Abstract
1. A1 adenosine receptors were investigated by radioligand binding and functional studies in slices and particulate preparations from guinea-pig cerebral cortex. 2. Binding of the adenosine receptor antagonist radioligand, 8-cyclopentyl-[3H]-1,3-dipropylxanthine (DPCPX) to guinea-pig cerebral cortical membranes exhibited high density (1410 +/- 241 fmol mg-1 protein) and high affinity (Kd 3.8 +/- 0.3 nM). 3. [3H]-DPCPX binding to guinea-pig cerebral cortical membranes was displaced in a monophasic manner by adenosine receptor antagonists with the rank order of affinity (Ki values, nM): DPCPX (6) < xanthine amine congener (XAC, 153) < PD 115,199 (308). 4. Agonist displacement of [3H]-DPCPX binding was biphasic and exhibited the following rank order at the low affinity site (Ki values): 2-chloro-N6-cyclopentyl-adenosine (CCPA, 513 nM) = N6-R-phenylisopropyladenosine (R-PIA, 526 nM) = N6-cyclopentyladenosine (CPA, 532 nM) < 2-chloroadenosine (2CA, 3.2 microM) = 5'-N-ethylcarboxamidoadenosine (NECA, 4.6 microM) < N6-S-phenylisopropyladenosine (S-PIA, 19.9 microM). 5. In cerebral cortical slices, [3H]-DPCPX binding was displaced by antagonists and agonists in an apparently monophasic manner with the rank order of affinity (Ki values, nM): DPCPX (14) < XAC (45) < R-PIA (266) < PD 115,199 (666) < S-PIA (21000). 6. Cyclic AMP accumulation stimulated by 30 microM forskolin in guinea-pig cerebral cortical slices was inhibited by R-PIA, CCPA and CPA up to 1 microM in a concentration-dependent fashion with IC50 values of 14, 18, and 22 nM, respectively. All three analogues inhibited the forskolin response to a similar extent (82-93% inhibition).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

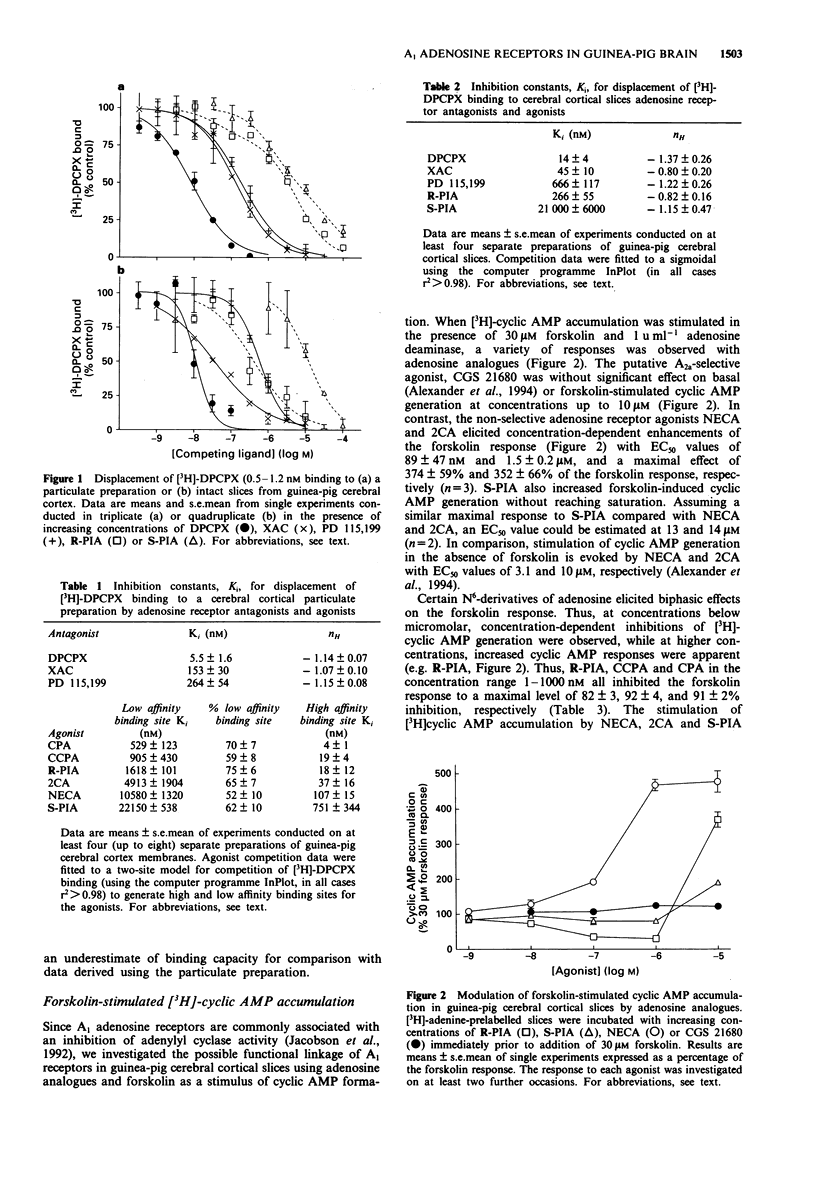



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
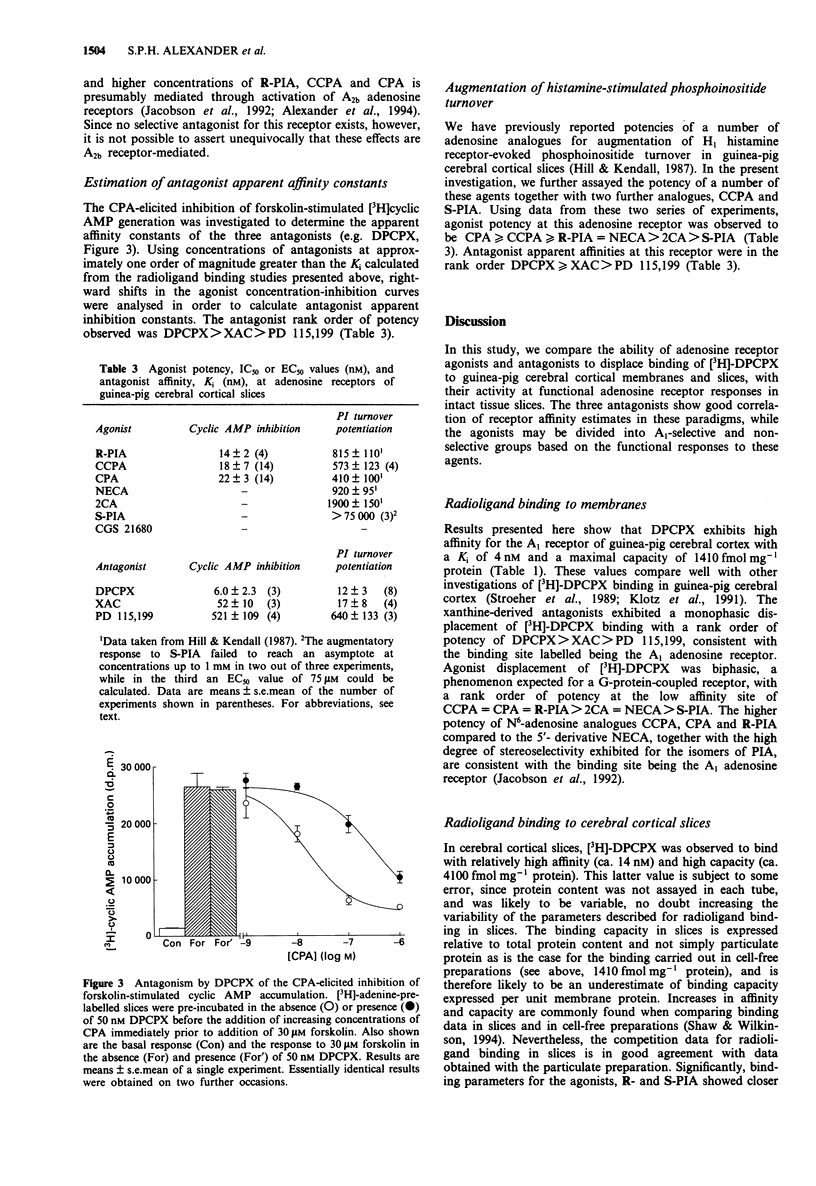
- Alexander S. P., Kendall D. A., Hill S. J. Differences in the adenosine receptors modulating inositol phosphates and cyclic AMP accumulation in mammalian cerebral cortex. Br J Pharmacol. 1989 Dec;98(4):1241–1248. doi: 10.1111/j.1476-5381.1989.tb12670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander S. P., Losinski A., Kendall D. A., Hill S. J. A comparison of A2 adenosine receptor-induced cyclic AMP generation in cerebral cortex and relaxation of pre-contracted aorta. Br J Pharmacol. 