamine oxidase activities in the human and rat kidney. Acta Physiol Scand. 1992 Aug;145(4):363–367. doi: 10.1111/j.1748-1716.1992.tb09376.x. [DOI] [PubMed] [Google Scholar]
- Jose P. A., Raymond J. R., Bates M. D., Aperia A., Felder R. A., Carey R. M. The renal dopamine receptors. J Am Soc Nephrol. 1992 Feb;2(8):1265–1278. doi: 10.1681/ASN.V281265. [DOI] [PubMed] [Google Scholar]
- Kopin I. J. Catecholamine metabolism: basic aspects and clinical significance. Pharmacol Rev. 1985 Dec;37(4):333–364. [PubMed] [Google Scholar]
- Pestana M., Soares-da-Silva P. The renal handling of dopamine originating from L-dopa and gamma-glutamyl-L-dopa. Br J Pharmacol. 1994 Jun;112(2):417–422. doi: 10.1111/j.1476-5381.1994.tb13088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saura J., Kettler R., Da Prada M., Richards J. G. Quantitative enzyme radioautography with 3H-Ro 41-1049 and 3H-Ro 19-6327 in vitro: localization and abundance of MAO-A and MAO-B in rat CNS, peripheral organs, and human brain. J Neurosci. 1992 May;12(5):1977–1999. doi: 10.1523/JNEUROSCI.12-05-01977.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siragy H. M., Felder R. A., Howell N. L., Chevalier R. L., Peach M. J., Carey R. M. Evidence that intrarenal dopamine acts as a paracrine substance at the renal tubule. Am J Physiol. 1989 Sep;257(3 Pt 2):F469–F477. doi: 10.1152/ajprenal.1989.257.3.F469. [DOI] [PubMed] [Google Scholar]
- Soares-da-Silva P., Fernandes M. H., Albino-Teixeira A., Azevedo I., Pestana M. Brief transient ischemia induces long-term depletion of norepinephrine without affecting the aromatic amino acid decarboxylase and monoamine oxidase activities in the rat kidney. J Pharmacol Exp Ther. 1992 Feb;260(2):902–908. [PubMed] [Google Scholar]
- Stöcker W., Hempel K. Inactivation and excretion of dopamine by the cat kidney in vivo. Naunyn Schmiedebergs Arch Pharmacol. 1976 Nov;295(2):123–126. doi: 10.1007/BF00499443. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Nakane H., Kawamura M., Yoshizawa M., Takeshita E., Saruta T. Excretion and metabolism of dopa and dopamine by isolated perfused rat kidney. Am J Physiol. 1984 Sep;247(3 Pt 1):E285–E290. doi: 10.1152/ajpendo.1984.247.3.E285. [DOI] [PubMed] [Google Scholar]
- Wedeen R. P., Weiner B. The distribution of p-aminohippuric acid in rat kidney slices. I. Tubular localization. Kidney Int. 1973 Apr;3(4):205–213. doi: 10.1038/ki.1973.33. [DOI] [PubMed] [Google Scholar]