1994 Jan;111(1):185–190. doi: 10.1111/j.1476-5381.1994.tb14042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns R. F., Fergus J. H., Badger E. W., Bristol J. A., Santay L. A., Hartman J. D., Hays S. J., Huang C. C. Binding of the A1-selective adenosine antagonist 8-cyclopentyl-1,3-dipropylxanthine to rat brain membranes. Naunyn Schmiedebergs Arch Pharmacol. 1987 Jan;335(1):59–63. doi: 10.1007/BF00165037. [DOI] [PubMed] [Google Scholar]
- Cartmell J., Kemp J. A., Alexander S. P., Hill S. J., Kendall D. A. Inhibition of forskolin-stimulated cyclic AMP formation by 1-aminocyclopentane-trans-1,3-dicarboxylate in guinea-pig cerebral cortical slices. J Neurochem. 1992 May;58(5):1964–1966. doi: 10.1111/j.1471-4159.1992.tb10077.x. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Daly J. W., Butts-Lamb P., Padgett W. Subclasses of adenosine receptors in the central nervous system: interaction with caffeine and related methylxanthines. Cell Mol Neurobiol. 1983 Mar;3(1):69–80. doi: 10.1007/BF00734999. [DOI] [PubMed] [Google Scholar]
- Dickenson J. M., Hill S. J. Intracellular cross-talk between receptors coupled to phospholipase C via pertussis toxin-sensitive and insensitive G-proteins in DDT1MF-2 cells. Br J Pharmacol. 1993 Jul;109(3):719–724. doi: 10.1111/j.1476-5381.1993.tb13633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie T. V., Fredholm B. B. Adenosine modulation of synaptic responses in rat hippocampus: possible role of inhibition or activation of adenylate cyclase. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1985;19:259–272. [PubMed] [Google Scholar]
- Fredholm B. B., Jonzon B., Lindgren E., Lindström K. Adenosine receptors mediating cyclic AMP production in the rat hippocampus. J Neurochem. 1982 Jul;39(1):165–175. doi: 10.1111/j.1471-4159.1982.tb04715.x. [DOI] [PubMed] [Google Scholar]
- Fredholm B. B., Jonzon B., Lindström K. Effect of adenosine receptor agonists and other compounds on cyclic AMP accumulation in forskolin-treated hippocampal slices. Naunyn Schmiedebergs Arch Pharmacol. 1986 Feb;332(2):173–178. doi: 10.1007/BF00511409. [DOI] [PubMed] [Google Scholar]
- Gerwins P., Nordstedt C., Fredholm B. B. Characterization of adenosine A1 receptors in intact DDT1 MF-2 smooth muscle cells. Mol Pharmacol. 1990 Nov;38(5):660–666. [PubMed] [Google Scholar]
- Hernández F., Kendall D. A., Alexander S. P. Adenosine receptor-induced second messenger production in adult guinea-pig cerebellum. Br J Pharmacol. 1993 Nov;110(3):1085–1090. doi: 10.1111/j.1476-5381.1993.tb13925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill S. J., Kendall D. A. Studies on the adenosine-receptor mediating the augmentation of histamine-induced inositol phospholipid hydrolysis in guinea-pig cerebral cortex. Br J Pharmacol. 1987 Jul;91(3):661–669. doi: 10.1111/j.1476-5381.1987.tb11260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iredale P. A., Alexander S. P., Hill S. J. Coupling of a transfected human brain A1 adenosine receptor in CHO-K1 cells to calcium mobilisation via a pertussis toxin-sensitive mechanism. Br J Pharmacol. 1994 Apr;111(4):1252–1256. doi: 10.1111/j.1476-5381.1994.tb14880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson K. A., van Galen P. J., Williams M. Adenosine receptors: pharmacology, structure-activity relationships, and therapeutic potential. J Med Chem. 1992 Feb 7;35(3):407–422. doi: 10.1021/jm00081a001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz K. N., Keil R., Zimmer F. J., Schwabe U. Guanine nucleotide effects on 8-cyclopentyl-1,3-[3H]dipropylxanthine binding to membrane-bound and solubilized A1 adenosine receptors of rat brain. J Neurochem. 1990 Jun;54(6):1988–1994. doi: 10.1111/j.1471-4159.1990.tb04902.x. [DOI] [PubMed] [Google Scholar]
- Klotz K. N., Vogt H., Tawfik-Schlieper H. Comparison of A1 adenosine receptors in brain from different species by radioligand binding and photoaffinity labelling. Naunyn Schmiedebergs Arch Pharmacol. 1991 Feb;343(2):196–201. doi: 10.1007/BF00168610. [DOI] [PubMed] [Google Scholar]
- Lohse M. J., Klotz K. N., Lindenborn-Fotinos J., Reddington M., Schwabe U., Olsson R. A. 8-Cyclopentyl-1,3-dipropylxanthine (DPCPX)--a selective high affinity antagonist radioligand for A1 adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol. 1987 Aug;336(2):204–210. doi: 10.1007/BF00165806. [DOI] [PubMed] [Google Scholar]
- Londos C., Cooper D. M., Wolff J. Subclasses of external adenosine receptors. Proc Natl Acad Sci U S A. 1980 May;77(5):2551–2554. doi: 10.1073/pnas.77.5.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons W. J., Ramkumar V., Stiles G. L. The new cardiotonic agent sulmazole is an A1 adenosine receptor antagonist and functionally blocks the inhibitory regulator, Gi. Mol Pharmacol. 1988 Apr;33(4):441–448. [PubMed] [Google Scholar]
- Prater M. R., Taylor H., Munshi R., Linden J. Indirect effect of guanine nucleotides on antagonist binding to A1 adenosine receptors: occupation of cryptic binding sites by endogenous vesicular adenosine. Mol Pharmacol. 1992 Nov;42(5):765–772. [PubMed] [Google Scholar]
- Ramkumar V., Stiles G. L. A novel site of action of a high affinity A1 adenosine receptor antagonist. Biochem Biophys Res Commun. 1988 Jun 30;153(3):939–944. doi: 10.1016/s0006-291x(88)81318-4. [DOI] [PubMed] [Google Scholar]
- Ramkumar V., Stiles G. L. Reciprocal modulation of agonist and antagonist binding to A1 adenosine receptors by guanine nucleotides is mediated via a pertussis toxin-sensitive G protein. J Pharmacol Exp Ther. 1988 Sep;246(3):1194–1200. [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Schiemann W. P., Walther J. M., Buxton I. L. On the ability of endogenous adenosine to regulate purine nucleoside receptor binding of antagonists in smooth muscle membranes. J Pharmacol Exp Ther. 1990 Nov;255(2):886–892. [PubMed] [Google Scholar]
- Schubert P., Reddington M., Kreutzberg G. W. On the possible role of adenosine as a modulatory messenger in the hippocampus and other regions of the CNS. Prog Brain Res. 1979;51:149–165. doi: 10.1016/S0079-6123(08)61303-5. [DOI] [PubMed] [Google Scholar]
- Schwabe U., Trost T. Characterization of adenosine receptors in rat brain by (-)[3H]N6-phenylisopropyladenosine. Naunyn Schmiedebergs Arch Pharmacol. 1980 Sep;313(3):179–187. doi: 10.1007/BF00505731. [DOI] [PubMed] [Google Scholar]
- Shaw C. A., Wilkinson M. Receptor characterization and regulation in intact tissue preparations. Pharmacological implications. Biochem Pharmacol. 1994 Mar 29;47(7):1109–1119. doi: 10.1016/0006-2952(94)90381-6. [DOI] [PubMed] [Google Scholar]
- Stiles G. L. A1 adenosine receptor-G protein coupling in bovine brain membranes: effects of guanine nucleotides, salt, and solubilization. J Neurochem. 1988 Nov;51(5):1592–1598. doi: 10.1111/j.1471-4159.1988.tb01129.x. [DOI] [PubMed] [Google Scholar]
- Ströher M., Nanoff C., Schütz W. Differences in the GTP-regulation of membrane-bound and solubilized A1-adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol. 1989 Jul;340(1):87–92. doi: 10.1007/BF00169212. [DOI] [PubMed] [Google Scholar]
- van Calker D., Müller M., Hamprecht B. Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J Neurochem. 1979 Nov;33(5):999–1005. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]


